Abstract
Background and aim
The impact of 25-OH vitamin D on sustained viral response (SVR) to antiviral therapy and on fibrosis progression in hepatitis C is debated. We assessed the impact of 25-OH vitamin D concentration on the efficacy of antiviral therapy in naïve genotype 1 hepatitis C virus (HCV)-infected patients.
Methods
The study population consisted of treatment-naïve genotype 1 patients enrolled in a randomised controlled trial. A total of 516 patients received peginterferon α-2a 180 µg/week plus ribavirin 800 mg/day for 24 weeks. There were 349 patients with undetectable HCV RNA (<50 IU/ml) at week 24 (W24) who were randomised to continue dual therapy (n = 173) or to continue peginterferon alone (n = 176) until week 48. 25-OH vitamin D concentration was measured at baseline in frozen serum.
Results
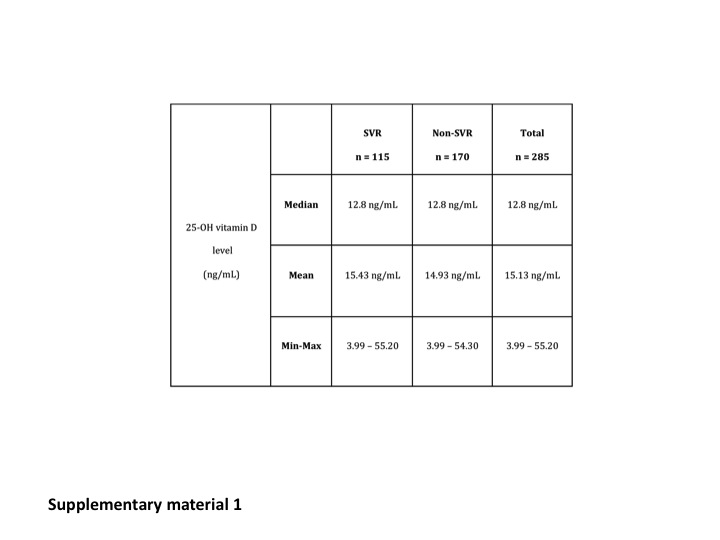
A total of 461 patients could be analysed for virologic response at W24, and 285 (119 non-responders at W24 + 166 responders who continued dual therapy until W48) for the impact of SVR. There were 487 patients who could be analysed for fibrosis progression. Metavir fibrosis scores (centralised analysis) were: F1 30%, F2 34%, F3 27% and F4 9%. Median 25-OH vitamin D concentrations were similar in virologic responders (13.5 ng/ml) and in non-responders at W24 (12.6 ng/ml), as well as in patients with SVR (12.8 ng/ml) and without SVR (12.8 ng/ml, 3.99) at W72. Median 25-OH vitamin D concentrations were: F1: 14.30 ng/ml, F2: 13.50 ng/ml, F3: 13.30 ng/ml and F4: 12.80 ng/ml.
Conclusion
In this study, 25-OH vitamin D level has no impact on the efficacy of antiviral therapy in naïve genotype 1 HCV-infected patients.
Keywords: Vitamin D, hepatitis C, viral response, fibrosis
Introduction
Hepatitis C virus (HCV) infection is one of the main causes of chronic liver disease worldwide. It can lead to extensive fibrosis, cirrhosis and hepatocellular carcinoma. The number of chronically infected patients worldwide is estimated to be about 160 million. Most of them are unaware of their infection.1,2 The prevalence of hepatitis C is highly variable according to geographic area but it seems higher in developing countries. The prevalence rates are as follow: less than 2.5% in North America and Western Europe, around 3.2% in Africa, 1.5% to 5% in Eastern Europe, 2.5% to 4.9% in the Western Pacific region and 1% to more than 12% in the Middle East and Central Asia.1
Despite recent major therapeutic breakthroughs,3,4 the standard of care remains the combination of pegylated interferon (IFN) alfa plus ribavirin in the majority of countries. Indeed, for most infected patients the direct-acting agents are inaccessible because of their costs. Thus, finding new ways to improve virologic response, at lower cost, is of high interest.
