Abstract
Catch-up growth in adult, is increasingly recognized as an important causative factor for the extremely prevalent insulin resistance-related diseases especially in developing countries/territories. We aimed to investigate the alteration of bile acids level, phosphorylation and sumoylation of its interacting protein, bile acid receptor/farnesoid X receptor and their downstream signaling pathway, as well as insulin sensitivity and lipid profile in catch-up growth in adult rats. Male Sprague-Dawley rats were randomly allocated into four groups for two sampling points: caloric restriction group, catch-up growth in adult refed with normal chow and their normal chow controls for four or eight weeks (N4, N8 individually).We found that total serum bile acids and farnesoid X receptor phosphorylation increased without significant changes in farnesoid X receptor sumoylation and its downstream small heterodimer partner expression at the end of caloric restriction stage, while the visceral fat decreased and insulin resistance never occurred in these animals; After refeeding, total serum bile acids, farnesoid X receptor phosphorylation and sumoylation, as well as Cyp7a1, SREBP-1c mRNA levels were higher with significant decrease in small heterodimer partner expression, which is associated fat accumulation, and drastic insulin resistance in whole body and skeletal muscle. Our findings demonstrated that the fat accumulation and insulin resistance are associated with increases of bile acids, alteration of farnesoid X receptor phosphorylation, and sumoylation and its downstream signaling pathway. These changes of bile acids, farnesoid X receptor phosphorylation and sumoylation, as well as their downstream signaling might be of importance in the etiology of fat accumulation and insulin resistance in catch-up growth in adult.
Keywords: Farnesoid X receptor, phosphorylation, sumoylation, catch-up growth in adult, fat accumulation, insulin resistance
Introduction
Catch-up growth, a phenomenon that embodies an accelerated recovery after weight loss or growth retardation, is well recognized to increase risks for insulin resistance (IR)-related diseases in later life, which could occur in prenatal, childhood, or adult life.1 In recent decades, with the rapid economic development, developing countries/territories are in transition from an era of food scarcity to one of abundance and generally have undergone nutrition promotion, which is the most common cause of catch-up growth in adult (CUGA).2,3 Furthermore, these changes are associated with an increasingly widespread prevalence of IR-related diseases such as type 2 diabetes4,5 and considered as one of the most important causative factors for the epidemic IR-related diseases around the world.1,6
In our previous study, we found that visceral fat accumulation and IR developed shortly while refeeding after caloric restriction in CUGA.7,8 Actually, the etiology of fat accumulation and IR in CUGA is not totally clear, although it is reported that skeletal muscle (SkM) mitochondrial abnormality,9 suppressed thermogenesis10 and thrifty metabolism11 might be critically involved. CUGA is usually developed by refeeding after caloric restriction in animals and the energy intake in CUGA is essentially never more than that in its normal chow control.7,8 Therefore, the increased efficiency in nutrient absorption and energy storage is increasingly considered as the crucial causative factor in the pathogenesis of fat accumulation and IR, which are characteristics of CUGA.12 Lately, It is reported that bile acids (BAs) and its interacting protein, bile acid receptor/farnesoid X receptor (BAR/FXR), play a vital role in nutrient absorption and energy storage and were considered to be vital in the etiology of IR-related diseases.13 BAs are end products of cholesterol metabolism and contribute to the solubilization and absorption of lipids and fat-soluble vitamins. It is also well recognized that BAs function as signaling molecules and activate specific receptors, including FXR.14 BA-activated FXR induces hepatic expression of the small heterodimer partner (SHP) and affects its downstream signaling molecular involved in nutrient absorption and energy storage, such as Cyp7a1 and SREBP-1c. Moreover, FXR transcriptional activity is regulated by its protein modifications such as phosphorylation and sumoylation.15,16 The main objective of the present study is to report the detailed changes in BAs level, hepatic FXR phosphorylation and sumoylation, BA-activated FXR downstream signaling in liver, lipid profile, and IR in CUGA rats. We also tentatively postulate that the increase in fat accumulation and IR were due to the alteration of FXR phosphorylation and sumoylation, as well as its subsequent changes of downstream signaling pathway in liver.
