Abstract
Sodium–glucose co-transporter 2 (SGLT2) inhibitors, such as canagliflozin, are used in patients with Type 2 diabetes mellitus (T2DM). In clinical studies, canagliflozin significantly reduced A1C, bodyweight and blood pressure, and was generally well tolerated with no increased risk of hypoglycemia. Most common adverse effects observed were genital mycotic infections and urinary tract infections, and increased urination. Approximately 10% of women treated with canagliflozin experienced a genital mycotic infection compared with 3% treated with placebo; those with a prior history were at greater risk. Approximately 9% of women treated with canagliflozin reported a urinary tract infection compared with 7% treated with placebo. Most adverse events were considered mild to moderate in intensity and responded to standard therapy. Treatment with canagliflozin was effective and generally well tolerated in both women (and men) with T2DM.
Keywords: canagliflozin, efficacy, safety, SGLT2, T2DM, women's health
Diabetes is a growing public health issue in the USA and around the world. At least 29 million people – of which 13.4 million women – in the USA have diabetes [1]. As a leading cause of kidney disease, blindness and excess mortality, diabetes presents challenges to healthcare and society [1]. Women are also at risk for complications and comorbidities that are exacerbated by diabetes, such as urinary tract infections (UTIs), genital mycotic infections (GMIs) and urinary frequency symptoms [2–5].
National diabetes treatment guidelines recommend that clinicians consider and balance treatment efficacy, associated risk for hypoglycemia and adverse effects when choosing therapy [6,7]. Since many patients require more than one antihyperglycemic agent, combining those with different mechanisms of action is a recommended approach [6].
Sodium–glucose co-transporter 2 (SGLT2) inhibitors are a newer class of oral antihyperglycemic agents with a unique mechanism of action that targets renal glucose handling to promote excretion of glucose [8]. At present, three SGLT2 inhibitors – canagliflozin, dapagliflozin and empagliflozin – are approved in the USA for use as an adjunct to diet and exercise in patients with Type 2 diabetes mellitus (T2DM). In general, SGLT2 inhibitors have demonstrated improved glycemic control, with A1C reductions ranging from 0.5 to 1.2% in placebo-controlled clinical studies [9]. Furthermore, the highest approved clinical dose of canagliflozin (300 mg) provided greater reductions in A1C versus sitagliptin or glimepiride in 52-week clinical studies [10,11]. In addition, clinical studies have shown that SGLT2 inhibitors reduce bodyweight and systolic blood pressure (SBP), are generally well tolerated and are associated with a low risk for hypoglycemia [7,12]. The most common adverse effects are related to the mechanism of action and include GMIs and UTIs, particularly among women, and increased urination [7,12]. This review examines the efficacy and safety of canagliflozin with a focus on issues of particular importance to women's health.
Role of the kidney & SGLT2 in T2DM
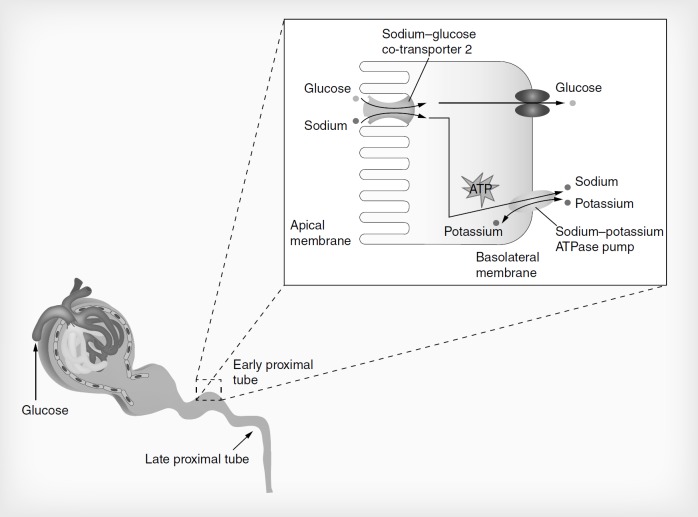
Because glucose is central to normal physiologic processes – yet toxic in excess – the human body has developed an intricate balancing mechanism to maintain glucose homeostasis. The kidneys are integral to this mechanism, playing a key role in both the production and elimination of glucose [13]. To maintain healthy glucose levels, the kidneys produce approximately 20% of endogenous glucose in the fasting state, filter and reabsorb glucose from the bloodstream and eliminate excess glucose through urination [8,13–14]. The balance between reabsorption and excretion in particular is crucial to glycemic control [14].
Glucose is reabsorbed actively in the kidney through the action of SGLT1 and SGLT2, and passively through the action of glucose transporters GLUT1 and GLUT2 present in the proximal tubules of the kidney (Figure 1) [13]. SGLT2 – a high-capacity, low-affinity transporter – is present in the proximal segment of the proximal tubule and is responsible for more than 90% of glucose reabsorption [8,13]. The remaining 10% of glucose reabsorption occurs through SGLT1 – a low-capacity, high-affinity transporter – present in the distal segment of the proximal tubule. SGLT1 is also located in the small intestine, where it is responsible for the reabsorption of glucose and galactose [8,13]. Compared with healthy individuals, SGLT2 transporters are overexpressed in patients with T2DM; this overexpression increases the system's capacity to reabsorb filtered glucose by approximately 20%. Animal and human studies indicate that chronic hyperglycemia upregulates expression of SGLT2 [13,14]. This overexpression of SGLT2 in T2DM increases the level at which glucose is excreted – the renal threshold for glucose (RTG) – contributing to hyperglycemia [13]. It follows that inhibition of SGLT2 should lower RTG to enable more glucose to be excreted, thereby reducing hyperglycemia. In animal models of diabetes, the nonselective SGLT inhibitor phlorizin was shown to reduce hyperglycemia [15]. However, the nonselective inhibition of SGLT1 resulted in gastrointestinal side effects. The insight that selective SGLT2 inhibition should have similar antihyperglycemic effects with a lower risk of gastrointestinal side effects led to clinical development of SGLT2 inhibitors [8].
