Abstract
Duck hepatitis A virus (DHAV) (Picornaviridae) causes an infectious disease in ducks which results in severe losses in duck industry. However, the proper antiviral supportive drugs for this disease have not been discovered. Polysaccharide is the main ingredient of Astragalus that has been demonstrated to directly and indirectly inhibit RNA of viruses replication. In this study, the antiviral activities of Astragalus polysaccharide (APS) and its derivatives against DHAV were evaluated and compared. APS was modified via the sodium trimetaphosphate and sodium tripolyphosphate (STMP-STPP) method and chlorosulfonic acid-pyridine method to obtain its phosphate (pAPS) and sulfate (sAPS), respectively. The infrared structures of APS, pAPS, and sAPS were analyzed with the potassium bromide disc method. Additionally, the antiviral activities were evaluated with the MTT ((4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide) method in vitro and the artificial inoculation method in vivo. The clinical therapy effects were evaluated by mortality rate, liver function-related biochemical indicators, and visual changes in pathological anatomy. The anti-DHAV proliferation effects of APS, pAPS, and sAPS on the viral multiplication process in cell and blood were observed with the reverse transcription-polymerase chain reaction method. The results revealed that pAPS inhibited DHAV proliferation more efficiently in the entire process of viral multiplication than APS and sAPS. Moreover, only pAPS significantly improved the survival rate to 33.5% and reduced the DHAV particle titer in the blood as well as liver lesions in clinical trials. The results indicated that pAPS exhibited greater anti-DHAV activity than APS and sAPS both in vitro and in vivo.
Keywords: Duck hepatitis A virus, Astragalus membranaceus, polysaccharide, phosphorylated polysaccharide, sulfated polysaccharide, antiviral activity
Introduction
Duck hepatitis A virus (DHAV) caused an acute, contagious, rapidly spreading, and highly lethal infectious disease that was first recorded in New York, USA in 1949.1 Currently, this disease remains one of the most severe diseases in the duck industry. In traditional Chinese medicine, polysaccharides are among the most important active ingredients that exhibit antiviral activity. Recent efforts have primarily been dedicated to the development of polysaccharides and their derivatives that function as inhibitors of RNA viruses.2 There have been studies indicated that chemical modifications, such as sulfation, can enhance the antiviral activities of polysaccharides. For example, the sulfated polysaccharides of Caesalpinia ferrea inhibited the replication of poliovirus in vitro.3
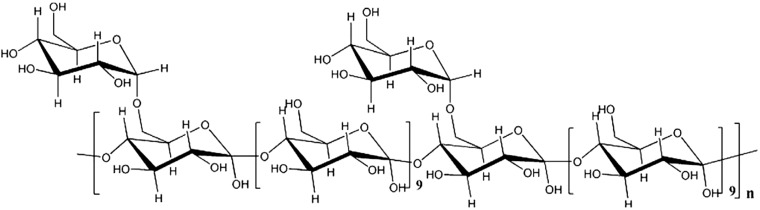
Astragalus membranaceus is the member of the Leguminosae family and is widely distributed in temperate regions.4,5 Throughout the history of Chinese herb use, the dried root of A. membranaceus has primarily been used to treat the common cold, diarrhea, fatigue, and anorexia.6 Astragalus polysaccharide (APS), which is characterized as a (1→4)-linked dextran backbone with a (1→6)-linked branch every 10 residues7 (Figure 1), is the primary bioactive ingredient of A. membranaceus. Kallon et al.8 reported that APS could significantly inhibit the H9N2 virus in chick embryo fibroblasts monolayers. Therefore, APS was hypothesized to exhibit antiviral activities against DHAV.
Figure 1.
The chemical structure of APS
Early research also reported that sulfated APS (sAPS) possessed stronger anti-HIV activity than APS,9 which had been broadly used for numerous animal diseases, including infectious bursal disease virus and porcine reproductive and respiratory syndrome.10 However, the sulfation process is characterized by difficult requirements, a complex operation and serious environmental pollution. Moreover, the experiment of Chen et al.11 revealed that sAPS could not efficiently reduce the mortality of the ducklings infected with DHAV. While the phosphorylation modification method discovered by Xiong et al.12 is easy to maintain, less labor, and is environmentally friendly. Besides, phosphorylated icariin can improve the survival rate of the ducklings infected with DHAV and exhibits a better curative effect than icariin. In this study, APS was modified by using the sodium trimetaphosphate and sodium tripolyphosphate (STMP-STPP) method which was first used by Muhammad et al. to modify the sago starch,13 and the product’s anti-DHAV activity was compared with that of sAPS and APS in vitro and in vivo.
Materials and methods
Reagents and virus
STMP (lot no. L1226014) was purchased from the Aladdin Company. STPP (lot no. 20120706) was manufactured by the Hushi Company. Pyridine (Pyr, lot no. 20130112) was procured from the Chengdu Kelong Chemical Industry Company. Chlorosulfonic acid (lot no. 080421) was purchased from the Shanghai Ling Feng Chemical Company. RNAiso Plus Reagent (lot no. 9108), PrimeScript™ RT Master Mix Kits (lot no. AK3101), and SYBR® Premix Ex Taq™ (Tli RNaseH Plus) Kits (lot no. AK4704) were purchased from Takara. DHAV (LQ2 strain, LD50: 5 × 10−3) was supplied by the Shandong Institute of Poultry in China.
Dulbecco’s modified Eagle’s medium (DMEM, Gibco) supplemented with penicillin (100 IU/mL−1), streptomycin (100 IU/mL−1) and 10% fetal bovine serum was used as the nutritive medium. The maintenance medium (MM) was identical to the nutritive medium with the exception that the fetal bovine serum was reduced to 1%. Dulbecco’s Hank’s balanced salt solution (D-Hank’s) was used to wash the tissues and cells. All reagents for the in vitro experiments were pH-adjusted to 7.4 using 5.6% NaHCO3 and stored at 4℃. Trypsin (Amresco) was dissolved to 0.2% with D-Hank’s and stored at −20℃. The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT, Amresco) was dissolved in calcium- and magnesium-free phosphate-buffered saline at 5 mg/mL−1 and stored at 4℃. These reagents were filtered through 0.22-µm syringe filters. The other chemicals used in the experiments were analytical grade.