Materials and methods
Animals and diets
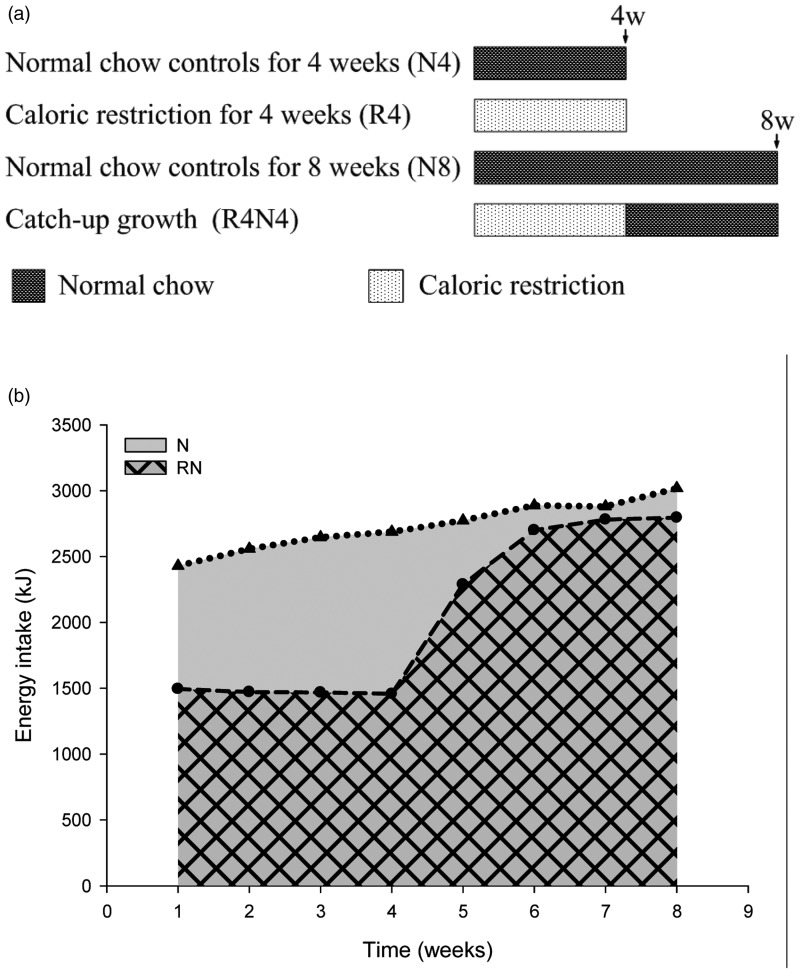
Male Sprague-Dawley rats (aged 6 weeks, weighing 140–180 g) were obtained from Laboratory Animal Center of Tongji Medical College, Huazhong University of Science and Technology. All rats were maintained in stainless steel cages individually, with free access to water and subjected to controlled lighting (lights on 06:00–18:00), temperature (22 ± 3℃) and relative humidity (50 ± 10%). All rats randomly allocated into four groups for two different sampling points with eight rats (N = 8) in each group as illustrated in Figure 1(a): R4 (maintained on caloric restriction for four weeks) and its controls N4 (fed ad libitum with normal chow for four weeks) for four-week sampling point; R4N4 (CUGA refed with weight-matched normal chow for four weeks after four weeks of caloric restriction) for eight-week sampling point, and N8 (fed ad libitum with normal chow for eight weeks as normal controls). They were fed with a standard rodent chow (13.68%, 64.44%, and 21.88% of calories derived respectively from fat, carbohydrate, and protein) as scheduled above (shown in Figure 1(a)) once per day, which was provided by the Laboratory Animal Center mentioned above. The food intake of CUGA rats was restricted to 60% of that eaten by their ad libitum-fed companions of the same weight while caloric restriction. The CUGA animals were pair-fed with their weight-matched, ad libitum-fed controls after refeeding as described previously.7,8 All the experimental procedures for the use of animals in research involved this study were approved by the Animal Ethics Committee of our university and in accordance with Laboratory Animal Care Guidelines of Hubei Province.
Figure 1.
Feeding regime (a) and energy intake (b). The catch-up growth in adult (CUGA) rats were developed by refeeding after caloric restriction. The controls (NC) were fed with normal chow diet ad libitum. The CUGA rats (RN) were fed as much as 60% of their weight-matched controls during caloric restriction and 100% of their weight-matched controls after refeeding (RN vs. NC)
Energy intake
Food intake of animals was measured every day in the evening as planned originally. The energy intake was calculated from the following formula
| (1) |
where Y is energy intake(kJ/day), X is food intake (g/day), and a is energy density (kcal/g).12
Body weight, Lee index, and body fat percentage
The body weight of all the rats was measured prior to the start of the hyperinsulinemic-euglycemic clamp. The nasoanal lengths were measured before their sacrifice. Lee index was calculated by the following equation12,17
| (2) |
where a is weight (g) and b is nasoanal length (mm), respectively.
Subcutaneous (groin), perirenal and epididymal white adipose tissues were carefully collected and weighed. The sum of perirenal and epididymal fat was considered as visceral AT.12 Body fat percentage of various AT compartments was calculated by dividing the weight of the determined fat per animal by the carcass weight.
