Abstract
Baccharis trimera, popularly known as “carqueja”, is a native South-American plant possessing a high concentration of polyphenolic compounds and therefore high antioxidant potential. Despite the antioxidant potential described for B. trimera, there are no reports concerning the signaling pathways involved in this process. So, the aim of the present study was to assess the influence of B. trimera on the modulation of PKC signaling pathway and to characterize the effect of the nicotinamide adenine dinucleotide phosphate oxidase enzyme (NOX) on the generation of reactive oxygen species in SK Hep-1 cells. SK-Hep 1 cells were treated with B. trimera, quercetin, or rutin and then stimulated or not with PMA/ionomycin and labeled with carboxy H2DCFDA for detection of reactive oxygen species by flow cytometer. The PKC expression by Western blot and enzyme activity was performed to evaluate the influence of B. trimera and quercetin on PKC signaling pathway. p47phox and p47phox phosphorylated expression was performed by Western blot to evaluate the influence of B. trimera on p47phox phosphorylation. The results showed that cells stimulated with PMA/ionomycin (activators of PKC) showed significantly increased reactive oxygen species production, and this production returned to baseline levels after treatment with DPI (NOX inhibitor). Both B. trimera and quercetin modulated reactive oxygen species production through the inhibition of PKC protein expression and enzymatic activity, also with inhibition of p47phox phosphorylation. Taken together, these results suggest that B. trimera has a potential mechanism for inhibiting reactive oxygen species production through the PKC signaling pathway and inhibition subunit p47phox phosphorylation of nicotinamide adenine dinucleotide phosphate oxidase.
Keywords: PKC, nicotinamide adenine dinucleotide phosphate oxidase, reactive oxygen species, Baccharis trimera, quercetin, rutin
Introduction
Oxidative stress has been implicated in the onset and progression of many chronic diseases, such as cancer, diabetes, neurodegenerative, and cardiovascular diseases.1 Alterations in the redox state affect the signaling pathways for biological processes and damage cellular functions.2 Reactive oxygen species (ROS) are continuously produced as a by-product of cellular metabolism, playing an important role in cellular signaling and homeostasis.3 However, in excess, ROS production might exhibit noxious effects on cells through direct interactions with DNA, RNA, lipids, and proteins, and this damage has been associated with the etiology of several diseases.4,5
Exogenous antioxidants, synthetic or natural, have been used as alternative therapeutic approaches for treating stress-related diseases, as these compounds likely combat the damaging effects of ROS.6 The majority of natural antioxidants are polyphenols, which exhibit powerful antioxidant activity as reactive species scavengers and induce the gene expression of antioxidant enzymes.7,8
Baccharis trimera, popularly known as “carqueja”, is a native South-American plant, widely distributed in Argentina, Brazil, Paraguay, and Uruguay. This plant possesses a high concentration of polyphenolic compounds and therefore exhibits antioxidant potential.9 Among the phenolic compounds previously described for B. trimera, apigenin, isoquercetin, luteolin, nepetin, quercetin, 3-O-methylquercetin, and rutin have been reported.10
Despite the antioxidant potential described for B. trimera, there are no reports concerning the signaling pathways involved in this process. Nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, also called NOX in non-phagocytic cells, is the enzyme responsible for the production of ROS in various cell types.11–13 NOX is a multicomponent enzyme comprising different subunits: the subunits p22phox and gp91phox are located in the membrane, and the subunits p40phox, p47phox, and p67phox are located in the cytosol.14 The mechanisms involved in activation of NOX are complex and diverse; however, inhibiting the translocation of the cytosolic subunit to the membrane might lead to the inhibition of this enzyme. NOX activity might also be inhibited through the inhibition of p47phox subunit phosphorylation via PKC inhibitors.15 PKCs are serine/threonine protein kinases that play important roles in signal transduction, such as the activation of NOX.16 The PKC family can be divided into three classes (conventional, novel, and atypical). The conventional PKCs require diacylglycerol (DAG) and Ca2+ for activation. The activity of PKC can be pharmacologically modulated using exogenous components.17 In this context, calphostin C acts selectively on the regulatory domain of this enzyme, thereby inhibiting PKC activity.18 Phorbol esters, such as PMA, are DAG mimetics and PKC activators19; ionomycin is a Ca2+ ionophore,20 and together with PMA, this compound acts as an activator of conventional PKC. Polyphenols exhibit antioxidant activity and can inhibit NOX.15 Thus, the aim of the present study was to determine the phytochemical and cytotoxic profiles of B. trimera and investigate whether the inhibition of reactive oxygen species (ROS) mediated by B. trimera is dependent on PKC and/or NOX.
Experimental section
Reagents
Quercetin (#Q4951) and Rutin (#R5143); dimethylsulfoxide (DMSO) (#D5879) (Dulbecco’s Modified Eagle’s Medium (DMEM) (#D1152); Thiazolyl Blue Tetrazolium Bromide (MTT) (#M2128), Phorbol 12-myristate 13-acetate (PMA) (#P8139), Ionomycin calcium salt (#I3909), Calphostin C (#C6303), Diphenyleneiodonium chloride (DPI) (#D2926); Monoclonal anti-protein kinase C (#P5704); Policlonal anti-p47phox (#SAB4502810), Policlonal anti-phospho p47phox (#SAB4504289), Monoclonal anti β-actina (#A1978), anti-mouse IgG peroxidase (#A9044) and anti-rabbit IgG peroxidase (#A0545) were obtained from Sigma Aldrich (St Louis, MO). Amersham ECL Western Blotting Detection Reagent was obtained from GE HealthCare Life Sciences and Carboxy-H2DCFDA was obtained from Life Technologies.