Preparation of APS, sAPS, and pAPS
APS was extracted from A. membranaceus by water decoction and the ethanol precipitation method. The decoction pieces were decocted three times with 10-fold water for 1.5 h per cycle. The decoctions were combined and concentrated until the concentration of materia medica was 1 g/mL−1. Next, the concentrate was centrifuged to remove the impurities. Ethanol (95%) was added slowly to the supernatant to ensure that the final concentration was 75% (v/v). Following static settlement for 12 h, the precipitate was dried at 60℃ for 24 h. The crude APS was purified with the Sevage method and column chromatography with DEAE column and Sephacryl-S400.7 The phenol–sulfuric acid method was used to determine the polysaccharide content, which was 93.36%.
The chlorosulfonic acid-pyridine method was used to prepare the sAPS.14 The sulfating reagent was a compound of chlorosulfonic acid and pyridine (1:6) that was mixed in an ice bath. Four hundred milligrams of APS were added, and the solution was stirred for 1 h at 95℃. The reaction was terminated with 100 mL of ice water. When the solution cooled to room temperature, it was neutralized with saturated NaOH, dialyzed and lyophilized to obtain sAPS. The sAPS content was 97.06%, which was calculated as the sum of the polysaccharide content determined by the phenol–sulfuric acid method and the sulfur content determined by the barium chloride-gelatin method.15
pAPS was prepared with the STMP-STPP method.12 In brief, APS was dissolved in distilled water, and STMP and STPP were combined at a ratio of 5:2, then two solutions mentioned above were combined, and the reaction was performed in a 70℃ water bath kettle for 4 h. The product was dialyzed and lyophilized to obtain pAPS. The pAPS content was 99.06%, which was calculated as the sum of the polysaccharide content as determined with the phenol–sulfuric acid method and the phosphate content as determined with the ascorbic acid method.16
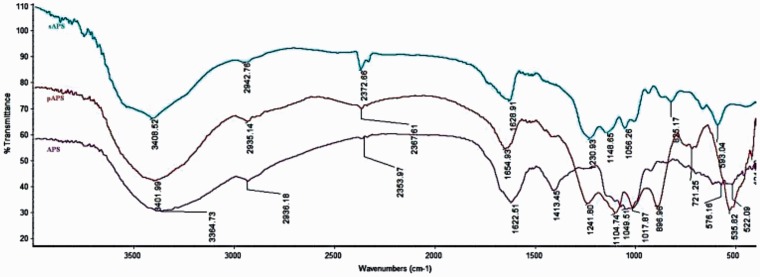
The FT-IR spectra of APS, sAPS, and pAPS were determined over the wavelength range of 400–4000 cm−1 and recorded using the potassium bromide disc method17 with a Nicolet FT-IR 360 spectrophotometer (Nicolet). The absorption peaks were analyzed with OMNIC software (Nicolet Instruments Corp.).
Anti-DHAV activity in vitro
The duck embryo hepatocytes (DEHs) were prepared from the livers of 14- or 15-day-old duck embryos, which were minced, digested with 0.2% trypsin and seeded at 0.8 ×10−6–1.2 × 10−6 cells/mL−1 in 96-well plates.18 The 96-well plates with monolayer DEHs were divided into cell control (CC), APS, sAPS, and pAPS groups. Three polysaccharides were, respectively, diluted to 5000 µg/mL−1, 1000 µg/mL−1, and 500 µg/mL−1 with MM and added into the 96-well plates at 100 µL. After incubation in 5% CO2 at 37℃ for 96 h, the cytoactivities of the DEHs monolayer were measured with the MTT method.19
APS, sAPS, and pAPS were diluted from maximum safe concentrations with MM. The 96-well plates with DEHs monolayer were divided into CC, virus control (VC), APS, sAPS, and pAPS groups. The virus was diluted with MM and then added to the DEHs monolayer of each group with the exception of the CC group at 100 µL per well. After incubation in 5% CO2 at 37℃ for 2 h, the virus dilutions were removed, and the plates were washed with D-Hank’s three times. One hundred microliters of the dilutions of the different dilutions were added to corresponding wells of the test groups, and 100 µL MM was added in the CC and the VC groups. Five repetitions were performed for each concentration. Subsequently, the cytoactivities of the DEHs monolayer was measured with the MTT ((4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide) method after incubation in 5% CO2 at 37℃ for 96 h. The virus inhibition rates were calculated according to the following formula: virus inhibition rate =(A570(Test Group) − A570(VC))/(A570(CC) − A570(VC)) ×100%.
Anti-DHAV proliferation assay in DEHs with reverse transcription-polymerase chain reaction
Virus adsorption
The monolayer DEHs in the 24-well plate were infected with DHAV at 400 µL per well with the exception of the CC group and maintained at 4℃ for 1 h. After removing the supernatant and washing cells with D-Hank’s three times, 400 µL per well of the dilutions were added into the plate at the peak antiviral concentration of each treatment. Three repetitions per treatment were performed. The plate was then stored at 4℃ for 1 h. The supernatant was removed, and the cell cultures were washed five times with phosphate buffered saline (PBS). Following the extraction of the total RNA, reverse transcription-polymerase chain reaction (RT-PCR) was employed to quantify the amount of the virus.
RT-PCR analysis: The total RNA was extracted from the monolayer DEHs using RNAiso Plus Reagent according to the manufacturer’s instructions, and the OD260/OD280 was verified to be between 1.8 and 2.1. The reverse transcription was performed at 37℃ for 15 min, 85℃ for 5 s, and 4℃ for 7 min in a PCR instrument (2720 Thermal Cycler PCR instrument Applied Biosystems, USA). Real-time PCR was performed at 95℃ for 30 s, 95℃ for 5 s (35 cycles), and 60℃ for 30 s in the PCR instrument (StepOnePlus™ Real Time PCR instrument, Applied Biosystems, USA) using a SYBR® Premix Ex Taq™ (Tli RNaseH Plus) Kit. The real-time PCR primers detailed in a previous study20 were as follows: DHAV forward, 5′-GCCACCCTTCCTGAGTTTGT-3′ (positions: 3336–3355) and reverse, 5′-TACCATTCCACTTCTCCTGCTT-3′ (positions: 3489–3510); and beta-actin forward, 5′-CTTTCTTGGGTATGGAGTCCTG-3′ (positions: 826–847) and reverse, 5′-TGATTTTCATCGTGCTGGGT-3′ (positions: 995–1014).
Viral replication
DHAV (400 µL) was added into each well of the 24-well plate with the exception of the well of the CC group, and the plate was maintained at 4℃ for 1 h. The cell cultures were washed three times with D-Hank’s. APS, pAPS, and sAPS were diluted to the most effective antiviral concentrations and added into the wells of the appropriate treatment groups at 400 µL per well. Each group had three repetitions. The plate was incubated at 37℃ in 5% CO2 for 12 h. After washing the plate with PBS three times and the extraction of the total RNA, RT-PCR was performed to quantify the amounts of virus following total RNA extraction.
