Abstract
Objective: Some ovarian malignancies may originate in the fallopian tube. The feasibility of ultrasonographically visualizing the fallopian tube is presented. Methods: In total, 549 normal women participated in the fallopian tube visualization trial, while ovarian visualization was studied in 43,521. Chi-square analysis, t-tests and multivariate analysis determined significance and interactions. Results: Ovaries were observed in 82.7% while fallopian tubes were detected in 77.2% of women and 85.2% of the time when an ovary was detected. Age, BMI or parity was not significantly different when one or both fallopian tubes were visualized. Elevated BMI had slightly greater influence than age in limiting visualization of the fallopian tubes in multivariate analysis. Conclusion: Fallopian tubes can often be identified sonographically. Ovarian visualization provides the strongest indicator favoring fallopian tube detection. Thus, ultrasonographic examinations for adnexal cancer could include evaluation of fallopian tubes even in women >60 years and in women with BMI ≥25.
Keywords: detection, fallopian tube, ovary, ultrasound
For several years, an expanding literature has implicated the fallopian tube fimbria as the point of origin of ovarian cancer [1–10]. Thus, malignancy can arise from the surface of the ovary, the fallopian tube and the mesothelial lining of the peritoneal cavity. Expanding the scope of surveillance to as many of these sources as possible in order to detect this disease in its earliest development may be of benefit. The frequency of surgically diagnosed fallopian tube malignancy is very rare (3.72/million women), about 3% of adnexal malignancies (119.9/million malignancies) [11]. In a sample of women at risk for ovarian cancer due to BRCA1/2 mutations who received risk-reducing salpingo-oophorectomy, occult malignancy was detected in 2.5% of patients [12,13]. Among the occult malignancies discovered, the frequency of fallopian tube malignancies has been reported over a wide range: 18.8–81.8% [2,12,13]. Consequently, the proportion of occult malignancies arising in the fallopian tube is of clinical significance. It is accepted that fallopian tubes are best visualized sonographically when thickened or filled with fluid due to pelvic inflammatory disease, torsion, ectopic pregnancy or tumors [14]. Demonstration of primary carcinoma in the fimbria by ultrasonography has been reported [15]. However, the notion predominates that fallopian tubes, which are approximately 10 cm long, are not consistently observable by ultrasonography in the absence of pathology [16], and often little or no effort is directed at including them in ultrasound studies of the adnexa. The results reported here show the facile visualization of normal fallopian tubes using transvaginal sonography and attest to the feasibility of including the fallopian tubes in routine ultrasonographic gynecologic examinations.
Methods
A sample of 549 women, enrolled in the University of Kentucky Ovarian Cancer Screening Program, was prospectively evaluated over the trial period January 2013–July 2014. The screening cohort contained 43,521 women that had prospectively received transvaginal ultrasound examinations of the ovaries in the screening program from 1987–2014. Approval was received from the University of Kentucky Institutional Review Board. Study eligibility, exclusions, instrumentation, protocol, criteria for designating an abnormality, data collection and storage were as previously reported [17–21]. In brief, criteria for eligibility were: asymptomatic women aged ≥50 years; and, asymptomatic women aged ≥25 years with a documented family history of ovarian cancer (OvCA) in at least one primary or secondary relative. Fallopian tube transvaginal sonography (TVS) was performed exclusively using a General Electric Voluson 730 ProV unit (WI, USA) with a 5–9-mHz vaginal probe to insure uniformity of results, and consisted of all new participants examined on this ultrasound unit during the 18-month trial period (January 2013–June 2014) so that the 549 women represented 100% of the women examined on this unit. This design avoided bias due to a previous screening result. Ovaries and fallopian tubes were measured in three dimensions. Ovarian volume was calculated using the prolate ellipsoid formula (length × width × height × 0.523) [22]. Criterion for positive fallopian tube visualization was a successful sagittal, coronal and transverse measurement. Genetic testing was not included in this trial. All study participants completed a questionnaire that included medical history, surgical history, last menstrual period/menopausal status, hormonal use and family history of cancer. Women with a known ovarian tumor or a personal history of ovarian cancer were excluded from the study, as were BRCA1 and/or BRCA2 mutation carriers and women with a history of prior abdominal surgery. Participants in the screening program received free annual screening.