Figure 1.
Renal glucose reabsorption in healthy individuals.
Reproduced with permission from [16].
Canagliflozin
Canagliflozin – a once-daily oral SGLT2 inhibitor – is highly selective for SGLT2 over SGLT1 [17]. Canagliflozin binds to SGLT2 in the proximal tubules, thus blocking reabsorption of glucose, lowering RTG and increasing urinary glucose excretion (UGE) [17,18]. In patients with T2DM, canagliflozin reduces RTG from approximately 240 mg/dl to between 70 and 90 mg/dl over 24 h, resulting in an increase in UGE of approximately 100 g/day [19]. At steady state in healthy subjects, mean 24-h UGE with canagliflozin 300 mg is approximately 25% higher than with dapagliflozin 10 mg [20].
Because of the mechanism of action, the glucose-lowering effect of canagliflozin is insulin independent, and thus not affected by baseline insulin resistance or compromised β-cell function often observed in patients with T2DM [21]. This suggests that SGLT2 inhibitors can be effective at any stage of T2DM, including the advanced stages in which insulin resistance is severe or β-cell function is severely compromised [13].
T2DM tends to affect individuals who are older and/or who have one or more additional cardiovascular risk factors or comorbidities. As such, they often require multiple medications, raising concerns about polypharmacy and drug–drug interactions. Because of its metabolic pathway, canagliflozin has no clinically relevant pharmacokinetic interactions with metformin, sulfonylureas, simvastatin or hydrochlorothiazide – all drugs commonly used in the management of T2DM [22–25]. For younger women with T2DM, it is reassuring that canagliflozin has no clinically relevant drug–drug interactions with oral contraceptives [22–23,25]. The pharmacokinetics of canagliflozin are not altered by administration of the drug after a high-fat meal and are similar regardless of gender, age or bodyweight [26].
Canagliflozin efficacy in Phase III trials
Canagliflozin has been studied across a range of patients with T2DM as monotherapy [27], as a component of dual therapy [10,28–30] and as a part of triple therapy [11,31–32]. In patients with T2DM with a mean baseline A1C of approximately 8.0%, treatment with canagliflozin 100 or 300 mg as monotherapy produced A1C reductions of 0.91 and 1.16%, respectively, relative to placebo, as well as significant reductions in fasting plasma glucose (FPG) and postprandial glucose (PPG) levels (Table 1) [27]. In this trial, 45 and 62% of patients achieved A1C less than 7.0% with canagliflozin 100 and 300 mg, respectively [27]. As an add-on to metformin, canagliflozin 300 mg provided greater reductions in A1C than glimepiride or sitagliptin after 52 weeks; canagliflozin 100 mg produced similar A1C reductions compared with glimepiride and sitagliptin [10,29]. When added to background metformin and a sulfonylurea or metformin and pioglitazone, both canagliflozin 100 and 300 mg significantly reduced A1C relative to placebo [31,32]. In another active-controlled study, treatment with canagliflozin 300 mg led to greater A1C reductions at 52 weeks than sitagliptin in patients with T2DM who were inadequately controlled with metformin and a sulfonylurea [11]. No difference in efficacy response was seen between female and male patients [33].
Table 1.
Summary of the clinical effects of canagliflozin compared with placebo.
| Clinical outcomes | Effect | Ref. |
|---|---|---|
| Diabetes related | ||
| A1C | ↓ | [10,11,27,29] |
| Blood pressure | ↓ | [11,28,29] |
| Bodyweight | ↓ | [10,11,28,29] |
| Fasting plasma glucose | ↓ | [27] |
| Postprandial glucose | ↓ | [27] |
| Hypoglycemia | ↔ | [11,28,34] |
| Adverse events | ||
| Bone mineral density | ↓ | [9,35,36] |
| Fractures | ↔ | [37] |
| Genital mycotic infections | ↑ | [10,11,34,38] |
| Urinary tract infections | ↔ | [12,39] |
↑: Statistically significant increase;
↓: No significant change;
↔: Statistically significant decrease.
As a result of excretion of the glucose and the associated calories, weight loss has been observed with SGLT2 inhibitors [16]. In multiple trials, canagliflozin treatment exerted a positive effect on bodyweight, with dose-related percent bodyweight reductions of approximately 3–5%. Two-thirds of the observed bodyweight reduction with canagliflozin 100 and 300 mg result from reductions in fat mass, with greater reductions in abdominal visceral fat relative to subcutaneous fat [10]. Canagliflozin provided greater reductions in bodyweight compared with sitagliptin (weight neutral) [11,29] and glimepiride (weight increase) [10]. Weight reductions were generally maintained during 2 years of follow-up [28,40].
SGLT2 inhibitors can cause a mild osmotic diuresis associated with increased UGE. In clinical studies, treatment with canagliflozin has been shown to result in decreases in SBP and diastolic blood pressure (DBP). As monotherapy or in combination with other agents, canagliflozin (at both 100 and 300 mg) is associated with dose-related reductions from baseline of approximately 3–5 mmHg SBP and 1–3 mmHg DBP, relative to placebo [41]. Changes in pulse rate were similar for canagliflozin and placebo in these trials. Canagliflozin also provided greater reductions in SBP and DBP compared with sitagliptin and glimepiride [11,28–29]. The reduction in blood pressure observed with canagliflozin was partly associated with weight loss [42].
Canagliflozin safety profile
Overall (all patients)
Canagliflozin, like other SGLT2 inhibitors, has been associated with an increased incidence of adverse effects related to its mechanism of action of increased glucose excretion, including GMIs, UTIs and osmotic diuresis [34]. Because GMIs and UTIs are adverse effects of particular importance in women with diabetes [2,5], they are discussed here in further detail.
