Abstract
Among female-specific cancers worldwide, ovarian cancer is the leading cause of death from gynecologic malignancy in the western world. Despite radical surgery and initial high response rates to first-line chemotherapy, up to 70% of patients experience relapses with a median progression-free survival of 12–18 months. There remains an urgent need for novel targeted therapies to improve clinical outcomes in ovarian cancer. This review aims to assess current understanding of targeted therapy in ovarian cancer and evaluate the evidence for targeting growth-dependent mechanisms involved in its pathogenesis. Of the many targeted therapies currently under evaluation, the most promising strategies developed thus far are antiangiogenic agents and PARP inhibitors.
Keywords: combination therapy, ovarian cancer, PARP inhibitors, targeted therapy, VEGF inhibitors
Among female-specific cancers worldwide, ovarian cancer is the leading cause of death from gynecologic malignancy in the western world [1]. It is estimated that 14,180 deaths from this disease will occur this year out of 21,290 women diagnosed, with a 5-year survival rate of approximately 30% in advanced-stage disease [2]. The current standard of care for ovarian cancer is a combination of optimal cytoreductive surgery and platinum-based chemotherapy with the carboplatin—paclitaxel regimen [3]. Despite radical surgery and initial high response rates to first-line chemotherapy, up to 70% of patients experience relapses with a median progression-free survival of 12–18 months [4]. Sensitivity to platinum-based chemotherapies also decreases with each subsequent relapse with the development of platinum-resistant and refractory disease [5]. As such, the long-term survival remains poor, with a high risk of recurrence. Furthermore, chemotherapeutic regimens for treatment of ovarian cancer adversely impact quality of life due to side effects, such as neurotoxicity, arthralgia and fatigue [6]. There remains an urgent need to establish novel targeted therapies and their routes of administration to improve clinical outcomes and tolerability in ovarian cancer treatment. In an age when great advances have been made in understanding the genetics and molecular biology of this heterogeneous disease, the introduction of novel targeted therapies will have a major impact on ovarian cancer management. Several are in the early stages of development, while other targeted agents have been examined in first-line therapy of ovarian cancer in clinical trials. These targets include VEGFR- and EGFR-signaling cascades [7,8]. Moreover, alternative routes of treatment have been proposed, such as intraperitoneal chemotherapy and nanotechnology-based therapy, which have shown promising results in early clinical trials [9,10]. The standard platinum-based treatment of ovarian cancer is evolving as intraperitoneal (ip.) chemotherapy has shown to be superior to intravenous (iv.) chemotherapy following optimal debulking surgery [11]. The aim of this review is to assess current understanding of targeted therapy in ovarian cancer, and evaluate the evidence for interfering with growth-dependent mechanisms involved in its pathogenesis. Targeted therapy directed at pertinent cancer cell growth and survival pathways will first be explored, singly and in combination with other anticancer and chemotherapeutic agents. The strengths and weaknesses of the evidence will be evaluated. Lastly, a summary of key findings will be made to identify possible changes in clinical care arising from findings of current studies.
Targeted therapeutic options in ovarian cancer
As a result of a greater understanding of molecular pathways involved in carcinogenesis and tumor growth, the following potential therapeutic targets have been identified for ovarian cancer; anti-VEGF/VEGFR angiogenic inhibitors, non-VEGF angiogenic inhibitors, PARP inhibitors, EGFR inhibitors, folate receptor inhibitor, IGFR inhibitors.
Anti-VEGF/VEGFR angiogenic inhibitors
Two primary strategies have been used to inhibit the VEGFR-signaling pathway, namely inhibition of the ligand (VEGF) with antibodies or soluble receptors, and inhibition of the receptor with tyrosine kinase inhibitors [12,13]. Of the VEGF targeting therapies, the most thoroughly investigated molecular targeted drug in ovarian cancer is bevacizumab. Bevacizumab is a recombinant monoclonal anti-VEGF antibody [14]. Several Phase II studies have shown bevacizumab is active in recurrent ovarian cancer and may be used singly or in combination with chemotherapy (Table 1). Currently, antiangiogenic agents are moving from Phase II to III clinical trials in ovarian cancer. The GOG-218 trial investigated the addition of bevacizumab every 3 weeks to standard three weekly carboplatin and paclitaxel in a randomized three-arm placebo controlled study [15]. The trial enrolled 1873 patients with stage 3–4 ovarian cancer who had residual disease following primary debulking surgery. In the two experimental arms, bevacizumab was given with chemotherapy and subsequently continued as maintenance treatment, while in the other arm, patients switched to placebo after chemotherapy. A substantial benefit in progression-free survival (PFS) was seen in the bevacizumab maintenance arm compared with the control arm at 10.3 and 14.1 months, respectively. A second Phase III trial (ICON-7) in 1528 high-risk early-stage or advanced ovarian cancer patients similarly examined addition of bevacizumab to standard carboplatin and paclitaxel followed by maintenance bevacizumab until disease progression [16]. The PFS at 36 months was substantially greater in patients receiving bevacizumab. Furthermore, an updated analysis of high-risk patients (stage 3 or 4 with >1 cm residual disease) at 42 months demonstrated a greater extent of benefit at 14.5 months for standard therapy in comparison with 18.1 months with combination treatment. In both trials, addition of bevacizumab was well-tolerated. Grade ≥2 hypertension (symptomatic increase by >20 mmHg (diastolic) or to >150/100) was observed in 16.5 and 22.9% in the two bevacizumab arms compared with 7.2% in the control arm. The incidence of other adverse effects such as gastrointestinal perforation and proteinuria was infrequent.
Table 1.
Anti-VEGF angiogenic inhibitors in ovarian cancer.
| Targeted agent | Chemotherapeutic agent | Dosing schedule | Phase | Participants (n) | Results | Adverse effects | Ref. |
|---|---|---|---|---|---|---|---|
| Bevacizumab | − | Bevacizumab 15 mg/kg iv. every 21 days until disease progression | II | 62 | Median PFS and OS were 4.7 and 17 months, respectively | Hypertension, vomiting, thromboembolism, dyspnea | [17] |
| Bevacizumab | − | Bevacizumab 15 mg/kg iv. every 21 days | II | 44 | Median PFS and OS was 4.4 and 10.7 months, respectively | Hypertension, proteinuria, bleeding | [18] |
| Bevacizumab | Cyclophosphamide | Bevacizumab 10 mg/kg iv. every 2 weeks and oral cyclophosphamide 50 mg/day | II | 70 | Median time to PFS and OS were 7.2 and 16.9 months, respectively | Hypertension, fatigue, pain, GIT bleeding | [19] |
| Bevacizumab | Irinotecan | Bevacizumab 5 mg/kg iv. every 2 weeks + irinotecan 60 mg/m2 weekly, repeated every 28 days, up to six cycles | II | 52 | Stable disease 42.3%, median PFS and OS were 8.0 and 13.8 months, respectively | Diarrhea, neutropenia, thrombocytopenia | [20] |
| Bevacizumab | Paclitaxel + carboplatin | Bevacizumab 7.5 mg/kg + paclitaxel 80 mg/m2 weekly + carboplatin AUC 6 iv. every 28 days for 6–8 cycles → bevacizumab for 1 year | II | 189 | Median PFS of 23.7 months, 1-year PFS of 85.6%, RR of 84.6% | Febrile neutropenia, thrombocytopenia, neuropathy | [21] |
| Bevacizumab | Oxaliplatin + docetaxel | Six cycles of oxaliplatin 85 mg/m2 + docetaxel 75 mg/m2 + bevacizumab 15 mg/kg every 3 weeks → bevacizumab 15 mg/kg every 3 weeks for 1 year | II | 132 | RR of 58.6%, median PFS and OS were 16.3 and 47.3 months, respectively | Neutropenia, leukopenia, hypertension, fatigue | [22] |
| Bevacizumab | Gemcitabine + carboplatin | Gemcitabine 1000 mg/m2 + carboplatin AUC 3 + bevacizumab 10 mg/kg iv. every 2 weeks for 6 cycles or up to 24 cycles if clinical benefit occurred | II | 45 | RR of 69%, median PFS of 13.3 months | Hypertension, fatigue | [23] |
| Sunitinib | − | Sunitinib 50 mg/day for 28 days followed by 14 days off drug | II | 73 | Median PFS and OS were 4.8 and 13.6 months, respectively | GIT bleeding, fatigue, nausea | [24] |
| Sunitinib | − | Sunitinib 50 mg/day, 4 of 6 weeks | 30 | Stable disease in 53%, five had >30% decrease in measurable disease, median PFS of 4.1 months | Fatigue, gastrointestinal symptoms, hand–foot syndrome, hypertension | [25] | |
| Sorafenib | − | Sorafenib 400 mg b.i.d | II | 71 | Two patients had partial response, 20 had stable disease, median PFS of 6 months | Rash, hand-foot syndrome, metabolic and GIT abnormalities | [26] |
| Sorafenib | − | Sorafenib 400 mg b.i.d | II | 246 | Median PFS of 12.7 months | Rash, hand–foot syndrome | [27] |
| Cediranib | Paclitaxel + carboplatin | Carboplatin AUC 5/6 + paclitaxel 175 mg/m2 + cediranib 20 mg/day followed by placebo (concurrent) or cediranib 20 mg/ day | 60 | 49 patients completed 6 cycles of chemotherapy | Diarrhea, hypertension, fatigue, rash | [28] | |
| BOOST (NCT01462890) | Paclitaxel + carboplatin | Bevacizumab 15 mg/kg, iv. and paclitaxel + carboplatin 175 mg/m2 iv. every 3 weeks | III | − | Ongoing | − | |
| MITO-16/ MANGO-2b (NCT01802749) | Paclitaxel, carboplatin | Bevacizumab 15 mg/kg, iv. and paclitaxel + carboplatin 175 mg/m2 iv. every 3 weeks | III | − | Ongoing | − | |
| GOG-0213 (NCT00565851) | Paclitaxel, carboplatin, gemcitabine hydrochloride | Paclitaxel + carboplatin/gemcitabine hydrochloride 175 mg/m2 iv. + bevacizumab 15 mg/kg iv. every 3 weeks | III | − | Ongoing | − | |
| PAZPET-1 (NCT01608009) | Paclitaxel | Pazopanib 800 mg q.d for 7 days → paclitaxel 80 mg/m2 weekly + pazopanib 800 mg q.d for 18 weeks → maintenance pazopanib 800 mg q.d until disease progression | III | − | Ongoing | − | |
| Carbo-Cox-2 (NCT01124435) | Carboplatin | Celecoxib 200 mg b.i.d for 28 days + carboplatin AUC 5 every 28 days | II | − | Ongoing | − | |
| Pazopanib (NCT00866697) | − | Pazopanib 800 mg daily for 24 months | III | − | Ongoing | − | |
| Sunitinib (NCT00768144) | − | Sunitinib 37.5 mg/day for 28 days | II | − | Ongoing | − | |
AUC: Area under curve; b.i.d: Two-times a day; GIT: Gastrointestinal; iv.: Intravenous; PFS: Progression-free survival; q.d: Once a day; OS: Overall survival; RR: Response rate.