Identifying the fallopian tubes & ovaries with transvaginal ultrasonography
The transducer was introduced vaginally and placed in the transverse plane focused on the most fundal portion of the uterus. From the most superior and lateral portion of the uterus, the transducer was adjusted so that images were acquired heading laterally to the right [23,24]. By continuing angling superior and right lateral, the fallopian tube appeared as the echoes headed lateral and posterior to the most distal portion. Landmarks for proving structure were the tubal vessels located posterior and parallel to the fallopian tube. Width measurements were made from the most proximal portion of the fallopian tube to the most distal. In order to measure the length and depth, the transducer was rotated into the longitudinal plane. In the longitudinal view, the fallopian tube had an oval appearance. An identical approach was taken in progression to the left in order to visualize the opposing fallopian tube. Continuation of the view aspect laterally presented the ovarian views, which were proven by association with ovarian blood vessels. Power Doppler was used to follow vessels and establish their identity. Archived blinded images were all reviewed by a physician in order to confirm or reject findings of the sonographer. Only when there was agreement by both, were positive results accepted in this study. A sonographic study was terminated in the absence of visualization following unsuccessful sonographic visualization after applying pressure to at least three regions of the abdominal surface and observing repositioning of bowel or complaints of pain from the participant. In the group reported on here, none of the nonvisualizations were associated with observed fibroids or ultrasonographic shadowing that would prevent signal penetration. Moreover, endometriosis was neither reported by any participants in the non-visualization group nor was it or resultant scarring evident in the ultrasound examination. We estimate that approximately 5 min were added to the ultrasound examination when fallopian tube visualization occurred and approximately 10 min when visualization could not be achieved.
Statistical methods
Significance was determined at the 0.05 level. Proportions were compared using chi-square statistics, and t-testing was performed on parametric variables. For the multivariate analysis, separate logistic regression models were applied to the outcomes visualization of one fallopian tube, or both tubes, or at least one tube, and each regressed against age and BMI. In all cases, responses were clustered at the individual woman level. The latter variables were first entered into the model as raw values (linear response for each variable) and then entered as a class variable: age in categories <60 years, 60–69 years and ≥70 years, and BMI in categories as <25, 25–29 and ≥30. The three outcomes of interest were: only one fallopian tube visualized, both fallopian tubes visualized and at least one fallopian tube visualized.
Results
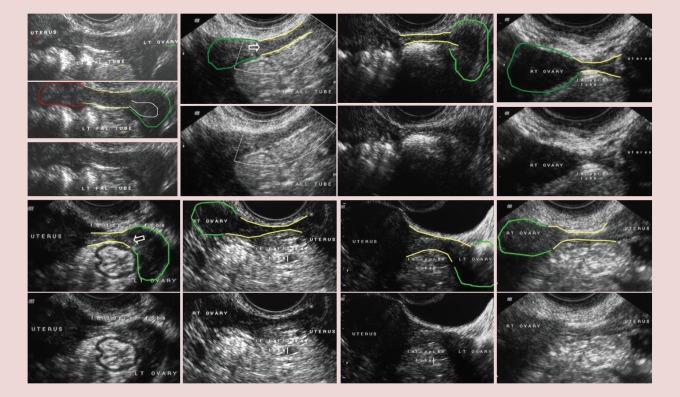
The ultrasonographic associations between ovary, fallopian tube with fimbria and uterus are shown in Figure 1. Faint echogenicity at the tubal–ovarian terminus reveals the fimbriated ends in the cases examined. Axial proportions differ in paired perspectives. In terms of fallopian tube and ovarian visualization, the groups in which ovaries or fallopian tubes were not visualized were significantly older and had a greater BMI (p < 0.001); however, they did not differ with respect to parity (Table 1). There were no significant differences in women that had one or both fallopian tubes visualized with respect to age (63.1 ± 0.5 vs 62.1 ± 0.6), BMI (24.5 ± 0.3 vs 26.2 ± 0.3) or parity (1.8 ± 0.01 vs 1.9 ± 0.6). Ovaries were observed in 82.7% of those examined (Table 2) and a fallopian tube was detected in 77.2% of the women examined (Table 2). When visualization of the ovary occurred, the visualization success rate for detecting the fallopian tube was 85.2% (Table 3) showing that the fallopian tube could be detected a high percentage of the time (85.2%) when an ovary was detectable (Table 3).
Figure 1.
Ultrasound image of the fallopian tube. Ultrasound perspectives of the fallopian tube (yellow outline), ovary (green outline), uterus (red outline) and fimbria (white outline and white arrow).
Table 1.