Estimation of insulin sensitivity in whole body and SkM using hyperinsulinemic-euglycemic clamp in conscious rats based on tail artery and vein catheterization technique
After a 10- to 12-h overnight fast, a 120-min hyperinsulinemic-euglycemic clamp was performed in conscious rats described previously.18,19 In brief, tail artery and vein catheterizations were performed with intravenous integrated catheters filled with heparin-saline solution under local anesthesia. The venous catheter was prepared for intravenous infusion of insulin and glucose, while the arterial catheter for blood sampling. A prime-continuous infusion of human insulin (Novolin R, Novo Nordisk, Tianjin, China) was maintained at a rate of 0.25 U/kg/h to raise plasma insulin for 120 min, and 25% glucose solution was infused at variable rates and periodically adjusted to clamp the plasma glucose levels at approximately 4.5 mmol/L. The average glucose infusion rate between the 60th and 120th min (GIR60-120) was used to evaluate systemic insulin sensitivity. Fasting plasma glucose was determined using a glucose oxidase kit (Beijing Chemical Industry, Beijing, China).20 Plasma insulin level was estimated using a rat insulin ELISA kit (Linco Research, St Charles, MO, USA). In addition, IR was assessed with the HOMA-IR index, calculated using the following formula
| (3) |
where a is glucose (mmol/L) and b is insulin (mU/L).21,22
Insulin-mediated glucose transport activity was employed to estimate the insulin sensitivity in SkM as described previously. Briefly, in 2-deoxy-D-glucose (2-DG, sigma-Aldrich, St Louis, MO, USA) was administered as a bolus (2 mmol/kg in saline) through the venous cannula 45 min before the end of the clamps.23 The rats were sacrificed with a lethal dose (150 mg/kg) of intraperitoneally injected pentobarbitone sodium at the end of hyperinsulinemic-euglycemic clamp and 2-deoxyglucose uptake in SkM in vivo during hyperinsulinemic-euglycemic clamp was determined using a non-radioisotope enzymatic assay as described previously.7
Determination of serum triglyceride and BAs
Serum triglyceride (TG) was assayed as described previously.24 Blood for serum BAs analysis was drawn at 07:00–09:00, through the vena caudalis of the rats three days before the day of the hyperinsulinemia-euglycemia clamp in order to exclude the possible impact on the results of insulin sensitivity determination in these animals. The 3α-hydroxysteroid dehydrogenase method was employed for the analysis of total serum BAs. The reaction was determined at 546 nm using a chemistry analyzer.25
Reverse transcription and real-time PCR analysis
Total RNA was extracted according to the manufacturer’s protocol and RNA concentration was analyzed by spectrophotometry as described previously.7 The cDNA samples were prepared from 2 µg of total RNA sample. The real-time PCR amplification cycles were performed using oligonucleotide primers as follows:26,27 SHP, forward 5′-CTCGGTTTGCATACAGTGTTTGAC-3′ and reverse 5′-GCATATTGGCCTGGAGGTTTT; Cyp7a1, forward 5′-GCTTTACAGAGTGCTGGCCAA-3′ and reverse 5′-CTGTCTAGTACCGGCAGGTCATT-3′; SREBP-1c, forward 5′-GCGGACGCAGTCTGGG-3′ and reverse 5′-ATGAGCTGGAGCATGTCTTCAAA-3′; β-actin, forward 5′-ACGAGGCCCAGAGCAAGAG-3′ and reverse 5′-GGTGTGGTGCCAGATCTTCTC-3′. The expression levels of mRNA were assessed after normalization with β-actin as an endogenous control gene.
Immunoprecipitation and immunoblot Analysis
Tissue extracts were prepared and immunoprecipitation was performed as reported.28 Antibodies used for immunoprecipitation and immunoblot assays were obtained from Santa Cruz Biotechnology (FXR), Cell Signaling Technology (PKB/Akt, phospho-PKB/Akt (Ser473) and β-actin), or Abcam (SUMO-1, phosphoserine (phospho-Ser)).
Statistical analysis
Groups at the same sampling points were compared using two-tailed unpaired Student’s t-test with SPSS 11.5 software (SPSS, Chicago, IL). Results are presented as means ± S.E.M. and P values of less than 0.05 were considered statistically significant.
Results
Energy intake
The average energy intake of rats was ∼60% of that eaten by their weight-matched normal chow controls during CR and 100% after refeeding. However, the total energy intake of CUGA rats is much less than their controls (Figure 1(b)).
Body weight, Lee index, body fat percentage, and serum TG
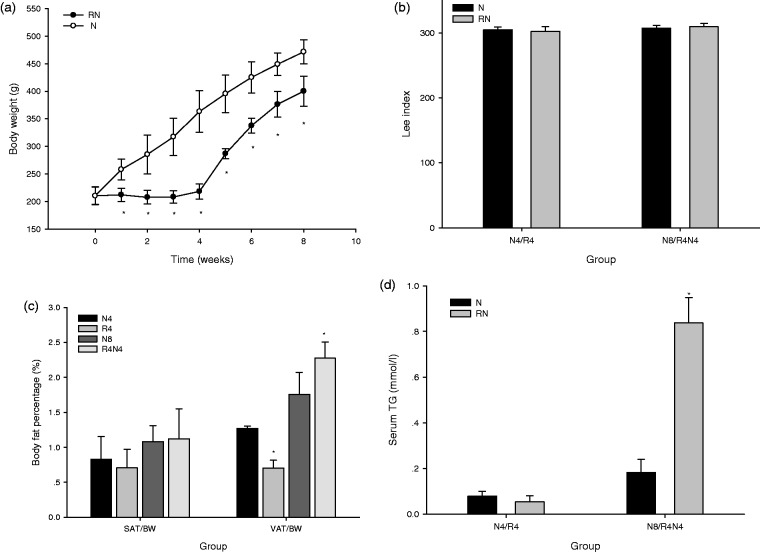
The body weight of rats in RN (during CR and after refeeding) was remarkably lower than their normal chow controls (P < 0.05) (Figure 2(a)). However, their Lee index showed no significant difference in R4 and R4N4 compared with their controls (Figure 2(b)). No obvious difference was found in the body fat percentage of subcutaneous AT (SAT/BW) in R4 and R4N4 compared with that in N4 and N8 (P > 0.05); on the contrary, the body fat percentage of visceral AT (VAT/BW) was notably lower in R4 than that in N4, while greater in R4N4 than that in N8 (P < 0.05) (Figure 2(c)). The serum TG was greater in R4N4 compared with N8 (P < 0.05) (Figure 2(d)).