A greater incidence of osmotic diuresis-related events such as pollakiuria (increased urinary frequency, the most frequently occurring of these events) and polyuria (increased urinary volume) is reported with canagliflozin compared with placebo [41]. These events tended to occur within the first 6 weeks of initiating treatment [41]. In pooled analyses of 26-week clinical studies, pollakiuria was shown to occur in 4.2, 3.1 and 0.6% of patients treated with canagliflozin 100 mg, canagliflozin 300 mg and placebo, respectively [34,41]. Similarly, in 1-year active-comparator studies, a higher proportion of patients receiving canagliflozin 100 and 300 mg experienced osmotic diuresis-related events compared with those who received glimepiride [10] or sitagliptin [29]. Patients should be educated to recognize the difference between frequent urination, which is related to a higher carbohydrate diet on an SGLT2 inhibitor, and dysuria, which could be a sign of a UTI.
Despite the transient increase in adverse events associated with osmotic diuresis, volume-depletion events – most commonly hypotension, postural dizziness and orthostatic hypotension – were generally uncommon with canagliflozin, and occurred at rates similar to those seen with placebo. In a pooled analysis of four placebo-controlled trials, treatment-related events occurred in 0.5–0.7% of canagliflozin-treated patients and 0.3% of patients receiving placebo [41]. However, in a broader pooled analysis, a few factors were identified that showed an increased risk of volume-related events in patients treated with canagliflozin 300 mg: older age (≥75 years of age), moderate renal impairment (estimated glomerular filtration rate [eGFR] <60 ml/min/m2), and concomitant treatment with loop diuretics [34]. Assessing patients for pre-existing volume-related issues and monitoring during treatment is useful for managing these adverse effects.
Because of its insulin-independent mechanism of action, canagliflozin alone is associated with a low risk of hypoglycemia, with no significant increase in overall or severe hypoglycemia reported in placebo-controlled studies of canagliflozin when used either as monotherapy or with other antihyperglycemic agents not typically associated with hypoglycemia (e.g., metformin or pioglitazone) [34]. Hypoglycemia rates are similar for canagliflozin 300 mg and sitagliptin 100 mg (despite a greater A1C reduction with canagliflozin), and lower with canagliflozin than glimepiride [11,28]. Co-administration with insulin or insulin secretagogues (such as sulfonylureas) increases overall hypoglycemia risk, which is expected for antihyperglycemic agents with a low risk for hypoglycemia; this suggests that adjustment of the insulin or secretagogue dose may be required [30,34,43].
Treatment with canagliflozin results in small changes in low-density lipoprotein cholesterol (LDL-C) levels. Dose-related increases in LDL-C of 4.5 and 8.0% relative to placebo occur with canagliflozin 100 and 300 mg, respectively, and LDL-C monitoring is recommended during therapy. Significant increases in high-density lipoprotein cholesterol (HDL-C) and small decreases in triglycerides are seen consistently in patients treated with canagliflozin, and may be related to improved glycemic control and reductions in bodyweight [34]. Based on data from longer-term trials, the increases in HDL-C and LDL-C appear to occur during the first 6 months of treatment and remain relatively stable thereafter [28,29]. The impact of these lipid changes, along with other changes (e.g., lower A1C, bodyweight, blood pressure, among others), is currently under evaluation in a cardiovascular safety study (CANVAS; ClinicalTrials.gov: NCT01032629) [44].
There have been reports describing concerns that SGLT2 inhibition might have negative effects on bone mineral density (BMD) and increase fracture risk in patients with T2DM [35]. This is of particular concern for older adult patients with T2DM, who by virtue of having diabetes are at higher risk for osteoporosis and fracture [6]. After 2 years of treatment with either canagliflozin 100 or 300 mg in older patients (aged 55–80 years) with T2DM, small but significant reductions (approximately 1% relative to placebo, which translate into a T-score change of −0.1 after 2 years) in BMD were observed in the total hip, with no significant changes in the femoral neck, lumbar spine and distal forearm BMD [9,36]. The change in total hip BMD was significantly associated with the reduction in bodyweight observed with canagliflozin [35].
No increase in fractures was seen with canagliflozin (1.1%) versus noncanagliflozin (1.3%) in a pooled analysis of eight randomized clinical trials, excluding the results from the CANVAS interim analysis [37]. In the interim analysis of CANVAS, which randomized patients with higher cardiovascular risk (mean baseline age was 62 years, duration of diabetes 13 years; hemoglobin A1c 8.2%, FPG 9.3 mmol/l and BMI 32 kg/m2; 57% had a history of atherosclerotic vascular disease), a non-dose-dependent increase in bone fractures was found with canagliflozin (doses pooled: 4.0%) versus placebo (2.6%) [37]. Fractures were observed as early as 12 weeks after treatment initiation, were more likely to be low trauma (e.g., fall from no more than standing height) and affect the upper extremities (e.g., hand and wrist). The increase in fractures may be mediated by falls or other extrinsic factors in the higher-risk population. The effects of canagliflozin on fractures is being monitored in ongoing outcomes studies including CANVAS, CANVAS-R (ClinicalTrials.gov: NCT01989754) and CREDENCE (ClinicalTrials. gov: NCT02065791) – a trial to assess the effects of canagliflozin on renal-related outcomes.
Serious events of diabetic ketoacidosis (DKA), all requiring hospitalization, have been reported to the US FDA Adverse Events Reporting System in association with all SGLT2 inhibitors. Warnings about the potential for DKA have been added to the labeling for SGLT2 inhibitor medications in this class [45]. DKA has occurred in euglycemic patients taking SGLT2 inhibitors [46]. In pooled analysis of more than 17,000 patients with T2DM enrolled in clinical studies with canagliflozin, the overall incidence of DKA and related events was low (<0.1%) and similar across canagliflozin and noncanagliflozin treatment groups [47].