Fallopian tube and ovary detection by transvaginal ultrasonography with respect to age, body habitus and parity.
| Visualization condition | n | Age (years) | BMI | Parity | |||
|---|---|---|---|---|---|---|---|
| Mean ± SEM | Median (range) | Mean ± SEM | Median (range) | Mean ± SEM | Median (range) | ||
| Total women examined | 549 | 63.5 ± 0.37 | 64 (26–85) | 26.2 ± 0.21 | 25.1 (15.3–48.6) | 1.9 ± 0.05 | 2 (0–7) |
| Women with no fallopian tubes visualized | 125 | 67.1 ± 0.74* | 67 (33–85) | 28.6 ± 0.47* | 28.7 (16.6–47.4) | 2.1 ± 0.12 | 2 (0–7) |
| Women with no ovaries visualized | 95 | 67.1 ± 0.88* | 67 (33–85) | 28.7 ± 0.55* | 28.5 (16.6–47.4) | 2 ± 0.13 | 2 (0–7) |
| Women with only one fallopian tube visualized | 172 | 63.1 ± 0.54 | 63 (42–80) | 24.5 ± 0.29 | 23.9 (17–40.4) | 1.8 ± 0.09 | 2 (0–5) |
| Women with only one ovary visualized | 105 | 65.7 ± 0.73 | 66 (50–82) | 26.9 ± 0.45 | 26.7 (18.8–37.4) | 2.1 ± 0.12 | 2 (0–5) |
| Women with one or two fallopian tubes visualized | 424 | 62.5 ± 0.42 | 64 (26–85) | 25.5 ± 0.22 | 24.8 (15.3–48.6) | 1.8 ± 0.06 | 2 (0–6) |
| Women with one or two ovaries visualized | 454 | 62.8 ± 0.41 | 63 (26–85) | 25.7 ± 0.21 | 24.9 (15.3–48.6) | 1.9 ± 0.06 | 2 (0–6) |
| Women with both fallopian tubes visualized | 252 | 62.1 ± 0.6 | 63 (26–85) | 26.2 ± 0.3 | 25.2 (15.3–48.6) | 1.9 ± 0.6 | 2 (0–6) |
| Women with both ovaries visualized | 349 | 61.9 ± 0.47 | 62 (26–85) | 25.3 ± 0.24 | 24.5 (15.3–48.6) | 1.8 ± 0.06 | 2 (0–6) |
p < 0.001.
SEM: Standard error of mean.
Table 2.
Fallopian tube and ovary detection by transvaginal ultrasonography.
| Visualization condition | n | % | p-value |
|---|---|---|---|
| Total women examined | 549 | 100.0 | |
| Women with no fallopian tubes visualized | 125 | 22.8 | |
| Women with no ovaries visualized | 95 | 17.3 | <0.05 |
| Women with only one fallopian tube visualized | 172 | 33.0 | |
| Women with only one ovary visualized | 105 | 19.1 | <0.001 |
| Women with one or two fallopian tubes visualized | 424 | 77.2 | |
| Women with one or two ovaries visualized | 454 | 82.7 | <0.05 |
| Women with both fallopian tubes visualized | 252 | 45.9 | |
| Women with both ovaries visualized | 349 | 63.6 | <0.001 |
Significance testing: visualization of fallopian tubes vs ovaries by Chi-square.
Table 3.
Detection frequency of fallopian tubes and ovaries by transvaginal ultrasonography.
| Summary of visualization | n | % |
|---|---|---|
| Total women examined | 549 | 100.0 |
| Surgically absent ovaries | 10 | 0.9 |
| Nonvisualized ovaries | 295 | 26.9 |
| Ovaries detected | 793 | 100.0 |
| Fallopian tubes visualized | 676 | 85.2 |
Total possible ovaries that could be included = 1098 (549 × 2) with correction for undetected and absent ovaries reducing the number to 305 (10 + 295) = 793 documentable ovaries present which becomes the maximum possible ovaries that were present (i.e., 100%). Fallopian tube visualization was normalized by the number of ovaries that were present (1098 − 305 = 793).
Detection of fallopian tubes did not differ from the detection of the ovaries across age categories (Table 4: F1 vs O1, F2 vs O2 and F3 vs O3). Both fallopian tubes could be detected in only ∼5% of women ≥75 years (Table 4, F3). This low rate of visualization for both fallopian tubes coincided with low detection of both ovaries in women ≥75 years (Table 4). In the women surveyed for fallopian tubes, failure to detect the fallopian tubes ([76 + 25]/125 = 80.8%) or ovaries ([57 + 20]/95) = 81.1%) was highest in women over 60 years of age (Table 4). Thus, age is a factor in the visualization of both the ovaries and fallopian tubes. An anomaly exists in the fallopian tube dataset presented here, which indicates that detection of tubes and ovaries (one or both) is higher for women who are 61–74 years old as compared with those that are 51–60 years old. This is not supported by ovarian visualization over all examinations in the entire screened population (Table 4, see bold upper case). In Table 4, it should be noted that summaries for all examinations reflect results from repeat examinations, while summaries for women are for a single ultrasound examination and will account for the differences in the distributions of results for women versus examinations.