Figure 2.
Body weight (a), Lee index (b), body fat percentage (c), and serum TG (d). The body weight in CUGA rats (RN) is remarkably lower than their age-matched controls (NC). No significant difference was observed in Lee index or the body fat percentage of subcutaneous AT (SAT/BW) in R4 compared with N4, in R4N4 compared with N8 (P > 0.05), while the body fat percentage of visceral AT (VAT/BW) was lower in R4 than that in N4 and greater in R4N4 than that in N8 (P < 0.05). The serum TG was greater in R4N4 than that in N8 (P < 0.05). (*P < 0.05 vs. age-matched normal chow controls, all data are mean ± SE)
Fasting plasma glucose, systemic and peripheral (SkM) insulin sensitivity
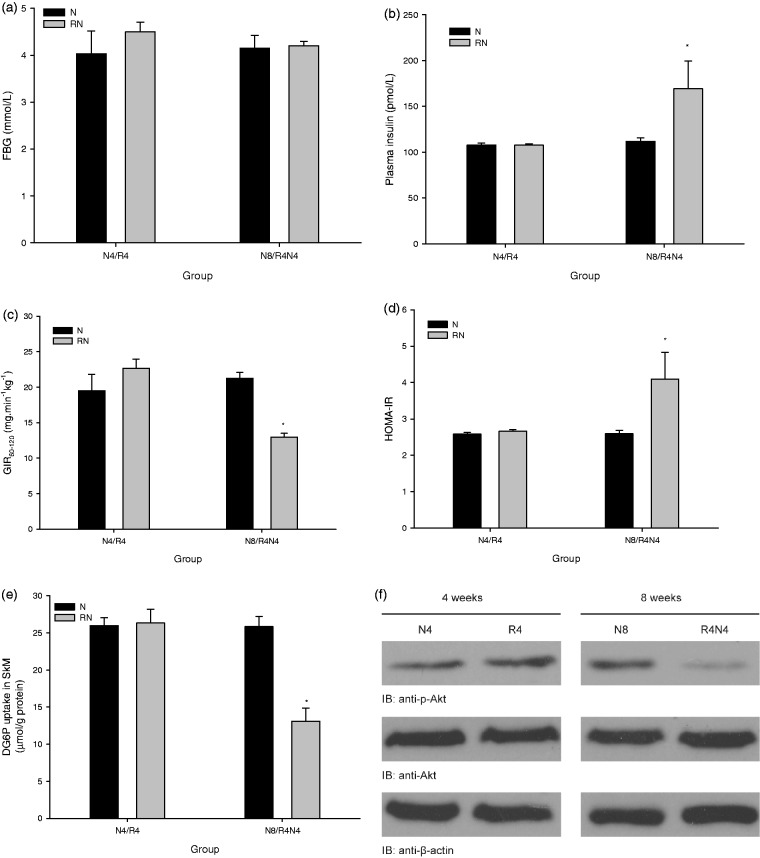
The fasting plasma glucose showed no significant difference in R4 and R4N4 compared with their controls in N4 and N8 (P > 0.05) (Figure 3(a)). The fasting plasma insulin (P < 0.05) (Figure 3(b)) and the HOMA-IR index (P < 0.05) (Figure 3(c)) increased, as well as the average GIR60-120 at hyperinsulinemic-euglycemic clamp decreased (P < 0.05) (Figure 3(d)) in R4N4 compared with N8, but they were similar in R4 to those in N4 (P > 0.05). We further found that the insulin-mediated 2-DG uptake (Figure 3(e)) and Ser473 phosphorylation of PKB/Akt (Figure 3(f)) in SkM in R4N4 were significantly lower compared with N8 (P < 0.05), while no significant difference was found in either of them between R4 and N4.
Figure 3.
Fasting plasma glucose, systemic and peripheral (SkM) insulin sensitivity. The fasting plasma glucose was similar in R4 and R4N4 compared with their controls in N4 and N8 (P > 0.05) (a). The fasting plasma insulin (b) and the HOMA-IR index (c) increased, as well as the average GIR60-120 at hyperinsulinemic-euglycemic clamp decreased (d) in R4N4 compared with N8 (P < 0.05), but they showed no significant difference in R4 compared with N4 (P > 0.05). The insulin-mediated 2-DG uptake (e) and Ser473 phosphorylation of PKB/Akt (f) in SkM were remarkably lower in R4N4 compared with N8 (P < 0.05), while no obvious difference was found between R4 and N4 (P > 0.05) (*P < 0.05 vs. age-matched normal chow controls, all data are mean ± SE)
Serum BAs level
The total serum BAs (Figure 4(a)) increased in R4 than that in N4 (P < 0.05). Additionally, this increase sustained after refeeding in R4N4 compared with N8 (P < 0.05).