Vitamin D is a fat-soluble secosteroid with pleiotropic effects. Recent molecular genetic techniques including genomics revealed that 25-hydroxy (25-OH) vitamin D controls more than just calcium homeostasis. It has widespread effects on cellular differentiation and proliferation, and plays a role in the immune system.5 A role for 1,25-OH-vitamin D3 as a mediator of normal function of both the innate and adaptive immune systems has been established.6 25-OH vitamin D deficiency is very common in chronic hepatitis C.7–9 Recent reports provided conflicting results on the impact on the efficacy of antiviral hepatitis C therapy10–15 and on fibrosis progression.10,16,17
The aim of this study was to assess the impact of 25-OH vitamin D concentration on the virologic response and on fibrosis progression in a post-hoc analysis of a randomised, controlled trial.
Materials and methods
The study population were genotype 1, naïve, mono-infected patients enrolled in a study assessing the effect of ribavirin in genotype 1 patients with chronic hepatitis C initially responding to pegylated IFN alfa 2a plus ribavirin.18 All patients received pegylated IFN alfa-2a (Pegasys; Roche, Basel, Switzerland), 180 µg once weekly subcutaneously, plus ribavirin (Copegus; Roche) 400 mg twice daily orally, for 24 weeks. At week 24 (W24), HCV RNA was analysed by means of a qualitative polymerase chain reaction (PCR) assay (Cobas Amplicor HCV v2.0; Roche Molecular Systems) with a lower limit of detection of 50 IU/ml. The patients were seen at W26 with the result of their HCV RNA detection. Patients with undetectable HCV RNA at W24 were randomised at W26 to a further 22 weeks of treatment (i.e. until W48) with either pegylated IFN alfa-2a 180 µg once weekly without ribavirin, or continuation of pegylated IFN alfa-2a 180 µg once weekly in combination with ribavirin 400 mg twice daily. The remaining patients who had detectable HCV RNA at W24 stopped therapy because they had a low chance of achieving a sustained viral response (SVR) with continued treatment.
All patients had a liver biopsy finding consistent with chronic hepatitis C obtained within 18 months before therapy (Metavir scores of at least A1 and F1, as assessed by a single pathologist).
25-OH vitamin D concentration was measured at baseline in serum, frozen at −80℃ with a chemiluminescent immunoassay which quantified 25-OH vitamin D and other hydroxylated vitamin D metabolites. The assay was used according to the manufacturer’s recommendation (LIAISON® 25 OH Vitamin D TOTAL Assay DiaSorin, Antony, France). Concentrations < 4 ng/ml were given as 3.99 ng/ml. Patients lacking a 25-OH vitamin D serum concentration before initiation of the treatment were excluded from the correlation analysis.
Correlation between the baseline 25-OH vitamin D concentration and virologic response at W24 and the fibrosis score respectively were analysed in the whole population whereas the correlation with SVR was assessed only in the non-responders at W24 and the responders at W24 randomised in the dual-therapy continuation arm.
The protocol was approved by the local ethics committee (Comité Consultatif de Protection des Personnes dans la Recherche Biomédicale) of the University Hospital of Nancy on 24 October 2000. All patients provided written informed consent. The study was conducted according to the guidelines of the Declaration of Helsinki, under provisions of Good Clinical Practices, or both. Data were collected by the study group and analysed by the authors with the help of the sponsor. The authors had unlimited access to the data and no limitation on publication was imposed by any party.
Statistical analyses
Continuous variables were described as medians, means, interquartiles and range, and categorical variables were reported as frequency and percentages. Differences between groups were assessed using the Kruskal-Wallis test or the Wilcoxon test for non-normally distributed variables. Differences between groups were assessed using the Chi2 test or Fisher’s exact test for categorical data. Receiver operating characteristic (ROC) curves were applied to find the best cut-off values of 25-OH vitamin D in order to distinguish virologic responders from non-responders.
A p value < 0.05 was considered statistically significant. All calculations were performed using SAS V9.1.3 software (SAS Institute Inc, Cary, NC, USA).
Results
Baseline characteristics of patients and 25-OH vitamin D concentration
Initially 516 patients were enrolled in the trial.18 Baseline characteristics of the patients are shown in Table 1. The baseline serum 25-OH vitamin D level was available in 489 patients. In this population, the median concentration was 13.4 ng/ml (mean 15.86 ng/ml, range 3.99–57.8 ng/ml). Patients were divided in two groups, those living in the North or South of France. The South of France is a sunnier geographic location. 25-OH vitamin D level was significantly higher in patients from the South of France, with a median of 16.80 ng/ml compared to 12.20 ng/ml in the North of France (p < 0.001).