Table 4.
Detection of fallopian tubes and ovaries as a function of age.
| Visualization condition | n | Age (years), n (%) | Grouping | ||||
|---|---|---|---|---|---|---|---|
| 25–40 | 41–50 | 51–60 | 61–74 | ≥75 | |||
| Total women examined for fallopian tubes | 549 | 8 (1.5) | 16 (2.9) | 162 (29.5) | 308 (56.1) | 55 (10) | |
| Women with no fallopian tubes visualized | 125 | 1 (0.8) | 1 (0.8) | 22 (17.6) | 76 (60.8) | 25 (20) | F1 |
| Women with no ovaries visualized | 95 | 1 (1.1) | 1 (1.1) | 16 (16.8) | 57 (60) | 20 (21.1) | O1 |
| Examinations with no ovaries visualized | 15,248 | 173 (1.1) | 1166 (7.6) | 5097 (33.4) | 6584 (43.2) | 2228 (14.6) | |
| Women with only one fallopian tube visualized | 172 | 2 (1.2) | 5 (2.9) | 64 (37.2) | 84 (48.8) | 17 (9.9) | |
| Women with only one ovary visualized | 105 | 0 | 1 (1) | 27 (25.7) | 64 (61) | 13 (12.4) | |
| Examinations with only one ovary visualized† | 19,266 | 302 (1.6) | 1717 (8.9) | 6979 (36.2) | 8024 (41.6) | 2244 (11.6) | |
| Women with one or two fallopian tubes visualized | 424 | 7 (1.7) | 15 (3.5) | 140 (33) | 232 (54.7) | 30 (7.1) | F2 |
| Women with one or two ovaries visualized | 454 | 7 (1.5) | 15 (3.3) | 146 (32.2) | 251 (55.3) | 35 (7.7) | O2 |
| Examinations with one or two ovaries visualized† | 80,852 | 2951 (3.6) | 9097 (11.3) | 29,594 (36.6) | 30,441 (37.7) | 8769 (10.8) | |
| Women with both fallopian tubes visualized | 252 | 5 (2) | 10 (4) | 76 (30.2) | 148 (58.7) | 13 (5.2) | F3 |
| Women with both ovaries visualized | 349 | 7 (2) | 14 (4) | 119 (34.1) | 187 (53.6) | 22 (6.3) | O3 |
| Examinations with both ovaries visualized† | 61,586 | 2649 (4.3) | 7380 (12) | 22,615 (36.7) | 22,417 (36.4) | 6525 (10.6) | |
F1 vs F3: p < 0.0001; O1 vs 03 p < 0.0001;
F1 vs 01: p = 0.998; F2 vs O2 p = 0.994;F3 vs O3 p = 0.786;
F1 vs F2: p < 0.0001;O1 vs O3: p < 0.001.
Total population set examinations reflect repeat examination results.
In total, 51,350 examinations were performed on women with a BMI <30 while 21,757 examinations were on women with a BMI ≥30. Approximately the same proportion of examinations were on women with BMI ≥30 who were ≤50 years old (31.1%) as were on those that were >50 years old (29.5%). The detection of the fallopian tubes did not differ from the detection of the ovaries with regard to BMI (Table 5: F1 vs O1, F2 vs O2 and F3 vs O3). However, the BMI distribution of women in which no fallopian tubes were visualized was significantly different from women in which both fallopian tubes were detected (Table 5: F1 vs F3; p < 0.0001) as was the BMI distribution of women in which ovaries were not visualized as compared with when both were visualized (Table 5: O1 vs O3; p < 0.0001), showing that a failure to detect the fallopian tubes ([44 + 50]/125 + 75.2%) or ovaries ([34 + 34]/95 + 71.6%) was highest in overweight women (BMI >25) and that the detection of both fallopian tubes ([72 + 25]/252 + 38.5%) or both ovaries ([110 + 43]/349 = 43.8%) was lowest in overweight women, Table 5. These results indicate that BMI has an influence on the ability to visualize the ovaries and fallopian tubes; however, one or both fallopian tubes were still visualized in 45.7% of cases with BMI ≥25 or about 3% less than ovarian visualization, suggesting that attempts to visualize the fallopian tube in women with BMI ≥25 will be worthwhile.
Table 5.