Figure 4.
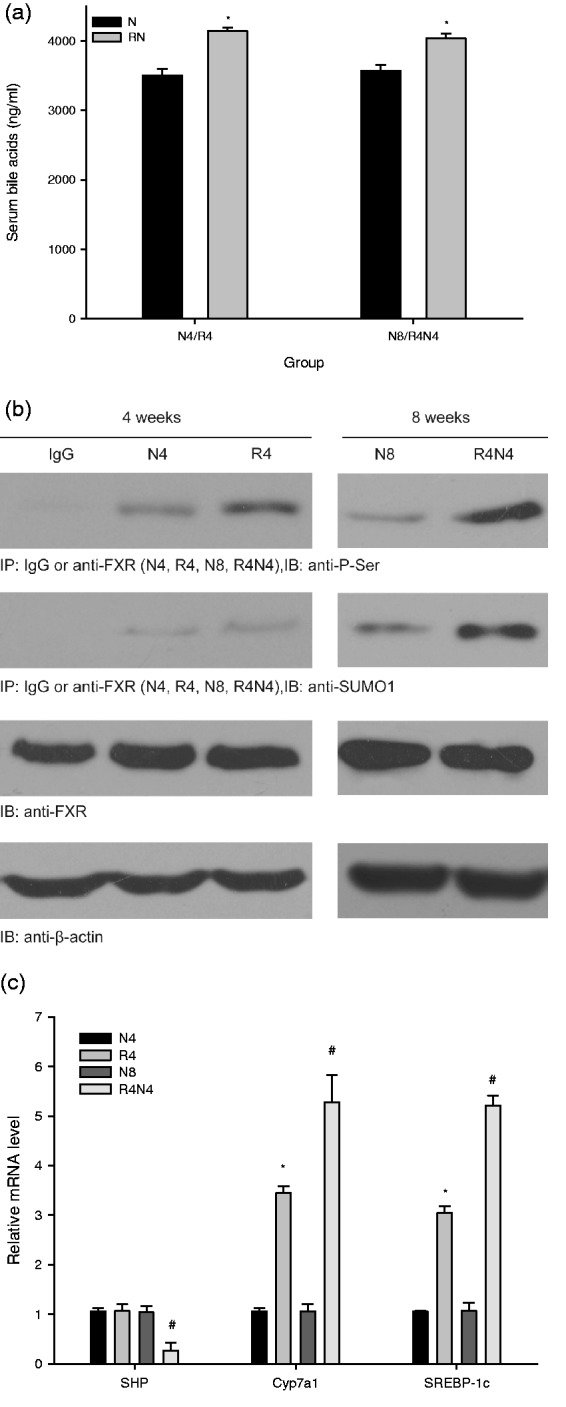
Alteration of the total serum bile acids (a), FXR sumoylation and phosphorylation (b), and the mRNA expression of SHP, Cyp7a1 and SREBP-1c (c) in liver. The total serum bile acids increased in R4 compared with N4 and in R4N4 compared with N8 (P < 0.05). FXR phosphorylation increased in R4 compared with N4 and was even higher in R4N4 compared with N8 (P < 0.05). FXR sumoylation elevated in R4N4 compared with N8 (P < 0.05), while no significant difference (P > 0.05) was observed in R4 compared with N4. The mRNA expression of SHP in liver was significantly downregulated in R4N4 compared with that in N8 (P < 0.05), without obvious changes (P > 0.05) in R4 compared with N4. Meanwhile, the mRNA expression of Cyp7a1 and SREBP-1c was dramatically up-regulated (P < 0.05) in R4 compared with N4 and remained even higher in R4N4 compared with N8 (P < 0.05) (*P < 0.05 vs. N4, #P < 0.05 vs. N8, all data are mean ± SE)
FXR sumoylation and phosphorylation in Liver
FXR phosphorylation was increased in R4 compared with N4 and became even higher in R4N4 compared with N8. Interestingly, FXR sumoylation was elevated in R4N4 compared with N8, while no significant different was observed in R4 compared with N4 (Figure 4(b)).
Alteration of SHP, Cyp7a1 and SREBP-1c mRNA expression level in liver
The mRNA expression level of SHP in liver was significantly downregulated in R4N4 compared with that in N8, without obvious changes in R4 compared with their age-matched controls (Figure 4(c)). Moreover, the mRNA expression level of Cyp7a1, SREBP-1c were dramatically up-regulated (P < 0.05) in R4 compared with N4, and remained even higher in R4N4 compared with N8 (P < 0.05).
Discussion
In this study, we showed that the total serum BAs and FXR phosphorylation increased without significant changes in FXR sumoylation and SHP expression at the end of caloric restriction stage, while the visceral fat decreased and IR never occurred in these animals. After refeeding, total serum BAs, FXR phosphorylation and sumoylation, as well as Cyp7a1, SREBP-1c mRNA levels were higher with significant decrease in SHP expression, which is associated with fat accumulation, and drastic IR in whole body and SkM.