In relapsed disease, both the OCEANS and AURELIA studies have evaluated addition of bevacizumab to chemotherapy and demonstrated an improvement in PFS. In AURELIA, for patients with relapsed platinum-resistant ovarian cancer, median PFS was 3.4 months with chemotherapy alone versus 6.7 months in conjunction with bevacizumab [29]. Likewise, in the OCEANS trial, addition of bevacizumab to carboplatin and gemcitabine in patients with relapsed platinum-sensitive ovarian cancer prolonged PFS at 12.4 months in the combination therapy group in comparison with 8.4 months in the chemotherapy group [30]. The AURELIA study revealed a 2.2% risk for gastrointestinal perforation with the addition of bevacizumab, however the risk for perforation was lower than expected, given that patients with ovarian cancer are at a higher risk for perforation than other solid organ malignancies. Overall, increased risk for perforation with addition of bevacizumab is small and does not outweigh its clinical benefit. Likewise, preliminary results from a Phase II study showed similar response rates and safety profile in patients treated with aflibercept, a VEGF monoclonal antibody [31]. Following these encouraging findings, Phase III trials are in progress involving VEGF inhibitors singly or in combination with chemotherapy (Table 1).
The success with use of bevacizumab for treatment of ovarian cancer has provided a useful platform for the introduction of other antiangiogenic agents. Targeting the intracellular tyrosine kinase component of VEGFR has been assessed in Phase II studies of pazopanib, sunitinib, sorafenib and cediranib (Table 1). They have demonstrated activity in patients with recurrent ovarian cancer, resulting in tumor responses and stabilization of disease, delaying tumor progression. In particular, pazopanib is an angiogenic multikinase inhibitor with broad spectrum activity against all three VEGF receptors, PDGFR and c-Kit [32]. This was demonstrated in a Phase III study of 940 women with advanced ovarian cancer where pazopanib prolonged disease-free survival by 5.6 months compared with placebo [33]. The PFS was 17.9 months for the patients receiving pazopanib and 12.3 months for the placebo group after 24 months. Pazopanib may be an effective agent as maintenance therapy, with manageable adverse events including nausea and neutropenia [34]. One key limitation of clinical studies involving newer targeted agents in ovarian cancer is the relatively small number of patients enrolled. Larger studies are required to provide more definitive demonstration of efficacy in combination with chemotherapy for the treatment of ovarian cancer. Furthermore, reported outcomes in the different trials included various response and survival measures. Hence, methodological differences between clinical studies and nonstandardized methods in evaluation of patient outcomes warrant caution when interpreting their findings.
Combinations of targeted antiangiogenic agents are also being explored. A Phase I and II study of bevacizumab and sorafenib showed six Response Evaluation Criteria in Solid Tumors (RECIST) partial responses in 13 ovarian cancer patients, with response duration from 4 to 22 months [35]. However, severe toxicities were reported with combination of bevacizumab and sorafenib, including grade 4 hypertension, proteinuria and two fistula formation at sites of disease response. These adverse events led to use of lower doses of both agents in a subsequent Phase II study [36]. By contrast, preliminary results from a Phase I study of bevacizumab and vascular disrupting agent (VDA) combretastatin 4A phosphate showed no additive toxicity and evidence for efficacy was encouraging, offering a potential treatment approach to be further evaluated [37].
Non-VEGF angiogenic inhibitors
Targeting the angiopoietin axis with non-VEGF inhibitors is an alternate strategy in ovarian cancer and is still undergoing early clinical trials [38]. Trebananib, a peptide-Fc fusion protein (peptibody) inhibiting the interaction of angiopoietin-1 and -2 to the Tie2 receptor, has been evaluated in combination with paclitaxel in recurrent ovarian cancer [39]. The results of a Phase III trial have been promising. Participants were treated with paclitaxel alone or paclitaxel and trebananib [40]. Notably, PFS was significantly longer in the combination therapy group at 7.2 months compared with 5.4 months for those treated with paclitaxel alone. Angiogenic inhibition via Tie2/angiopoietin pathway inhibition may offer effective treatment for advanced recurrent ovarian cancer. Further exploration within the TRINOVa-3 trial of trebananib in combination with carboplatin and paclitaxel is underway.
PARP inhibitors
PARP is a key enzyme involved in the repair of DNA single-strand breaks using the base excision repair pathway [41]. PARP inhibition results in accumulation of DNA single-strand breaks, which lead to DNA double-strand breaks at replication forks [42]. Double-strand breaks are effectively repaired in normal cells by homologous recombination (HR) DNA repair mechanisms [43]. In the absence of functional BRCA1 or BRCA2 proteins, alternative DNA repair pathways such as nonhomologous end joining are used, resulting in chromosomal instability and cell death [44]. As such, women with inherited mutations in BRCA1 or BRCA2 are at significantly higher risk of developing ovarian cancer, where lifetime risks of ovarian cancer are 54 and 23% for BRCA1 and BRCA2 mutation carriers, respectively [45]. PARP inhibitors in BRCA mutation carriers specifically exploit the concept of synthetic lethality by combining base excision repair inhibition with a defective HR DNA repair pathway [46]. Hence, BRCA tumors are particularly susceptible to PARP and offer a promising approach to targeted therapy.
Clinical trials in recurrent ovarian cancer have demonstrated single-agent activity of PARP inhibitors [47–49]. The first Phase I trial of olaparib was evaluated in patients with BRCA mutations and was well-tolerated with grade ≤2 toxicities of nausea, vomiting and fatigue [47]. Pharmacodynamic studies showed significant PARP1 inhibition in tumor tissues at a dose level of 100 mg daily and higher [48]. Moving forward, three randomized Phase II trials incorporating olaparib monotherapy have been reported [49–51]. In the first, women with recurrent, BRCA-deficient epithelial ovarian cancer were randomized between olaparib at 200 mg twice daily, olaparib at 400 mg twice daily, and pegylated liposomal doxorubicin (PLD) [52]. Initial results show a median PFS of 6.5, 8.8 and 7.1 months, respectively. The highest rate of response was in the high-dose olaparib group at 31%. In a second Phase II trial, olaparib at 400 mg twice daily was compared with placebo in a cohort of women with recurrent serous epithelial ovarian cancer as maintenance therapy after complete response to platinum therapy [51]. The study showed olaparib maintenance therapy significantly prolonged PFS compared with placebo in patients with BRCA-mutated ovarian cancer with PFS of 11.2 and 4.3 months, respectively. The most common adverse events in these trials were mild and included nausea, vomiting and anemia. In addition, a recent study investigating the combination of olaparib and cediranib in recurrent ovarian cancer associated with a BRCA gene mutation reported a response rate (RR) of 80% with PFS of 18 months [53]. In comparison, for patients who received only olaparib, RR was 48% with PFS of 9 months. Notably, although side effects were more common for women taking the combination therapy, they were manageable with reduction of treatment doses.
Several Phase II and III trials are currently evaluating olaparib in combination with chemotherapy [54–56]. PARP inhibition in combination with DNA-damaging agents may enhance the effects of chemotherapy and potentially delay treatment resistance [57]. A recent Phase II trial demonstrated olaparib in conjunction with paclitaxel and carboplatin followed by maintenance monotherapy significantly improved PFS compared with paclitaxel and carboplatin alone [58]. The greatest clinical benefit was seen in BRCA-mutated patients, and the treatment regimen had a favorable toxicity profile. Combinations of olaparib with other chemotherapeutic agents are underway (NCT01445418, NCT01237067, NCT00516724, NCT01081951). In addition to olaparib, additional randomized trials of other PARP inhibitors are in clinical development (Table 2). For example, niraparib, a novel inhibitor of PARP1 and PARP2, demonstrated a 40% RR in BRCA-mutated ovarian cancer in a Phase I trial [59]. Niraparib is being further explored in a randomized placebo-controlled Phase III trial as maintenance therapy in patients with platinum-sensitive BRCA-mutated ovarian cancer. Other PARP inhibitors including veliparib and rucaparib have shown similar efficacy in ovarian cancer patients.
Table 2.