Viral release
DHAV (400 µL) was added into each of the 24-well with a monolayer DEHs with the exception of the CC group, and the plate was then maintained at 4℃ for 30 h. The infected DEHs monolayer was washed with D-Hank’s and treated with the polysaccharide dilutions at the peak antiviral concentrations at 400 µL per well and three repetitions per group. Next, the plate was maintained at 37℃ in 5% CO2 for 4 h. The supernatants were gathered in no-enzyme tubes and mixed with 1.0 × 106 DEHs. RT-PCR was performed to quantify the amounts of virus following total RNA extraction.
Clinical anti-DHAV effect
Three hundred three-day-old ducklings (Anas platyrhynchos, purchased from Chaoyang hatchery, Anhui, China), excluding those in the blank control (BC) group (60 feathers), were intramuscularly injected with 0.2 mL (10 LD50) DHAV and randomly divided into the following four groups: APS, sAPS, pAPS, and VC. The BC group (isolation reared) was intramuscularly injected with 0.2 mL physiological saline. Following the injections with DHAV, the corresponding experimental drugs in aqueous solution were given by oral administration once per day for five days. The dosage of polysaccharides for each duckling was 3 mg (net). The numbers of surviving ducks were accurately recorded every 12 h after the DHAV challenge, and dead ducklings that did not exhibit pathological changes were excluded. To monitor the blood virus contents in the early (4th h and 8th h) and late (54th h) phases of the disease, blood samples were randomly taken from five feathers per group at each time point after virus challenge and treated with heparin anticoagulation. And then they were used to detect the DHAV nucleic acid contents by the method used in vitro assay. The survival rate of each group was calculated until no further deaths were observed. The dead ducklings were dissected, and the pathological changes in the livers were observed.
The activities of alanine aminotransferase (ALT), aspartate aminotransferase (AST), lactate dehydrogenase (LDH), and alkaline phosphatase (ALP) in serum of each group and the contents of albumin (ALB), total protein (TP), and globulin (GLO) in serum of each groups were tested at 4hpi, 8hpi, and 54hpi by using Automatic Biochemistry Analyzer (7180 Automatic Biochemistry Analyzer, HITACHI, Japan).
The animal experiment was completed in accordance with the guide of the EU Directive 2010/63/EU for animal experiments and was accepted by the Nanjing Agricultural University Animal Care Committee. The surviving ducklings were executed according to the national regulations, and the dead ducklings were disposed of according to local standard protocols. All steps complied with the AVMA Guidelines for the Euthanasia of Animals (2013 edition).
Statistical analysis
The relative gene expressions were analyzed with the 2−ΔΔCT method. The A570 and relative gene expression data were expressed as the means ± the S.E. Duncan’s multiple range test was applied to analyze the differences between groups using the SPSS 16.0 software. The chi-square test was used for the survival rate analysis. Significant differences were considered at the level of P < 0.05.
Results
Infrared spectrum analysis
The infrared spectra of APS, sAPS, and pAPS are depicted in Figure 2. The characteristic absorption peaks of polysaccharide appeared in the APS, sAPS, and pAPS samples. The absorption band at approximately 3400 cm−1 was the result of hydroxyl stretching vibration. The peak in the region of 3000–2800 cm−1 was due to C-H stretching vibration. The absorption peak that appeared at 1600 cm−1 was a result of COO− stretching vibration. In addition to the three signal absorption bands of polysaccharide, APS also exhibited an absorption peak at 1400–1000 cm−1 that was related to C-H, C-O, and C-C bending vibration. Regarding sAPS, the asymmetrical S = O stretching vibration at 1230.93 cm−1 and the symmetrical C-O-S vibration at 817 cm−1 were verified in the FT-IR spectrum. The pAPS spectrum exhibited the following three additional absorption peaks: a peak at 1240 cm−1 caused by P = O stretching vibration, a peak at 1018 cm−1 attributed to a P-OH bond, and band at 896 cm−1 that corresponded to P-O-C bonds.
Figure 2.
Infrared spectroscopy of APS, pAPS, and sAPS. The FT-IR spectra of APS, sAPS, and pAPS were determined over the wavelength range of 400–4000 cm−1. The curve in blue represents sAPS; the curve in red represents pAPS; the curve in purple represents APS. (A color version of this figure is available in the online journal.)
Antiviral activity in vitro
The direct anti-DHAV effects of APS, sAPS, and pAPS were compared in the hepatocytes which were the primary host cells of DHAV. The maximum safe concentrations of APS, sAPS, and pAPS were tested on DEHs before research. The results showed that the maximum safe concentration of APS was 625 µg/mL−1, that of sAPS was 7.813 µg/mL−1 and pAPS was 31.250 µg/mL−1 (Table 1). Then, DEHs infected with DHAV were treated with the diluted polysaccharides. The results revealed APS, sAPS, and pAPS inhibit DHAV at different concentrations. The most effective concentration of APS was 78.125 µg/mL−1, with a viral inhibition rate of 71.7%. Meanwhile, the antiviral concentration ranges of sAPS and pAPS were 3.906 µg/mL−1 to 0.488 µg/mL−1 and 7.813 µg/mL−1 to 0.488 µg/mL−1, respectively. Among all of the treatment groups, the pAPS group exhibited the highest viral inhibition rate of 85.1% at 7.813 µg/mL−1 and the widest range of antiviral concentrations (Table 2).
Table 1.
The A570 values of APS, pAPS, and sAPS on DEHs
| Concentration (μgċmL−1) | APS | pAPS | sAPS | ||
|---|---|---|---|---|---|
| Concentration (μgċmL−1) | Concentration (μgċmL−1) | ||||
| 5000 | 0.274 ± 0.045a | 1000 | 0.144 ± 0.002b | 500 | 0.393 ± 0.077c |
| 2500 | 0.305 ± 0.020a | 500 | 0.233 ± 0.011a | 250 | 0.410 ± 0.007c,d |
| 1250 | 0.357 ± 0.025efg | 250 | 0.360 ± 0.014g | 125 | 0.430 ± 0.014cdh |
| 625 | 0.354 ± 0.009fg | 125 | 0.552 ± 0.009f | 62.5 | 0.442 ± 0.018bdh |
| 312.5 | 0.367 ± 0.036efg | 62.5 | 0.598 ± 0.010e | 31.25 | 0.453 ± 0.007bh |
| 156.25 | 0.387 ± 0.031ef | 31.25 | 0.595 ± 0.001e | 15.625 | 0.483 ± 0.011ab |
| 78.125 | 0.392 ± 0.018e | 15.625 | 0.615 ± 0.006e | 7.813 | 0.505 ± 0.011ag |
| 39.06 | 0.369 ± 0.013efg | 7.8125 | 0.596 ± 0.006e | 3.906 | 0.520 ± 0.016afg |
| 19.53 | 0.386 ± 0.016ef | 3.90625 | 0.610 ± 0.006e | 1.953 | 0.536 ± 0.031fg |
| 9.765 | 0.353 ± 0.019fg | 0.977 | 0.561 ± 0.015ef | ||
| 4.883 | 0.346 ± 0.017g | 0.488 | 0.586 ± 0.032e | ||
| CC | 0.390 ± 0.012ef | CC | 0.609 ± 0.006e | CC | 0.507 ± 0.019ag |
Data in same column without same superscript (a–f) differ significantly(P < 0.05).