Preparation of B. trimera hydroethanolic extract
The aerial parts of B. trimera were collected during August 2011 in the city of Ouro Preto, Minas Gerais, Brazil. The specimen, voucher number OUPR 22.127, was identified by Professor Viviane R Scanlon and deposited in the Herbarium José Badini – UFOP. The methodology for the extract preparation was based on the work of Grance et al.,21 with modifications according to Pádua et al.22 Briefly, the dry plant material (50 g) was immersed in water and 70% ethanol 1:1 (v/v) for 24 h. Subsequently, the solvent was evaporated in a rotavapor, and the crude extract was used to dilute the samples (10–100 µg ml−1) used in these experiments.
Preparation of quercetin and rutin solutions
Quercetin and rutin were first solubilized in DMSO and further dissolved in PBS (pH 7.3). These solutions were prepared at different concentrations (10–100 µM) on the day of experiment. The final concentration of DMSO in all experiments was 0.2%.
LC-DAD-ESI-MS analyses
The hydroethanolic extract was resuspended in a solution 98: 2 (methanol: water) and then filtered through syringe filter PVDV 0.20 µm. It was subsequently injected into the liquid chromatograph. Analyses were performed using an UPLC Acquity (Waters) ion trap mass spectrometer equipped with an atmospheric pressure chemical ionization (APCI) interface operated in the following conditions: positive and negative ion mode; capillary voltage, 3500 V; capillary temperature, 320℃; source voltage, 5 kV; vaporizer temperature, 320℃; corona needle current, 5 mA; and sheath gas, nitrogen, 27 psi. Analyses were run in the full scan mode (100–2000 Da). The ESI-MS/MS analyses were additionally performed in an UPLC Acquity (Waters) with helium as the collision gas, and the collision energy was set at 30 eV. Chromatographic separation was done on ACQUITY UPLC BEH RP-18 (1.7 µm, 50 × 2 mm i.d.) (Waters). The mobile phase consisted of water 0.1% formic acid (solvent A) and acetonitrile 0.1% formic acid (solvent B). The elution protocol was 0–11 min, linear gradient from 5% to 95% B. The flow rate was 0.3 mL min−1, and the sample injection volume was 4.0 µL. The UV spectra were registered from 190 to 450 nm. Mass spectrometry analysis was performed on quadrupole instrument fitted with an electrospray source in the negative mode (Figure 1). Ion spray voltage: −4 kV; orifice voltage: −60 V.
Figure 1.
RP-UPLC-DAD profiles of hydroethanolic extract of Baccharis trimera. Identified compounds: 1. Coumaroylquinic acid; 2. Coumaroylquinic acid; 3. 5-O-feruloylquinc acid; 4. 3-O-Isoferuloylquinc acid; 5. Coumaroylquinic acid; 6. 5-O-Isoferuloylquinc acid; 7. 6(8)-C-furanosyl-8(6)-C-hexosyl-flavone; 8. 6(8)-C-hexosyl-8(6)-C-furanosyl-flavone; 9. 3,4-di-O-caffeoylquinc acid; 10. 3,5-di-O-caffeoylquinc acid; 11. 4,5-di-O-caffeoylquinc acid; 12. Quercetin; 13. 3′,5-dihydroxy-4′,7-dimethoxyflavone; 14. 3′,5-dihydroxy-4′,6,7-trimethoxyflavone. Analysis conditions: see in experimental section
Cell culture
The SK Hep-1 cells were a kind gift from Dr. Miriam Martins Chaves from the Federal University of Minas Gerais. SK Hep-1 cells were grown in a monolayer culture in DMEM culture medium (Sigma) supplemented with 5% (v/v) bovine fetal serum (Invitrogen Co Ltd, Carlsbad, CA, USA), 100 IU penicillin ml−1, 100 µg streptomycin ml−1, and 0.25 µg amphotericin B ml−1 (Sigma). The cells were cultivated in 75 cm2 bottles and incubated at 37℃ in a humidified atmosphere of 5% CO2.
MTT assay
The MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay was performed according to the methods of Mosmann.23 Briefly, after pre-incubation with medium for 18 h, the SK Hep-1 cells (5.0 × 103/well) were incubated with B. trimera hydroethanolic extract (0–100 µg ml−1), quercetin or rutin (0–100 µM) for 12 h at 37℃ in a humidified atmosphere of 5% CO2. Subsequently, the medium was replaced with MTT solution (5.0 mg ml−1) and the plates were incubated for 1 h at 37℃. The MTT solution was removed, DMSO was added, and the absorbance was read at 570 nm (Biotek EL 808). The cell viability was assessed relative to the control (cells without any treatment – only medium).
Determination of ROS production
After confirming that B. trimera hydroethanolic extract, quercetin and rutin at the evaluated concentrations were not cytotoxic to SK Hep-1 cells using the MTT assay, the treatments for the subsequent analyses were performed.
SK Hep-1 cells were pre-incubated for two different times (30 min or 6 h) using three different concentrations of B. trimera (10, 25 and 50 µg ml−1), rutin or quercetin (10, 25, and 50 μM). After pre-incubation, the unstimulated cells were washed and used for further assays.