Table 1.
Patient characteristics at baseline
| All enrolled and treated patients (n = 516) | |
|---|---|
| Sex: male/female | 306/210 |
| Age, years | 46.2 ± 15 |
| Weight, kg | 70.8 ± 11.5 |
| Body mass index, kg/m2 | 24.6 ± 4.4 |
| Mode of infection: n (%) | |
| Transfusion | 180 (34.9) |
| Injection drug use | 155 (30.0) |
| Other/unknown | 181 (35.1) |
| Serum ALT level (IU/l) | 94.2 ± 66.9 |
| Log serum HCV-RNA level | 6.2 ± 0.8 |
| HCV RNA > 800,000 IU/ml: n (%) | 354 (70.2) |
| Fibrosis score: n (%) | |
| F1 | 150 (29.70) |
| F2 | 176 (34.85) |
| F3 | 135 (26.73 |
| F4 | 44 (8.72) |
| Missing | 4 |
ALT: alanine aminotransferase; HCV: hepatitis C virus.
Baseline 25-OH vitamin D concentration and the virologic response at W24 and with SVR
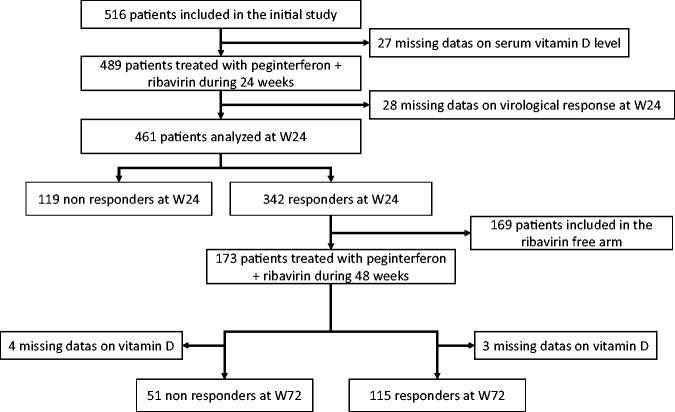
Among the 489 patients with available baseline 25-OH vitamin D concentration, the virologic response at W24 was missing in 28 patients. Thus, 461 patients were eligible for the correlation study with the virologic response at W24. A total of 342 of the 461 patients (74%) were HCV RNA negative at W24 of combination therapy. They were randomised into two arms, one continuing combination therapy (n = 173), the second arm treated with peginterferon only (n = 169). Patients included in the second arm were excluded from the correlation analysis with SVR. Among the 173 patients continuing combination therapy for a total of 48 weeks, 118 achieved an SVR, and 55 patients failed to achieve SVR. 25-OH vitamin D was missing in three of the 118 patients achieving SVR and in four of the 55 patients failing to achieve SVR. The patients’ disposition is summarised in Figure 1.
Figure 1.
Flowchart.
The median 25-OH vitamin D concentrations were similar in the virologic responders (13.5 ng/ml) and in non-responders at W24 (12.6 ng/ml). Median 25-OH vitamin D concentrations were also similar in patients with (12.8 ng/ml) and without SVR (12.8 ng/ml) at W72. Results are summarised in Table 2 and Supplementary Material Figure 1.
Table 2.
Baseline 25-OH vitamin D level and virologic response at W24
| 25-OH vitamin D level (ng/ml) | Responders n = 342 | Non-responders n = 119 | Total n = 461 | |
|---|---|---|---|---|
| Median | 13.5 ng/ml | 12.6 ng/ml | 13.4 ng/ml | |
| Mean | 16.19 ng/ml | 14.7 ng/ml | 15.86 ng/ml | |
| Minimum–Maximum | 3.99–57.80 | 3.99–54.30 | 3.99–57.80 |
25-OH: 25-hydroxy; W24: week 24.