APS: astragalus polysaccharide; pAPS: phosphate astragalus polysaccharide; sAPS: sulfate astragalus polysaccharide; CC: cell control.
Table 2.
The A570 values and virus inhibitory rate in anti-DHV-1 activity test in vitro
| Group | Concentration | A570 | Virus inhibitory rate/% | Group | Concentration | A570 | Virus inhibitory rate/% | Group | Concentration | A570 | Virus inhibitory rate/% |
|---|---|---|---|---|---|---|---|---|---|---|---|
| APS (µg mL−1) | 625 | 0.334 ± 0.012a | 0.5 | pAPS (µgċmL−1) | 31.250 | 0.361 ± 0.018ab | 29.2 | sAPS (µgċmL−1) | 7.813 | 0.414 ± 0.030cd | 17.0 |
| 312.5 | 0.347 ± 0.019ae | 15.7 | 15.625 | 0.364 ± 0.035ab | 33.3 | 3.906 | 0.460 ± 0.028aef | 64.1 | |||
| 156.25 | 0.352 ± 0.013ae | 20.9 | 7.813 | 0.402 ± 0.016ef | 85.1 | 1.953 | 0.469 ± 0.042ef | 73.4 | |||
| 78.125 | 0.399 ± 0.011f | 71.7 | 3.906 | 0.386 ± 0.004ae | 63.5 | 0.977 | 0.454 ± 0.005abe | 58.6 | |||
| 39.06 | 0.354 ± 0.010ae | 22.7 | 1.953 | 0.380 ± 0.003ae | 54.8 | 0.488 | 0.451 ± 0.019abce | 54.9 | |||
| 19.53 | 0.364 ± 0.022e | 34.1 | 0.977 | 0.383 ± 0.005ae | 58.9 | 0.244 | 0.428 ± 0.015abcd | 31.4 | |||
| 9.765 | 0.372 ± 0.006e | 42.5 | 0.488 | 0.370 ± 0.018a | 41.6 | 0.122 | 0.417 ± 0.015bcd | 20.6 | |||
| VC | 0.332 ± 0.037a | 0 | VC | 0.340 ± 0.004b | 0 | VC | 0.397 ± 0.021d | 0 | |||
| CC | 0.426 ± 0.019f | 100 | CC | 0.413 ± 0.002f | 100 | CC | 0.495 ± 0.024f | 100 | |||
APS: astragalus polysaccharide; pAPS: phosphate astragalus polysaccharide; sAPS: sulfate astragalus polysaccharide; VC: virus control; CC: cell control.
Data in same column without same superscript (a–f) differ significantly (P < 0.05).
Anti-DHAV proliferation assays with APS, pAPS, and sAPS in the DEHs
Influence on DHAV adsorption
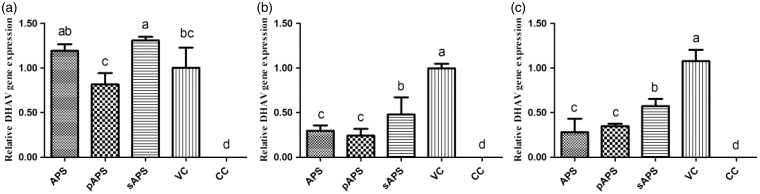
Figure 3(a) depicts the effects on APS, pAPS, and sAPS on the adsorption of DHAV on the DEHs. No DHAV expression was detected in the CC group. The relative DHAV gene expression of the pAPS group was notably lower than that of the APS group and sAPS group (P < 0.05) and non-significantly lower than that of the VC group. The DHAV expression of APS group has no significant difference compared with that of the VC group and sAPS group.
Figure 3.
The anti-DHAV proliferation of APS, pAPS, and sAPS in DEHs. (a) Analysis of relative DHAV gene expression in virus adsorption; (b) the relative DHAV gene expression in process of virus replication; and (c) the quantity of DHAV gene expression when virus released from cells. Data without same superscripts (a–d) differ significantly (P < 0.05).
APS: astragalus polysaccharide; pAPS: phosphate astragalus polysaccharide; sAPS: sulfate astragalus polysaccharide; VC: virus control; CC: cell control; DHAV: duck hepatitis A virus
Influences of APS, pAPS, and sAPS on DHAV replication
For viral replication in vitro, the relative gene expressions of DHAV in the APS (0.30), pAPS (0.24), and sAPS (0.48) groups were markedly lower than that of the VC group (P < 0.05). The APS and the pAPS groups exhibited more obvious effects than the sAPS group (P < 0.05). Although there was no significant difference between the APS and pAPS groups, the DHAV expression was greater in the APS group (Figure 3(b)). No viral gene expression was observed in the CC group.
Influences of APS, pAPS, and sAPS on DHAV release
As demonstrated in Figure 3(c), the levels of viral release in the APS, pAPS, and sAPS groups (relative DHAV gene expressions of 0.28, 0.35, and 0.57, respectively) were dramatically lower than that in the VC group (P < 0.05). Among treatment groups, the viral release from the DEHs in the sAPS group was obviously greater than those in the APS and the pAPS groups (P < 0.05).
Therapeutic effect in vivo
Table 3 displays the survival rate of each group. There was no death in the BC group. The survival rates in the APS, sAPS, and VC groups were not significantly different. However, the survival rate of the pAPS group was markedly higher than those of the other three challenged groups (P < 0.05).
Table 3.