In order to verify that B. trimera and positive controls were capable of inhibiting ROS in a PKC-dependent manner, we used phorbol myristate acetate (PMA) and ionomycin as pathway activators. Thus, after washing, the cells were treated with PMA (50 nM), a DAG analog and potent activator of PKC, and ionomycin (100 nM), which increases the release of calcium, and incubated for an additional 30 min. Subsequently, the cells were washed 2× in Hanks solution and used in subsequent assays. The cells (5 × 105) were resuspended in DMEM and treated as previously described. Diphenyleneiodonium chloride (DPI) (20 µM), a NADPH oxidase inhibitor, was used as a negative control during the first incubation (30 min or 6 h). Carboxy-H2DCFDA (10 µM) was added to assess ROS production in all samples and incubated for 30 min during the last incubation (in combination with PMA and Ionomycin or alone). In the final washing, the supernatant was discarded, and the cells were resuspended in 200 µL of fixing solution. The data acquisition was performed using a BD FACSCalibur™ flow cytometer and CellQuest™ software. Fluorescence images were obtained to illustrate ROS production in SK Hep-1 cells. The cells were treated as described above, fixed with paraformaldehyde and analyzed using fluorescence microscopy.
Western blot analysis
Western blotting assay was performed to analyze PKC protein expression and p47phox and p47phox phosphorylated expression. The B. trimera hydroethanolic extract (50 µg ml−1) and quercetin (50 μM) were analyzed. A total of 5 × 106 cells were treated as previously described, and calphostin C (100 nM), a highly specific inhibitor of protein kinase C, was used as a negative control for PKC expression, during pre-incubation for 6 h. As negative control for p47phox phosphorylated, DPI was used as negative control. After pre-incubation, the cells were stimulated with PMA and ionomycin for 30 min. The cells were lysed in 500 µL of lysis buffer containing 1.0 mM Tris-HCl; 0.5 M EDTA; 5.0 M NaCl; DTT; Nonidet P40; protease inhibitor cocktail, and phosphatase inhibitor (Sigma-Aldrich, St. Louis, MO, USA - Sigma®). The samples were sonicated five times for 30 pulses, followed by centrifugation at 15.6 × g for 3 h. The supernatant was removed and used for protein measurement and preparation for gel electrophoresis. The BCA protein quantification method was used. A 50 µg protein sample was analyzed using 10% polyacrylamide gel electrophoresis, followed by transferred a nitrocellulose membrane according to Sambrook et al.,24 with modifications. Subsequently, membranes for PKC, for p47phox, and for p47phox phosphorylated were incubated with primary antibody (anti-protein kinase C, anti-p47phox, anti-phospho p47phox (pSer359) antibody, respectively) at a dilution factor of 1:1000 overnight at 5℃. Subsequently, membranes were washed with TBST (500 mmol l−1 NaCl, 20 mmol l−1 Tris-HCl and 0.4% Tween 20, pH 7.4) and incubated with the secondary antibody (anti-mouse IgG − peroxidase antibody for PKC analyze, and anti-rabbit IgG peroxidase antibody for p47phox analyses) for 1 h at 4℃. To visualize the bands, the membrane was exposed to Luminol Enhancer Solution (GE Healthcare, Buckinghamshire, United Kingdom) for 1 min, followed by a 30-s exposure to Hyperfilm-ECL (GE Healthcare) and the intensity of the bands was quantified using Quantity One software (Bio-Rad, Berkeley, California).
PKC activity
PKC activity was analyzed using the PKC kinase activity kit (Enzo Life Sciences – ADI-EKS-420 A). Briefly, the cells were treated as previously described and after centrifugation, lysis buffer (Tris-HCl 1.0 mM; EDTA 0.5 M; NaCl 5.0 M; DTT; Nonidet P40; Protease inhibitor cocktail; Sigma-Aldrich, St. Louis, MO, USA – Sigma®) was added to each sample, followed by sonication five times for 30 s. The samples were centrifuged for 3 h at 15.6 × g. The supernatant was removed and the proteins were quantified using BCA. The samples (30 µL) were added to wells of a PKC Substrate Microtiter Plate, and the reaction was initiated after adding 10 µL of diluted ATP to each well. The plate was incubated for 90 min at 30℃. Phosphospecific substrate antibody was added to wells, and the plate was incubated again for 60 min. The wells were washed four times with 100 µL of Wash Buffer, and anti-rabbit IgG:HRP-conjugated secondary antibody was added to each well, followed by incubation at room temperature for 30 min. The plate was washed four times, and TMB Substrate was added to each wells, followed by incubation for 30 min. Approximately 20 µL of acid stop solution was added, and the absorbance was measured at 450 nm using a microplate reader (Biotek EL 808).
Statistical analyzes
Two independent experiments were conducted in triplicate. The values are presented as the mean values and standard error. Statistical analysis was performed using analysis of variance (ANOVA), followed by Dunnett’s or Bonferroni’s post-test using Graph Prism (version 5.0). Statistical significance was considered at P < 0.05.
Results
LC-DAD-ESI-MS analysis of hydroethanolic extract
In the hydroethanolic extract of B. trimera, we identified five flavonoids and nine chlorogenic acids by LC-DAD-ESI-MS. The RP-UPLC-DAD fingerprint is shown in Figure 1. The structures of the identified compounds are shown in Figure 2 and their spectrum data are listed in Table 1.
Figure 2.