PARP inhibitors in ovarian cancer.
| Targeted agent | Phase | Dosing schedule | Participants (n) | Results | Adverse effects | Ref. |
|---|---|---|---|---|---|---|
| Olaparib | 1 | Olaparib 400 mg b.i.d, 21-day cycles | 59 | RR of 41 and 43% among patients with BRCA1/2 mutation | Neutropenia, lekopenia, anemia | [47] |
| Olaparib | Olaparib 400 mg b.i.d, 28-day cycles | 19 | Partial response of 37%, one patient remains on olaparib monotherapy without progression | Diarrhea, nausea, neutropenia | [48] | |
| Olaparib | I | Olaparib 400 mg b.i.d, 28-day cycles | 28 | RR of 44%, clinical benefit rate of 61%, 2 patients had stable disease for >24 weeks | Hypertension, fatigue | [49] |
| Olaparib | I | Olaparib 400 mg b.i.d, 28-day cycles | 298 | RR of 31.1%, stable disease ≥8 weeks observed in 42% of patients | Fatigue, nausea, vomiting, anemia | [52] |
| Olaparib + cediranib | II | Cediranib 20 mg/day + olaparib 100 mg b.i.d (standard 3 + 3 dose escalation design) → cediranib 20 mg/day + olaparib 200 mg b.i.d → cediranib 30 mg/day + olaparib 400 mg b.i.d | 46 | Median PFS of 17.7 months RR of 44%, clinical benefit rate of 61 %, 2 patients had stable disease for >24 weeks, median PFS of 17.7 months | Fatigue, diarrhea, hypertension | [50] |
| Veliparib | I | Veliparib 60 mg/day, 21-day cycles | 35 | Seven patients had partial responses, additional six patients had disease stabilization for at least six cycles | Myelosuppression | [60] |
| Veliparib | I | Veliparib 70 mg/day, 21-day cycles | 68 | RR of 23%, clinical benefit rate of 58% | Neutropenia, thrombocytopenia, peripheral neuropathy | [61] |
| Rucaparib | I | Rucaparib 12 mg/m2 iv. every 28 days | 40 | RR of 17.4%, median time to progression 3.5 months, median OS 9.9 months, 36% of patients progression-free at 6 months | Thrombocytopenia, neutropenia, anemia | [62] |
| Rucaparib | I | Rucaparib 12 mg/m2 iv. every 28 days | 32 | RR of 20%, median time to progression 5.5 months, median OS 9 months, 30% of patients progression-free at 6 months | Thrombocytopenia, anemia, neutropenia | [63] |
| (Talazoparib) NCT02326844) | I | Talazoparib 1 mg/day, 28-day cycles until disease progression | − | Ongoing | − | [64] |
b.i.d: Two-times a day; iv.: Intravenous; OS: Overall survival; PFS: Progression-free survival; RR: Response rate.
The use of PARP inhibitors could also be extended to sporadic ovarian cancers with HR defects due to loss of function of DNA repair proteins, including RAD51, ATM and ATR [65]. These sporadic tumors appear to phenocopy BRCA1- or BRCA2-deficient tumors although they do not possess germline mutations in either gene, a phenomenon termed ‘BRCAness' [66]. Further studies are required to identify patients with HR-defective tumors who are most likely to benefit from this new therapy. A randomized placebo-controlled trial of olaparib as maintenance therapy in patients with sporadic ovarian cancer is ongoing (NCT00753545).
EGFR inhibitors
The EGFR is overexpressed in up to 70% of ovarian cancers and is associated with poor prognosis and chemoresistance [67]. Responses to EGFR inhibitors in recurrent ovarian cancer are infrequent and dependent on a mutation in the EGFR catalytic domain [68]. Studies of EGFR tyrosine kinase inhibitors (erlotinib and gefitinib) and monoclonal antibodies against EGFR (cetuximab, panitumumab and matuzumab) have shown only modest efficacy (Table 3). For example, a Phase II trial of 837 patients with ovarian cancer treated with anti-HER2 monoclonal antibody, trastuzumab, showed only 7.3% of the 41 ERBB2-positive patients responded to treatment [69]. Furthermore, the European Organisation for Research and Treatment of Cancer (EORTC) evaluated the efficacy of maintenance erlotinib following first-line chemotherapy in 835 ovarian cancer patients unselected for EGFR expression [70]. The study reported that maintenance of erlotinib did not improve progression-free or overall survival (OS). Overall, clinical studies using EGFR antagonists in ovarian cancer have shown limited success.
Table 3.
EGFR inhibitors in ovarian cancer.
| Targeted agent | Phase | Dosing schedule | Participants (n) | Results | Adverse effects | Ref. |
|---|---|---|---|---|---|---|
| Gefitinib | II | Gefitinib 500 mg/day on 28-day cycles until progressive disease, unacceptable toxicity or withdrawal | 16 | No complete or partial responses, nine patients (37%) had stable disease for >2 months | Diarrhea, rash, asymptomatic elevated hepatic transaminases | [71] |
| Gefitinib | II | Gefitinib 500 mg/day on 28-day cycles until progressive disease or unacceptable toxicity | 27 | Four patients progression-free >6 months, one objective response (4%) | Diarrhea, rash | [72] |
| Gefitinib | II | Tamoxifen 40 mg/day + gefitinib 500 mg/day until progression or unacceptable toxicity | 56 | Six patients had stable disease, median time-to-progression was 58 days, median survival was 253 days | Diarrhea, rash, nausea, fatigue | [73] |
| Erlotinib | III | Maintenance erlotinib 150 mg/day for 2 years or to observation | 835 | Median PFS and OS were 12.7 and 50.8 months, respectively | Diarrhea, rash, abdominal pain | [70] |
| Erlotinib | I | Erlotinib 150 mg/day | 30 | Stable disease in two patients, median PFS of 2 months | Diarrhea, rash, nausea, fatigue | [74] |
| Erlotinib | lb | Carboplatin AUC 5 + docetaxel 75 mg/m2, followed by erlotinib 75–100 mg/day every 21 days | 45 | 52% objective response rate | Diarrhea, rash | [75] |
| Erlotinib | II | Carboplatin AUC 5 every 21 days + erlotinib 150 mg/day | 50 | 57% objective response rate | Diarrhea, rash, vomiting | [76] |
| Cetuximab | II | Initial dose of cetuximab 400 mg/m2, then 250 mg/m2 weekly for two 3-week cycles | 25 | Median PFS and 1-year survival rate were 2.1 months and 54.8%, respectively | Rash, abdominal pain | [77] |
| Cetuximab | II | Initial iv. dose of cetuximab 400 mg/m2 followed by weekly iv. infusions of cetuximab 250 mg/m2 + paclitaxel 175 mg/m2 + carboplatin AUC 6 every 21 days for six cycles | 41 | Median PFS 14.4 months, PFS at 18 months was 38.8% | Febrile neutropenia, rash, hypersensitivity reaction | [78] |
| Panitumumab | I | Panitumumab 4.8 mg/kg every 2 weeks | 32 | Three partial responses, ten stable disease ≥6 months | Rash/pruritis, nausea, vomiting, fatigue | [79] |
| Panitumumab | II | Panitumumab 6 mg/kg day 1 and day 15 + PLD 40 mg/m2 every 4 weeks | 46 | PFS and OS were 2.7 and 8.1 months, respectively | Skin toxicity, fatigue, vomiting | [80] |
| Matuzumab | II | Matuzumab 800 mg/week iv. | 37 | No formal responses, seven patients on therapy >3 months with stable disease | Rash, acne, dry skin, diarrhea | [81] |
AUC: Area under curve; iv.: Intravenous; OS: Overall survival; PFS: Progression-free survival; PLD: Pegylated liposomal doxorubicin.
Folate receptor inhibitors
The αFR is overexpressed in ovarian cancer and represents a potent target for therapy [82]. An overexpression might confer a tumor growth advantage by increasing folate availability to cancer cells where the degree of αFR expression has been shown to correlate with the grade of malignancy [83]. Farletuzumab, a monoclonal antibody to αFR, inhibits the growth of cells that overexpress αFR and activates antibody-dependent cell-mediated cytotoxicity and complement-mediated cytotoxicity [84]. In a Phase II study of 54 patients with platinum-sensitive relapsed disease, in which farletuzumab was given in combination with chemotherapy, there were encouraging signs of benefit [85]. Specifically, 37 patients showed normalization of CA-125 levels while 12 demonstrated a longer period of remission than their previous remission. Moving forward, larger randomized trials of farletuzumab are anticipated.
αFR is also being investigated as a selective drug target for a series of new quinazoline anti-folates. These include BGC945, a potent inhibitor of thymidylate synthase and highly selective for αFR [86]. Encouraging data from Phase II trials showed an improvement in PFS from 2.7 to 5 months. Similarly, selectively therapy targeting the folate receptor is being developed by using EC145, a conjugate of desacetylvinblastine monohydrazide linked through a peptide spacer to folate receptor targeting moiety [87]. The first study, PRECEDENT comparing EC145 and PLD with PLD alone showed an improvement in PFS of 20% [88]. Folate targeted agents have shown promising antitumor activity in ovarian malignancy and their continual development remains an active area.
IGFR inhibitors
IGF-1 is involved in inhibition of apoptosis, tumor progression and metastases [89]. Support for a role of IGF-I in ovarian cancer progression arose from a recent study which showed high free IGF-I protein expression in ovarian tumor tissue was independently associated with disease progression [90]. Moreover, IGF-I mRNA expression levels were positively associated with ovarian cancer progression, suggesting endocrine and paracrine regulations of IGF-I activity are involved in this disease [91]. As such, IGF-1 is a potential effective therapeutic target. In particular, aMG 479 is a monoclonal antibody that is a potent inhibitor of the IGF-1 receptor and a randomized Phase II study of aMG 479 added to first-line chemotherapy in patients with optimally debulked ovarian cancer is underway (NCT00719212).
