Abstract
Aim: Evaluate efficacy/safety of bremelanotide (BMT), a melanocortin-receptor-4 agonist, to treat female sexual dysfunctions in premenopausal women. Methods: Patients randomized to receive placebo or BMT 0.75, 1.25 or 1.75 mg self-administered subcutaneously, as desired, over 12 weeks. Primary end point was change in satisfying sexual events/month. Secondary end points included total score changes on female sexual function index and female sexual distress scale-desire/arousal/orgasm. Results: Efficacy data, n = 327. For 1.25/1.75-mg pooled versus placebo, mean changes from baseline to study end were +0.7 versus +0.2 satisfying sexual events/month (p = 0.0180), +3.6 versus +1.9 female sexual function index total score (p = 0.0017), −11.1 versus −6.8 female sexual distress scale-desire/arousal/orgasm total score (p = 0.0014). Adverse events: nausea, flushing, headache. Conclusion: In premenopausal women with female sexual dysfunctions, self-administered, as desired, subcutaneous BMT was safe, effective, and well tolerated (NCT01382719).
Keywords: bremelanotide, female sexual arousal disorder, female sexual dysfunction, FSD, hypoactive sexual desire disorder, HSDD
Female sexual dysfunctions (FSD) are a range of distressing, multifactorial conditions for which few treatment options exist. In the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision, the conditions are classified as disorders of sexual desire (hypoactive sexual desire disorder [HSDD]), sexual arousal (female sexual arousal disorder [FSAD]), orgasm (delay or absence) or sexual pain (dyspareunia or vaginismus) [1]. Other conceptualizations view the conditions as overlapping comorbidities [2,3]. Indeed, the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, replaces HSDD and FSAD with a new diagnostic category, female sexual interest/arousal disorder [4]. In a US national household survey of adult sexual behavior conducted in 1992, the overall prevalence of sexual dysfunction among women 18–59 years old was 43% [5]. Moreover, all types of sexual dysfunction (low sexual desire, arousal disorder and sexual pain) exhibited strong associations with low feelings of satisfaction and happiness. Another US national household survey, conducted in 2006 [6], reported the prevalence of ‘any sexual problem’ among women ≥18 years old to be 43%. The prevalence of ‘any distressing sexual problem’ (with a score ≥15 on the female sexual distress scale [FSDS] [7]) was 12.0%. Women in the 45–64-year-old group also had a higher prevalence of distressing low sexual desire (12.3%) and arousal (7.5%) than women 18–44 years old or ≥65 years old [6]. Distress is a defining and common characteristic of these disorders and may lead to consultation and treatment seeking, especially if safe and effective pharmacotherapy is available.
Bremelanotide (BMT) is a novel cyclic 7-aminoacid melanocortin-receptor agonist with high affinity for the type-4 receptor [8], giving it a potential to modulate brain pathways involved in sexual response [9]. For example, melanocortinergic neurons may stimulate dopamine release in the medial preoptic area, a locus implicated in the sexual behavior of both sexes of several species [10,11]. In hormone-primed female rats, BMT administered systemically, by infusion into the lateral ventricles or directly into the medial preoptic area significantly and selectively increased the appetitive sexual behaviors used by females to entice males into sexual activity, such as solicitations, hops and darts and mounting [10,11]. Clinical studies have documented the penile erectogenic efficacy of BMT in healthy men and in men with erectile dysfunction [12,13]. In a placebo-controlled feasibility study of its potential use for FSD, significantly more premenopausal women with FSAD reported moderate or high sexual desire after intranasal dosing of a BMT formulation, with a trend toward greater feelings of genital arousal [14]. A 2-month Phase II trial evaluated an intranasal BMT formulation in pre- and post-menopausal women with FSAD. Significant treatment effects were observed for satisfying sexual events (SSEs), desire and arousal [15].
The present Phase IIB trial was designed to explore the safety and efficacy of subcutaneous BMT in a well-characterized population of premenopausal women with HSDD, FSAD or both, and identify appropriate doses for Phase III registration trials. Based on prior clinical studies [12–15] and pharmacokinetic modeling, subcutaneous doses of 0.75, 1.25 and 1.75 mg were selected. The trial was supported by a data safety monitoring board and was registered with ClinicalTrials. gov (identifier: NCT01382719).
Methods & data analysis
Study participants
All patients were required to be premenopausal [16], nonpregnant women at least 21 years old with FSAD, HSDD or a combination of these disorders for at least the preceding 6 months, as diagnosed by a qualified clinician using a diagnostic interview plus a total score >18 on the female sexual distress scale-desire/arousal/orgasm (FSDS-DAO) [17] and <26.5 on the female sexual function index (FSFI) [18]. Inclusion also required previous sexual functionality for at least 2 years, a current stable, monogamous relationship of at least 6-month duration with a male or female partner and willingness to be sexually active at least once per month. If a change in partner occurred, the patient would be discontinued.
Patients were excluded for unstable or uncontrolled medical conditions; lifelong sexual dysfunction; pelvic inflammatory disease, cervical dysplasia, clinically significant cervicitis, chronic or complicated urinary tract infection or chronic dyspareunia (not attributable to vaginal dryness) within the preceding 12 months; clinically significant condyloma or more than one outbreak of genital herpes within the preceding 6 months; active moderate to severe vaginitis or a clinically significant vaginal infection; current gynecologic conditions that might interfere with study procedures; a history of unresolved sexual trauma or abuse; a history of hysterectomy or any other procedure that affects the menstrual cycle; FSD secondary to untreated endocrine disease; current psychotherapy for FSD; treatment of depression or psychosis within the preceding 6 months; use of antidepressants or antipsychotics within the preceding 3 months; and use of any drug or nutritional supplement known to affect sexual arousal or desire. Patients were also excluded for a current diagnosis of uncontrolled hypertension, repeated observation of a systolic blood pressure (SBP) ≥140 mmHg or diastolic blood pressure (DBP) ≥90 mmHg or change in any antihypertensive therapy within the preceding 6 weeks.