We observed that total serum BAs level increased at the end of four weeks caloric restriction. Fu and Klaassen29 found that caloric restriction can cause increases in BAs level in mice, which is consistent with our findings. We further found that total serum BAs sustained in a high level after refeeding. It is reported that BAs are end-products of cholesterol metabolism in liver and of utmost importance in facilitating cholesterol elimination in liver and dietary fat absorption in intestine.15,29 Thus, the increases in BAs during CR and after refeeding might increase the capability of dietary fat absorption in intestine, which might be an important explanation for the fat accumulation in these animals after refeeding with sufficient nutrient supply but no fat deposition during CR because of undernutrition.
It is well known that the effects of BAs are mediated through the interaction of BAs with membrane or intracellular proteins, including FXR.15 FXR is a master regulator of lipid and glucose homeostasis and activated by primary and secondary BAs.30 The transcriptional activity of FXR is negatively regulated by its phosphorylation and sumoylation.15,16 We observed that FXR phosphorylation increased without significant changes in FXR sumoylation at the end of caloric restriction stage, when we did not find fat accumulation, IR, and any obvious changes in SHP expression. Fu and Klaassen29 found that CR increases Cyp7a1 expression and no significant changes in SHP expression were observed in their study, which is consistent with our present investigation. Herein, the concurrent increases in total BAs level and their probable positive effects to BA-activating FXR signaling, as well as the increase in FXR phosphorylation, might be an important explanation to the unchanged SHP expression. Our data indicated that Cyp7a1 mRNA expression increased mildly during CR, which is consistent with the findings reported by Fu, Z. D. et al. and might be the cause of elevation of BAs.29 Even though our results showed the SREBP-1c mRNA expression was up-regulated modestly during CR, the nutrient deficiency in this stage might be the key point of no fat accumulation and IR in these rats. Interestingly, in our study, no obvious alteration was observed in SHP mRNA but remarkable changes were found in Cyp7a1 and SREBP-1c mRNA levels during CR. That Cyp7a1 and SREBP-1c are regulated by complicated multifactor mechanisms might be an important explanation of the discrepancy.31 After refeeding, our observations demonstrated that FXR phosphorylation and sumoylation increased, SHP expression decreased, and Cyp7a1, SREBP-1c mRNA expression were upregulated significantly in liver, which were associated fat accumulation, and drastic IR in whole body and SkM. The changes of FXR-SHP signaling pathway could be explained by that the transcriptional activity of FXR is negatively regulated by its phosphorylation and sumoylation.15,16 As the rate limiting enzyme and the major site of regulation of bile acid synthesis, Cyp7a1 increased in CUGA and is likely to be the key cause of the elevation of BAs and their consequent facilitated energy absorption.13 Additionally, the increase of SREBP-1c, which is an important transcriptional regulator of lipogenesis, is probably crucial in the pathogenesis of fat accumulation in CUGA animals and plays a pivotal role in the consequent IR.32
Yet, it should be noted that for practical reasons involving the unavailability of agents, we were not able to determine energy absorption in intestine, as well as the activity of Cyp7a1 and SREBP1c in liver in time unfortunately, although their mRNA expression level are closely and positively correlates with their activity.33–35 Moreover, we did not test the methodologies involved in this report in a cross-sectional or longitudinal study in catch-up growth following economic transition from poverty to higher income status, in spite of the fact that the relevance between bile acid signaling pathways and obesity as well as its related IR is well recognized in recent years. Nevertheless, the alterations in bile acid signaling pathways probably leading to IR and fat accumulation observed in the refed rats might be relevant to obesity, IR and type 2 diabetes in catch-up growth population.
Notwithstanding these limitations, we found that the visceral fat accumulation and IR were associated with increases in BAs, FXR phosphorylation and sumoylation, and alteration of their downstream signaling pathway. Herein, we tentatively put forward the hypothesis that FXR phosphorylation and sumoylation, mediating elevation in Cyp7a1 and SREBP1c expression, is likely to be an important trigger for the fat accumulation and IR in CUGA rats.
Acknowledgements
We thank all those involved in this work, with particular gratitude to Dr. Hui-Qing Li, Xiu-Ling Deng, Wen-Fang Xia and Yun-Fei Liao for generous advice and fruitful discussions. This work was supported by National Natural Science Foundation of China (81471069, 81100562, 81170737, 81400820).