Genital mycotic infections
GMIs are a relatively common experience for women. The most frequently occurring GMI among women, vulvovaginal candidiasis (VVC), affects nearly 75% women at least once during a lifetime [5]. Women with T2DM are more likely than healthy women to be colonized with Candida spp.; the point prevalence of Candida colonization among women with T2DM is reported to be 20–29% [5]. These women are also more likely to develop VVC. A study of >122,000 women treated in primary care practice found that women with T2DM had an 81% higher risk of VVC than women without diabetes [5,48]. Hyperglycemia contributes to GMIs by providing an environment that facilitates fungal growth, impairs neutrophils and complement proteins, and promotes the virulence of yeasts [5,49]. The risk for developing VVC (or any type of infection) in women with T2DM increases with progressively poorer glycemic control [48,50]. Glucosuria, a symptom of uncontrolled hyperglycemia, predisposes women to VVC, likely because increased glucose in the urine creates conditions conducive to yeast adherence, germination and growth. This is relevant to treatment with SGLT2 inhibitors as their mechanism of action increases UGE. In clinical trials, GMIs were reported more frequently during treatment with all SGLT2 inhibitors than with placebo or active comparators [49,51–52].
Diabetes-associated GMIs are less frequent in men. Candida balanitis, a common GMI among men, occurs at a higher rate among men with diabetes than among those without diabetes (16 vs 5.8% in a veterans aging cohort). It is extremely rare among circumcised men [5].
Genital mycotic infections with canagliflozin
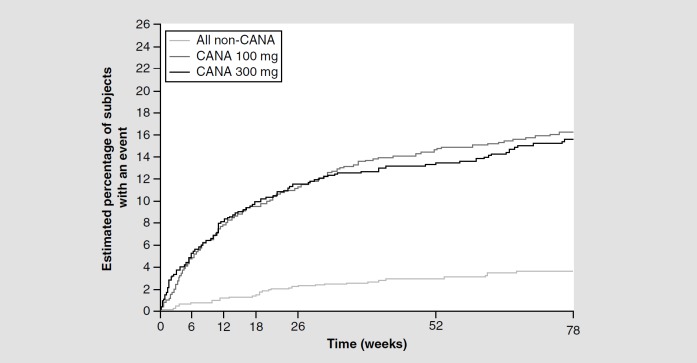
The incidence of GMIs was higher among women and men treated with canagliflozin compared with placebo or active comparators (sitagliptin or glimepiride), but was not shown to be dose related (Table 2) [10–11,38]. Overall, approximately 10% of women treated with canagliflozin developed a GMIs of mild-to-moderate intensity compared with 3% of those treated with placebo in the clinical trials. Women who had a GMIs with canagliflozin tended to report them early (i.e., during the first 3–6 months of treatment), with a decline in risk with time (Figure 2) [38]. No life-threatening GMIs or hospitalization occurred in women in these trials, and greater than 99% of GMIs were considered mild to moderate in intensity [38]. All GMIs responded to standard antifungal treatment, and in many cases were self-treated by the patient; very few GMIs led to treatment discontinuation (0.5–0.9%) [34,38]. The majority of women (78.5%) who developed a GMIs with canagliflozin had a single episode; 18.3% had two episodes and 3.2% had three or more episodes [34,38]. Women with a prior history of VVC were 2.5 times more likely to experience a GMIs during canagliflozin treatment than women with no prior history [38]. Thus, physicians should inquire on previous history of VVC in patients. Furthermore, women who reported a GMIs during canagliflozin treatment tended to be younger (premenopausal), had a slightly higher BMI and were more often colonized with Candida spp. at baseline, compared with those who did not develop a GMIs [38,53].
Table 2.
Rates of genital mycotic infection in a pooled analysis of patients receiving canagliflozin in clinical trials.
| Placebo | Canagliflozin 100 mg | Canagliflozin 300 mg | |
|---|---|---|---|
| Women | n = 312 | n = 425 | n = 430 |
| Any GMI | 3.2 | 10.4 | 11.4 |
| Genital fungal infection | 0.3 | 0 | 0 |
| Vaginal infection | 0.6 | 1.2 | 1.6 |
| Vulvitis | 0 | 0 | 0.5 |
| Vulvovaginal candidiasis | 1.0 | 1.6 | 2.8 |
| Vulvovaginal mycotic infection | 1.3 | 5.9 | 5.3 |
| Vulvovaginitis | 0 | 1.9 | 1.6 |
| Men | n = 334 | n = 408 | n = 404 |
| Any GMI | 0.6 | 4.2 | 3.7 |
| Balanitis | 0 | 2.2 | 1.7 |
| Candida balanitis | 0.3 | 0.5 | 0.5 |
| Balanoposthitis | 0.3 | 1.0 | 0.7 |
| Genital fungal infection | 0 | 0.5 | 0.7 |
Figure 2.
Kaplan–Meier plot of time to first female genital mycotic infection adverse event.
CANA: Canagliflozin.
Reproduced with permission from [38].
Men treated with canagliflozin experienced more GMIs than placebo, but the overall incidence of GMIs was lower compared with women regardless of treatment. The most relevant risk factors for developing GMIs among men were being uncircumcised, prior history of GMIs and a longer duration of T2DM [38]. Two serious GMIs occurred in men, one each with canagliflozin 100 mg and canagliflozin 300 mg, and involved hospitalization for circumcision (phimosis) [38].