Limitations & challenges
Despite promising results of established targeted agents, including PARP and VEGF inhibitors, there remain several challenges to further refine their clinical development. These include the identification of the correct population to treat as well as a clearer understanding of mechanisms underlying drug resistance. In particular, PARP inhibitors have demonstrated maximal effect in germline BRCA-associated tumors and sporadic cases deficient in repair of DNA damage. While testing for germline BRCA mutations is available, there currently is no validated biomarker for HR-deficient ovarian cancer predictive of response to PARP inhibition [92]. The clinical benefit of PARP inhibitors may not be limited to germline BRCA mutation carriers but a wider group of patients with BRCA dysfunction [93]. It is imperative to develop appropriate companion diagnostic tests to enable patient selection and identify reliable biomarkers for accurate prognosis of targeted therapies. With the growing availability and scope of multiplex-gene testing and massive parallel sequencing, patients with mutations in HR-related genes are being identified and may be suitable PARP inhibitor candidates.
In addition to difficulties in identifying appropriate patient candidates, there are patients with HR-deficient tumors who do not respond or develop resistance to PARP inhibition [94]. This suggests tumors can have both de novo and acquired resistance to PARP inhibition [95]. Given the multiplicity of aberrant pathways involved in ovarian cancer, it is unlikely inhibition of a single cascade will be sustainable. For example, there are data to suggest that exposure to DNA damaging agents leads to re-expression of BRCA1 by genetic reversion [96]. This causes a partial restoration of HR-mediated DNA repair and renders cells less sensitive to PARP inhibition [97]. Another mechanism of resistance involves increased expression of multidrug resistant (Mdr1a/b) genes which encode the drug efflux transporter P-glycoprotein [98]. Elevated expression of this target results in the need for increasing drug concentrations required for effective inhibition. Likewise, tumors may also adapt to evade blockade of angiogenesis by VEGF inhibitors through upregulation of proangiogenic signals, such as matrix metalloproteinase and SDF-1α [99]. Furthermore, differences between different PARP and VEGF inhibitors have yet to be fully defined. Multiple PARP inhibitors appear to be active in epithelial ovarian cancer in Phase II and III trials. However, there are no clinical data comparing one PARP inhibitor with another in the clinical arena. Although olaparib is associated with considerable clinical benefit, preclinical studies suggest that selectivity of various PARP inhibitors may be different and have an impact on patient outcome. Recent data demonstrated potency in trapping PARP differs markedly among niraparib, olaparib and veliparib, and patterns of trapping were not correlated with the catalytic inhibitory properties for each drug [100]. As such, niraparib may not share the same mechanism of action as olaparib and veliparib. These results suggest drug inhibitors are not as targeted in practice as they are during initial development [101]. Molecular profiling of tumor and normal tissues will enable better understanding of the effects of inhibiting the target in tumor and host tissue. Hence, further studies will be needed to clarify differences in pharmacokinetics and efficacy between these related drugs.
Additional challenges facing the success of targeted therapy include identification of biomarkers to guide management and assess response. The complexity of signaling cascades and lack of specificity of small molecules make it difficult to predict which therapy will be successful or identify appropriate patient populations. Although a range of predictive biomarkers have been proposed, such as the plasma levels of circulating VEGFA, soluble VEGFR and basic fibroblast growth factor, none have proven to be robust [102,103]. A potential alternative is to use functional imaging techniques, such as diffusion contrast-enhanced magnetic resonance imaging and fluoro-d-glucose positron emission tomography [104]. Hence, use of new targeted agents will be improved by the development of multiple biomarkers to identify patients most likely to benefit and monitor treatment efficacy.
Conclusion
In conclusion, ovarian cancer remains a therapeutic challenge due to advanced disease at presentation and limited success of traditional treatment approaches. Understanding molecular changes driving ovarian cancer is critical for selection of appropriate candidate agents and success of these agents in improving clinical outcome. This allows for the development of effective targeted therapeutic approaches demonstrated by the various clinical trials discussed above. These therapies facilitate a shift in ovarian cancer management from empirical cytotoxic therapies to individualized approavhes targeted against specific pathological features of each tumor.
Future perspective
Several emerging targeted therapies have been highlighted in this review. Of the various targeted therapies under evaluation in Phase II and III studies, the most promising strategies developed thus far are antiangiogenic agents and PARP inhibitors. Therapies targeting specific molecular features as strategies in the treatment of ovarian cancer have been clearly demonstrated with PARP inhibitors. Specifically, this has been exemplified by addition of olaparib in the maintenance treatment of women with platinum-sensitive BRCA1/2-mutated relapsed ovarian cancer. In particular, BRCA mutations have been associated with improved survival and increased responsiveness to PARP inhibitors. Moving forward, there may be introduction of treatments targeted to specific groups of patients, on the basis of robust predictive biomarkers. For example, pharmacodynamic assays that measure PARP activity in peripheral mononuclear blood cells could provide useful information on biological activity [99]. As more is known regarding the molecular subgroups of ovarian carcinoma as well as acquired and inherent resistance to PARP inhibition, treatment can be increasingly tailored to the individual patient to maximize OS.
In addition to PARP inhibitors, angiogenic inhibitors may similarly be incorporated into clinical practice in the future. One of the most important cytokines responsible for tumor-mediated angiogenesis is VEGF. Efforts to block this pathway have arisen as attractive strategies for ovarian cancer treatment. The most promising antiangiogenic agent to date is bevacizumab. As discussed above, studies have shown a significant improvement in PFS with concurrent use of bevacizumab and chemotherapy in comparison with chemotherapy alone. However, several studies have demonstrated mixed results with addition of bevacizumab to chemotherapy. The GOG-218 study reported improvement in OS with bevacizumab which was not statistically significant, with median OS of 38.6 months on standard chemotherapy compared with 42.1 months on combined therapy [15]. Similarly, in the ICON-7 study, there was no OS difference with the combination treatment regimen in the overall study population with a mean survival of 44.6 months with standard chemotherapy compared with 45.5 months with addition of bevacizumab [16]. Notably, there was an OS benefit in a high-risk subset of 502 patients with inoperable or suboptimally cytoreduced stage III or IV disease, with mean OS of 34.5 months in the chemotherapy alone group compared with 39.3 months with bevacizumab. When considering the balance of clinical benefit, quality of life preservation and tolerability of bevacizumab in combination with chemotherapy, this treatment regimen could be appropriate as a front-line option for advanced ovarian cancer supported by consistent clinical evidence. Although adverse events are not commonly observed with use of bevacizumab, those that occur can usually be managed with close monitoring and dose adjustment. In addition, significant activity demonstrated with concurrent targeted treatment suggests it could be an alternate therapeutic approach to standard chemotherapy. Strategies such as combining multiple antiangiogenic agents or the concurrent use of antiangiogenic agents with chemotherapy may overcome resistance [105]. Combinatorial targeted therapies could involve either vertical or horizontal pathway blockade and is useful in counteracting negative feedback loops. Notably, the combination of bevacizumab and sorafenib is an example of vertical pathway blockade [106]. This combination is noteworthy for its substantial efficacy and favorable safety profile compared with either of the single agents. Moreover, use of PARP inhibitors with antiangiogenic agents may circumvent increased VEGFR2 phosphorylation and subsequent activation of endothelial cell survival, seen in PARP inhibitor monotherapy [107]. Care must be taken to appropriately manage toxicities demonstrated with combination therapy, especially with increased myelosuppression seen with these regimens. This may involve patient stratification based on altered oncogenic pathways or intermittent dosing strategies. New targeted approaches, including immune checkpoint inhibitors, are also being examined and have shown promising potential [108,109]. This includes nivolumab which is a human IgG4 monoclonal antibody that targets PD-1 and stimulates antitumor immune responses. A Phase II study of nivolumab has demonstrated encouraging clinical efficacy and tolerability in patients with platinum-resistant ovarian cancer with median PFS of 3.5 months and OS of 20.0 months [108]. Further clinical trials are underway to establish the clinical use of these targeted agents. Alternate routes of administration may also be considered to ensure effective delivery of drugs to the intended site of action. The advantages of administering chemotherapy into the peritoneal cavity are supported by both preclinical and clinical trials [110–113]. In comparison with iv. treatment, ip. administration achieves a fourfold increase in drug concentration within the abdominal cavity [110]. In addition, long-term results from two studies demonstrated the benefits of ip. administration of chemotherapy over iv. administration following surgery in patients with advanced ovarian cancer [113,114]. The data arise from a 10-year follow-up of patients involved in GOG trials 114 and 172.
Executive summary
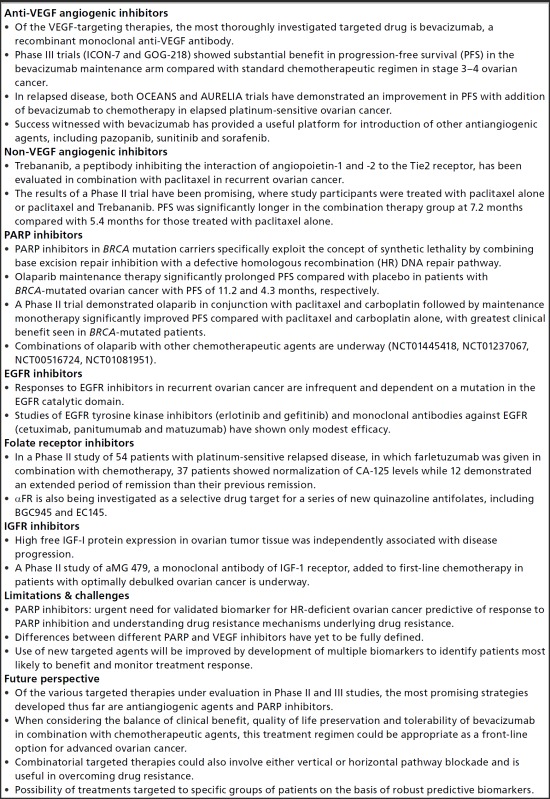
- Anti-VEGF angiogenic inhibitors
- Of the VEGF-targeting therapies, the most thoroughly investigated targeted drug is bevacizumab, a recombinant monoclonal anti-VEGF antibody.