Detection of fallopian tubes and ovaries as a function of BMI.
| Visualization condition | n | BMI, n (%) | Grouping | |||
|---|---|---|---|---|---|---|
| <18.5 | 18.5–24.9 | 25–29.9 | ≥30 | |||
| Total women examined | 549 | 8 (1.5) | 253 (46.1) | 181 (33) | 107 (19.5) | |
| Women with no fallopian tubes visualized | 125 | 1 (0.8) | 30 (24) | 44 (35.2) | 50 (40) | F1 |
| Women with no ovaries visualized | 95 | 1 (1.1) | 26 (27.4) | 34 (35.8) | 34 (35.8) | O1 |
| Examinations with no ovaries visualized† | 12,275 | 258 (2.1) | 3978 (32.4) | 4413 (36) | 3626 (29.5) | |
| Women with only one fallopian tube visualized | 172 | 1 (0.6) | 74 (43) | 65 (37.8) | 32 (18.6) | |
| Women with only one ovary visualized | 105 | 0 | 38 (36.2) | 37 (35.2) | 30 (28.6) | |
| Examinations with only one visualized† | 15,652 | 324 (2.1) | 5464 (34.9) | 5684 (36.3) | 4180 (26.7) | |
| Women with one or two fallopian tubes visualized | 424 | 7 (1.7) | 223 (52.6) | 137 (32.3) | 57 (13.4) | F2 |
| Women with one or two ovaries visualized | 454 | 7 (1.5) | 227 (50) | 147 (32.4) | 73 (16.1) | O2 |
| Examinations with one or two ovaries visualized† | 67,868 | 1374 (2) | 24,813 (36.6) | 24,074 (35.5) | 17,607 (25.9) | |
| Women with both fallopian tubes visualized | 252 | 6 (2.4) | 149 (59.1) | 72 (28.6) | 25 (9.9) | F3 |
| Women with both ovaries visualized | 349 | 7 (2) | 189 (54.2) | 110 (31.5) | 43 (12.3) | O3 |
| Examinations with both ovaries visualized† | 52,216 | 1050 (2) | 19,349 (37.1) | 18,390 (35.2) | 13,427 (25.7) | |
F1 vs F3: p < 0.0001.
01 vs 03: p < 0.0001.
F1 vs 01: p = 0.7695.
F2 vs 02: p = 0.514.
F3 vs 03: p = 0.3904.
F1 vs F2: p < 0.0001.
01 vs 03: p < 0.0001.
Total population set examinations reflect repeat examination results.
Multivariate analysis
Since obesity can be expected to be limiting to longevity, multivariate analysis was performed to isolate the effects of age and body habitus on fallopian tube visualization. Both age and BMI were significant predictors of the events: both fallopian visualized (odds ratio [OR]AGE: 1.038 [95% CI: 1.017–1.060]; ORBMI: 1.134 [95% CI: 1.088–1.182]) and at least one fallopian visualized (ORAGE: 1.088 [95% CI: 1.057–1.120]; ORBMI: 1.179 [95% CI: 1.175–1.236]), while neither age (ORAGE: 1.013 [95% CI: 0.99–1.034]) nor BMI (ORBMI: 1.0 [95% CI: 0.96–1.030]) were significant predictors of the event only one fallopian visualized. These results permit the exclusion of the event only one fallopian tube from further considerations.
To determine if either age or BMI is more important, it could be argued in favor of BMI because its p-value for the event both visualized is smaller than that for age, although both p-values are small (p < 0.0004 for age and p < 0.0001 for BMI). However, a better alternative argument relies on the C statistic. The C statistic is an indicator of how well the logistic model fits the data with C + 1 being a perfect prediction and C + 0.5 being the equivalent of guessing when using the model to predict the outcome. For the outcome both fallopian tubes visualized, C statisticBMI: 0.665 comes closer to the logistic model (C statistic: 0.680) with both variables than C statisticAGE: 0.574 and, hence, is clearly the better of the two outcomes tested. This is less clear for the outcome at least one fallopian tube visualized (C statisticBMI: 0.679 or C statisticAGE: 0.657) when compared with the logistic model for both variables (C statistic: 0.769). Thus, if using only one of the two variables in a logistic model, BMI would have the greater weight.
Because the multivariate model assumes that the probability of visualization is linear with regard to age and BMI, age was broken into intervals:
<60 years (n = 168);
60–69 years (n = 252);
≥70 years (n = 130).
While BMI was broken into the intervals:
<25 (n = 262);
25–29 (n = 181);
≥30 (n = 107).