Management of genital mycotic infections in canagliflozin-treated patients
Patients with uncontrolled hyperglycemia should be managed to achieve glycemic goals, which in turn may reduce their GMI risk [5]. GMI that emerge during canagliflozin treatment are similar to those observed in noncanagliflozin-treated women, and female patients with T2DM have the same risk factors for VVC. These risk factors include the use of high-estrogen oral contraceptives, intrauterine devices, diaphragms with spermicide, and antibiotics [5]. History and clinical presentation are adequate for most diagnoses [5]. History should include targeted questions about health status, recent medication use, vaginal symptoms, previous VVC episodes, hygiene and use of over-the-counter self-administered medications. Diabetes-related questions should ascertain the T2DM medications used and the degree of glycemic control achieved using current therapies [5]. Symptoms are the same as those in women without T2DM, including vulvar itching, erythema, irritation and vaginal discharge that may be curdy (VVC) or yellowish [5].
In clinical trials, women who develop VVC while being treated with canagliflozin have similar response to typical antifungal agents such as over-the-counter azoles, intravaginal agents and fluconazole [5]. Those with severe infection or very poorly controlled hyperglycemia may need two doses of fluconazole or a longer course of topical azole therapy (≥5 days). If non-Candida spp. are identified, a topical nonfluconazole azole for ≥7 days is the recommended initial therapy [5]. In a study of canagliflozin-treated women with T2DM (n = 198), two of nine infections with culture results were identified as Candida glabrata; no women experienced recurrence after a standard course of antifungal therapy [53].
Urinary tract infections
UTIs are the most commonly encountered bacterial infections in the USA; from 40 to 50% of women will experience at least one symptomatic UTIs during their lifetime [2,50,54]. T2DM itself is a risk factor for UTIs; older age, longer duration of disease, poor glycemic control, presence of complications and diabetic kidney disease further increase the risk of UTIs [55–59]. In postmenopausal women with T2DM, UTIs risk is increased by disease duration of at least 10 years (relative risk: 2.6; 95% CI: 1.3–5.1), and treatment with insulin (relative risk: 3.7; 95% CI: 1.8–7.3) [60]. Treatment with SGLT2 inhibitors, including canagliflozin, increased the risk of developing a UTI (odds ratio: 1.42, 95% CI: 1.06–1.90) [12].
Urinary tract infections with canagliflozin
During canagliflozin clinical trials, a moderate, non-dose-dependent increase in UTI rate was observed in both women and men. In these trials, UTIs occurred in 5.9 and 4.3% of patients treated with canagliflozin 100 and 300 mg, respectively, and 4.0% of patients who received placebo [34]. In both the canagliflozin (pooled doses) and placebo groups, UTIs occurred more often among women (8.7 vs 7.7%, respectively) than among men (1.4 vs 0.6%, respectively). However, the rate of recurrent UTIs was similar with canagliflozin and placebo [34]. In active-comparator studies, the incidence of UTIs was generally similar for canagliflozin versus sitagliptin [11,29].
In clinical trials, UTIs occurring with canagliflozin treatment predominantly involved mild-to-moderate symptoms and responded to standard antimicrobial therapy. Upper UTIs, including urosepsis and pyelonephritis, were rare (0.1%) and there was no increase in their incidence with canagliflozin versus placebo [34,39]. The pattern of mild-to-moderate UTIs and low risk for upper UTIs remained consistent during 2 years of follow-up [28,40]. In patients with moderate renal impairment and in older patients, an increase in UTIs was seen with canagliflozin 300 mg (7.9 and 8.1%, respectively) compared with canagliflozin 100 mg (5.6 and 5.8%, respectively) or placebo (5.6 and 5.1%, respectively) [39].
In postmarketing surveillance by the FDA, 19 cases of urosepsis or pyelonephritis were reported after using/taking SGLT2 inhibitors. As a result, the FDA required the manufacturers of SGLT2 inhibitors to update prescribing information with a warning highlighting the potential for these types of adverse events [61].
Canagliflozin recommended dosing
The starting dose of canagliflozin is 100 mg/day for all patients with an eGFR of at least 45 ml/min/1.732. If more glycemic control is needed, and provided the patient's eGFR is at least 60 ml/min/1.732 and 100 mg dose is tolerated, the dose can be increased to 300 mg/day. Canagliflozin should not be initiated for patients with eGFR <45 ml/min/1.732, and is contraindicated in patients with eGFR <30 ml/min/1.732. Renal function should be assessed prior to starting canagliflozin and periodically during treatment. If the eGFR is persistently <45 ml/min/1.732, canagliflozin should be discontinued [62].
Conclusion
Canagliflozin is associated with reductions in blood glucose, bodyweight and blood pressure. The most common adverse effects observed in clinical trials were an increased incidence of GMIs and increased urination. Overall, canagliflozin is generally well tolerated and provides a unique mechanism of action in the treatment of diabetes in women.
Executive summary
- Canagliflozin
- Sodium-glucose co-transporter 2 inhibitors are a class of oral antihyperglycemic agents that work through an insulin-independent mechanism by inhibiting renally mediated glucose reabsorption.
- Canagliflozin, the first sodium-glucose co-transporter 2 inhibitor approved in the USA, is a once-daily drug that improves glycemic control with a low risk of hypoglycemia in patients with Type 2 diabetes mellitus.
- Canagliflozin safety profile
- In clinical use, canagliflozin is also associated with reductions in blood glucose, bodyweight and blood pressure.
- Canagliflozin is generally well tolerated; the most common adverse effects observed in clinical trials are an increased incidence of genital mycotic infections and increased urination.
- Given the unique mechanism of action and demonstrated efficacy and safety as monotherapy or in combination with metformin, other oral antihyperglycemic agents or basal insulin, canagliflozin may be a useful treatment option in patients with Type 2 diabetes mellitus regardless of their stage of disease.