- Phase III trials (ICON-7 and GOG-218) showed substantial benefit in progression-free survival (PFS) in the bevacizumab maintenance arm compared with standard chemotherapeutic regimen in stage 3–4 ovarian cancer.
- In relapsed disease, both OCEANS and AURELIA trials have demonstrated an improvement in PFS with addition of bevacizumab to chemotherapy in elapsed platinum-sensitive ovarian cancer.
- Success witnessed with bevacizumab has provided a useful platform for introduction of other antiangiogenic agents, including pazopanib, sunitinib and sorafenib.
- Non-VEGF angiogenic inhibitors
- Trebananib, a peptibody inhibiting the interaction of angiopoietin-1 and -2 to the Tie2 receptor, has been evaluated in combination with paclitaxel in recurrent ovarian cancer.
- The results of a Phase II trial have been promising, where study participants were treated with paclitaxel alone or paclitaxel and Trebananib. PFS was significantly longer in the combination therapy group at 7.2 months compared with 5.4 months for those treated with paclitaxel alone.
- PARP inhibitors
- PARP inhibitors in BRCA mutation carriers specifically exploit the concept of synthetic lethality by combining base excision repair inhibition with a defective homologous recombination (HR) DNA repair pathway.
- Olaparib maintenance therapy significantly prolonged PFS compared with placebo in patients with BRCA-mutated ovarian cancer with PFS of 11.2 and 4.3 months, respectively.
- A Phase II trial demonstrated olaparib in conjunction with paclitaxel and carboplatin followed by maintenance monotherapy significantly improved PFS compared with paclitaxel and carboplatin alone, with greatest clinical benefit seen in BRCA-mutated patients.
- Combinations of olaparib with other chemotherapeutic agents are underway (NCT01445418, NCT01237067, NCT00516724, NCT01081951).
- EGFR inhibitors
- Responses to EGFR inhibitors in recurrent ovarian cancer are infrequent and dependent on a mutation in the EGFR catalytic domain.
- Studies of EGFR tyrosine kinase inhibitors (erlotinib and gefitinib) and monoclonal antibodies against EGFR (cetuximab, panitumumab and matuzumab) have shown only modest efficacy.
- Folate receptor inhibitors
- In a Phase II study of 54 patients with platinum-sensitive relapsed disease, in which farletuzumab was given in combination with chemotherapy, 37 patients showed normalization of CA-125 levels while 12 demonstrated an extended period of remission than their previous remission.
- αFR is also being investigated as a selective drug target for a series of new quinazoline antifolates, including BGC945 and EC145.
- IGFR inhibitors
- High free IGF-I protein expression in ovarian tumor tissue was independently associated with disease progression.
- A Phase II study of aMG 479, a monoclonal antibody of IGF-1 receptor, added to first-line chemotherapy in patients with optimally debulked ovarian cancer is underway.
- Limitations & challenges
- PARP inhibitors: urgent need for validated biomarker for HR-deficient ovarian cancer predictive of response to PARP inhibition and understanding drug resistance mechanisms underlying drug resistance.
- Differences between different PARP and VEGF inhibitors have yet to be fully defined.
- Use of new targeted agents will be improved by development of multiple biomarkers to identify patients most likely to benefit and monitor treatment response.
- Future perspective
- Of the various targeted therapies under evaluation in Phase II and III studies, the most promising strategies developed thus far are antiangiogenic agents and PARP inhibitors.
- When considering the balance of clinical benefit, quality of life preservation and tolerability of bevacizumab in combination with chemotherapeutic agents, this treatment regimen could be appropriate as a front-line option for advanced ovarian cancer.
- Combinatorial targeted therapies could also involve either vertical or horizontal pathway blockade and is useful in overcoming drug resistance.
- Possibility of treatments targeted to specific groups of patients on the basis of robust predictive biomarkers.
After 876 women from the two trials had undergone primary surgical cytoreduction, they were randomized to receive either ip. or iv. chemotherapy. There was a significant improvement in OS with the ip. route compared with iv. administration. Specifically, median OS with ip. therapy was 61.8 months compared with 51.4 months for patients treated with iv. chemotherapy. This difference resulted in a 23% decreased risk for death. Moreover, ip. therapy was also associated with improved survival among those patients with gross residual disease. Similarly, a review of ip. chemotherapy in women undergoing treatment for advanced ovarian cancer reported a 21% decrease in the risk of death in patients undergoing combined ip. and iv. therapy compared with those undergoing iv. therapy alone [115]. Additional trials are underway to define the optimal number of cycles of ip. chemotherapy while minimizing treatment-related toxicity and infection risk. Furthermore, recent advances in nanotechnology enable various types of nanoparticles to improve the therapeutic efficacy of anticancer drugs [116,117]. Their properties can be designed for targeted delivery to tumors and remain a new area of study to modulate ip. therapy [116]. These include multifunctional polymer micelles, lipid nanoparticles and polymeric nanoparticles. The delivery and therapeutic efficacy of majority of nanoparticles are still under investigation, and studies are primarily limited to preclinical stages currently [118–120]. For example, a preclinical study using a lipidoid ip. delivery system to deliver small interfering RNA (siRNA) to PARP1 in a BRCA1-deficient murine ovarian cancer model demonstrated impaired cell growth in vitro and extended OS of mice bearing BRCA1-deficient tumors [118]. The further development of such delivery systems and introduction into clinical trials is a highly promising method to target a host of anticancer targets and potentially modulate ip. therapy. Nanotechnology has the potential to overcome the current chemotherapeutic barriers in ovarian cancer treatment and multidrug resistance [121,122]. Hence, defining the appropriate combination of drugs and dosing schedules catered to individual patients is essential to achieve meaningful yet tolerable target inhibition. Ongoing clinical trials to define strategies of use and ideal patient populations will facilitate successful use of these drugs. With encouraging results from targeted approaches demonstrated in other malignancies, it is with much anticipation to examine their outcomes in ovarian cancer.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
- Papers of special note have been highlighted as: • of interest; •• of considerable interest [Google Scholar]
- 1.Hunn J, Rodriguez GC. Ovarian cancer: etiology, risk factors, and epidemiology. Clin. Obstet. Gynecol. 55(1), 3–23 (2012). [DOI] [PubMed] [Google Scholar]
- 2.Cramer DW. The epidemiology of endometrial and ovarian cancer. Hematol. Oncol. Clin. North Am. 26(1), 1–12 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bolton KL, Chenevix-Trench G, Goh C, et al. Association between BRCA1 and BRCA2 mutations and survival in women with invasive epithelial ovarian cancer. JAMA 307(4), 382–389 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goff BA. Advanced ovarian cancer: what should be the standard of care? J. Gynecol. Oncol. 24(1), 83–91 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sieh W, Köbel M, Longacre TA, et al. Hormone-receptor expression and ovarian cancer survival: an ovarian Tumor Tissue Analysis consortium study. Lancet Oncol. 14(9), 853–862 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cristea M, Han E, Salmon L, Morgan RJ. Practical considerations in ovarian cancer chemotherapy. Ther. Adv. Med. Oncol. 2(3), 175–187 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vaughan S, Coward JI, Bast RC, et al. Rethinking ovarian cancer: recommendations for improving outcomes. Nat. Rev. Cancer 11(10), 719–725 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burger RA, Brady MF, Bookman MA, et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N. Engl. J. Med. 365(26), 2473–2483 (2011). [DOI] [PubMed] [Google Scholar]
- 9.Kwa M, Jandial D. Modulation of intraperitoneal (IP) chemotherapy in ovarian cancer. Transl. Cancer Res. 4(1), 60–69 (2015). [Google Scholar]
- 10.Engelberth SA, Hempel N, Bergkvist M. Development of nanoscale approaches for ovarian cancer therapeutics and diagnostics. Crit. Rev. Oncog. 19(0), 281–315 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trimble EL, Thompson S, Christian MC, Minasian L. Intraperitoneal chemotherapy for women with epithelial ovarian cancer. Oncologist 13(4), 403–409 (2008). [DOI] [PubMed] [Google Scholar]