The multivariate model was retrofitted with these intervals and for the outcome that both fallopian tubes were visualized. It was observed that women aged ≥70 years differed (were worse or less likely to have both fallopian tubes visualized) when compared with women under 60 years of age (OR: 1.84 [95% CI: 1.125–3.011]). Comparisons of BMI showed that while BMI 25–29 was worse (OR: 2.451 [95% CI: 1.655–9.082]) than BMI <25, the BMI interval ≥30 (OR: 5.362 [95% CI: 3.166–3.011]) was even worse when compared with BMI <25.
When the multivariate model was retrofitted with these intervals for the outcome that at least one fallopian tube was visualized, it was observed that the OR did vary by age since visualization in women under 60 years of age differed from visualization in those of age 60–69 years (OR: 2.655 [95% CI: 1.478–4.769]) and those ≥70 years (OR: 5.315 [95% CI: 2.810–10.052]), while under 25 BMI differed from both 25–29 (OR: 2.499 [95% CI: 1.487–4.201]) and ≥30 (OR: 7.562 [95% CI: 4.320–13.237]). Thus, the likelihood of visualizing at least one fallopian tube gets worse with both increasing age and increasing BMI.
Discussion
The work reported here presents a strong argument that when sonographic detection of an ovary is possible, visualization of the normal fallopian tube can be performed in a large majority (85%) of these cases. Prior work confined to younger infertility patients and not stratified for age, body habitus and ovarian visualization reported sonographic visualization of the fallopian tubes with similar frequency as reported here [25]. Increasing age and body habitus negatively influence visualizations. However, we show here that the fallopian tubes can still be detected in over 60% of women who are >60 years of age and in women with BMI ≥25 at a frequency that is similar to or only marginally less than ovarian detection (∼59%). Importantly, visualization of the ovaries is the strongest factor indicating that visualization of the fallopian tubes is likely. In addition, the geometrical arrangements of the fallopian tubes, ovaries and uterus are likely to be limiting, especially when the uterus gets positioned between these structures or the fallopian tube bends behind the uterus. Because occult malignancy has been reported with high frequency in specimens from risk-reducing salpingo-oophorectomies [2,12,13], we believe that including the fallopian tubes in ultrasonographic surveillance has the potential for detecting adnexal carcinomas at earlier points of onset. Ultrasound detection of fimbrial malignancy has been reported as a proof of concept [15]. The work presented here provides proof of concept that fallopian tube visualization by ultrasound is very often possible. It has not yet been determined if malignancy detected by fallopian tube surveillance will lead to an advantage over early stage detection of malignancy achieved by monitoring only the ovary. However, at present it seems prudent to avoid ignoring this possibility. The present report neither attempts to characterize, categorize or classify sonographic findings of the fallopian tubes, nor does it include abnormal findings like malignancy, but has a singular focus on fallopian tube detectability. The adequacy of ultrasound to detect primary malignancy in the ductal fimbria has been established [15]. Here we report that the 5–10 min extension of adnexal ultrasound studies to include fallopian tubes will frequently be successful and does not add a time or cost burden.
Some consideration of the origin of ovarian cancer in the fimbria of the fallopian tube as a model is appropriate. A popular hypothesis is that invasive or serous tubal intraepithelial carcinoma [6] is responsible for seeding the peritoneal cavity with malignant cells originating in the fimbriated ends of the fallopian tubes [26]. A presumption of this model is that it presents only microscopic disease that is expected to evade sonographic detection. However, just as ultrasonography can detect early stage malignancy in the ovary that is highly curable with surgery alone [27], we hypothesize that there can be cases which have progressed beyond serous tubal intraepithelial carcinoma in the distal fallopian tube that will be detected by ultrasonography and represent an early development of disease that has a favorable prognosis for survival.
Not all cases of ovarian cancer are associated with fimbrial malignancy and this leads to several considerations:
Not all ovarian cancers originate in the fimbria;
After spreading beyond their origination point in the fimbria, originating foci of primary fimbrial malignancy disappear.
We admit that in such cases, ultrasonography of the distal fallopian tube will reveal little, but neither will histology.
However, it is possible that the proximity of the fimbriated end to the peritoneal cavity facilitates the appearance of nonovarian peritoneal malignancy. In these cases, including the fallopian tubes in ultrasonographic studies of the adnexa may discern distal tube abnormalities that could identify a precursor event to eventual peritoneal cancer and lead to much earlier discovery of peritoneal disease. We assert that all too many times the fallopian tubes are overlooked under the presumption that their detection is very unlikely. The work presented here shows that this presumption is unwarranted, and we advocate including the fallopian tubes in pelvic ultrasound studies.
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
Executive summary
- The nature of the problem
- Abdominal cancers can arise from the surface of the ovary, the fallopian tube and the mesothelial lining of the peritoneal cavity.