Study design
Each study site was reviewed by a central or local Institutional Review Board or ethics committee. Prior to study procedures, written informed consent was obtained from each patient. After a no-treatment month to confirm diagnoses, each patient self-administered, under clinical research staff supervision, a single-blind, in-clinic placebo dose, followed by 1-week monitoring. Patients then entered a 4-week period of single-blind, at-home placebo self-dosing, to establish each patient's baseline. By an interactive voice/web response system, patients were then randomized in a 1:1:1:1 ratio, with stratification by diagnosis (FSAD, HSDD or mixed FSAD/HSDD), to double-blind placebo or BMT 0.75, 1.25 or 1.75 mg. The double-blind treatment began with two self-administered in-clinic study-drug doses spaced approximately 1 week apart. This was followed by 12 weeks of at-home, as-desired self-dosing approximately 45 min prior to anticipated sexual activity (not exceeding one dose per day or 16 doses during a 4-week period). During this study phase, patients were assessed every 4 weeks. Throughout the study, BMT or matching placebo was provided in prefilled syringes as an aqueous solution (0.3 ml volume) for subcutaneous injection into the anterior thigh or abdomen.
Main outcome measures
The primary efficacy end point was each patient's change, from baseline to end of study (EOS), in the number of SSEs, as measured by a response of ‘Yes’ to question 10 (Q10) of the Female Sexual Encounter Profile-Revised questionnaire (FSEP-R) [19]. The end point was calculated as the number of events during the last 4 weeks of treatment with Q10 = Yes minus the number of baseline events with Q10 = Yes. Q10 reads “Did you consider this sexual encounter satisfactory for you?” The questionnaire was completed at home using an electronic diary within 24 h after each sexual encounter, regardless of whether study drug had been used.
Secondary end points included change from baseline to EOS in total FSFI score, FSFI domain scores for desire and arousal, total FSDS-DAO score and individual FSDS-DAO item #14 (arousal) and #13 (desire) scores. The FSFI is a validated 19-item self-assessment of sexual feelings and responses, each on a five- or six-choice scale [18]. Increased scores indicate improvement. The FSDS-DAO is a validated 15-item self-assessment of sexual feelings and problems, each on a five-choice scale [17]. Decreased scores indicate improvement. Each questionnaire has been extensively utilized to measure female sexual health.
Safety assessments
Throughout the study, all patients were asked to report adverse events. Safety assessments also included physical examination, vital signs, 12-lead ECG and clinical laboratory tests.
BP monitoring
At each of the three in-clinic study-drug dosing administrations, BP was monitored manually for 2 h and 24-h ambulatory BP monitoring (ABPM) was initiated with a 15-min interrogation interval. Intensive BP monitoring was included to address US FDA concerns expressed after earlier studies of an intranasal BMT formulation [20]. Patients were withdrawn from the study if they sustained elevated values, predefined as SBP ≥150–170 mmHg/DBP ≥95–105 mmHg in two to four consecutive readings (15 min apart), or a change from baseline in SBP ≥30 mmHg/DBP ≥15 mmHg in two to four consecutive readings (15 min apart).
Statistical analyses
Efficacy was analyzed by Van Elteren tests stratified by diagnosis, which were performed at an α = 0.05 significance level on data from all randomized patients who used at least one at-home dose of double-blind study drug and were assessed after at least the first 4-week interval of at-home use (modified intent-to-treat [mITT] population). The primary efficacy analysis compared placebo and the two highest BMT dose levels pooled (1.25 and 1.75 mg) with respect to the primary end point. To maintain a 0.05 significance level, statistical testing was performed hierarchically on the primary and then each secondary end point, in a prespecified order. Safety was assessed descriptively in all recipients of at least one in-clinic dose of double-blind study drug (safety population).
Sample size calculation
A sample size of 100 patients on placebo and 100 in each of the two highest-dose BMT groups, or 200 pooled, was predicted to have 80% power to detect a difference of 1.4 SSEs, using a two-sided Wilcoxon–Mann–Whitney test at the 0.05 significance level and assuming an standard deviation (SD) of 4 (based on a previous study [15]).
Results
Participant flow
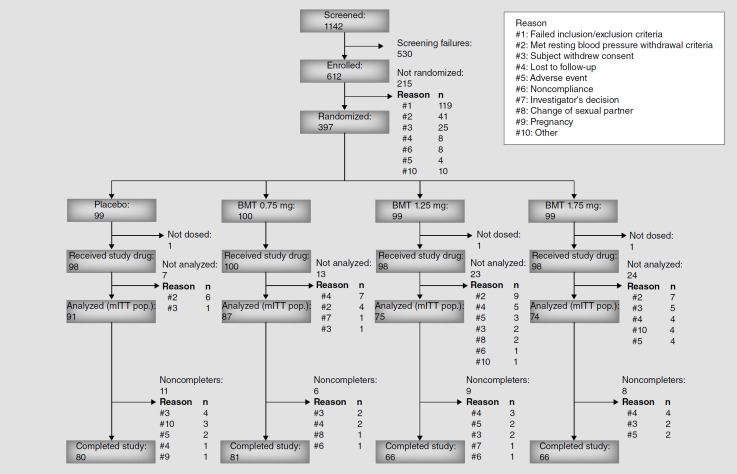
In all, 1142 patients were screened at 67 US and Canadian clinical sites. Among them, 612 patients were enrolled, 397 were randomized, 394 received at least one in-clinic dose of double-blind study drug (safety population), 327 provided data after 4 weeks of double-blind treatment (mITT population) and 293 completed the study. The study began in June 2011 with the final patient visit in September 2012. Patient flow throughout the study is diagrammed by treatment group in Figure 1.
Figure 1.
Patient disposition.
BMT: Bremelanotide; mITT: Modified intent-to-treat; Pop.: Population.
Patients' characteristics
Baseline characteristics of all double-blind study-drug recipients are summarized by treatment group in Table 1, upper panel. Overall, the mean age was 37 years (range: 21–53 years), and 71% were white. Most patients had regular menses (79%) and were not using oral contraceptives (87%). In the mITT population, 74% had diagnoses of both FSAD and HSDD, 23% had solely a diagnosis of HSDD and 3% solely a diagnosis of FSAD. Baseline values of FSD measures in the mITT population are summarized in Table 2, upper panel. By treatment group, the mean number of SSEs during the 4 weeks preceding randomization ranged from 1.5 to 1.9.
Table 1.