- 12.Kim A, Ueda Y, Naka T, Enomoto T. Therapeutic strategies in epithelial ovarian cancer. J. Exp. Clin. Cancer Res. 31(1), 14 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jitawatanarat P, Ma WW. Update on antiangiogenic therapy: aflibercept and regorafenib. J. Gastro. Oncol. 4(2), 231–238 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hall M, Gourley C, McNeish I, et al. Targeted anti-vascular therapies for ovarian cancer: current evidence. Br. J. Cancer 108(2), 250–258 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perren TJ, Swart AM, Pfisterer J, et al. A Phase 3 trial of bevacizumab in ovarian cancer. N. Engl. J. Med. 365(26), 2484–2496 (2011). [DOI] [PubMed] [Google Scholar]
- •• Study demonstrated that bevacizumab improved progression-free survival in women with ovarian cancer [Google Scholar]
- 16.Burger RA, Brady MF, Bookman MA, et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N. Engl. J. Med. 365(26), 2473–2483 (2011). [DOI] [PubMed] [Google Scholar]
- 17.Burger RA, Sill MW, Monk BJ, Greer BE, Sorosky JI. Phase II trial of bevacizumab in persistent or recurrent epithelial ovarian cancer or primary peritoneal cancer: a gynecologic oncology group study. J. Clin. Oncol. 25(33), 5165–5171 (2007). [DOI] [PubMed] [Google Scholar]
- 18.Cannistra SA, Matulonis UA, Penson RT, et al. Phase II study of bevacizumab in patients with platinum-resistant ovarian cancer or peritoneal serous cancer. J. Clin. Oncol. 25(33), 5180–5186 (2007). [DOI] [PubMed] [Google Scholar]
- 19.Garcia AA, Hirte H, Fleming G, et al. Phase II clinical trial of bevacizumab and low-dose metronomic oral cyclophosphamide in recurrent ovarian cancer: a trial of the California, Chicago, and Princess Margaret Hospital Phase II Consortia. J. Clin. Oncol. 26(1), 76–82 (2008). [DOI] [PubMed] [Google Scholar]
- 20.Liu Y, Ren Z, Xu S, Bai H, Ma N, Wang F. Low-dose-intensity bevacizumab with weekly irinotecan for platinum-and taxanes-resistant epithelial ovarian cancer. Cancer Chemother. Pharmacol. 75(3), 645–651 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gonzalez-Martin A, Gladieff L, Tholander B. Efficacy and safety results from OCTAVIA, a single-arm Phase II study evaluating front-line bevacizumab, carboplatin and weekly paclitaxel for ovarian cancer. Eur. J. Cancer 49(18), 3831–3838 (2013). [DOI] [PubMed] [Google Scholar]
- • Study evaluated the efficacy and safety of front-line bevacizumab plus weekly paclitaxel and carboplatin administered every 3 weeks [Google Scholar]
- 22.Herzog TJ, Monk BJ, Rose PG. A Phase II trial of oxaliplatin, docetaxel, and bevacizumab as first-line therapy of advanced cancer of the ovary, peritoneum, and fallopian tube. Gynecol. Oncol. 132(3), 517–525 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eisenhauer EL, Zanagnolo V, Cohn DE. A Phase II study of gemcitabine, carboplatin and bevacizumab for the treatment of platinum-sensitive recurrent ovarian cancer. Gynecol. Oncol. 134(2), 262–266 (2014). [DOI] [PubMed] [Google Scholar]
- 24.Baumann KH, du Bois A, Meier W, et al. A Phase II trial (AGO 2.11) in platinum-resistant ovarian cancer: a randomized multicenter trial with sunitinib (SU11248) to evaluate dosage, schedule, tolerability, toxicity and effectiveness of a multitargeted receptor tyrosine kinase inhibitor monotherapy. Ann. Oncol. 23(9), 2265–2271 (2012). [DOI] [PubMed] [Google Scholar]
- 25.Biagi JJ, Oza AM, ChalChal HI, et al. A Phase II study of sunitinib in patients with recurrent epithelial ovarian and primary peritoneal carcinoma: an NCIC Clinical Trials Group Study. Ann. Oncol. 22(2), 335–340 (2011). [DOI] [PubMed] [Google Scholar]
- 26.Herzog TJ, Scambia G, Kim BG, et al. A randomized Phase II trial of maintenance therapy with sorafenib in front-line ovarian carcinoma. Gynecol. Oncol. 130(1), 25–30 (2013). [DOI] [PubMed] [Google Scholar]
- 27.Matei D, Sill MW, Lankes HA, et al. Activity of sorafenib in recurrent ovarian cancer and primary peritoneal carcinomatosis: a gynecologic oncology group trial. J. Clin. Oncol. 29(1), 69–75 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raja FA, Griffin CL, Qian W, et al. Initial toxicity assessment of ICON6 : a randomised trial of cediranib plus chemotherapy in platinum-sensitive relapsed ovarian cancer. Br. J. Cancer 105(7), 884–889 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pujade-Lauraine E, Hilpert F, Weber B, et al. Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: the AURELIA open-label randomized Phase III trial. J. Clin. Oncol. 32(13), 1302–1308 (2014). [DOI] [PubMed] [Google Scholar]
- •• A Phase III trial investigated efficacy and safety of bevacizumab with gemcitabine (G) and carboplatin (C) compared with GC in platinum-sensitive recurrent ovarian, primary peritoneal or fallopian tube cancer [Google Scholar]
- 30.Aghajanian C, Blank SV, Goff BA, et al. OCEANS: a randomized, double-blind, placebo-controlled Phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. J. Clin. Oncol. 30(17), 2039–2045 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tew WP, Colombo N, Ray-Coquard I, et al. Intravenous aflibercept in patients with platinum-resistant, advanced ovarian cancer: results of a randomized, double-blind, Phase 2, parallel-arm study. Cancer 120(3), 335–343 (2014). [DOI] [PubMed] [Google Scholar]
- 32.Davidson BA, Secord AA. Profile of pazopanib and its potential in the treatment of epithelial ovarian cancer. Int. J. Womens Health 6, 289–300 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.du Bois A, Floquet A, Kim JW, et al. Incorporation of pazopanib in maintenance therapy of ovarian cancer. J. Clin. Oncol. 32(30), 3374–3382 (2014). [DOI] [PubMed] [Google Scholar]
- 34.Azad NS, Posadas EM, Kwitkowski VE, et al. Combination targeted therapy with sorafenib and bevacizumab results in enhanced toxicity and antitumor activity. J. Clin. Oncol. 26(22), 3709–3714 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mina LA, Yu M, Johnson C, Burkhardt C, Miller KD, Zon R. A Phase II study of combined VEGF inhibitor (bevacizumab + sorafenib) in patients with metastatic breast cancer: Hoosier Oncology Group Study BRE06-109. Invest. New Drugs 31(5), 1307–1310 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nathan P, Zweifel M, Padhani AR, et al. Phase I trial of combretastatin A4 phosphate (CA4P) in combination with bevacizumab in patients with advanced cancer. Clin. Cancer Res. 18(12), 3428–3439 (2012). [DOI] [PubMed] [Google Scholar]
- 37.Monk BJ, Poveda A, Vergote I, et al. Anti-angiopoietin therapy with trebananib for recurrent ovarian cancer (TRINOVA-1), a randomised, multicentre, double-blind, placebo-controlled Phase 3 trial. Lancet Oncol. 15(8), 799–808 (2014). [DOI] [PubMed] [Google Scholar]
- 38.Vergote I, Schilder RJ, Pippitt CH, et al. A Phase 1b study of trebananib in combination with pegylated liposomal doxorubicin or topotecan in women with recurrent platinum-resistant or partially platinum-sensitive ovarian cancer. Gynecol. Oncol. 135(1), 25–33 (2014). [DOI] [PubMed] [Google Scholar]
- 39.Vergote I, Oaknin A, Baurain JF, et al. A Phase 1b, open-label study of trebananib in combination with paclitaxel and carboplatin in patients with ovarian cancer receiving interval or primary debulking surgery. Eur. J. Cancer 50(14), 2408–2416 (2014). [DOI] [PubMed] [Google Scholar]
- 40.Monk BJ, Poveda A, Vergote I, et al. Anti-angiopoietin therapy with trebananib for recurrent ovarian cancer (TRINOVA-1): a randomised, multicentre, double-blind, placebo-controlled Phase 3 trial. Lancet Oncol. 15, 799–808 (2014). [DOI] [PubMed] [Google Scholar]
- 41.Coward JI, Middleton K, Murphy F. New perspectives on targeted therapy in ovarian cancer. Int. J. Womens Health 7, 189–203 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Della Pepa C, Tonini G, Pisano C, et al. Ovarian cancer standard of care: are there real alternatives? Chin. J. Cancer 34(1), 17–27 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scott CL, Swisher EM, Kaufmann SH. Poly (ADP-ribose) polymerase inhibitors: recent advances and future development. J. Clin. Oncol. 33(12), 1397–1406 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tangutoori S, Baldwin P, Sridhar S. PARP inhibitors: a new era of targeted therapy. Maturitas 81(1), 5–9 (2015). [DOI] [PubMed] [Google Scholar]
- 45.Ward A, Khanna KK, Wiegmans AP. Targeting homologous recombination, new pre-clinical and clinical therapeutic combinations inhibiting RAD51. Cancer Treat. Rev. 41(1), 35–45 (2015). [DOI] [PubMed] [Google Scholar]
- 46.Audeh MW, Carmichael J, Penson RT, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer: a proof-of-concept trial. Lancet 376(9737), 245–251 (2010). [DOI] [PubMed] [Google Scholar]
- 47.Fong PC, Yap TA, Boss DS, et al. Poly(ADP)-ribose polymerase inhibition: frequent durable responses in BRCA carrier ovarian cancer correlating with platinum-free interval. J. Clin. Oncol. 28(15), 2512–2519 (2010). [DOI] [PubMed] [Google Scholar]
- 48.Yamamoto N, Nokihara H, Yamada Y, et al. A Phase I, dose-finding and pharmacokinetic study of olaparib (AZD2281) in Japanese patients with advanced solid tumors. Cancer Sci. 103(3), 504–509 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sonnenblick A, de Azambuja E, Azim HA, Jr, Piccart M. An update on PARP inhibitors- moving to the adjuvant setting. Nat. Rev. Clin. Oncol. 12(1), 27–41 (2015). [DOI] [PubMed] [Google Scholar]