Identified compounds in hydroethanolic extract of Baccharis trimera by LC-DAD-ESI-MS
Caf: caffeoyl; Fer: feruloyl; Isofer: isoferuolyl; Coum: coumaroyl
Table 1.
Substances identified in the hydroethanolic extract of Baccharis trimera by LC-DAD-ESI-MS
| Peak | Compound | RT (min) | UV (nm) | LC-MS [M – H]- (m/z) (Fragmentation m/m) |
|---|---|---|---|---|
| 1 | Coumaroylquinic acid | 1.39 | 315 | 337.51 (191.3; 173.1; 163.3; 118.9) |
| 2 | Coumaroylquinic acid | 1.41 | 316 | 337.82 (191.2; 173.2; 136.8; 118.9) |
| 3 | 5-O-feruloylquinc acid | 1.95 | 322 | 367.37 (192.9; 173.4; 149.3; 133.9; 117.1) |
| 4 | 3-O-Isoferuloylquinc acid | 2.03 | 323 | 367.42 (193.1; 173.1; 136.8; 133.9) |
| 5 | Coumaroylquinic acid | 2.04 | 314 | 337.56 (191.1; 173.2; 163.1; 118.9) |
| 6 | 5-O-Isoferuloylquinc acid | 2.43 | 321 | 367.52 (191.3; 176.5; 155.4; 133.5; 127.2) |
| 7 | 6(8)-C-furanosyl-8(6)-C-hexosyl-flavone | 2.60 | 274; 331 | 563.37 (545.0; 503.0; 472.9; 453.9; 383.2; 359.9; 323.3; 295.9) |
| 8 | 6(8)-C-hexosyl-8(6)-C-furanosyl-flavone | 2.74 | 273; 326 | 563.31 (473.1; 442.7; 383.2; 353.1; 325.0; 282.0) |
| 9 | 3,4-di-O-caffeoylquinc acid | 2.81 | 324 | 515.26 (353.2; 191.1; 178.9; 172.8; 161.1; 154.8; 136.0) |
| 10 | 3,5-di-O-caffeoylquinc acid | 2.93 | 323 | 515.0 (323.2; 190.7; 178.6; 172.8; 161.1; 154.7; 135.0) |
| 11 | 4,5-di-O-caffeoylquinc acid | 3.20 | 325 | 515.07 (352.9; 191.1; 178.8; 173.1; 135.1) |
| 12 | Quercetin | 3.82 | 255; 369 | 301.23 (283.0; 255.3; 227.3; 191.4; 183.3; 181.1; 174.2; 150.7143.0; 120.8) |
| 13 | 3′,5-dihydroxy-4′,7-dimethoxyflavone | 5.32 | 282; 329 | 313.09 (298.0; 282.7; 270.6; 254.7; 183.0; 129.1; 99.2) |
| 14 | 3′,5-dihydroxy-4′,6,7-trimethoxyflavone | 5.50 | 275; 340 | 343.31 (328.1; 313.2; 297.9; 285.0; 269.8; 255.0) |
B. trimera hydroethanolic extract does not alter the viability of SK Hep-1 cells
The MTT assay showed no significant difference in the viability of SK Hep-1 cells treated with the hydroethanolic extract of B. trimera (Bt) at concentrations of 10, 25, and 50 µg mL−1, and the viability of these cells was maintained above 86%. At 100 µg mL−1, the B. trimera extract significantly reduced cell viability compared with the control. There was no significant difference in the viability of cells incubated with quercetin (Que) or rutin (Rut) at all concentrations evaluated, and these cells maintained viability greater than 86% (Figure 3).
Figure 3.
Viability of SK Hep-1 cells exposed to Baccharis trimera, quercetin and rutin for 12 h. The MTT assay was performed to analyze cell viability. The viability of the control group (cells not exposed to extract, quercetin, or rutin) was considered as 100%, and the other values were compared with the control group. The results are expressed as the means ± SEM. ***P < 0.001 for 100 µg mL−1 of Baccharis trimera hydroethanolic extract, which decreased cell viability. One-way ANOVA, followed by Bonferroni’s post-test was used for statistical analysis. This test was performed in sextuplicate from three independent experiments
Measurement of ROS generation with carboxy-H2DCFDA
B. trimera hydroethanolic extract inhibits ROS production in unstimulated cells
We examined the influence of B. trimera hydroethanolic extract and positive controls quercetin (Que) and rutin (Rut) on the modulation of ROS production in unstimulated cells (basal) at two incubation times, 30 min (Figure 4(a) to (c)) and 6 h (Figure 4(d) to (f)). The results show no significant difference in ROS production in cells incubated with 10 µg mL−1 B. trimera (Bt) hydroethanolic extract for 30 min. The same profile was observed for the positive controls, Que, and Rut (Figure 4(a)). However, a significant decrease in ROS production was observed in cells incubated with 25 µg mL−1 Bt or 25 µM Que (Figure 4(b)). Moreover, a significant decrease in ROS production was observed in cells incubated with 50 µg mL−1 Bt or 50 µM Que or Rut (Figure 4(c)). After incubation for 6 h, only 25 and 50 µg mL−1 concentrations of Bt or 50 µM Que were sufficient to inhibit ROS production (Figure 4(e) and (f)).
Figure 4.