Authors contributions
All authors participated in the design and interpretation of the studies, analysis of the data and review of the manuscript; XH, QZ, JZ, WK, H-HZ, T-SZ, J-YZ, and JM conducted the experiments; L-LC, XH and CW wrote the manuscript. The authors had no conflict of interest to declare.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Chen LL, Yang WH, Zheng J, Zhang JY, Yue L. Influence of catch-up growth on islet function and possible mechanisms in rats. Nutrition 2011; 27: 456–62. [DOI] [PubMed] [Google Scholar]
- 2.van Abeelen AF, Elias SG, Bossuyt PM, Grobbee DE, van der Schouw YT, Roseboom TJ, Uiterwaal CS. Famine exposure in the young and the risk of type 2 diabetes in adulthood. Diabetes 2012; 61: 2255–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhutta ZA, Salam RA. Global nutrition epidemiology and trends. Ann Nutr Metab 2012; 61(Suppl 1): 19–27. [DOI] [PubMed] [Google Scholar]
- 4.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 2004; 27: 1047–53. [DOI] [PubMed] [Google Scholar]
- 5.Weigensberg MJ, Goran MI. Type 2 diabetes in children and adolescents. Lancet 2009; 373: 1743–44. [DOI] [PubMed] [Google Scholar]
- 6.Chen LL, Yang WH, Zheng J, Hu X, Kong W, Zhang HH. Effect of catch-up growth after food restriction on the entero-insular axis in rats. Nutr Metab (Lond) 2010; 7: 45–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hu X, Chen LL, Zheng J, Kong W, Zhang HH, Zeng TS, Zhang JY, Li HQ, Hu D, Liao YF. Increases in systemic and local stress: a probable mechanism of visceral fat accumulation and insulin resistance in adult catch-up growth rats? Exp Biol Med (Maywood) 2013; 238: 57–65. [DOI] [PubMed] [Google Scholar]
- 8.Chen LL, Hu X, Zheng J, Kong W, Zhang HH, Yang WH, Zhu SP, Zeng TS, Zhang JY, Deng XL, Hu D. Lipid overaccumulation and drastic insulin resistance in adult catch-up growth rats induced by nutrition promotion after undernutrition. Metabolism 2011; 60: 569–78. [DOI] [PubMed] [Google Scholar]
- 9.Crescenzo R, Lionetti L, Mollica MP, Ferraro M, D'Andrea E, Mainieri D, Dulloo AG, Liverini G, Iossa S. Altered skeletal muscle subsarcolemmal mitochondrial compartment during catch-up fat after caloric restriction. Diabetes 2006; 55: 2286–93. [DOI] [PubMed] [Google Scholar]
- 10.Dulloo AG. Regulation of fat storage via suppressed thermogenesis: a thrifty phenotype that predisposes individuals with catch-up growth to insulin resistance and obesity. Horm Res 2006; 65(Suppl 3): 90–7. [DOI] [PubMed] [Google Scholar]
- 11.Summermatter S, Marcelino H, Arsenijevic D, Buchala A, Aprikian O, Assimacopoulos-Jeannet F, Seydoux J, Montani JP, Solinas G, Dulloo AG. Adipose tissue plasticity during catch-up fat driven by thrifty metabolism: relevance for muscle-adipose glucose redistribution during catch-up growth. Diabetes 2009; 58: 2228–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Navarro-Alvarez N, Soto-Gutierrez A, Chen Y, Caballero-Corbalan J, Hassan W, Kobayashi S, Kondo Y, Iwamuro M, Yamamoto K, Kondo E, Tanaka N, Fox IJ, Kobayashi N. Intramuscular transplantation of engineered hepatic tissue constructs corrects acute and chronic liver failure in mice. J Hepatol 2010; 52: 211–9. [DOI] [PubMed] [Google Scholar]
- 13.Fang S, Suh JM, Reilly SM, Yu E, Osborn O, Lackey D, Yoshihara E, Perino A, Jacinto S, Lukasheva Y, Atkins AR, Khvat A, Schnabl B, Yu RT, Brenner DA, Coulter S, Liddle C, Schoonjans K, Olefsky JM, Saltiel AR, Downes M, Evans RM. Intestinal FXR agonism promotes adipose tissue browning and reduces obesity and insulin resistance. Nat Med 2015; 21: 159–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perino A, Schoonjans K. Another Shp on the horizon for bile acids. Cell Metab 2014; 20: 203–5. [DOI] [PubMed] [Google Scholar]
- 15.Lien F, Berthier A, Bouchaert E, Gheeraert C, Alexandre J, Porez G, Prawitt J, Dehondt H, Ploton M, Colin S, Lucas A, Patrice A, Pattou F, Diemer H, Van Dorsselaer A, Rachez C, Kamilic J, Groen AK, Staels B, Lefebvre P. Metformin interferes with bile acid homeostasis through AMPK-FXR crosstalk. J Clin Invest 2014; 124: 1037–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Balasubramaniyan N, Luo Y, Sun AQ, Suchy FJ. SUMOylation of the farnesoid X receptor (FXR) regulates the expression of FXR target genes. J Biol Chem 2013; 288: 13850–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Balbo SL, Grassiolli S, Ribeiro RA, Bonfleur ML, Gravena C, Brito Mdo N, Andreazzi AE, Mathias PC, Torrezan R. Fat storage is partially dependent on vagal activity and insulin secretion of hypothalamic obese rat. Endocrine 2007; 31: 142–8. [DOI] [PubMed] [Google Scholar]