Patients' characteristics (safety population).
| Characteristic | Placebo group | BMT groups |
|||
|---|---|---|---|---|---|
| 0.75 mg | 1.25 mg | 1.75 mg | 1.25/1.75 mg pooled | ||
| All patients | |||||
| n | 97† | 100 | 99† | 98 | 196 |
| Age (years), mean (SD) | 37.0 (7.7) | 37.6 (7.8) | 35.7 (7.2) | 37.0 (7.6) | 36.4 (7.4) |
| Race, n (%): – White | 75 (77) | 71 (71) | 65 (66) | 70 (71) | 135 (69) |
| – Black | 19 (20) | 25 (25) | 32 (32) | 23 (23) | 55 (28) |
| – Other | 3 (3) | 4 (4) | 2 (2) | 5 (5) | 7 (4) |
| Weight at screening (lbs), mean (SD) | 164.4 (42.1) | 168.2 (37.9) | 174.0 (43.2) | 179.2 (45.9)‡ | 176.5 (44.5)§ |
| BMI at screening (kg/m2), mean (SD) | 27.7 (6.2) | 28.5 (6.6)¶ | 29.2 (7.1) | 29.9 (7.2)‡ | 29.5 (7.1)§ |
| FSD diagnosis, n (%): | |||||
| – FSAD | 4 (4) | 3 (3) | 3 (3) | 2 (2) | 5 (3) |
| – HSDD | 24 (25) | 20 (20) | 24 (24) | 24 (24) | 48 (24) |
| – Mixed | 69 (71) | 77 (77) | 72 (73) | 72 (73) | 144 (73) |
| Menses frequency regular, n (%) | 72 (74) | 75 (75) | 86 (87) | 79 (81) | 165 (84) |
| Used oral contraception within the 30 days before screening, n (%) | 12 (12) | 15 (15) | 11 (11) | 15 (15) | 26 (13) |
| Patients with primarily or solely HSDD | |||||
| n | 81 | 87 | 87 | 85 | 172 |
| Age (years), mean (SD) | 36.3 (7.7) | 37.6 (7.9) | 35.9 (7.1) | 37.4 (7.6) | 36.6 (7.4) |
| Race, n (%): – White | 66 (82) | 63 (72) | 59 (68) | 64 (75) | 123 (72) |
| – Black | 13 (16) | 22 (25) | 27 (31) | 16 (19) | 43 (25) |
| – Other | 2 (3) | 2 (2) | 1 (1) | 5 (6) | 6 (4) |
| Weight at screening (lbs), mean (SD) | 162.8 (40.9) | 171.7 (38.2) | 174.8 (43.0) | 174.4 (45.3)# | 174.6 (44.0)†† |
| BMI at screening (kg/m2), mean (SD) | 27.5 (5.9) | 29.1 (6.6)‡‡ | 29.3 (7.1) | 29.2 (7.0)# | 29.3 (7.1)†† |
| HSDD diagnosis, n (%): | |||||
| – Primary | 57 (70) | 67 (77) | 63 (72) | 61 (72) | 124 (72) |
| – Sole | 24 (30) | 20 (23) | 24 (28) | 24 (28) | 48 (28) |
| Menses frequency regular, n (%) | 60 (74) | 63 (72) | 75 (86) | 67 (79) | 142 (83) |
| Used oral contraception within the 30 days before screening, n (%) | 10 (12) | 11 (13) | 9 (10) | 14 (17) | 23 (13) |
During visit 2 (prior to randomization) one patient inadvertently received a treatment kit containing BMT 1.25 mg in place of the assigned placebo. In accordance with the study protocol, this individual was considered a 1.25 mg BMT patient within the safety population.
n = 97.
n = 196.
n = 98.
n = 84.
n = 171
n = 85.
BMT: Bremelanotide; FSAD: Female sexual arousal disorder; FSD: Female sexual dysfunction; HSDD: Hypoactive sexual desire disorder; SD: Standard deviation.
Table 2.
Female sexual dysfunction measures: mean (standard deviation) baseline† values (modified intent-to-treat population population).
| Measure | Placebo group | BMT groups |
|||
|---|---|---|---|---|---|
| 0.75 mg | 1.25 mg | 1.75 mg | 1.25/1.75 mg pooled | ||
| All patients | |||||
| n | 91 | 87 | 75 | 74 | 149 |
| Prespecified measures‡: | |||||
| – SSEs during the 28 days before | 1.7 (1.9) | 1.9 (2.1)§ | 1.5 (1.6) | 1.8 (2.6)¶ | 1.6 (2.1)# |
| randomization (FSEP-R Q10) | |||||
| – FSDS-DAO arousal score†† | 2.3 (1.1) | 2.1 (1.0) | 2.3 (1.0) | 2.4 (0.9) | 2.3 (1.0) |
| – FSDS-DAO desire score†† | 2.6 (1.0) | 2.4 (0.9) | 2.5 (1.0) | 2.7 (0.9) | 2.6 (1.0) |
| – FSFI total score‡‡ | 21.9 (5.9) | 22.8 (5.4) | 21.5 (5.4) | 21.7 (5.0) | 21.6 (5.2) |
| – FSFI arousal score§§ | 3.0 (1.3) | 3.2 (1.2) | 2.9 (1.2) | 2.9 (1.1) | 2.9 (1.3) |
| – FSFI desire score§§ | 2.4 (1.0) | 2.6 (1.1) | 2.5 (0.9) | 2.4 (1.0) | 2.5 (0.9) |
| – FSDS-DAO total score¶¶ | 32.1 (12.8) | 30.5 (12.4) | 32.7 (13.8) | 33.3 (12.7) | 33.0 (13.2) |
| Exploratory measures: | |||||
| – Level of sexual arousal (FSEP-R Q6)## | 1.4 (0.7) | 1.4 (0.7)§ | 1.2 (0.7) | 1.3 (0.7)¶ | 1.3 (0.7)# |
| – Satisfaction with arousal (FSEP-R Q7)††† | 1.1 (0.8) | 1.2 (0.8)§ | 1.0 (0.7) | 1.0 (0.8)¶ | 1.0 (0.7)# |
| – Level of sexual desire (FSEP-R Q3)## | 1.1 (0.7) | 1.2 (0.7)§ | 1.1 (0.7) | 1.0 (0.7)¶ | 1.1 (0.7)# |
| – Satisfaction with desire (FSEP-R Q4)††† | 0.9 (0.8) | 0.9 (0.8)§ | 0.8 (0.8) | 0.8 (0.8)¶ | 0.8 (0.8)# |
| Patients with primarily or solely HSDD‡‡‡ | |||||
| n | 76 | 76 | 66 | 63 | 129 |
| Exploratory measures: | |||||
| – SSEs during the 28 days before randomization (FSEP-R Q10) | 1.6 (1.8) | 1.8 (2.0)§§§ | 1.5 (1.4) | 1.9 (2.7)¶¶¶ | 1.6 (2.1)### |
| – FSFI total score‡‡ | 22.2 (6.1) | 22.8 (5.6) | 21.6 (5.5) | 21.5 (4.9) | 21.6 (5.2) |
| – FSFI desire score§§ | 2.4 (1.0) | 2.6 (1.1) | 2.5 (0.9) | 2.3 (0.9) | 2.4 (0.9) |
| – FSDS-DAO total score¶¶ | 32.9 (13.0) | 31.1 (12.4) | 32.5 (14.0) | 33.7 (12.3) | 33.1 (13.1) |
| – FSDS-DAO desire score†† | 2.6 (1.0) | 2.5 (0.9) | 2.5 (1.0) | 2.7 (0.95) | 2.6 (1.0) |
Each patient's single-blind placebo month.