ROS production in unstimulated SK Hep-1 cells. The results are expressed as the means ± SEM. (a–c) The cells were exposed to treatments (Baccharis trimera, quercetin and rutin) for 30 min. (d–f) The cells were treated with Baccharis trimera, quercetin or rutin for 6 h. This test was performed in sextuplicate from three independent experiments. *P < 0.05, **P < 0.001, ***P < 0.0001 for values significantly different from C (control cells)
B. trimera hydroethanolic extract inhibits ROS production through the PKC pathway
In order to evaluate the influence of the PKC pathway on ROS production, the cells were incubated with the PKC activators, PMA (50 nM), and ionomycin (100 nM). The results showed that cells incubated with PMA and ionomycin showed an increase in ROS production compared with the respective control (unstimulated cells) (Figure 5(a) to (f)). To evaluate the influence of NOX on ROS production, the cells were incubated with DPI (20 µM), a NADPH oxidase inhibitor. The results showed a significant reduction in ROS production compared with stimulated cells (PMA + iono) (Figure 5(a) to (f)), demonstrating the importance of the NOX for the stimulation of ROS production through the PKC pathway.
Figure 5.
Influence of the PKC/NADPH oxidase pathway in ROS production in SK Hep-1 cells. The results are expressed as the means ± SEM. (a–c) The cells were exposed to treatments for 30 min and stimulated with PMA + Iono for 30 min. (d–f) The cells were treated with Baccharis trimera, quercetin or rutin for 6 h and incubated for 30 min with PMA + iono. This test was performed in sextuplicate from three independent experiments. *P < 0.05 for values significantly different from C (control cells, without stimulus). #P < 0.05, ##P < 0.001, ###P < 0.0001 for values significantly different from C (PMA + Iono). (g) Cells stained with Carboxy- H2DCFDA and visualized through fluorescence microscopy. Cells – SK Hep-1 without fluorescence. ROS – cells analyzed with fluorescence labeled with Carboxy-H2DCFDA. C: Control cells; PMA/iono: positive control cells (C + PMA/Iono). DPI: Negative control cells (C + DPI + PMA/Iono). Bt50: Baccharis trimera-treated cells stimulated with PMA + Iono. Que50: Quercetin-treated cells stimulated with PMA + iono. (A color version of this figure is available in the online journal.)
In order to assess the effect of B. trimera hydroethanolic extract on the modulation of ROS production through PKC, the cells were pre-incubated with B. trimera extract (10, 25, or 50 µg mL−1), quercetin, or rutin (positive controls; 10, 25 or 50 µM), followed by incubation with PMA + ionomycin (PKC activators). No significant difference in ROS production was observed in cells incubated with Bt, Que, or Rut at any concentration for 30 min (Figure 5(a) to (c)). However, after incubation for 6 h, we observed a significant reduction in ROS production in cells pre-incubated with Bt (50 µg mL−1) or Que (25 and 50 µM) (Figure 5(e) and (f)) and then stimulated with PMA + ionomycin. Panel G shows fluorescence microscopy images of SK-Hep 1 cells treated with hydroethanolic extract of B. trimera, qualitatively demonstrating its effect in reducing ROS production. We observe an increase in fluorescence in cells stimulated with PMA + ionomycin compared with that in control cells (unstimulated). When cells are pre-incubated with DPI, we observed a decrease in fluorescence compared with that in the experimental group. The same effect was observed in relation to the hydroethanolic extract (50 µg mL−1) of B. trimera and quercetin (50 μM), thus confirming the effect of these compounds on the modulation of ROS via PKC.
B. trimera hydroethanolic extract inhibits PKC protein expression and PKC activity
PKC is responsible for p47phox phosphorylation and NOX activation, with a consequent increase in ROS production. In the present study, we demonstrated that SK Hep-1 cells incubated with PMA/iono (activators for conventional PKC) showed increased PKC protein expression, although this increase was not statistically significant, and cells incubated with calphostin C (selective inhibitors of the PKC) showed a significant decrease in PKC expression. Moreover, treatment with Bt (50 µg mL−1) and Que (50 µM) significantly decreased the expression of this protein to levels similar to those observed for the negative control (Figure 6(a)). The results showed that treatment with PMA+iono significantly increased PKC activity, whereas calphostin C significantly inhibited the activity of this kinase. Surprisingly, Bt (50 µg mL−1) and Que (50 µM) significantly inhibited PKC activity (Figure 6(b)).
Figure 6.
Reduction of PKC protein expression and inhibition of PKC activity through Baccharis trimera extract and quercetin. (a) Densitometric scanning of PKC after normalization with β-actin. Results of the Western blotting assay showing PKC activation through PMA + Ionomycin in SK Hep-1 cells and the effect of Baccharis trimera hydroethanolic extract and quercetin on this protein. *P < 0.05 for values significantly different from C (PMA + iono). The analyses were performed using Quantity One image analysis software (Bio Rad). (b) PKC activity in cells exposed to Baccharis trimera extract and quercetin, followed by stimulation with PMA + ionomycin was commercially assessed using a kit purchased from Enzo Life Sciences. As a positive control, PKC was supplied in the kit. #P < 0.0001 for values significantly different from C (control cells); **P < 0.001 and ***P < 0.0001 for values significantly different from C (PMA + Iono). The results are expressed as the means ± SEM. Statistically significant differences were determined using One-way ANOVA and Dunnett’s post-test. These analyses were performed in triplicate
B. trimera hydroethanolic extract inhibits p47phox phosphorylation
As mentioned above, the phosphorylation of p47phox subunit is required for activation of NOX enzyme, increasing the production of intracellular ROS. In addition to evaluating the involvement of PKC pathway in enzyme activation, we also evaluated the influence of B. trimera extract on p47phox phosphorylation. It was observed that B. trimera extract did not alter p47phox expression (Figure 7(a)), however was able to decrease the expression of p47phox phosphorylated, showing that B. trimera (50 µg mL−1), as quercetin (50 µM), its positive control, also influence the phosphorylation of p47phox subunit (Figure 7(b)), decreasing NOX activation.