- 50.Gelmon KA, Tischkowitz M, Mackay H, et al. Olaparib in patients with recurrent high-grade serous or poorly differentiated ovarian carcinoma or triple-negative breast cancer: a Phase 2, multicentre, open-label, non-randomised study. Lancet Oncol. 12(9), 852–861 (2011). [DOI] [PubMed] [Google Scholar]
- 51.Ledermann J, Harter P, Gourley C, et al. Olaparib maintenance therapy in patients with platinum-sensitive relapsed serous ovarian cancer: a preplanned retrospective analysis of outcomes by BRCA status in a randomised Phase 2 trial. Lancet Oncol. 15(8), 852–861 (2014). [DOI] [PubMed] [Google Scholar]
- 52.Kaye SB, Lubinski J, Matulonis U, et al. Phase II, open-label, randomized, multicenter study comparing the efficacy and safety of olaparib, a poly(ADP-ribose) polymerase inhibitor, and pegylated liposomal doxorubicin in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer. J. Clin. Oncol. 30(4), 372–379 (2012). [DOI] [PubMed] [Google Scholar]
- • Study assessed the comparative efficacy and safety of olaparib and pegylated liposomal doxorubicin (PLD) in patients with ovarian cancer that recurred within 12 months of prior platinum therapy and with confirmed germline BRCA1 or BRCA2 mutations [Google Scholar]
- 53.Liu JF, Barry WT, Birrer M, et al. Combination cediranib and olaparib versus olaparib alone for women with recurrent platinum-sensitive ovarian cancer: a randomised Phase 2 study. Lancet Oncol. 15(11), 1207–1214 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ang JE, Gourley C, Powell CB, et al. Efficacy of chemotherapy in BRCA1/2 mutation carrier ovarian cancer in the setting of PARP inhibitor resistance: a multi-institutional study. Clin. Cancer Res. 19(19), 5485–5493 (2013). [DOI] [PubMed] [Google Scholar]
- 55.Ledermann J, Harter P, Gourley C, et al. Olaparib maintenance therapy in platinum-sensitive relapsed ovarian cancer. N. Engl. J. Med. 366(15), 1382–1392 (2012). [DOI] [PubMed] [Google Scholar]
- 56.Lee JM, Hays JL, Annunziata CM, et al. Phase I/Ib study of olaparib and carboplatin in BRCA1 or BRCA2 mutation-associated breast or ovarian cancer with biomarker analyses. J. Natl Cancer Inst. 106(6), 89 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tutt A, Robson M, Garber JE, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and advanced breast cancer: a proof-of-concept trial. Lancet 376(9737), 235–244 (2010). [DOI] [PubMed] [Google Scholar]
- 58.Oza AM, Cibula D, Benzaquen AO, et al. Olaparib combined with chemotherapy for recurrent platinum-sensitive ovarian cancer: a randomised Phase 2 trial. Lancet Oncol. 16(1), 87–97 (2015). [DOI] [PubMed] [Google Scholar]
- 59.Sandhu SK, Schelman WR, Wilding G, et al. The poly(ADP-ribose) polymerase inhibitor niraparib (MK4827) in BRCA mutation carriers and patients with sporadic cancer: a Phase 1 dose-escalation trial. Lancet Oncol. 14(9), 882–892 (2013). [DOI] [PubMed] [Google Scholar]
- 60.Kummar S, Oza AM, Fleming GF, et al. Randomized trial of oral cyclophosphamide and veliparib in high-grade serous ovarian, primary peritoneal, or fallopian tube cancers, or BRCA-mutant ovarian cancer. Clin. Cancer Res. 21(7), 1574–1582 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kummar S, Kinders R, Gutierrez ME, et al. Phase 0 clinical trial of the poly (ADP-ribose) polymerase inhibitor ABT-888 in patients with advanced malignancies. J. Clin. Oncol. 27(16), 2705–2711 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Plummer R, Lorigan P, Steven N, et al. A Phase II study of the potent PARP inhibitor, rucaparib (PF-01367338, AG014699), with temozolomide in patients with metastatic melanoma demonstrating evidence of chemopotentiation. Cancer Chemother. Pharmacol. 71(5), 1191–1199 (2013). [DOI] [PubMed] [Google Scholar]
- 63.Molife LR, Roxburgh P, Wilson RH, et al. A Phase I study of oral rucaparib in combination with carboplatin. J. Clin. Oncol. 31, 2586–2586 (2013).23733761 [Google Scholar]
- 64.Lee JM, National Cancer Institute BMN 673 (Talazoparib), an Oral PARP Inhibitor, in People With Deleterious BRCA1/2 Mutation-Associated Ovarian Cancer Who Have Had Prior PARP Inhibitor Treatment. In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US) https://clinicaltrials.gov/ct2/show/NCT02326844 [Google Scholar]
- 65.Lee JM, Ledermann JA, Kohn EC. PARP inhibitors for BRCA1/2 mutation-associated and BRCA-like malignancies. Ann. Oncol. 25(1), 32–40 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Muggia F, Safra T. ‘BRCAness’ and its implications for platinum action in gynecologic cancer. Anticancer Res. 34(2), 551–556 (2014). [PMC free article] [PubMed] [Google Scholar]
- 67.Raja FA, Chopra N, Ledermann JA. Optimal first-line treatment in ovarian cancer. Ann. Oncol. 23(10), 118–127 (2012). [DOI] [PubMed] [Google Scholar]
- 68.Bookman MA, Darcy KM, Clarke-Pearson D, Boothby RA, Horowitz IR. Evaluation of monoclonal humanized anti-HER2 antibody, trastuzumab, in patients with recurrent or refractory ovarian or primary peritoneal carcinoma with overexpression of HER2: a Phase II trial of the Gynecologic Oncology Group. J. Clin. Oncol. 21(2), 283–290 (2013). [DOI] [PubMed] [Google Scholar]
- 69.Bookman MA, Darcy KM, Clarke-Pearson D, et al. Evaluation of monoclonal humanized anti-HER2 antibody, trastuzumab, in patients with recurrent or refractory ovarian or primary peritoneal carcinoma with overexpression of HER2: a Phase II trial of the Gynecologic Oncology Group. J. Clin. Oncol. 21(2), 283–290 (2003). [DOI] [PubMed] [Google Scholar]
- 70.Vergote IB, Jimeno A, Joly F, et al. Randomized Phase III study of erlotinib versus observation in patients with no evidence of disease progression after first-line platin-based chemotherapy: a European Organisation for Research and Treatment of Cancer-Gyneacological Cancer Group, and Gyneacologic Cancer Intergroup study. J. Clin. Oncol. 32(4), 320–326 (2014). [DOI] [PubMed] [Google Scholar]
- 71.Posadas EM, Liel MS, Kwitkowski V, et al. A Phase II and pharmacodynamic study of gefitinib in patients with refractory or recurrent epithelial ovarian cancer. Cancer 109(7), 1323–1330 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schilder RJ, Sill MW, Chen X, et al. Phase II study of gefitinib in patients with relapsed or persistent ovarian or primary peritoneal carcinoma and evaluation of epidermal growth factor receptor mutations and immunohistochemical expression: a Gynecologic Oncology Group Study. Clin. Cancer Res. 11(15), 5539–5548 (2005). [DOI] [PubMed] [Google Scholar]
- 73.Wagner U, du Bois A, Pfisterer J, et al. Gefitinib in combination with tamoxifen in patients with ovarian cancer refractory or resistant to platinum-taxane based therapy – a Phase II trial of the AGO Ovarian Cancer Study Group (AGO-OVAR 2.6). Gynecol. Oncol. 105(1), 132–137 (2007). [DOI] [PubMed] [Google Scholar]
- 74.Bauman J, Verschraegen C, Belinsky S, et al. A Phase I study of 5-azacytidine and erlotinib in advanced solid tumor malignancies. Cancer Chemother. Pharmacol. 69(2), 547–554 (2012). [DOI] [PubMed] [Google Scholar]
- 75.Vasey PA, Gore M, Wilson R, et al. A Phase Ib trial of docetaxel, carboplatin and erlotinib in ovarian, fallopian tube and primary peritoneal cancers. Br. J. Cancer 98(11), 1774–1780 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chambers SK, Clouser MC, Baker AF, et al. Overexpression of tumor vascular endothelial growth factor A may portend an increased likelihood of progression in a Phase II trial of bevacizumab and erlotinib in resistant ovarian cancer. Clin. Cancer Res. 16(21), 5320–5328 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schilder RJ, Pathak HB, Lokshin AE, et al. Phase II trial of single agent cetuximab in patients with persistent or recurrent epithelial ovarian or primary peritoneal carcinoma with the potential for dose escalation to rash. Gynecol. Oncol. 113(1), 21–27 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Konner J, Schilder RJ, DeRosa FA, et al. A Phase II study of cetuximab/paclitaxel/carboplatin for the initial treatment of advanced-stage ovarian, primary peritoneal, or fallopian tube cancer. Gynecol. Oncol. 110(2), 140–145 (2008). [DOI] [PubMed] [Google Scholar]
- 79.Vlahovic G, Meadows KL, Uronis HE, et al. A Phase I study of bevacizumab, everolimus and panitumumab in advanced solid tumors. Cancer Chemother. Pharmacol. 70(1), 95–102 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Steffensen KD, Waldstrom M, Pallisgard N, et al. Panitumumab and pegylated liposomal doxorubicin in platinum-resistant epithelial ovarian cancer with KRAS wild-type: the PaLiDo study, a Phase II nonrandomized multicenter study. Int. J. Gynecol. Cancer 23(1), 73–80 (2013). [DOI] [PubMed] [Google Scholar]
- 81.Seiden MV, Burris HA, Matulonis U, et al. A Phase II trial of EMD72000 (matuzumab), a humanized anti-EGFR monoclonal antibody, in patients with platinum-resistant ovarian and primary peritoneal malignancies. Gynecol. Oncol. 104(3), 727–731 (2007). [DOI] [PubMed] [Google Scholar]
- 82.Ledermann JA, Canevari S, Thigpen T. Targeting the folate receptor: diagnostic and therapeutic approaches to personalize cancer treatments. Ann. Oncol. 26(10), 2034–2043 (2015). [DOI] [PubMed] [Google Scholar]