Financial & competing interests disclosure
P Kushner serves as a consultant for AstraZeneca, Pfizer, Eli Lilly and Janssen Pharmaceuticals; and is a member of the Speaker Bureau for AstraZeneca and Janssen Pharmaceuticals. Canagliflozin has been developed by Janssen Research & Development, LLC, in collaboration with Mitsubishi Tanabe Pharma Corporation. The author has no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Medical writing and editorial assistance in the preparation of this manuscript was provided by Sandrine Buisson and Michael van der Veer of Excerpta Medica. Support for assistance was funded by Janssen Scientific Affairs, LLC.
References
- Papers of special note have been highlighted as: • of interest; •• of considerable interest. [Google Scholar]
- 1.Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2014 (2014). Estimates of Diabetes and its Burden in the United States; www.cdc.gov/diabetes [Google Scholar]
- 2.Foxman B. Epidemiology of urinary tract infections: incidence, morbidity, and economic costs: Am. J. Med. 11 3, S5–S13 (2002). [DOI] [PubMed] [Google Scholar]
- • It describes a broader effect and impact of urinary tract infection on everyday life. [Google Scholar]
- 3.Irwin DE, Milsom I, Hunskaar S, et al. Population-based survey of urinary incontinence, overactive bladder, and lower urinary tract symptoms in five countries: results of the EPIC study. Eur. Urol. 50, 1306–1314 (2006). [DOI] [PubMed] [Google Scholar]
- 4.McGrother CW, Donaldson MMK, Hayward T, et al. Urinary storage symptoms and comorbidities: a prospective population cohort study in middle-aged and older women. Age Ageing 35, 16–24 (2006). [DOI] [PubMed] [Google Scholar]
- 5.Nyirjesy P, Sobel JD. Genital mycotic infections in patients with diabetes. Postgrad. Med. 125, 1–14 (2013). [DOI] [PubMed] [Google Scholar]
- 6.American Diabetes Association. Standards of medical care in diabetes. Diabetes Care 38(Suppl. 1), S1–S93 (2015). [Google Scholar]
- 7.Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in Type 2 diabetes, 2015: a patient-centered approach. Update to a Position Statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 38, 140–149 (2015). [DOI] [PubMed] [Google Scholar]
- 8.Fujita Y, Inagaki N. Renal sodium glucose co-transporter 2 inhibitors as a novel therapeutic approach to treatment of Type 2 diabetes: clinical data and mechanism of action. J. Diabetes Invest. 5, 265–275 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- • Extensive overview of sodium–glucose cotransporter 2 inhibitors in Type 2 diabetes. [Google Scholar]
- 9.Meininger G, Canovatchel W, Polidori D, et al. Canagliflozin for the treatment of adults with Type 2 diabetes. Diabet. Manage. 5, 183–201 (2015). [Google Scholar]
- •• Most recent overview of canagliflozin in treatment of Type 2 diabetes. [Google Scholar]
- 10.Cefalu WT, Leiter LA, Yoon K-H, et al. Efficacy and safety of canigliflozin versus glimepiride in patients with Type 2 diabetes inadequately controlled with metformin [CANTATA-SU]: 52 week results from a randomised, double-blind, Phase 3 non-inferiority trial. Lancet 382, 941–950 (2013). [DOI] [PubMed] [Google Scholar]
- 11.Schernthaner G, Gross JL, Rosenstock J, et al. Canagliflozin compared with sitagliptin for patients with Type 2 diabetes who do not have adequate glycemic control with metformin plus sulfonylurea. A 52-week randomized trial. Diabetes Care 36, 2508–2515 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nauck MA. Update on developments with SGLT2 inhibitors in the management of Type 2 diabetes. Drug Des. Devel. Ther. 8, 1335–1380 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DeFronzo RA, Davidson JA, Del Prato S. The role of the kidneys in glucose homeostasis: a new path toward normalizing glycaemia. Diabet. Obes. Metab. 14, 5–14 (2012). [DOI] [PubMed] [Google Scholar]
- 14.Gerich JE. Role of the kidney in normal glucose homeostasis and in the hyperglycaemia of diabetes mellitus: therapeutic implications. Diabet. Med. 27, 136–142 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rossetti L, Smith D, Shulman GI, Papachristou D, DeFronzo RA. Correction of hyperglycemia with phlorizin normalizes tissue sensitivity to insulin in diabetic rats. J. Clin. Invest. 79, 1510–1515 (1987). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosenwasser RF, Sultan S, Sutton D, Choksi R, Epstein BJ. SGLT-2 inhibitors and their potential in the treatment of diabetes. Diabetes Metab. Syndr. Obes. 6, 453–467 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuriyama C, Xu JZ, Lee SP, et al. Analysis of the effect of canagliflozin on renal glucose reabsorption and progression of hyperglycemia in zucker diabetic fatty rats. J. Pharmacol. Exp. Ther. 351, 423–431 (2014). [DOI] [PubMed] [Google Scholar]
- 18.Sha S, Devineni D, Ghosh A, et al. Canagliflozin, a novel inhibitor of sodium glucose co-transporter 2, dose dependently reduces calculated renal threshold for glucose excretion and increases urinary glucose excretion in healthy subjects. Diabetes Obes. Metab. 13, 669–672 (2011). [DOI] [PubMed] [Google Scholar]
- 19.Devineni D, Curtin CR, Polidori D, et al. Pharmacokinetics and pharmacodynamics of canagliflozin, a sodium glucose co-transporter 2 inhibitor, in subjects with Type 2 diabetes mellitus. J. Clin. Pharmacol. 53, 601–610 (2013). [DOI] [PubMed] [Google Scholar]