Listed in the order prespecified by the study protocol for hierarchical statistical testing.
n = 85.
n = 73.
n = 148.
From 0 (best possible score) to 4 (worst possible score).
From 2 (worst possible total score) to 36 (best possible total score).
From 0 (worst possible score) to 6 (best possible score).
From 0 (best possible total score) to 60 (worst possible total score).
From 0 (no desire or arousal) to 3 (high desire or arousal).
From 0 (not at all satisfied) to 3 (completely satisfied).
HSDD with or without decreased arousal.
n = 74.
n = 62.
n = 128.
BMT: Bremelanotide; FSDS-DAO: Female sexual distress scale-desire/arousal/orgasm; FSEP-R: Female sexual encounter profile-revised; FSFI: Female sexual function index; HSDD: Hypoactive sexual desire disorder; SSE: Satisfying sexual event.
Efficacy
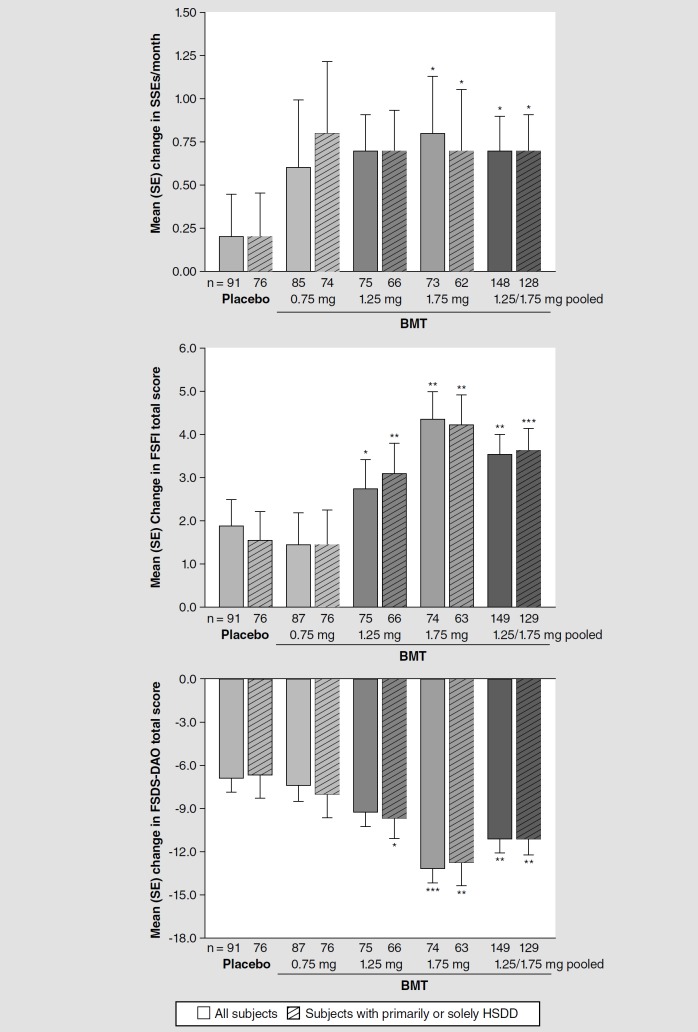
BMT-treated patients showed statistically significant and clinically important improvements in the primary end point and many secondary end points. In the mITT population, the mean (SD) change in number of SSEs from baseline to EOS was +0.7 (2.4) events/month for BMT 1.25/1.75 mg pooled, compared with +0.2 (2.3) for placebo (p = 0.0180). Among secondary outcome measures, the mean change in FSFI total score was +3.6 (5.7) versus +1.9 (5.9; p = 0.0017) and the mean change in FSDS-DAO total score was −11.1 (12.0) versus −6.8 (13.6; p = 0.0014). In preplanned responder analyses anchored to historical estimates of clinically important change, significantly greater proportions of patients in the pooled 1.25/1.75-mg groups (and in the 1.75-mg group alone) showed responses on these end points, compared with proportions in the placebo group. For 1.25/1.75 mg pooled, the responder proportions were 51 versus 37% for SSEs/month (p = 0.0381, Cochran–Mantel–Haenszel test), 47 versus 29% for FSFI total score (p = 0.0051) and 64 versus 45% for FSDS-DAO total score (p = 0.0032).
Figure 2 displays the mean changes in number of SSEs, FSFI total score and FSDS-DAO total score by treatment group. For other prespecified outcome measures, Table 3 lists the mean changes by treatment group. The first secondary measure to undergo hierarchical testing, FSDS-DAO arousal score, showed a statistically insignificant change for 1.25/1.75 mg pooled; thus all secondary end points were considered exploratory and p-values are therefore nominal. However, statistically significant improvement versus placebo was identified at 1.25/1.75 mg for total score and for desire score on both the FSDS-DAO and the FSFI, and at 1.75 mg for all FSDS-DAO and FSFI outcomes.
Figure 2.
Mean (standard error) change in satisfying sexual events/month: female sexual function index total score: and female sexual distress scale-desire/arousal/orgasm total score from double-blind baseline to end of study in all patients and in patients with hypoactive sexual desire disorder as their primary or sole female sexual dysfunction diagnosis (modified intent-to-treat population) (see facing page). Van Elteren test stratified by diagnosis.
*p < 0.05 versus placebo.
**p < 0.01 versus placebo.
***p < 0.001 versus placebo.
BMT: Bremelanotide; FSDS-DAO: Female sexual distress scale-desire/arousal/orgasm; FSFI: Female sexual function index; HSDD: Hypoactive sexual desire disorder; SE: Standard error; SSE: Satisfying sexual event.
Table 3.