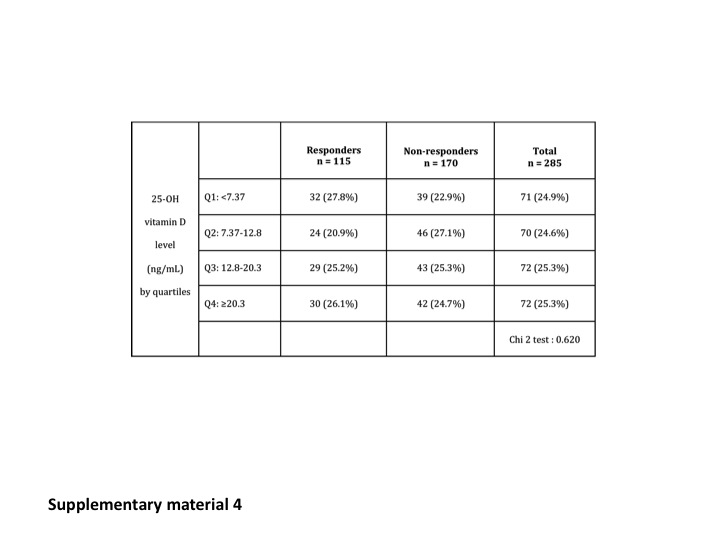
We could not identify a level of 25-OH vitamin D predictive of the virologic response by ROC curves analysis (Supplementary Material Figures 2 and 3). Virologic responses were also similar in the different subgroups divided according to the quartiles of 25-OH vitamin D concentration at W24 and W72. Results are summarised in Table 3 and Supplementary Material Figure 4.
Table 3.
Virologic response at W24 according to the quartiles of 25-OH vitamin D concentration
| 25-OH vitamin D level (ng/ml) by quartiles | Responders n = 342 | Non-responders n = 119 | Total n = 461 | |
|---|---|---|---|---|
| Q1: <7.95 | 84 (24.6%) | 30 (25.2%) | 114 (24.7%) | |
| Q2: 7.95–13.4 | 85 (24.9%) | 31 (26.1%) | 116 (25.2%) | |
| Q3: 13.4–21.8 | 80 (24.3%) | 35 (29.4%) | 115 (24.9%) | |
| Q4: ≥21.8 | 93 (27.2%) | 23 (19.3%) | 116 (25.2%) | |
| Chi2 test: 0.318 |
W24: week 24; 25-OH: 25-hydroxy.
Subgroup analysis according to geographic origin (North vs South of France) did not reveal an influence of 25-OH vitamin D concentration on SVR in either of the two regions.
Baseline 25-OH vitamin D concentration and fibrosis score
For this analysis, the 25-OH vitamin D concentration was available in 487 patients. The median 25-OH vitamin D concentrations according to fibrosis were as follow: F1: 14.3 ng/ml, F2: 13.5 ng/ml, F3: 13.3 ng/ml and F4: 12.8 ng/ml. No difference was seen between the different groups. Results are summarised in Table 4.
Table 4.
Baseline 25-OH vitamin D level and fibrosis score
| 25-OH vitamin D level (ng/ml) | F1 n = 147 | F2 n = 168 | F3 n = 130 | F4 n = 42 | Total n = 487 | |
|---|---|---|---|---|---|---|
| Median | 14.3 ng/ml | 13.5 ng/ml | 13.3 ng/ml | 12.8 ng/ml | 13.4 ng/ml | |
| Average | 16.04 ng/ml | 16.51 ng/ml | 15.13 ng/ml | 14.69 ng/ml | 15.84 ng/ml | |
| Minimum–Maximum | 3.99–53.30 | 3.99–55.20 | 3.99–57.80 | 3.99–34.40 | 3.99–57.80 | |
| p = 0.677 |
25-OH: 25-hydroxy.
Discussion
Our study is the largest study performed in genotype 1 naïve patients assessing the impact on 25-OH vitamin D of virologic response and fibrosis progression. In our study, 25-OH vitamin D level before antiviral treatment has no impact on the efficacy of antiviral therapy in naïve genotype 1 HCV-infected patients. Moreover, no significant association was found with the fibrosis progression.