- •• Key publication on the mode of action of the sodium–glucose cotransporter 2 inhibitor canagliflozin. [Google Scholar]
- 20.Sha S, Polidori D, Farrell K, et al. Pharmacodynamic differences between canagliflozin and dapagliflozin: results of a randomized, double-blind, crossover study. Diabetes Obes. Metab. 17, 188–197 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matthews M, Zinman B, Tong C, et al. Glycemic efficacy of canagliflozin is largely dependent of baseline beta-cell function or insulin sensitivity. Diabetes 63(Suppl. 1), A285 (2014). [Google Scholar]
- 22.Scheen AJ. Drug–drug interactions with sodium–glucose cotransporter Type 2 (SGLT2) inhibitors, new oral glucose-lowering agents for the management of Type 2 diabetes mellitus. Clin. Pharmacokinet. 53, 295–304 (2014). [DOI] [PubMed] [Google Scholar]
- 23.Devineni D, Vaccaro N, Polidori D, Rusch S, Wajs E. Effects of hydrochlorothiazide on the pharmacokinetics, pharmacodynamics, and tolerability of canagliflozin, a sodium glucose co-transporter 2 inhibitor, in healthy participants. Clin. Ther. 36, 698–710 (2014). [DOI] [PubMed] [Google Scholar]
- 24.Devineni D, Manitpisitkul P, Vaccaro N, et al. Effect of canagliflozin, a sodium glucose co-transporter 2 inhibitor, on the pharmacokinetics of oral contraceptives, warfarin, and digoxin in healthy participants. Int. J. Clin. Pharmacol. Ther. 53, 41–53 (2015). [DOI] [PubMed] [Google Scholar]
- 25.Devineni D, Manitpisitkul P, Murphy J, et al. Effect of canagliflozin on the pharmacokinetics of glyburide, metformin, and simvastatin in healthy individuals. Clin. Pharmacol. Drug Dev. 4, 226–236 (2014). [DOI] [PubMed] [Google Scholar]
- 26.Scheen AJ. Evaluating SGLT2 inhibitors for Type 2 diabetes: pharmacokinetic and toxicological considerations. Expert Opin. Drug Metab. Toxicol. 10, 647–663 (2014). [DOI] [PubMed] [Google Scholar]
- 27.Stenlöf K, Cefalu WT, Kim K-A, et al. Efficacy and safety of canagliflozin monotherapy in subjects with Type 2 diabetes mellitus inadequately controlled with diet and exercise. Diabetes Obes. Metab. 15, 372–382 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leiter LA, Yoon K-H, Arias P, et al. Canagliflozin provides durable glycemic improvements and body weight reduction over 104 weeks versus glimepiride in patients with Type 2 diabetes on metformin: a randomized, double-blind, Phase 3 study. Diabetes Care 38, 355–364 (2015). [DOI] [PubMed] [Google Scholar]
- 29.Lavalle-González FJ, Januszewicz A, Davidson J, et al. Efficacy and safety of canagliflozin compared with placebo and sitagliptin in patients with Type 2 diabetes on background metformin monotherapy: a randomised trial. Diabetologia 56, 2582–2592 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Neal B, Perkovic V, de Zeeuw D, et al. Efficacy and safety of canagliflozin, an inhibitor of sodium cotransporter 2, when used in conjunction with insulin therapy in patients with Type 2 diabetes. Diabetes Care 38, 403–411 (2015). [DOI] [PubMed] [Google Scholar]
- 31.Forst T, Guthrie R, Goldenberg R, et al. Efficacy and safety of canagliflozin over 52 weeks in patients with Type 2 diabetes on background metformin and pioglitazone. Diabetes Obes. Metab. 16, 467–477 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilding JP, Charpentier G, Hollander P, et al. Efficacy and safety of canagliflozin in patients with Type 2 diabetes mellitus inadequately controlled with metformin and sulphonylurea: a randomised trial. Int. J. Clin. Pract. 67, 1267–1282 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gilbert RE, Weir MR, Fioretto P, et al. Impact of age and estimated glomerular filtration rate on the glycaemic efficacy and safety of canagliflozin: a pooled analysis of clinical studies. Canadian J. Diabetes (2016) (In press). [DOI] [PubMed]
- 34.Usiskin K, Kline I, Fung A, Mayer C, Meininger G. Safety and tolerability of canagliflozin in patients with Type 2 diabetes mellitus: pooled analysis of Phase 3 study results. Postgrad. Med. 126, 16–34 (2014). [DOI] [PubMed] [Google Scholar]
- 35.Taylor SI, Blau JE, Rother KI. Possible adverse effects of SGLT2 inhibitors on bone. Lancet Diabetes Endocrinol. 3, 8–10 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bilezikian JP, Watts NB, Usiskin K, et al. Evaluation of bone mineral density and bone mineral biomarkers in patients with Type 2 diabetes treated with canagliflozin. J. Clin. Endocrinol. Metab. 101(1), 44–51 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Watts NB, Bilezikian JP, Usiskin K, et al. Effects of canagliflozin on fracture risk in patients with Type 2 diabetes mellitus. J. Clin. Endocrinol. Metab. 101(1), 157–166 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- • Summary of recent US FDA indicated real-world complication with use of sodium–glucose co-transporter 2 inhibitors. [Google Scholar]
- 38.Nyirjesy P, Sobel JD, Fung A, et al. Genital mycotic infections with canagliflozin, a sodium glucose co-transporter 2 inhibitor, in patients with Type 2 diabetes mellitus: a pooled analysis of clinical studies. Curr. Med. Res. Opin. 30, 1109–1119 (2014). [DOI] [PubMed] [Google Scholar]
- 39.Nicolle LE, Capuano G, Fung A, Usiskin K. Urinary tract infection in randomized Phase III studies of canagliflozin, a sodium glucose co-transporter 2 inhibitor. Postgrad. Med. 126, 7–17 (2014). [DOI] [PubMed] [Google Scholar]