Figure 7.
p47phox and p47phox phosphorylated expression in SK Hep-1 cells exposed to Baccharis trimera and quercetin. (a) Densitometric scanning of p47phox and phospho p47phox after normalization with β-actin. Results of Western blotting assay showing that p47phox expression are not altered by PMA + iono exposure nor with Baccharis trimera and quercetin treatments for 6 h. (b) p47phox phosphorylated expression was reduced by Baccharis trimera and quercetin treatments. *P < 0.05 for values significantly different from C (PMA + iono). The analyses were performed using Quantity One image analysis software (Bio Rad). The results are expressed as the means ± SEM. Statistically significant differences were determined using One-way ANOVA and Dunnett’s post-test. These analyses were performed in triplicate
Discussion
B. trimera plants are rich in phenolic compounds, including quercetin and rutin.10 Analysis by LC-DAD-ESI-MS, realized in the hydroethanolic extract, detected quercetin, flavones (6(8)-C-furanosyl-8(6)-C-hexosyl-flavone, 6(8)-C-hexosyl-8(6)-C-furanosyl-flavone, 3′,5-dihydroxy-4′,7-dimethoxyflavone, 3′,5-dihydroxy-4′,6,7-trimethoxyflavone) and chlorogenic acids (coumaroylquinic acids, 5-O-feruloylquinc acid, 3-O-Isoferuloylquinc acid, 5-O-Isoferuloylquinc acid, 3,4-di-O-caffeoylquinc acid; 3,5-di-O-caffeoylquinc acid; 11. 4,5-di-O-caffeoylquinc acid) (Table 1 and Figure 2). Therefore, in the present study, we used quercetin and rutin compounds as positive controls for B. trimera hydroethanolic extract. Despite the antioxidant potential of B. trimera, toxic effects have been reported for different plant extracts.21,25 The results of the present study showed that Bt exhibited no cytotoxic effect at any concentration after incubation for 12 h. Nogueira et al.26 also did not observe the cytotoxic effects of the ethanol extract of B. trimera on rat hepatoma cells and human embryo kidney epithelial cells after incubation for 72 and 96 h, respectively. Only a fraction of the aqueous extract induces cytotoxic effects at high doses in kidney cells. Similarly, quercetin and rutin showed no cytotoxic effects at any concentration evaluated. Yang et al.27 observed a decrease in the viability of rat brain microvessel endothelial cells (BMECs) with high concentrations of quercetin (500 µM) and rutin (750 µM). Thus, all concentrations below these values were considered safe, consistent with the results presented herein.
B. trimera (25 and 50 µg mL−1), quercetin (25 and 50 µM), and rutin (50 µM) inhibited basal ROS production after incubation for 30 min, and after 6 h, the inhibition of ROS production was only observed with high concentrations of Bt (25 and 50 µg mL−1) and Que (50 µM). B. trimera exhibits excellent potential to modulate ROS production compared with quercetin and rutin. These results suggest that the inhibitory effect of this plant on the ROS production is associated with the synergic effect of the components of the extract, such as quercetin and other flavones. Some antioxidant activities of plants have been attributed to other unidentified compounds or synergistic effects between the constituents of the plant extracts.28
Moreover, the modulation of ROS production in unstimulated cells might reflect mechanisms of direct neutralization. Indeed, the neutralization of free radicals of flavonoids has been associated with hydrogen donation.29
However, the main goal of the present study was to elucidate whether B. trimera extract modulates ROS production through the inhibition of cellular signaling pathways, PKC, and NOX. The results of the present study showed that SK Hep-1 cells incubated with PMA/iono (PKC activators) exhibited increased ROS production via PKC signaling, which once activated, can promote the activation of NOX. To confirm the hypothesis that the most of ROS production in SK Hep-1 cells is dependent on NOX, the cells were incubated with DPI, an inhibitor of this enzyme. The results showed a significant decrease in ROS production, and these values were similar to those observed in unstimulated cells (basal). Pre-treatment with Bt and Que was effective to modulate ROS production in PMA/iono-treated cells.It is known that quercetin and related flavonoids are broad protein kinase inhibitors, including PKC30; thus, we elucidated the effects of B. trimera and quercetin on the PKC signaling pathway. Romero et al.31 found that quercetin decreases ROS production through the inhibition of PKC activity. Other studies have demonstrated that quercetin diminished the activation of PKC and ROS in HepG2 cells.32 Thus, several studies suggest the involvement of quercetin in modulating of expression and/or activity PKC and NOX; however, there is no report in the literature about the possible signaling mechanisms of B. trimera responsible for the reduction of ROS. In our study, we demonstrated for the first time that B. trimera was able to inhibit protein expression and the activity of the PKC enzyme. In addition, our goal was to verify that the B. trimera was also able to down-regulate subunit p47phox phosphorylation of NOX without modifying the expression level of this subunit, elucidating a possible signaling pathway responsible for the antioxidant activity of B. trimera.
Thus, the results of the present study provide the first evidence that B. trimera decreased the protein expression and enzymatic activity of PKC as a potential mechanism for the inhibition of NOX activity through of down-regulation p47phox phosphorylation and consequently ROS production.