Clinical survival of ducklings infected with DHAV
| Group | Sample (feather) | Survival (feather) | Survival rate (%) |
|---|---|---|---|
| APS | 45 | 7 | 15.6a |
| pAPS | 45 | 15 | 33.3b |
| sAPS | 45 | 3 | 6.7a |
| VC | 45 | 7 | 15.6a |
| BC | 45 | 45 | 100c |
APS: astragalus polysaccharide; pAPS: phosphate astragalus polysaccharide; sAPS: sulfate astragalus polysaccharide; VC: virus control; BC: blank control.
Different superscript (a–c) of survival rate indicated significant difference (P < 0.05).
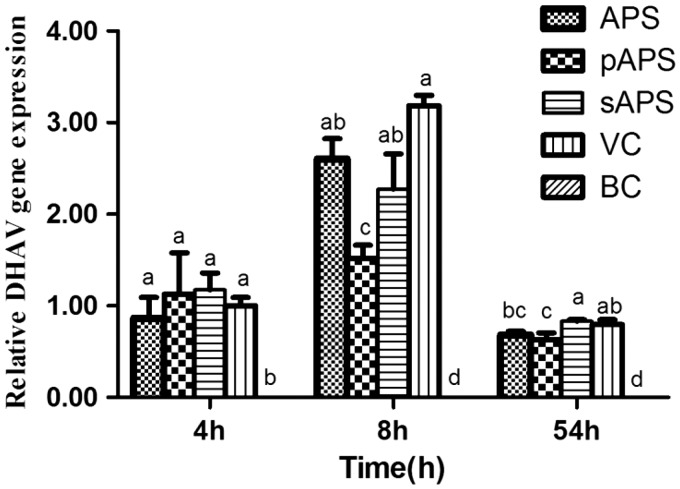
Figure 4 illustrates the proliferation of DHAV in the blood of all treatment groups. In the early stage of infection, the proliferations of DHAV in the treatment groups were similar to that of the VC group at the 4th h. Subsequently, proliferation in the pAPS group was markedly lower than that of the VC group at the 8th h (P < 0.05). The DHAV proliferations in the APS and sAPS groups were lower than that of the VC group with no significant difference. In the late stage of infection, the virus content of the pAPS group remained significantly lower than that of the VC group (P < 0.05). The DHAV proliferation of the APS group was slightly lower than that of the VC group, and the content of DHAV of the sAPS group was greater than that of the VC group. No DHAV was detected in the BC group at any sampling time.
Figure 4.
The effect of APS, pAPS, and sAPS on proliferation of DHAV in blood. Data at same time point without same superscripts (a–d) differ significantly (P < 0.05).
APS: astragalus polysaccharide; pAPS: phosphate astragalus polysaccharide; sAPS: sulfate astragalus polysaccharide; VC: virus control; BC: blank control; DHAV: duck hepatitis A virus
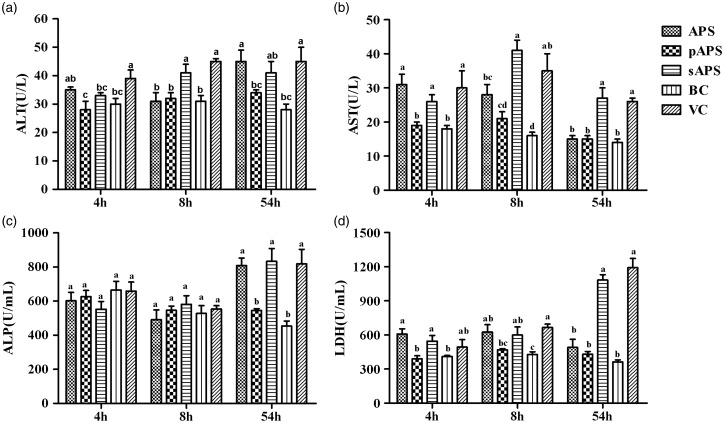
The activities of liver function-related enzymes are exhibited in Figure 5. The content of ALT, ALP, AST, and LDH of the VC group significantly increased at all sampling time points compared with those of the BC group (P < 0.05). The activities of ALT, AST, and LDH of the pAPS group were evidently lower than those of the VC group at all time points. In sAPS group, only the content of ALT was remarkably lower than that of the VC group at the 4th h. For remaining indexes, there was no significant difference between the sAPS group and the VC group. Excepting the content of ALP at 8 h and the content of AST and LDH at 96 h, which were significantly lower than those of the VC group (P < 0.05), it was similar to the APS group that no observable difference existed between the VC group and the APS group.
Figure 5.
The activity of liver function-related enzymes in each groups. (a) The activity of ALT (U/L) in serum; (b) the activity of AST (U/L) in serum; (c) the activity of ALP (U/mL) in serum; and (d) the activity of LDH (U/mL) in serum. Data at same time point without same superscripts (a–d) differ significantly (P < 0.05).
ALT: alanine aminotransferase; AST: aspartate aminotransferase; LDH: lactate dehydrogenase; ALP: alkaline phosphatase; APS: astragalus polysaccharide; pAPS: phosphate astragalus polysaccharide; sAPS: sulfate astragalus polysaccharide; VC: virus control; BC: blank control
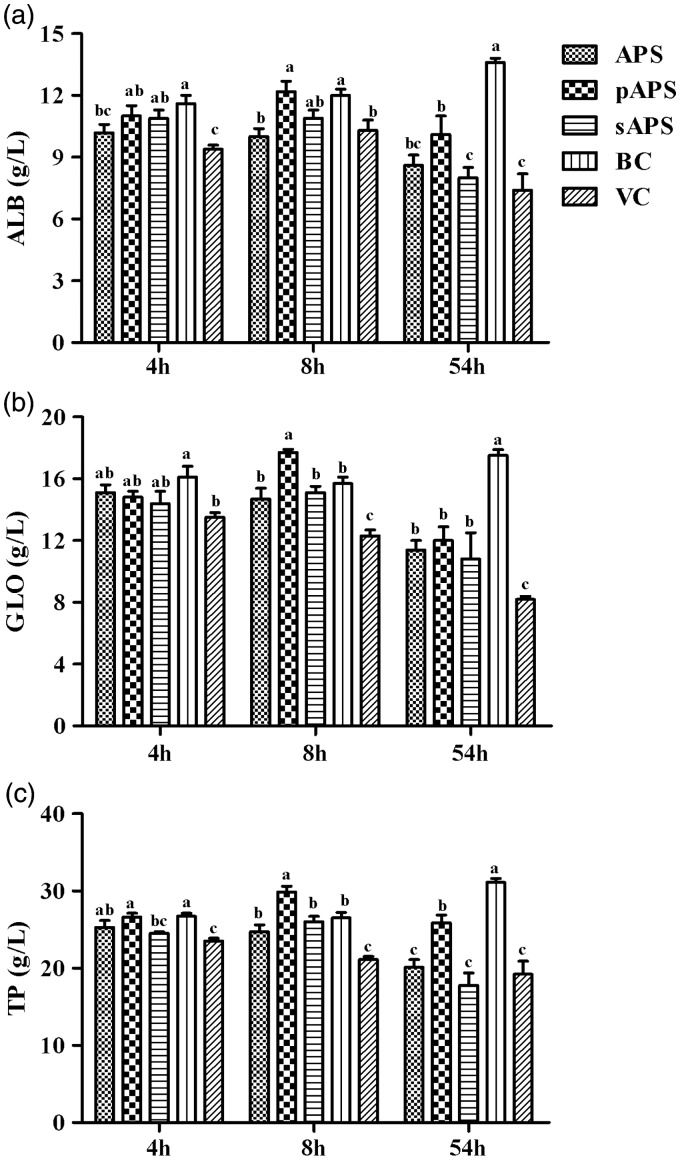
Figure 6 shows the proteins in serum associated with the liver function. The contents of TP, ALB, and GLO were markedly lower than those of the BC group at all time points. The contents of these three proteins in the pAPS group were significantly higher than those of the VC group at all sampling time except for the content of GLO at 4 h which was higher than that of the VC group without significance. In the sAPS group, the contents of TP, ALB, and GLO were higher than those of the VC group at the 8th h, and the content of GLO was higher than the VC group at the 54th h. The content of TP at all time points and GLO at the 8th h and the 96th h in APS group was significantly higher than those of the VC group (P < 0.05).