- • Overview of incidence of urinary tract infections in all recent canagliflozin studies. [Google Scholar]
- 40.Bode B, Stenlöf K, Harris S, et al. Long-term efficacy and safety of canagliflozin over 104 weeks in patients aged 55–80 with Type 2 diabetes. Diabetes Obes. Metab. 17, 294–303 (2015). [DOI] [PubMed] [Google Scholar]
- 41.Weir MR, Januszewicz A, Gilbert RE, et al. Effect of canagliflozin on blood pressure and adverse events related to osmotic diuresis and reduced intravascular volume in patients with Type 2 diabetes mellitus. J. Clin. Hypertens. 16, 875–882 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cefalu WT, Stenlöf K, Leiter LA, et al. Effects of canagliflozin on body weight and relationship to HbA1c and blood pressure changes in patients with Type 2 diabetes. Diabetologica 58, 1183–1187 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fulcher G, Matthews DR, Perkovic V, et al. Efficacy and safety of canagliflozin used in conjunction with sulfonylurea in patients with Type 2 diabetes mellitus: a randomized, controlled trial. Diabetes Ther. 6, 289–330 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Neal B, Perkovic V, de Zeeuw D, et al. Rationale, design, and baseline characteristics of the canagliflozin cardiovascular assessment study (CANVAS) – a randomized placebo-controlled trial. Am. Heart J. 155, 217–223 (2013). [DOI] [PubMed] [Google Scholar]
- 45.Food and Drug Adminstration Drug Safety Communication. FDA revises labels of SGLT2 inhibitors for diabetes to include warnings about too much acid in the blood and serious urinary tract infections. www.fda.gov/Drugs
- 46.Rosenstock J, Ferrannini E. Euglycemic diabetic ketoacidosis: a predictable, detectable, and preventable safety concern with SGLT2 inhibitors. Diabetes Care 38, 1638–1642 (2015). [DOI] [PubMed] [Google Scholar]
- 47.Erondu N, Desai M, Ways K, Meininger G. Diabetic ketoacidosis and related events in the Canagliflozin Type 2 Diabetes Clinical Program. Diabetes Care 38, 1680–1686 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hirji I, Andersson SW, Guo Z, Hammar N, Gomez-Caminero A. Incidence of genital infection among patients with Type 2 diabetes in the UK General Practice Research Database. J. Diabet. Compl. 26, 501–505 (2012). [DOI] [PubMed] [Google Scholar]
- 49.Geerlings S, Fonseca V, Castro-Diaz D, List J, Parikh S. Genital and urinary tract infections in diabetes: impact of pharmacologically-induced glucosuria. Diabetes Res. Clin. Pract. 103, 373–381 (2014). [DOI] [PubMed] [Google Scholar]
- 50.Burekovic A, Dizdarevic-Bostandzic A, Godinjak A. Poorly regulated blood glucose in diabetic patients-predictor of acute infections. Med. Arh. 68, 163–166 (2014). [PubMed] [Google Scholar]
- 51.Johnsson KM, Ptaszynska A, Schmitz B, Sugg J, Parikh SJ, List JF. Vulvovaginitis and balanitis in patients with diabetes treated with dapagliflozin. J. Diabet. Compl. 27, 479–484 (2013). [DOI] [PubMed] [Google Scholar]
- 52.Yang XP, Lai D, Zhong XY, Shen HP, Huang YL. Efficacy and safety of canagliflozin in subjects with Type 2 diabetes: systematic review and meta-analysis. Eur. J. Clin. Pharmacol. 70, 1149–1158 (2014). [DOI] [PubMed] [Google Scholar]
- 53.Nyirjesy P, Zhao Y, Ways K, Usiskin K. Evaluation of vulvovaginal symptoms and Candida colonization in women with Type 2 diabetes treated with canagliflozin, a sodium glucose co-transporter 2 inhibitor. Curr. Med. Res. Opin. 28, 1173–1178 (2012). [DOI] [PubMed] [Google Scholar]
- 54.Mody L, Juthani-Mehta M. Urinary tract infections in older women: a clinical review. JAMA 311, 844–854 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Arrellano-Valdez F, Urrutia-Osorio M, Arroyo C, Soto-Vega S. A comprehensive review of urologic complications in patients with diabetes. Springerplus 3, 549 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Boyko EJ, Fihn SD, Scholes D, Chen CL, Normand EH, Yarbro P. Diabetes and the risk of acute urinary tract infection among postmenopausal women. Diabetes Care 25, 1778–1783 (2002). [DOI] [PubMed] [Google Scholar]
- 57.Geerlings SE. Urinary tract infections in patients with diabetes: epidemiology, pathogenesis, and treatment. Int. J. Antimicrob. Agent 31S, S54–S57 (2008). [DOI] [PubMed] [Google Scholar]
- 58.Minf MF, Kamoun M, Kacem FH, et al. Complicated urinary tract infections associated with diabetes mellitus: pathologenesis, diagnosis, and management. Indian J. Endocr. Metab. 17, 442–445 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nitzan O, Elias M, Chazan B, Saliba W. Urinary tract infections in patients with Type 2 diabetes mellitus: review of prevalence, diagnosis, and management. Diabetes Metab. Syndr. Obes. 8, 129–36 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Boyko EJ, Fihn SD, Scholes D, Abraham L, Monsey B. Risk of urinary tract infection and asymptomatic bacteriuria among diabetic and nondiabetic postmenopausal women. Am. J. Epidemiol. 161, 557–564 (2005). [DOI] [PubMed] [Google Scholar]
- 61.Food and Drug Adminstration Drug Safety Communication. FDA revises labels of SGLT2 inhibitors for diabetes to include warnings about too much acid in the blood and serious urinary tract infections – Update. www.fda.gov/Drugs/DrugSafety
- 62.Invokana [canagliflozin], Janssen (US), US prescribing information (2015). www.invokanahcp.com/prescribing-information.pdf