Efficacy outcomes: mean (standard deviation) changes from baseline† to end of study (modified intent-to-treat population).
| Outcome measure | Placebo group | BMT groups |
|||||
|---|---|---|---|---|---|---|---|
| 0.75 mg | 1.25 mg | 1.75 mg | 1.25/1.75 mg pooled | ||||
| All patients | |||||||
| n | 91 | 87 | 75 | 74 | 149 | ||
| Prespecified measures‡: | |||||||
| – SSEs (events per 28 days, from FSEP-R Q10) | + 0.2 (2.3) | + 0.6 (3.6)§ | +0.7 (1.8) | + 0.8 (2.9)*¶ | + 0.7 (2.4)*# | ||
| – FSDS-DAO arousal score | −0.5 (1.3) | −0.5 (1.2) | −0.5 (0.9) | −0.9 (1.1)* | −0.7 (1.0) | ||
| – FSDS-DAO desire score | −0.6 (1.3) | −0.5 (1.1) | −0.7 (1.0) | −1.1 (1.2)** | −0.9 (1.1)* | ||
| – FSFI total score | + 1.9 (5.9) | +1.5 (6.9) | +2.8 (5.7)* | + 4.4 (5.6)** | +3.6 (5.7)** | ||
| – FSFI arousal score | +0.5 (1.4) | +0.4 (1.5) | +0.7 (1.1) | + 1.1 (1.2)** | +0.9 (1.1) | ||
| – FSFI desire score | + 0.4 (1.1) | +0.3 (1.1) | +0.6 (0.8) | + 1.0 (1.0)** | +0.8 (0.9)** | ||
| – FSDS-DAO total score | −6.8 (13.6) | −7.4 (13.5) | −9.2 (10.8) | −13.1 (12.9)*** | −11.1 (12.0)** | ||
| Exploratory measures: | |||||||
| – Level of sexual arousal (FSEP-R Q6) | −0.1 (0.9) | + 0.1 (1.0)§ | + 0.2 (0.9)* | + 0.4 (0.8)*** | + 0.3 (0.9)***# | ||
| – Satisfaction with arousal (FSEP-R Q7) | 0.0 (1.0) | + 0.1 (1.0)§ | + 0.5 (1.0)* | + 0.6 (0.8)***¶ | + 0.5 (0.9)***# | ||
| – Level of sexual desire (FSEP-R Q3) | 0.0 (0.9) | 0.0 (0.9)§ | +0.3 (0.8)* | + 0.4 (0.8)***¶ | + 0.4 (0.8)***# | ||
| – Satisfaction with desire (FSEP-R Q4) | +0.1 (1.0) | + 0.3 (0.9)§ | + 0.4 (0.8)* | +0.6 (0.8)**¶ | + 0.5 (0.8)**# | ||
| Patients with primarily or solely HSDD†† | |||||||
| n | 76 | 76 | 66 | 63 | 129 | ||
| Exploratory measures: | |||||||
| – SSEs (events per 28 days, from FSEP-R Q10) | +0.2 (2.2) | + 0.8 (3.60)‡‡ | +0.7 (1.9) | + 0.7 (2.8)*§§ | +0.7 (2.3)*¶¶ | ||
| – FSFI total score | + 1.6 (5.9) | +1.5 (7.2) | +3.1 (5.5)** | +4.2 (5.4)** | +3.7 (5.5)*** | ||
| – FSFI desire score | + 0.4 (1.1) | +0.3 (1.1) | +0.6 (0.8) | + 1.0 (0.9) | +0.8 (0.9) | ||
| – FSDS-DAO total score | −6.6 (14.1) | −8.0 (13.4) | −9.6 (11.2)* | −12.7 (12.6)** | −11.1 (12.0)** | ||
| – FSDS-DAO desire score | 0.6 (1.2) | −0.5 (1.0) | −0.7 (1.0) | −1.0 (1.1) | −0.9 (1.1) | ||
Van Elteren test stratified by diagnosis.
p < 0.05 versus placebo.
p < 0.01 versus placebo.
p < 0.001 versus placebo.
Each patient's single-blind placebo month.
Listed in the order prespecified by the study protocol for hierarchical statistical testing.
n = 85.
n = 73.
n = 148.
HSDD with or without decreased arousal.
n = 74.
n = 62.
n = 128.
BMT: Bremelanotide; FSDS-DAO: Female sexual distress scale-desire/arousal/orgasm; FSEP-R: Female sexual encounter profile-revised; FSFI: Female sexual function index; HSDD: Hypoactive sexual desire disorder; SSEs: Satisfying sexual events.
Efficacy per episode (exploratory analyses)
Episodic experience with BMT was assessed by patients' responses to FSEP-R items after each sexual encounter. Among such responses, patients rated their level of sexual arousal, their satisfaction with arousal, their level of sexual desire and their satisfaction with desire. On all these items, 4-week averages at EOS showed statistically significant improvement from baseline at 1.25 and 1.75 mg (see Table 3).
Efficacy in HSDD (exploratory analyses)
In the mITT population, 281 patients (86%) had HSDD as either their primary or their sole FSD diagnosis (206 and 75 patients, respectively). Their baseline characteristics (Table 1, lower panel) and baseline FSD measures (Table 2, lower panel) resembled those across the entire study population. At EOS, their mean improvements in SSEs/month and in FSFI and FSDS-DAO scores also resembled those in the broader study (see Figure 2 & Table 3).
Safety
Treatment-emergent adverse events (TEAEs) reported during double-blind study-drug treatment are summarized in Table 4. At all BMT doses, the most common TEAEs were nausea, flushing and headache. Of these events, only nausea had a slight dose-dependence. Seventy BMT users (24% of 297) and seven placebo users (7% of 97) reported injection-site reactions, defined as encompassing irritation, rash, urticaria, swelling, pruritus, warmth, erythema, hematoma, hemorrhage, induration, nodule or pain. Among these patients, four BMT users and one placebo user had injection-site reactions classified as moderate. No injection-site reactions were classified as severe, and none were accompanied by signs or symptoms of a more generalized hypersensitivity response. During double-blind treatment, three BMT users and one placebo user reported serious TEAEs. Each patient had a history of the noted condition (asthma exacerbation, ventral incisional hernia and noncardiac chest pain in the BMT users and lumbar–disk herniation in the placebo user). No serious TEAEs were attributed to BMT.
Table 4.