Figure 6.
The content of liver function-related proteins in each group. (a) The content of ALB (g/L) in serum; (b) the content of GLO (g/L) in serum; and (c) the content of TP (g/L) in serum. Data at same time point without same superscripts (a–d) differ significantly (P < 0.05).
APS: astragalus polysaccharide; pAPS: phosphate astragalus polysaccharide; sAPS: sulfate astragalus polysaccharide; VC: virus control; BC: blank control; ALB: albumin; TP: total protein; GLO: globulin
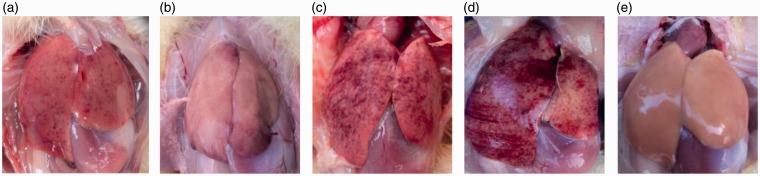
The pathological anatomies of the livers obviously reflected the effects of the different polysaccharides on the infected ducklings which were infected with DHAV, and the results conformed to those of the clinical research detailed above (Figure 7). Figure 7(d) displays a liver from the VC group that exhibited apparent lesions including diffuse petechia, extravasated blood, and hepatomegaly. Regarding the treatment groups, the pathological changes in the pAPS group were less extensive than those in the APS and sAPS groups (Figure 7(a) to (c)). Visual examination of the BC group duckling livers revealed no pathological changes (Figure 7(e)).
Figure 7.
Clinical pathological anatomy of ducklings’ livers. (a)–(e) respectively referred to APS group, pAPS group, sAPS group, VC group, and BC group. (A color version of this figure is available in the online journal.)
Discussion
Duck virus hepatitis caused by DHAV is a disease characterized by a rapid onset, high fatality rate, and widespread infection.1 When an infected duckling is identified, it should be killed immediately to avoid huge economic losses to the duck industry. APS is highly popular in traditional Chinese medicine polysaccharide research for its essential role in treatment of many diseases.6 In recent studies, diverse modification methods, such as sulfation and phosphorylation have been applied to this polysaccharide aiming at improving polysaccharide bioactivities.21 Both of these two modification methods have been reported to enhance the antiviral activities of active constituents in traditional Chinese medicine.14,22 The chlorosulfonic acid-pyridine method is a common method for achieving sulfation modifications. Huang et al.14 employed the chlorosulfonic acid-pyridine method to sulfate a polysaccharide from Astragalus. Additionally, the STMP-STPP method has been widely used for the edible polysaccharide modification.13,23 To achieve phosphorylation modifications of polysaccharides, Muhammad et al. utilized a mixture of 2% STMP and 5% STPP at pHs between 6 and 11 to modify sago starch, which finally improved water solubility of sago starch.13 And the phosphorylated icariin which is modified by icariin with the STMP-STPP method could improves the survival rate of ducklings infected with DHAV by 10% compared with non-phosphorylated icariin.12
In vitro, the maximum safe concentration of pAPS (31.250 µg/mL−1) was higher than that of sAPS (7.813 µg/mL−1), which indicated that the phosphorylation modification method was less toxic than sulfation modification (Table 1). The effective concentration range of antiviral activities of pAPS was wider than those of APS and sAPS. The greatest virus inhibition rate of pAPS at the optimal concentration was higher than those of APS and sAPS at optimal concentrations (Table 2). In research about antiviral activities of polysaccharides on virus different multiplication process, the RT-PCR assay has higher accuracy, is more autonomous and results have less contamination, thus it was applied to examine the activities of APS, pAPS, and sAPS.24 Ding and Zhang25 determined the sequence of DHAV, which also provides a solid foundation for this study. The DHAV proliferation process included virus adsorption, RNA replication, and virus release.26 Regarding the process of virus adsorption, the relative DHAV gene expressions in the APS and sAPS groups revealed that these polysaccharides were unable to inhibit viral adsorption. At the 12th h, which represents the process of viral replication (Figure 3(b)), the relative DHAV gene expressions of the pAPS group were lower than those of the VC group, APS group, and sAPS group, and there were notably differences compared with the sAPS group and VC group (P < 0.05). Regarding the viral release stage in which new virions are released from cells to infect other cells and reproduce the virus, the DHAV gene expression in treatment groups markedly reduced compared with that of the VC group (P < 0.05) (Figure 3(c)). These results suggest that pAPS inhibited DHAV proliferation more effectively than the other two polysaccharides throughout the entire process. Although APS and sAPS inhibited the replication and release of the virus in the DEHs, the viral contents of both groups in the adsorption process were greater than that of the VC group. APS and sAPS failed to display perfect anti-DHAV abilities over the entire process because the viral invasion of the cells was too extensive.