- 18.Shang J, Chen LL, Xiao FX, Sun H, Ding HC, Xiao H. Resveratrol improves non-alcoholic fatty liver disease by activating AMP-activated protein kinase. Acta Pharmacol Sin 2008; 29: 698–706. [DOI] [PubMed] [Google Scholar]
- 19.Chen LL, Hu X, Zheng J, Zhang HH, Kong W, Yang WH, Zeng TS, Zhang JY, Yue L. Increases in energy intake, insulin resistance and stress in rats before Wenchuan earthquake far from the epicenter. Exp Biol Med (Maywood) 2010; 235: 1216–23. [DOI] [PubMed] [Google Scholar]
- 20.Chen L, Zhang J, Zhang Y, Wang Y, Wang B. Improvement of inflammatory responses associated with NF-kappa B pathway in kidneys from diabetic rats. Inflamm Res 2008; 57: 199–204. [DOI] [PubMed] [Google Scholar]
- 21.Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care 2004; 27: 1487–95. [DOI] [PubMed] [Google Scholar]
- 22.Maiztegui B, Borelli MI, Raschia MA, Del Zotto H, Gagliardino JJ. Islet adaptive changes to fructose-induced insulin resistance: beta-cell mass, glucokinase, glucose metabolism, and insulin secretion. J Endocrinol 2009; 200: 139–49. [DOI] [PubMed] [Google Scholar]
- 23.Yamamoto N, Kawasaki K, Sato T, Hirose Y, Muroyama K. A nonradioisotope, enzymatic microplate assay for in vivo evaluation of 2-deoxyglucose uptake in muscle tissue. Anal Biochem 2008; 375: 397–9. [DOI] [PubMed] [Google Scholar]
- 24.Chen LL, Zhang JY, Wang BP. Renoprotective effects of fenofibrate in diabetic rats are achieved by suppressing kidney plasminogen activator inhibitor-1. Vascul Pharmacol 2006; 44: 309–15. [DOI] [PubMed] [Google Scholar]
- 25.Kohli R, Setchell KD, Kirby M, Myronovych A, Ryan KK, Ibrahim SH, Berger J, Smith K, Toure M, Woods SC, Seeley RJ. A surgical model in male obese rats uncovers protective effects of bile acids post-bariatric surgery. Endocrinology 2013; 154: 2341–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Monte MJ, Fernandez-Tagarro M, Marin JJ. Transient changes in the expression pattern of key enzymes for bile acid synthesis during rat liver regeneration. Biochim Biophys Acta 2005; 1734: 127–35. [DOI] [PubMed] [Google Scholar]
- 27.Alberdi G, Rodriguez VM, Miranda J, Macarulla MT, Arias N, Andres-Lacueva C, Portillo MP. Changes in white adipose tissue metabolism induced by resveratrol in rats. Nutr Metab (Lond) 2011; 8: 29–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chan CB, Liu X, Jung DY, Jun JY, Luo HR, Kim JK, Ye K. Deficiency of phosphoinositide 3-kinase enhancer protects mice from diet-induced obesity and insulin resistance. Diabetes 2010; 59: 883–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fu ZD, Klaassen CD. Increased bile acids in enterohepatic circulation by short-term calorie restriction in male mice. Toxicol Appl Pharmacol 2013; 273: 680–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jiao Y, Lu Y, Li X. Farnesoid X receptor: a master regulator of hepatic triglyceride and glucose homeostasis. Acta Pharmacol Sin 2015; 36: 44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Song Y, Xu C, Shao S, Liu J, Xing W, Xu J, Qin C, Li C, Hu B, Yi S, Xia X, Zhang H, Zhang X, Wang T, Pan W, Yu C, Wang Q, Lin X, Wang L, Gao L, Zhao J. Thyroid-stimulating hormone regulates hepatic bile acid homeostasis via SREBP-2/HNF-4alpha/CYP7A1 axis. J Hepatol 2015; 62: 1171–9. [DOI] [PubMed] [Google Scholar]
- 32.Han J, Li E, Chen L, Zhang Y, Wei F, Liu J, Deng H, Wang Y. The CREB coactivator CRTC2 controls hepatic lipid metabolism by regulating SREBP1. Nature 2015; 524: 243–6. [DOI] [PubMed] [Google Scholar]
- 33.Gupta S, Stravitz RT, Dent P, Hylemon PB. Down-regulation of cholesterol 7alpha-hydroxylase (CYP7A1) gene expression by bile acids in primary rat hepatocytes is mediated by the c-Jun N-terminal kinase pathway. J Biol Chem 2001; 276: 15816–22. [DOI] [PubMed] [Google Scholar]
- 34.Lundasen T, Andersson EM, Snaith M, Lindmark H, Lundberg J, Ostlund-Lindqvist AM, Angelin B, Rudling M. Inhibition of intestinal bile acid transporter Slc10a2 improves triglyceride metabolism and normalizes elevated plasma glucose levels in mice. PLoS One 2012; 7: e37787–e37787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ponugoti B, Kim DH, Xiao Z, Smith Z, Miao J, Zang M, Wu SY, Chiang CM, Veenstra TD, Kemper JK. SIRT1 deacetylates and inhibits SREBP-1C activity in regulation of hepatic lipid metabolism. J Biol Chem 2010; 285: 33959–70. [DOI] [PMC free article] [PubMed] [Google Scholar]