Treatment-emergent adverse events during double-blind treatment (safety population).
| TEAE | Placebo group (n = 97)†, n (%) | BMT groups, n (%) |
||
|---|---|---|---|---|
| 0.75 mg (n = 100) | 1.25 mg (n = 99)† | 1.75 mg (n = 98) | ||
| Any TEAE‡: | 49 (51) | 64 (64) | 61 (62) | 67 (68) |
| – Nausea | 3 (3) | 18 (18) | 22 (22) | 24 (24) |
| – Flushing | 0 | 17 (17) | 14 (14) | 17 (17) |
| – Headache | 3 (3) | 9 (9) | 9 (9) | 14 (14) |
| – Injection-site pain | 3 (3) | 6 (6) | 6 (6) | 7 (7) |
| – Upper respiratory tract infection | 4 (4) | 8 (8) | 5 (5) | 4 (4) |
| – Injection-site pruritus | 0 | 4 (4) | 4 (4) | 6 (6) |
| Any TEAE leading to withdrawal§: | 2 (2) | 0 | 5 (5) | 6 (6) |
| – Vomiting | 0 | 0 | 1 (1) | 2 (2) |
| – Nausea | 0 | 0 | 0 | 3 (3) |
| – Flushing | 0 | 0 | 1 (1) | 1 (1) |
During visit 2 (prior to randomization) one patient inadvertently received a treatment kit containing BMT 1.25 mg in place of the assigned placebo. In accordance with the study protocol, this individual was considered a 1.25 mg BMT patient within the safety population.
The types listed each had an incidence ≥5% among all BMT users.
The types listed each occurred in >1 BMT user. The listing excludes hypertension, which was coded as an adverse event in two placebo and three BMT users among the 26 protocol-mandated ambulatory blood-pressure monitoring-based withdrawals.
BMT: Bremelanotide; TEAE: Treatment-emergent adverse event.
Throughout the study, no patients had in-clinic, manual BP findings meeting the predefined criteria for BP-related withdrawal. By ABPM findings prior to randomization, 41 patients (8% of 489) met the criteria after in-clinic placebo administration, and were withdrawn. During double-blind treatment, 26 patients (6.5% of 397) met the criteria. These events were evenly distributed among treatment arms: 0.75 mg (n = 4), 1.25 mg (n = 9), 1.75 mg (n = 7) and placebo (n = 6). An additional 13 patients withdrew during double-blind treatment because of other TEAEs, most commonly vomiting, nausea or flushing (see Table 4). Throughout the study, physical examination, ECG and clinical laboratory findings showed no clinically significant trends. Overall, the study's ABPM data associated subcutaneous BMT administered at 1.25 or 1.75 mg with small, transient increases in ambulatory BP (∼3 mmHg in SBP and DBP, confined to the first 4 h postdose), accompanied by small (∼5%) decreases in heart rate. Cardiovascular safety findings will be presented more fully in a separate report.
Discussion
At 1.25/1.75 mg pooled, BMT was associated with a statistically and clinically significant improvement in SSEs per month (the study's primary outcome measure), and with consistent improvements in FSFI total score and FSDS-DAO total score.
The design of this Phase IIB study was notable for its inclusion of patients with HSDD, FSAD or a combination of these conditions. HSDD was the primary diagnosis in 63% of the women and the sole diagnosis in another 23%. In exploratory analyses, the outcomes of BMT use in women with HSDD as either their primary or their sole diagnosis (i.e., HSDD with or without decreased arousal) resembled those in the study's full mITT population, including dose-related improvements on multiple FSD measures, with statistical significance versus placebo at 1.75 mg and at 1.25/1.75 mg pooled.
The design of the study was also notable for including, prior to randomization, a 4-week single-blind placebo self-dosing period. The observed placebo responses were not employed to exclude any patients from further study participation, as would be the case in a study with an enrichment design. Rather, they served to set rigorous baselines for the study's efficacy and ABPM analyses.
In general, BMT was safe and well tolerated. During double-blind treatment, nausea was reported by 64 BMT users, or 22% of 296, compared with three placebo users or 3% of 98, but caused the withdrawal of only three BMT users. Vomiting was reported by 12 BMT users, or 4%, compared with no placebo users, but caused the withdrawal of only three of these patients. Flushing was reported by 48 BMT users, or 16%, compared with no placebo users, but caused the withdrawal of only two patients. Among BMT users with injection-site reactions, 94% experienced only mild events. No injection-site reactions were severe, caused discontinuation or became generalized.
A number of medications have been studied as potential interventions in FSD (e.g., testosterone [21–26], flibanserin [27–32], bupropion [33] and agents specifically targeting vaginal vasocongestion and clitoral engorgement, such as phosphodiesterase inhibitors and prostaglandin E1 [2–3,14,34]). Recently, flibanserin, a 5-HT1A agonist requiring daily dosing, was approved by the FDA for treatment of acquired, generalized HSDD in premenopausal women [35]. All treatments, potential and approved, have individual safety and efficacy profiles that may make them inappropriate or inadequate for clinical use by some patients. For such persons, a medical therapy with an alternate mechanism of action and safety profile could be a valuable option. The additional flexibility of a therapy with as-desired dosing would also have value for patients who prefer not to use an agent that must be taken chronically. The continued clinical evaluation of BMT may provide an efficacious, well-tolerated, episodically dosed alternative for women with FSD.
Conclusion
In premenopausal women with HSDD, FSAD or both conditions, double-blind BMT self-administered subcutaneously on an at-home, as-desired basis over 12 weeks showed significant efficacy versus placebo, as demonstrated by an increase in the monthly number of SSEs (the study's primary outcome measure) and by multiple other measures, both episodic and longer-term (longitudinal), with robust consistency. Although SSEs are a downstream measure of treatment effect [36], the study's end points also included direct demonstration of increased sexual desire and arousal and decreased distress. Effective dosing with 1.25 and 1.75 mg was established. The dose of 0.75 mg did not differ from placebo (ineffective dose). The findings of this large Phase IIB trial provide further evidence of the safety and efficacy of BMT in premenopausal patients with FSAD and/or HSDD, and suggest that ongoing Phase III registration trials may confirm BMT as a significant as-desired treatment in this important, underserved patient population.
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
Executive summary
Female sexual dysfunction is a common and distressing condition with limited treatment options currently available.
A total of 397 women were randomized to receive placebo or bremelanotide (BMT) 0.75, 1.25 or 1.75 mg over 12 weeks. Efficacy data were available for 327 women.
Women with female sexual dysfunction treated with 1.25/1.75 mg BMT showed a statistically significant increase in the number of satisfying sexual events/month compared with patients treated with placebo. BMT 0.75 mg did not differ from placebo.
Significant increases were also observed in female sexual function index total score and female sexual distress scale-desire/arousal/orgasm total score.
Women with a primary or sole diagnosis of hypoactive sexual desire disorder showed responses to BMT similar to those in the full study population
The most common treatment-emergent adverse events were nausea, flushing and headache, with no marked dosage dependence.