These results were corresponded to those in vivo; only pAPS significantly improves the survival rate of ducklings after challenged by DHAV (P < 0.05) (Table 3). In vivo, DHAV completes replication cycle, including virus adsorption, replication, and release, between 6 and 8 h.20 The relative gene expressions of DHAV at the 8th and 54th h in vivo revealed that pAPS significantly inhibited viral proliferation to a greater extent than APS or sAPS (Figure 4). DHAV selected liver as the main target organ to reproduce more viruses. Therefore, the test of liver function and the extent of liver damage could also reflect the invasion of DHAV in vivo. The serum enzymes including ALT, AST, ALP, and LDH mainly consisted in hepatocytes. When the acute virus hepatitis occurred, the activities of these enzymes were increased in serum for the reason that hepatocytes were damaged during virus proliferation.27 In contrast, the contents of serum proteins (TP, ALB, and GLO) reduced after virus invasion.11 These results were verified by ocular anatomy that less symptomatic hemorrhages in the livers of the infected ducklings were found than APS and sAPS (Figure 7).
The position, degree, and group of substitution might be the reason for the differences of polysaccharide bioactivities. In a study of chemically modified polysaccharides from Polygonatum cyrtonema Hua, C-6 was substituted with a sulfur group, and different β-α-Fruf residues were substituted with a phosphor group.21 He et al.28 also reported that the substituted position of the sulfur group differs from that of the phosphor group in an exopolysaccharide from Lachnum The antiviral activity of sAPS was also related to its degree of substitution. The antiviral activity of the sulfated polysaccharide increased with the number of substituted sulfate groups. This pattern was confirmed in an experiment in which the anti-murine leukemia virus activity of a sulfated polysaccharide from Angelica sinensis was found to increase with elevation in the DS in vivo.29 In contrast, the phosphorylated polysaccharide was generally modified with a low degree of substitution and exhibited more effective bioactivity than the sulfated polysaccharide.21,28 In the present research, the polysaccharide chains of pAPS and sAPS were not degraded during the modifications. pAPS exhibits adsorption peaks related to P = O stretching vibration,23 P-OH and P-O-C bonds.13 Asymmetrical S = O stretching vibration and symmetrical C-O-S vibration have been detected in sAPS3 (Figure 2). Although the backbone chain of the polysaccharide is preserved in the sulfated and phosphorylated forms,21 differences in the degree of substitution, chain conformation, and functional groups between the phosphorylated and sulfated polysaccharides might have altered the antiviral activities of pAPS and sAPS. The extended chain conformation of a chemically modified polysaccharide can also influence its bioactivities.22,30 These diversities might ultimately result in differences in the antiviral activities of sulfated and phosphorylated polysaccharides. In addition, syndrome differentiation is the key to treatment determination in traditional Chinese medicine.31 Astragalus is a traditional Chinese herb and Qi-tonifying drug replenishing the basic energy needed by the body, which creates the opposite effects required in the treatment of duck virus hepatitis, a blood-heat disease that is accompanied by hemorrhaging in the liver and convulsions. Therefore, the therapeutic principles of duck virus hepatitis based on the theory of syndrome differentiation for the treatment of duck virus hepatitis include heat-clearing and detoxifying. Furthermore, the essence of traditional Chinese medicine is the emphasis on the effect of drug on holistic body instead of partial cells, which resulted in the unsatisfactory effect of APS.32 These hypotheses related to the structure–function relationships among different derivatives of APS and the Chinese medicine theory require additional research in the future.
Acknowledgments
This project was funded by the National Natural Science Foundation of China (Grant No. 31172355, 31572557), the Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), the Special Fund for Agro-scientific Research in the Public Interest (201303040 and 201403051), and the development plan of science and technology of Shandong Province (2012GGC15003). We appreciate all of the staff at the Institute of Traditional Chinese Veterinary Medicine of Nanjing Agricultural University for their experimental assistance.
Authors’ contributions
YW, JL, and YC participated in the design; YW wrote the manuscript and was responsible for the interpretation of the studies and analysis of the data; YW, YC, HD, JY, KM, and MS conducted the experiment. JL participated in the review of the manuscript.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Levine PP, Fabricant J. A hitherto-undescribed virus disease of ducks in North America. Cornell Veterinarian 1950; 40: 71–86. [Google Scholar]
- 2.Galhardi LCF, Yamamoto KA, Ray S, Ray B, Linhares REC, Nozawa C. The in vitro antiviral property of Azadirachta indica polysaccharides for poliovirus. J Ethnopharmacol 2012; 142: 86–90. [DOI] [PubMed] [Google Scholar]
- 3.Lopes N, Galhardi LCF, Espada SF, Pacheco AC, Ricardo NMPS, Linhares REC, Nozawa C. Sulfated polysaccharide of Caesalpinia ferrea inhibits herpes simple virus and poliovirus. Int J Biol Macromol 2013; 60: 93–9. [DOI] [PubMed] [Google Scholar]
- 4.Jia R, Cao LP, Xu P, Jeney G, Yin GJ. In vitro and in vivo hepatoprotective and antioxidant effects of Astragalus polysaccharides against carbon tetrachloride-induced hepatocyte damage in common carp (Cyprinus carpio). Fish Physiol Biochem 2011; 38: 871–81. [DOI] [PubMed] [Google Scholar]
- 5.Fan YP, Hu YL, Wang DY, Liu JG, Zhang J, Zhao XJ, Liu X, Liu C, Yuan J, Ruan SL. Effects of Astragalus polysaccharide liposome on lymphocyte proliferation in vitro and adjuvanticity in vivo. Carbohyd Polym 2012; 88: 68–74. [Google Scholar]