- 83.Vergote IB, Marth C, Coleman RL. Role of the folate receptor in ovarian cancer treatment: evidence, mechanism, and clinical implications. Cancer Metastasis Rev. 34(1), 41–52 (2015). [DOI] [PubMed] [Google Scholar]
- 84.Farrell C, Schweizer C, Wustner J, et al. Population pharmacokinetics of farletuzumab, a humanized monoclonal antibody against folate receptor alpha, in epithelial ovarian cancer. Cancer Chemother. Pharmacol. 70(5), 727–734 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Armstrong DK, White AJ, Weil SC, Phillips M, Coleman RL. Farletuzumab (a monoclonal antibody against folate receptor alpha) in relapsed platinum-sensitive ovarian cancer. Gynecol. Oncol. 129(3), 452–458 (2013). [DOI] [PubMed] [Google Scholar]
- 86.Gibbs DD, Theti DS, Wood N, et al. BGC 945, a novel tumor-selective thymidylate synthase inhibitor targeted to alpha-folate receptor-overexpressing tumors. Cancer Res. 65(24), 11721–11728 (2005). [DOI] [PubMed] [Google Scholar]
- 87.Lorusso PM, Edelman MJ, Bever SL, et al. Phase I study of folate conjugate EC145 (vintafolide) in patients with refractory solid tumors. J. Clin. Oncol. 30(32), 4011–4016 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Naumann RW, Coleman RL, Burger RA, et al. PRECEDENT: a randomized Phase II trial comparing vintafolide (EC145) and pegylated liposomal doxorubicin (PLD) in combination versus PLD alone in patients with platinum-resistant ovarian cancer. J. Clin. Oncol. 31(35), 4400–4406 (2013). [DOI] [PubMed] [Google Scholar]
- 89.Weroha SJ, Haluska P. IGF-1 receptor inhibitors in clinical trials – early lessons. J. Mammary Gland Biol. Neoplasia 13(4), 471–483 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.King ER, Zu Z, Tsang YT, et al. The insulin-like growth factor 1 pathway is a potential therapeutic target for low-grade serous ovarian carcinoma. Gynecol. Oncol. 123(1), 13–18 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Brokaw J, Katsaros D, Wiley A, et al. IGF-1 in epithelial ovarian cancer and its role in disease progression. Growth Factors 25(5), 346–354 (2007). [DOI] [PubMed] [Google Scholar]
- 92.O'Sullivan CC, Moon DH, Kohn EC, Lee JM. Beyond breast and ovarian cancers: PARP inhibitors for BRCA mutation-associated and BRCA-like solid tumors. Front. Oncol. 4, 42 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Michels J, Vitale I, Saparbaev M, Castedo M, Kroemer G. Predictive biomarkers for cancer therapy with PARP inhibitors. Oncogene 33(30), 3894–3907 (2014). [DOI] [PubMed] [Google Scholar]
- 94.Weil MK, Chen A. PARP inhibitor treatment in ovarian and breast cancer. Curr. Prob. Cancer 35(1), 7–50 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Montoni A, Robu M, Pouliot É, Shah GM. Resistance to PARP-inhibitors in cancer therapy. Front. Pharmacol. 4, 18 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bouwman P, Jonkers J. Molecular pathways: how can BRCA-mutated tumors become resistant to PARP inhibitors? Clin. Cancer Res. 20(3), 540–547 (2014). [DOI] [PubMed] [Google Scholar]
- 97.Frey MK, Pothuri B. Targeting DNA repair: poly (ADP-ribose) polymerase inhibitors. Transl. Cancer Res. 4(1), 84–96 (2015). [Google Scholar]
- 98.Karssen AM, Meijer OC, van der Sandt IC, De Boer AG, De Lange EC, De Kloet ER. The role of the efflux transporter P-glycoprotein in brain penetration of prednisolone. J. Endocrinol. 175(1), 251–260 (2002). [DOI] [PubMed] [Google Scholar]
- 99.Rodríguez D, Morrison CJ, Overall CM. Matrix metalloproteinases: what do they not do? New substrates and biological roles identified by murine models and proteomics. Mol. Cell. Res. 1803(1), 39–54 (2010). [DOI] [PubMed] [Google Scholar]
- 100.Murai J, Huang SN, Das BB, et al. Differential trapping of PARP1 and PARP2 by clinical PARP inhibitors. Cancer Res. 72(21), 5588–5599 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Krishnakumar R, Kraus WL. The PARP side of the nucleus: molecular actions, physiological outcomes, and clinical targets. Mol. Cell 39(1), 8–24 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Murukesh N, Dive C, Jayson GC. Biomarkers of angiogenesis and their role in the development of VEGF inhibitors. Br. J. Cancer 102(1), 8–18 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Liu G, Chen X. Vascular endothelial growth factor as an antiangiogenic target for cancer therapy. Curr. Drug Targets 11(8), 1000–1017 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ji J, Kinders RJ, Zhang Y, et al. Modeling pharmacodynamic response to the poly(ADP-ribose) polymerase inhibitor ABT-888 in human peripheral blood mononuclear cells. PLoS ONE 6(10), 26–42 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Garcia V Moreno, Basu B, Molife LR, Kaye SB. Combining antiangiogenics to overcome resistance: rationale and clinical experience. Clin. Cancer Res. 18(14), 3750–3761 (2012). [DOI] [PubMed] [Google Scholar]
- 106.Lee JM, Sarosy GA, Annunziata CM, et al. Combination therapy: intermittent sorafenib with bevacizumab yields activity and decreased toxicity. Br. J. Cancer 102(3), 495–499 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mathews MT, Berk BC. PARP-1 inhibition prevents oxidative and nitrosative stress-induced endothelial cell death via transactivation of the VEGF receptor 2. Arterioscler. Thromb. Vasc. Biol. 28, 711–717 (2008). [DOI] [PubMed] [Google Scholar]
- 108.Hamanishi J, Mandai M, Ikeda T, et al. Safety and antitumour activity of anti-PD-1 antibody, nivolumab, in patients with platinum-resistant ovarian cancer. J. Clin. Oncol. 33(34), 4015–4022 (2015) [DOI] [PubMed] [Google Scholar]
- 109.Varga A, Pihua-Paul SA, Ott PA, et al. Antitumor activity and safety of pembrolizumab in patients (pts) with PD-L1 positive advanced ovarian cancer: interim results from a Phase Ib study. J. Clin. Oncol. 33(15), 5510 (2015). [Google Scholar]
- 110.Dedrick RL, Myers CE, Bungay PM, DeVita VT. Pharmacokinetic rationale for peritoneal drug administration in the treatment of ovarian cancer. Cancer Treat. Rep. 62(1), 1–11 (1978). [PubMed] [Google Scholar]
- 111.Shah DK, Veith J, Bernacki RJ, et al. Evaluation of combined bevacizumab and intraperitoneal carboplatin or paclitaxel therapy in a mouse model of ovarian cancer. Cancer Chemother. Pharmacol. 68, 951–958 (2011). [DOI] [PubMed] [Google Scholar]
- 112.Vassileva V, Allen CJ, Piquette-Miller M. Effects of sustained and intermittent paclitaxel therapy on tumor repopulation in ovarian cancer. Mol. Cancer Ther. 7(3), 630–637 (2008). [DOI] [PubMed] [Google Scholar]
- 113.Armstrong DK, Bundy B, Wenzel L, et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N. Engl. J. Med. 354(1), 34–43 (2006). [DOI] [PubMed] [Google Scholar]
- • Study showed that intravenous (iv.) paclitaxel plus intraperitoneal cisplatin and paclitaxel improves survival in patients with optimally debulked stage III ovarian cancer as compared with iv. paclitaxel plus cisplatin [Google Scholar]
- 114.Markman M, Bundy BN, Alberts DS, et al. Phase III trial of standard-dose intravenous cisplatin plus paclitaxel versus moderately high-dose carboplatin followed by intravenous paclitaxel and intraperitoneal cisplatin in small-volume stage III ovarian carcinoma: an intergroup study of the Gynecologic Oncology Group, Southwestern Oncology Group, and Eastern Cooperative Oncology Group. J. Clin.Oncol. 19(4), 1001–1007 (2001). [DOI] [PubMed] [Google Scholar]
- • Study demonstrated high-dose iv. carboplatin followed by intraperitoneal paclitaxel and iv. cisplatin yielded a significant improvement in progression-free survival when compared with a standard regimen of iv. cisplatin and paclitaxel [Google Scholar]
- 115.Jaaback K, Johnson N, Lawrie TA. Intraperitoneal chemotherapy for the initial management of primary epithelial ovarian cancer. Cochrane Database Syst. Rev. (11), CD005340 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yallapu MM, Jaggi M, Chauhan SC. Scope of nanotechnology in ovarian cancer therapeutics. J. Ovarian Res. 3, 19–19 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kim PS, Djazayeri S, Zeineldin R. Novel nanotechnology approaches to diagnosis and therapy of ovarian cancer. Gynecol. Oncol. 120(3), 393–403 (2011). [DOI] [PubMed] [Google Scholar]
- 118.Goldberg MS, Xing D, Ren Y, et al. Nanoparticle-mediated delivery of siRNA targeting Parp1 extends survival of mice bearing tumors derived from Brca1-deficient ovarian cancer cells. Proc. Natl Acad. Sci. USA 108(2), 745–750 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Xiao K, Luo J, Fowler WL, et al. A self-assembling nanoparticle for paclitaxel delivery in ovarian cancer. Biomaterials 30(30), 6006–6016 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wang L, Neoh KG, Kang ET, Shuter B. Multifunctional polyglycerol-grafted Fe3O4@SiO2 nanoparticles for targeting ovarian cancer cells. Biomaterials 32(8), 2166–2173 (2011). [DOI] [PubMed] [Google Scholar]
- 121.Lu H, Li B, Kang Y, et al. Paclitaxel nanoparticle inhibits growth of ovarian cancer xenografts and enhances lymphatic targeting. Cancer Chemother. Pharmacol. 59(2), 175–181 (2007). [DOI] [PubMed] [Google Scholar]
- 122.Xiong X, Arvizo R, Saha S, et al. Sensitization of ovarian cancer cells to cisplatin by gold nanoparticles. Oncotarget 5(15), 6453–6465 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]