Conclusion
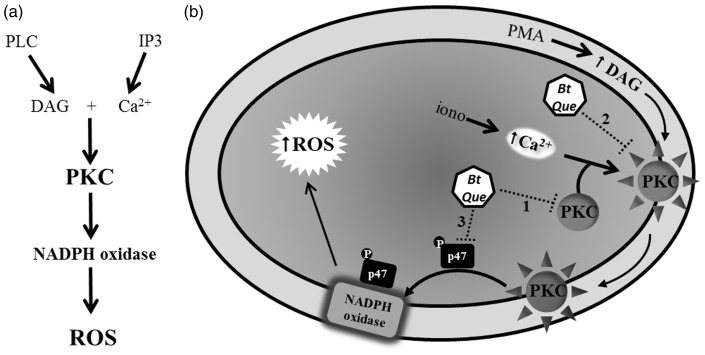
This study provides the first evidence of a signaling mechanism triggered through B. trimera to modulate ROS production. The inhibitory effect of B. trimera on ROS can be explained by two distinct mechanisms, which may or not generate synergistic effects. The first mechanism involves the inhibition of PKC protein expression and activity, and the second mechanism is associated with the down-regulation p47phox phosphorylation of NOX (Figure 8).
Figure 8.
Proposed mechanisms for the antioxidant effect of Baccharis trimera. (a) Represents the production of ROS through the PKC/NADPH oxidase signaling pathway. (b) Represents the mechanism of the Baccharis trimera-mediated modulation of ROS production. (1) The inhibition of PKC protein expression; (2) the inhibition of enzyme activity; and (3) down-regulation of p47phox phosphorylation. PLC: phospholipase C; IP3: inositol-1,4,5-trisphosphate; DAG: diacylglycerol; PKC: protein kinase C; Bt: Baccharis trimera; Que: quercetin; iono: ionomycin; PMA: phorbol ester
Acknowledgments
This research was supported through funding from the Foundation for Research of the State of Minas Gerais (FAPEMIG), National Council for Scientific and Technological Development (CNPq), Top Level Personnel Improvement Coordination (CAPES- PNPD), Phytochemistry Laboratory and Pharmaceutical Biology of the Faculty of Pharmacy, Federal University of Minas Gerais (UFMG) and Federal University of Ouro Preto (UFOP), Brazil.
Authors’ contributions
The corresponding author and all of the authors have read and approved the final submitted manuscript. All authors participated in the design, interpretation of the studies, and analysis of the data of the manuscript; Araújo, GR realized with other authors all the experiments, analyses, and writing. RACS, MJS, R-JJV, BMA, S-LD helped with experiments, G-SR and C-BW provided specific reagents and materials. SGHB and BGC realized the characterization of extract. CMM helped with reagents and supported the project. CDC is the leader of the project and wrote the manuscript.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.McCord JM. The evolution of free radicals and oxidative stress. Am J Med 2000; 108: 652–9. [DOI] [PubMed] [Google Scholar]
- 2.Tobwala S, Fan W, Hines CJ, Folk WR, Ercal N. Antioxidant potential of Sutherlandia frutescens and its protective effects against oxidative stress in various cell cultures. BMC Comp Altern Med 2014; 29: 271–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim BH, Choi JS, Yi EH, Lee JK, Won C, Ye SK, Kim MH. Relative antioxidant activities of quercetin and its structurally related substances and their effects on NF-κB/CRE/AP-1 signaling in murine macrophages. Mol Cells 2013; 35: 410–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee DJ, Kang SW. Reactive oxygen species and tumor metastasis. Mol Cells 2013; 35: 93–8. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 5.Rahman T, Hosen I, Islam M, Shekhar H. Oxidative stress and human health. Adv Biosci Biotechnol 2012; 3: 997–1019. [Google Scholar]
- 6.Papas AM. Diet and antioxidant status. Food and Chem Toxicol 1999; 37: 999–1007. [DOI] [PubMed] [Google Scholar]
- 7.Rice-Evans C. Flavonoid antioxidants. Cur Med Chem 2001; 8: 797–807. [DOI] [PubMed] [Google Scholar]
- 8.Krinsky NI. Mechanism of action of biological antioxidants. Proc Soc Exp Biol Med 1992; 200: 248–54. [DOI] [PubMed] [Google Scholar]
- 9.Miraballes M, Gámbaro A, Ares G. Sensory characteristics of antioxidant extracts from Uruguayan native plants: influence of deodorization by steam distillation. Food Sci Technol Intern 2013; 19: 485–92. [DOI] [PubMed] [Google Scholar]
- 10.Soicke H, Leng-Peschlow E. Characterization of flavonoids from Baccharis trimera and their antihepatotoxic properties. Planta Medica 1987; 53: 37–9. [DOI] [PubMed] [Google Scholar]
- 11.Meier B, Cross AR, Hancock JT, Kaup FJ, Jones OT. Identification of a superoxide-generating NADPH oxidase system in human fibroblasts. Biochem J 1991; 275: 241–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paik Y, Brenner DA. NADPH oxidase mediated oxidative stress in hepatic fibrogenesis. Korean J Hepatol 2011; 17: 251–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schramm A, Matusik P, Osmenda G, Guzik TJ. Targeting NADPH oxidases in vascular pharmacology. Vasc Pharmacol 2012; 56: 216–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leung PS, Chan YC. Role of oxidative stress in pancreatic inflammation. Antioxid Redox Signal 2009; 11: 135–65. [DOI] [PubMed] [Google Scholar]