Financial & competing interests disclosure
This study was funded by Palatin Technologies, Inc. AH Clayton has received royalties from Ballantine Books/Random House, Changes in Sexual Functioning Questionnaire, Guilford Publications; has received research support or consulting fees from Auspex, Forest Research Institute, Inc., now Actavis, Genomind, Lundbeck, Palatin Technologies, Inc., Pfizer Inc., S1 Biopharmaceuticals, Inc., and Sprout Pharmaceuticals, a division of Valeant; and has stocks, stock options, or ownership interest, excluding diversified mutual funds, from Euthymics and S1 Biopharmaceuticals, Inc. SE Althof has received research support or consulting fees from Abbott, Allergan, Astellas, Eli Lilly, Evidera, Ixchelsis, Palatin Technologies, Inc., Pfizer, Promescent, Sprout Pharmaceuticals, S1 Biopharmaceuticals, Strategic Science Technologies, Trimel Pharmaceuticals, Inc., and Vyrix. S Kingsberg has received research support or consulting fees from Palatin Technologies, Inc., Sprout Pharmaceuticals, Valeant, Novo Nordisk, Shionogi, Apricus, Emotional Brain, Pfizer Inc., Trimel Pharmaceuticals, Inc. (now Acerus), Nuelle, SST, TherapeuticsMD, Materna, and Viveve; and has stocks, stock options, or ownership interest, excluding diversified mutual funds, from Viveve. LR DeRogatis has received research support or consulting fees from Palatin Technologies, Inc. R Kroll has received research support or consulting fees from Bayer, Palatin Technologies, Inc., AbbVie, Shionogi, Trimel, Teva Pharmaceuticals, Actavis, and Endoceutics. I Goldstein has received research support or consulting fees from Emotional Brain, The Female Condom Company, Nuelle, Palatin Technologies, Inc., Shionogi, Strategic Science & Technologies, TherapeuticsMD. J Kaminetsky has received research support or consulting fees from NxThera Inc., Palatin Technologies, Inc., Lipocine Inc., Repros Theraputics, Apricus BioSciences, Endo Pharmaceuticals, Antares Pharmaceuticals, and has ownership interest in Sprout Pharmaceuticals. C Spana, J Lucas and R Jordan are employees and stockholders of Palatin Technologies, Inc. DJ Portman has received research support or consulting fees from Bayer, Palatin Technologies, Inc., Pfizer, Shionogi, Trimel, Sprout, Valeant, Endoceutics, Nuelle, and Noven Pharmaceuticals; is on the speakers bureau for Pfizer and Noven; and is currently CEO and shareholder of Sermonix Pharmaceuticals, LLC. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Editorial assistance was provided by L Elliott, of The Curry Rockefeller Group, LLC, which was funded by Palatin Technologies, Inc.
References
- Papers of special note have been highlighted as: • of interest; •• of considerable interest. [Google Scholar]
- 1.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition, Text Revision). American Psychiatric Association, Washington, DC, USA: (2000). [Google Scholar]
- 2.Basson R, Leiblum S, Brotto L, et al. Definitions of women's sexual dysfunction reconsidered: advocating expansion and revision. J. Psychosom. Obstet. Gynaecol. 24(4), 221–229 (2003). [DOI] [PubMed] [Google Scholar]
- 3.Cellek S, Giraldi A. Challenges in sexual medicine. Nat. Rev. Urol. 9(9), 537–542 (2012). [DOI] [PubMed] [Google Scholar]
- 4.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. (Fifth Edition) American Psychiatric Association, Arlington, VA, USA: (2013). [Google Scholar]
- 5.Laumann EO, Paik A, Rosen RC. Sexual dysfunction in the United States: prevalence and predictors. JAMA 281(6), 537–544 (1999). [DOI] [PubMed] [Google Scholar]
- 6.Shifren JL, Monz BU, Russo PA, Segreti A, Johannes CB. Sexual problems and distress in United States women: prevalence and correlates. Obstet. Gynecol. 112(5), 970–978 (2008). [DOI] [PubMed] [Google Scholar]
- • The most recent large-scale survey documenting in American women a high prevalence of self-reported sexual problems, with peak prevalence in middle age. [Google Scholar]
- 7.Derogatis LR, Rosen R, Leiblum S, Burnett A, Heiman J. The Female Sexual Distress Scale (FSDS): initial validation of a standardized scale for assessment of sexually related personal distress in women. J. Sex Marital Ther. 28(4), 317–330 (2002). [DOI] [PubMed] [Google Scholar]
- 8.Molinoff PB, Shadiack AM, Earle D, Diamond LE, Quon CY. PT-141: a melanocortin agonist for the treatment of sexual dysfunction. Ann. NY Acad. Sci. 994, 96–102 (2003). [DOI] [PubMed] [Google Scholar]
- •• Pioneering preclinical and clinical evidence of the capacities of bremelanotide (BMT) to modulate brain function and influence sexual response. [Google Scholar]
- 9.Wikberg JE, Muceniece R, Mandrika I, et al. New aspects on the melanocortins and their receptors. Pharmacol. Res. 42(5), 393–420 (2000). [DOI] [PubMed] [Google Scholar]
- 10.Pfaus JG, Shadiack A, Van Soest T, Tse M, Molinoff P. Selective facilitation of sexual solicitation in the female rat by a melanocortin receptor agonist. Proc. Natl Acad. Sci. USA 101(27), 10201–10204 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- •• The first preclinical evidence showing a selective BMT effect on appetitive sexual behaviors. [Google Scholar]
- 11.Pfaus J, Giuliano F, Gelez H. Bremelanotide: an overview of preclinical CNS effects on female sexual function. J. Sex. Med. 4(Suppl. 4), 269–279 (2007). [DOI] [PubMed] [Google Scholar]
- •• Preclinical research implicating the medial preoptic area in the behavioral effects of BMT, including drug delivered by subcutaneous injection. [Google Scholar]
- 12.Diamond LE, Earle DC, Rosen RC, Willett MS, Molinoff PB. Double-blind, placebo-controlled evaluation of the safety, pharmacokinetic properties and pharmacodynamic effects of intranasal PT-141, a melanocortin receptor agonist, in healthy males and patients with mild-to-moderate erectile dysfunction. Int. J. Impot. Res. 16(1), 51–59 (2004). [DOI] [PubMed] [Google Scholar]