- In recent years, the fimbrial portion of the fallopian tube has been implicated in the origin of some ovarian and primary peritoneal malignancies.
- Ultrasound is recognized as effective for examining ovarian structure; however, the notion predominates that ultrasound is ineffective for revealing the fallopian tubes in the absence of pathology related to enlargement, thickening or fluid retention.
- The present work finds that ultrasound effectively visualizes the fallopian tubes in a very large proportion of normal women indicating that expanding ultrasound examinations to include the fallopian tubes as an additional source of malignancy may achieve detection at an early stage.
- Experimental setting for the findings
- In total, 549 women enrolled in the University of Kentucky Ovarian Cancer Screening program (n = 43,621) were prospectively examined ultrasonographically for the visualization of fallopian tubes on their first encounter.
- In total, 43,621 women were prospectively examined ultrasonographically for visualization of the ovaries.
- The criterion for positive fallopian tube and ovarian visualization was successful measurement in all three dimensions.
- Summary of the findings
- Ovaries were ultrasonographically visualized in 82.7% of the women and fallopian tubes were seen in 77.2% of the women.
- When ovaries were visualized, fallopian tubes were also seen in 85.2% of the women.
- The likelihood of visualizing at least one fallopian tube gets lower with both increasing age and increasing BMI so that detection of the ovaries and fallopian tubes was lowest after the age 60 years and when BMI was ≥25.
- Fallopian tubes are still detectable in >60% of women aged >60 years and in women with BMI ≥25.
- Implications of the findings
- Attempts to detect the fallopian tubes are justified by visualization rates that parallel ovarian visualization rates across age and BMI, and these visualization rates are high.
- There is no significant burden of time or cost imposed by efforts to visualize the fallopian tubes.
- Questions worthy of discussion
- Will ultrasonographic surveillance of the fallopian tubes lead to early detection of ovarian and primary peritoneal malignancies?
-
–The most promising aspect of early detection led by fallopian tube surveillance is the possibility of becoming aware of occult primary peritoneal malignancy early in its onset.
-
–
- Will early detection of fimbrial malignancy offer any advantage over early detection of ovarian malignancy?
-
–It is possible that stage I ovarian malignancy detected by fallopian tube surveillance will have similar great outcomes to stage I malignancy detected by ovarian ultrasonography. It is also possible that fimbrial ultrasound surveillance will usher in more cases where peritoneal tumor burden discovered operatively is much smaller so that it can be more completely removed surgically or effectively treated intraperitoneally.
-
–
- What is needed next to take advantage of fallopian tube surveillance using ultrasonography?
-
–A body of knowledge must be developed to characterize abnormalities of the fimbria ultrasonographically and relate them to malignancy.
-
–
Financial & competing interests disclosure
This work was supported by grants from the Telford Foundation and the Department of Health and Human Services, Commonwealth of Kentucky. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
- 1.Alvarado-Cabrero I, Navani SS, Young RH, Scully RE. Tumors of the fimbriated end of the fallopian tube: a clinicopathologic analysis of 20 cases, including nine carcinomas. Int. J. Gynecol. Pathol. 16, 189–196 (1997). [DOI] [PubMed] [Google Scholar]
- 2.Colgan TJ, Murphy J, Cole DE, Narod S, Rosen B. Occult carcinoma in prophylactic oophorectomy specimens: prevalence and association with BRCA germline mutation status. Am. J. Surg. Pathol. 25, 1283–1289 (2001). [DOI] [PubMed] [Google Scholar]
- 3.Cass I, Holschneider C, Datta N, Barbuto D, Walts AE, Karlan BY. BRCA-mutation-associated fallopian tube carcinoma: a distinct clinical phenotype? Obstet. Gynecol. 106, 1327–1334 (2005). [DOI] [PubMed] [Google Scholar]
- 4.Medeiros F, Muto MG, Lee Y, et al. The tubal fimbria is a preferred site for early adenocarcinoma in women with familial ovarian cancer syndrome. Am. J. Surg. Pathol. 30, 230–236 (2006). [DOI] [PubMed] [Google Scholar]