- 15.Maraldi T. Natural compounds as modulators of NADPH oxidases. Oxid Med Cell Longev 2013; 2013: Article 271602–Article 271602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geraldes P, King GL. Activation of protein kinase C isoforms and its impact on diabetic complications. Circul Res 2010; 106: 1319–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abrial E, Lucas G, Scarna H, Haddjeri N, Lambás-Señas L. A role for the PKC signaling system in the pathophysiology and treatment of mood disorders: involvement of a functional imbalance? Mol Neurobiol 2011; 44: 407–19. [DOI] [PubMed] [Google Scholar]
- 18.Hofmann J. The potential for isoenzyme-selective modulation of protein kinase C. FASEB J 1997; 11: 649–69. [DOI] [PubMed] [Google Scholar]
- 19.Macrae K, Stretton C, Lipina C, Blachnio-Zabielska A, Baranowski M, Gorski J, Marley A, Hundal HS. Defining the role of DAG, mitochondrial function, and lipid deposition in palmitate-induced proinflammatory signaling and its counter-modulation by palmitoleate. J Lipid Res 2013; 54: 2366–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dang M, Dubbin K, D'Aiello A, Hartmann M, Lodish H, Herrlich A. Epidermal growth factor (EGF) ligand release by substrate specific a disintegrin and metalloproteases (ADAMs) involves different protein kinase C (PKC) isoenzymes depending on the stimulus. J Biol Chem 2011; 286: 17704–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grance SRM, Teixeira MA, Leite RS, Guimarães EB, de Siqueira JM, de Oliveira Filiu WF, de Souza Vasconcelos SB, do Carmo Vieira M. Baccharis trimera: effect on hematological and biochemical parameters and hepatorenal evaluation in pregnant rats. J Ethnopharmacol 2008; 117: 28–33. [DOI] [PubMed] [Google Scholar]
- 22.Pádua BC, Silva LD, Rossoni Junior JV, Humberto JL, Chaves MM, Silva ME, Pedrosa ML, Costa DC. Antioxidant properties of Baccharis trimera in the neutrophils of Fisher rats. J Ethnopharmacol 2010; 129: 381–6. [DOI] [PubMed] [Google Scholar]
- 23.Mosmann T. Rapid colorimetric assay for cellular growth and survival. Application to proliferation and cytotoxicity assays. J Immunol Meth 1983; 6: 55–63. [DOI] [PubMed] [Google Scholar]
- 24.Sambrook J, Fritschi EF, Maniatis T. Molecular cloning: a laboratory manual, New York: Cold Spring Harbor Laboratory Press, 1989. [Google Scholar]
- 25.Rodrigues CRF, Dias JH, Mello R, Richter MF, Picada JN, Ferraz AB. Genotoxic and antigenotoxic properties of Baccharis trimera in mice. J Ethnopharmacol 2009; 125: 97–101. [DOI] [PubMed] [Google Scholar]
- 26.Nogueira NP, Reis PA, Laranja G, Pinto AC, Aiub CA, Felzenszwalb I, Paes MC, Bastos FF, Bastos VL, Sabino KC, Coelho MG. In vitro and in vivo toxicological evaluation of extract and fractions from Baccharis trimera with anti-inflammatory activity. J Ethnopharmacol 2011; 138: 513–22. [DOI] [PubMed] [Google Scholar]
- 27.Yang Y, Bai L, Li X, Xiong J, Xu P, Guo C, Xue M. Transport of active flavonoids, based on cytotoxicity and lipophilicity: an evaluation using the blood-brain barrier cell and Caco-2 cell models. Toxicol In Vitro 2014; 28: 388–96. [DOI] [PubMed] [Google Scholar]
- 28.Komolafe K, Olaleye TM, Omotuyi OI, Boligon AA, Athayde ML, Akindahunsi AA, Teixeira da Rocha JB. In vitro antioxidant activity and effect of Parkia biglobosa bark extract on mitochondrial redox status. J Acupunct Meridian Stud 2014; 7: 202–10. [DOI] [PubMed] [Google Scholar]
- 29.Burda S, Oleszek W. Antioxidant and antiradical activities of flavonoids. J Agricult Food Chem 2001; 49: 2774–9. [DOI] [PubMed] [Google Scholar]
- 30.Middleton E, Jr, Kandaswami C, Theoharides TC. The effects of plat flavonoids on mammalian cells implications for inflammation, heart-disease, and cancer. Pharmacol Rev 2000; 52: 6673–751. [PubMed] [Google Scholar]
- 31.Romero M, Jiménez R, Sánchez M, López-Sepúlveda R, Zarzuelo MJ, O'Valle F, Zarzuelo A, Pérez-Vizcaíno F, Duarte J. Quercetin inhibits vascular superoxide production induced by endothelin-1: role of NADPH-oxidase, uncoupled eNOS and PKC. Atherosclerosis 2009; 202: 58–67. [DOI] [PubMed] [Google Scholar]
- 32.Maurya AK, Vinayak M. Anticarcinogenic action of quercetin by downregulation of phosphatidylinositol 3-kinase (PI3K) and protein kinase C (PKC) via induction of p53 in hepatocellular carcinoma (HepG2) cell line. Mol Biol Rep 2015; 42: 1419–29. [DOI] [PubMed] [Google Scholar]