- 13.Rosen RC, Diamond LE, Earle DC, Shadiack AM, Molinoff PB. Evaluation of the safety, pharmacokinetics and pharmacodynamic effects of subcutaneously administered PT-141, a melanocortin receptor agonist, in healthy male subjects and in patients with an inadequate response to Viagra. Int. J. Impot. Res. 16(2), 135–142 (2004). [DOI] [PubMed] [Google Scholar]
- 14.Diamond LE, Earle DC, Heiman JR, Rosen RC, Perelman MA, Harning R. An effect on the subjective sexual response in premenopausal women with sexual arousal disorder by bremelanotide (PT-141), a melanocortin receptor agonist. J. Sex. Med. 3(4), 628–638 (2006). [DOI] [PubMed] [Google Scholar]
- •• The initial clinical evidence of potential BMT benefit in female patients with impaired sexual arousal. [Google Scholar]
- 15.Levine SB, Brown C, Palace E, Fischkoff S, Schnorrbusch C. Phase 2B bremelanotide study in pre- and postmenopausal women with female sexual arousal disorder. Presented at: American College of Obstetricians and Gynecologists 56th Annual Clinical Meeting. New Orleans, LA, USA, 3–7 May 2008. [Google Scholar]
- •• Further clinical evidence of potential BMT benefit for impaired sexual arousal in women. [Google Scholar]
- 16.Soules MR, Sherman S, Parrott E, et al. Stages of Reproductive Aging Workshop (STRAW). J. Womens Health Gend. Based Med. 10(9), 843–848 (2001). [DOI] [PubMed] [Google Scholar]
- 17.Derogatis LR, Edelson J, Revicki DA. Reliability and validity of the female sexual distress scale-desire/arousal/orgasm instrument in a Phase 2B dose-ranging study of bremelanotide. Presented at: 167th Annual Meeting of the American Psychiatric Association. NY, USA, 3–7 May 2014. [Google Scholar]
- 18.Rosen R, Brown C, Heiman J, et al. The Female sexual function index (FSFI): a multidimensional self-report instrument for the assessment of female sexual function. J. Sex Marital Ther. 26(2), 191–208 (2000). [DOI] [PubMed] [Google Scholar]
- 19.Ferguson DM. Clinical trial development in female sexual dysfunction. J. Sex Marital Ther. 28(Suppl. 1), 77–83 (2002). [DOI] [PubMed] [Google Scholar]
- 20.King Pharmaceuticals and Palatin Technologies Delay Immediate Plans for Phase 3 Clinical Program with Bremelanotide for Erectile Dysfunction [press release] (2007). www.palatin.com/investorcenter/pressreleases.asp?offset=160
- 21.Wierman ME, Nappi RE, Avis N, et al. Endocrine aspects of women's sexual function. J. Sex. Med. 7(1 Pt 2), 561–585 (2010). [DOI] [PubMed] [Google Scholar]
- • A relatively recent survey of hormones and hormonal changes as components of female sexual health. [Google Scholar]
- 22.Braunstein GD. Safety of testosterone treatment in postmenopausal women. Fertil. Steril. 88(1), 1–17 (2007). [DOI] [PubMed] [Google Scholar]
- 23.Buster JE, Kingsberg SA, Aguirre O, et al. Testosterone patch for low sexual desire in surgically menopausal women: a randomized trial. Obstet. Gynecol. 105(5 Pt 1), 944–952 (2005). [DOI] [PubMed] [Google Scholar]
- 24.Simon J, Braunstein G, Nachtigall L, et al. Testosterone patch increases sexual activity and desire in surgically menopausal women with hypoactive sexual desire disorder. J. Clin. Endocrinol. Metab. 90(9), 5226–5233 (2005). [DOI] [PubMed] [Google Scholar]
- 25.Nachtigall L, Casson P, Lucas J, Schofield V, Melson C, Simon JA. Safety and tolerability of testosterone patch therapy for up to 4 years in surgically menopausal women receiving oral or transdermal oestrogen. Gynecol. Endocrinol. 27(1), 39–48 (2011). [DOI] [PubMed] [Google Scholar]
- 26.Reis SL, Abdo CH. Benefits and risks of testosterone treatment for hypoactive sexual desire disorder in women: a critical review of studies published in the decades preceding and succeeding the advent of phosphodiesterase type 5 inhibitors. Clinics (Sao Paulo) 69(4), 294–303 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thorp J, Simon J, Dattani D, et al. Treatment of hypoactive sexual desire disorder in premenopausal women: efficacy of flibanserin in the DAISY study. J. Sex. Med. 9(3), 793–804 (2012). [DOI] [PubMed] [Google Scholar]
- 28.Derogatis LR, Komer L, Katz M, et al. Treatment of hypoactive sexual desire disorder in premenopausal women: efficacy of flibanserin in the VIOLET Study. J. Sex. Med. 9(4), 1074–1085 (2012). [DOI] [PubMed] [Google Scholar]
- 29.Katz M, Derogatis LR, Ackerman R, et al. Efficacy of flibanserin in women with hypoactive sexual desire disorder: results from the BEGONIA trial. J. Sex. Med. 10(7), 1807–1815 (2013). [DOI] [PubMed] [Google Scholar]
- 30.Simon JA, Kingsberg SA, Shumel B, Hanes V, Garcia M, Jr, Sand M. Efficacy and safety of flibanserin in postmenopausal women with hypoactive sexual desire disorder: results of the SNOWDROP trial. Menopause 21(6), 633–640 (2014). [DOI] [PubMed] [Google Scholar]
- 31.Jayne C, Simon JA, Taylor LV, Kimura T, Lesko LM. Open-label extension study of flibanserin in women with hypoactive sexual desire disorder. J. Sex. Med. 9(12), 3180–3188 (2012). [DOI] [PubMed] [Google Scholar]
- 32.Sprout Pharmaceuticals Receives Clear Guidance from FDA on Path Forward to Resubmit NDA for Flibanserin [press release] (2014). www.drugs.com/nda/flibanserin_140211.html
- 33.Segraves RT, Clayton A, Croft H, Wolf A, Warnock J. Bupropion sustained release for the treatment of hypoactive sexual desire disorder in premenopausal women. J. Clin. Psychopharmacol. 24(3), 339–342 (2004). [DOI] [PubMed] [Google Scholar]
- 34.Chivers ML, Rosen RC. Phosphodiesterase type 5 inhibitors and female sexual response: faulty protocols or paradigms? J. Sex. Med. 7(2 Pt 2), 858–872 (2010). [DOI] [PubMed] [Google Scholar]
- 35.Gohil K. Pharmaceutical approval update. PT 40(10), 649–689 (2015). [PMC free article] [PubMed] [Google Scholar]
- 36.Kingsberg SA, Althof SE. Satisfying sexual events as outcome measures in clinical trial of female sexual dysfunction. J. Sex. Med. 8(12), 3262–3270 (2011). [DOI] [PubMed] [Google Scholar]