- 6.Jin ML, Zhao K, Huang QS, Shang P. Structural features and biological activities of thepolysaccharides from Astragalus membranaceus. Int J Biol Macromol 2014; 64: 257–66. [DOI] [PubMed] [Google Scholar]
- 7.Niu Y, Wang H, Xie Z, Whent M, Gao X, Zhang X, Zou S, Yao W, Yu L. Structural analysis and bioactivity of a polysaccharide from the roots of Astragalus membranaceus (Fisch) Bge var. mongolicus (Bge.) Hsiao. Food Chem 2011; 128: 620–6. [Google Scholar]
- 8.Kallon S, Li XR, Ji J, Chen CY, Xi QY, Chang S, Xue CY, Ma JY, Xie QM, Zhang YL. Astragalus polysaccharide enhances immunity and inhibits H9N2 avian influenza virus in vitro and in vivo. J Anim Sci Biotechnol 2013; 4: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu GG, Borjihan G, Baigude H, Nakashima H, Uryu T. Synthesis and anti-HIV activity of sulfated astragalus polysaccharide. Polym Advan Technol 2003; 14: 471–6. [Google Scholar]
- 10.Jiang JB, Wu CH, Gao H, Song JD, Li HQ. Effects of astragalus polysaccharides on immunologic function of erythrocyte in chickens infected with infectious bursa disease virus. Vaccine 2010; 28: 5614–6. [DOI] [PubMed] [Google Scholar]
- 11.Chen Y, Song MY, Wang YX, Xiong W, Zeng L, Zhang SB, Xu MY, Du HX, Liu JG, Wang DY, Wu Y, Hu YL. The anti-DHAV activities of Astragalus polysaccharide and its sulfatecompared with those of BSRPS and its sulfate. Carbohyd Polym 2015; 17: 330–45. [DOI] [PubMed] [Google Scholar]
- 12.Xiong W, Chen Y, Wang Y, Liu JG. Roles of the antioxidant properties of icariin and its phosphorylated derivative in the protection against duck virus hepatitis. BMC Vet Res 2014; 10: 226–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suflet DM, Nicolescu A, Popescu I, Chitanu GC. Phosphorylated polysaccharides. 3. Synthesis of phosphorylated curdlan and its polyelectrolyte behaviour compared with other phosphorylated polysaccharides. Carbohyd Polym 2011; 84: 1176–81. [Google Scholar]
- 14.Huang XY, Wang DY, Hu YL, Lu Y, Guo ZH, Kong XF, Sun JL. Effect of sulfated astragalus polysaccharide on cellular infectivity of infectious bursal disease virus. Int J Bio Macromol 2008; 42: 166–71. [DOI] [PubMed] [Google Scholar]
- 15.Dodgson K, Price R. A note on the determination of the ester sulphate content of sulphated polysaccharides. Biochem J 1962; 84: 106–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lowry OH, Roberts NR, Leiner KY, Wu ML, Farr AL. The quantitative histochemistry of brain 1 chemicalmethods. J Bio Chem 1954; 207: 1–17. [PubMed] [Google Scholar]
- 17.Meyer JD, Manning MC, Carpenter JF, Pharm J. Effects of potassium bromide disk formation on the infrared spectra of dried model proteins. J Pharm Sci 2004; 93: 496–506. [DOI] [PubMed] [Google Scholar]
- 18.Fu Y, Pan M, Wang XY, Xu YL, Yang HC, Zhang DB. Molecular detection and typing of duck hepatitis A virus directly from clinical specimens. Vet Microbiol 2008; 131: 247–57. [DOI] [PubMed] [Google Scholar]
- 19.Pan M, Yang XR, Zhao L, Ge XN, Guo X, Liu JH, Zhang DB, Yang HC. Duck hepatitis A virus possesses a distinct type IV internal ribosome entry site element of picornavirus. J Virol 2012; 86: 1129–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen Y, Xiong W, Zeng L, Wang DY, Liu JG, Wu Y. Comparison of bush sophora root polysaccharide and its sulfate’s anti-duck hepatitis A virus activity and mechanism. Carbohyd Polym 2014; 102: 333–40. [DOI] [PubMed] [Google Scholar]
- 21.Liu XX, Wan ZJ, Shi L, Lu XX. Preparation and antiherpetic activities of chemically modified polysaccharides from Polygonatum cyrtonema Hua. Carbohyd Polym 2011; 83: 737–42. [Google Scholar]
- 22.Chen X, Xu X, Zhang L, Zeng F. Chain conformation and anti-tumor activities of phosphorylated (1→3)-β-d-glucan from Poria cocos. Carbohyd Polym 2009; 78: 581–7. [Google Scholar]
- 23.Deng C, Fu HT, Xu JJ, Shang JY, Cheng YM. Physiochemical and biological properties of phosphorylated polysaccharides from Dictyophora indusiata. Int J Bio Macromol 2015; 72: 894–9. [DOI] [PubMed] [Google Scholar]
- 24.Luo YJ, Zhang GH, Xu XQ, Chen JH, Liao M. Molecular characterization and SYBR green I-based quantitative PCR for duck hepatitis virus type 1. Agr Sci China 2008; 7: 1140–6. [Google Scholar]
- 25.Ding CY, Zhang DB. Molecular analysis of duck hepatitis virus type 1. Virology 2007; 361: 9–17. [DOI] [PubMed] [Google Scholar]
- 26.Norder H, Palma AMD, Selisko B, Costenaro L, Papageorgiou N, Arnan C, Coutard B, Lantez V, Lamballerie XD, Baronti C, Sola M, Tan J, Neyts J, Canard B, Coll M, Gorbalenya AE, Hilgenfeld R. Picornavirus non-structural proteins as targets for new anti-virals with broad activity. Antivir Res 2011; 89: 204–18. [DOI] [PubMed] [Google Scholar]
- 27.Lu Y, Hu DM, Ma SB, Zhao X, Wang S, Wei G, Wang XF, Wen AD, Wang JW. Protective effect of wedelolactone against CCl4-induced acute liver injury in mice. Int Immunopharmacol 2016; 34: 44–52. [DOI] [PubMed] [Google Scholar]
- 28.He Y, Ye M, Jing L, Du Z, Surahio M, Xu H, Li J. Preparation, characterization and bioactivities of derivatives of an exopolysaccharide from Lachnum. Carbohyd Polym 2015; 117: 788–96. [DOI] [PubMed] [Google Scholar]
- 29.Yang TH, Jia M, Zhou S, Pan F, Mei QB. Antivirus and immune enhancement activities of sulfated polysaccharide from Angelica sinensis. Int J Biol Macromol 2012; 50: 768–72. [DOI] [PubMed] [Google Scholar]
- 30.Wang JL, Guo HY, Zhang J, Wang XF, Zhao BT, Yao J, Wang YP. Sulfated modification, characterization and structure–antioxidant relationships of Artemisia sphaerocephala polysaccharides. Carbohyd Polym 2010; 81: 897–905. [Google Scholar]
- 31.Zhao YF, He LY, Liu BY, Li J, Li FY, Huo RL, Jing XH. Syndrome classification based on manifold ranking for viral hepatitis. Chin J Integr Med 2014; 20: 394–9. [DOI] [PubMed] [Google Scholar]
- 32.Wu T, Zhang S, Guo S, Gu Y, Dou L, Wang Y, Zhang HS, Cao SC, Li Y, Zhong Y. Correspondence analysis between traditional Chinese medicine (TCM) syndrome differentiation and histopathology in colorectal cancer. Eur J Integr Med 2015; 7: 342–7. [Google Scholar]