- 5.Kindelberger DW, Lee Y, Miron A, et al. Intraepithelial carcinoma of the fimbria and pelvic serous carcinoma: evidence for a causal relationship. Am. J. Surg. Pathol. 31, 161–169 (2007). [DOI] [PubMed] [Google Scholar]
- 6.Crum CR, Drapkin R, Miron A, et al. The distal fallopian tube: a new model for pelvic serous carcinogenesis. Curr. Opin. Obstet. Gynecol. 19, 3–9 (2007). [DOI] [PubMed] [Google Scholar]
- 7.Landen CN, Birrer MJ, Sood AK. Early events in the pathogenesis of epithelial ovarian cancer. J. Clin. Oncol. 26, 995–1005 (2008). [DOI] [PubMed] [Google Scholar]
- 8.Lengyel E. Ovarian cancer development and metastasis. Am. J. Pathol. 177, 1053–1064 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kurman RJ, Shih L-M. The origin and pathogenesis of epithelial ovarian cancer: a proposed unifying theory. Am. J. Surg Pathol. 34, 433–443 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crum CP, Mckeon FD, Xian X. The oviduct and ovarian cancer: causality, clinical implications, and “targeted prevention”. Clin. Obstet. Gynecol. 55, 24–35 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goodman MT, Shvetsov YB. Incidence of ovarian, peritoneal, and fallopian tube carcinoma in the United States, 1995–2004. Cancer Epidemiol. Biomarkers. Prev. 18, 132–139 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Domchek SM, Friebel TM, Garber JE, et al. Occult ovarian cancers identified at risk-reducing salpingo-oophorectomy in a prospective cohort of BRCA1/2 mutation carriers. Breast Cancer Res. Treat. 124, 195–203 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sherman ME, Piedmonte M, Mai PL, et al. Pathologic findings at risk-reducing salpingo-oophorectomy: primary results from Gynecologic Oncology Group Trial GOG-0199. J. Clin. Oncol. 32, 3275–3283 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Benjaminov O, Atr M. Sonography of the abnormal fallopian tube. Am. J. Roentgenol. 183, 737–742 (2004). [DOI] [PubMed] [Google Scholar]
- 15.Huang W-C, Yang S-H, Yang J-M. Ultrasonographic manifestations of fallopian tube carcinoma in the fimbriated end. J. Ultrasound. Med. 24, 1157–1160 (2005). [DOI] [PubMed] [Google Scholar]
- 16.The normal fallopian tubes and fimbria. www.fetalultrasound.com/online/text/3-149.HTM
- 17.Ueland FR, DePriest P, DeSimone C, et al. The accuracy of examination under anesthesia and transvaginal sonography in evaluating ovarian size. Gynecol. Oncol. 99, 400–403 (2005). [DOI] [PubMed] [Google Scholar]
- 18.DePriest PD, Gallion HH, Pavilk EJ, Kryscio RJ, van Nagell JR. Transvaginal sonography as a screening method for the detection of early ovarian cancer. Gynecol. Oncol. 65, 408–414 (1997). [DOI] [PubMed] [Google Scholar]
- 19.Van Nagell JR, DePriest P, Reedy M, et al. The efficacy of transvaginal sonographic screening in asymptomatic women at risk for ovarian cancer. Gynecol. Oncol. 77, 350–356 (2000). [DOI] [PubMed] [Google Scholar]
- 20.Pavlik EJ, Saunders BA, Doran S, et al. The search for meaning – symptoms and transvaginal sonography screening for ovarian cancer. Cancer 115, 3689–3698 (2009). [DOI] [PubMed] [Google Scholar]
- 21.Pavlik EJ, DePriest PD, Gallion HH, et al. Ovarian volume related to age. Gynecol. Oncol. 77, 410–412 (2000). [DOI] [PubMed] [Google Scholar]
- 22.MacMahon PJ, Kennedy AM, Murphy DT, McNicholas MM. Modified prostate volume algorithm improves transrectal US volume estimation in men presenting for prostate brachytherapy. Radiology 250, 273–280 (2009). [DOI] [PubMed] [Google Scholar]
- 23.OBG management. www.obgmanagement.com/home/article
- 24.Ultrasoundpaedia: ultrasound of the uterus – normal. www.ultrasoundpaedia.com/normal-uterus/
- 25.Fleischer AC, Vasquez JM, Cullinan JA, Eisenberg E. Sonohysterography combined with sonosalpingography: correlation with endoscopic findings in infertility patients. J. Ultrasound. Med. 16, 381–384 (1997). [DOI] [PubMed] [Google Scholar]
- 26.Nezhat FR, Aposto Rl, Nezha CT, Pejovic T. New insights in the pathophysiology of ovarian cancer and implications for screening and prevention. Am. J. Obstet. Gynecol. 213, 262–267 (2015). [DOI] [PubMed] [Google Scholar]
- 27.Pavlik EJ, van Nagell JR. “Early detection of ovarian tumors using ultrasound.” Women's Health (Lond. Engl.) 9, 39–57 (2013). [DOI] [PubMed] [Google Scholar]