25-OH-vitamin D has pleiotropic effects.5 It is an innate antiviral agent and has anti-fibrotic effects.16,19,20 Several studies assessed the relationship between vitamin D concentration and achievement of SVR in patients with genotype 1 chronic hepatitis C. A total of 274 naïve genotype 1 patients, from the Cognitive Health in Ageing Register: Investigational, Observational, and Trial Studies in Dementia Research (CHARIOT) cohort study, were included in the Kitson et al. study.15 Baseline 25-OH vitamin D level was not independently associated with achievement of SVR.15 Moreover, 25-OH-vitamin D level did not show any significant association with treatment outcome among 391 genotype 1 patients followed prospectively.21 On the other hand, six studies found a correlation between vitamin D level and achievement of SVR.8–11,13,22,23 The largest is a retrospective study including 468 patients living in Germany, infected with all genotypes.8 Reduced 25-OH-vitamin D levels were associated with failure to achieve SVR in HCV genotype 1-, 2- and 3-infected patients.8 In 117 naïve genotype 1 chronic hepatitis C patients followed prospectively, 25-OH vitamin D levels were independently associated with the likelihood to achieve SVR.22 In recurrent hepatitis C, after liver transplantation, 25-OH vitamin D deficiency predicted an unfavourable response to antiviral treatment.11 A meta-analysis by García-Alvarez et al. retrieved 14 studies evaluating relationship of vitamin D status with advanced liver fibrosis in chronic hepatitis C treatment-naïve patients and sustained virologic response in patients under pegylated IFN alfa plus ribavirin therapy.24 They retrieved 11 studies that evaluated relationship with SVR, representing a total of 2672 patients. They noted a significant heterogeneity among studies, and only found a significant association with SVR for a vitamin D cut-off of 20 ng/ml (odds ratio (OR) = 0.53 (95% confidence interval (CI) = 0.31–0.91).24 After excluding the outliers, they found significant pooled ORs, for all patients and for genotype 1 and 4 patients, for vitamin D cut-off of 10 ng/ml and 20 ng/ml. More recently another meta-analysis concluded that baseline 25-OH vitamin D level was not associated with SVR to pegylated-IFN plus ribavirin therapy in chronic HCV infection, regardless of genotype.25
Our study did not find a correlation between 25-OH vitamin D level and fibrosis stage. Results previously published are contradictory.10,15,26–29 In the CHARIOT study, 25-OH vitamin D level was not independently associated with fibrosis stage, in 274 naïve genotype 1 patients, but a correlation was found with the histological activity.15 On the other hand, lower 25-OH vitamin D levels were independently associated with the severity of liver fibrosis in 197 Italian naïve genotype 1 patients.10 It is worthy to note that genetic variants involved in cholesterol synthesis, vitamin D hydroxylation and vitamin D transport were also associated with the severity of fibrosis.26 Three more studies assessed a correlation between 25-OH vitamin D status and fibrosis in chronic hepatitis C patients10,28,29 and one study in HCV/human immunodeficiency virus (HIV) co-infected patients.27 In the García-Alvarez et al. meta-analysis, seven studies were selected which evaluated the relationship of vitamin D status with advanced liver fibrosis in chronic hepatitis C treatment-naïve patients. Low vitamin D status was related to a diagnosis of advanced liver fibrosis, with the cut-offs of 10 ng/ml (OR = 2.37 (95% CI = 1.20–4.72)) and 30 ng/ml (OR = 2.22 (95% CI = 1.24–3.97)) being significant.24
Concerning the impact of vitamin D supplementation on virologic response, data are scarce. Three studies evaluated the impact of vitamin D supplementation on the virologic response in chronic hepatitis C.11,23,30 The early virologic response was better in a subgroup of patients receiving 1-OH vitamin D3 and having an interleukin (IL)-28B T/T genotype. The study compared 42 patients treated with a combination of peginterferon alfa, ribavirin, and 1-OH vitamin D3 to 42 matched controls treated with peginterferon alfa and ribavirin only.30 In another study, vitamin D supplementation improved the probability of achieving an SVR in patients with recurrent hepatitis C, and in the presence of a normal or near normal baseline vitamin D concentration.11 The last study focused on genotype 1 treatment-naïve patients. Adding vitamin D3 to conventional peginterferon alfa 2b/ribavirin therapy significantly improved the virologic response in a population of 72 consecutive patients.23 In this study 86% of the patients undergoing vitamin D supplementation achieved an SVR, vs 42% of control patients (p < 0.001), which means that vitamin D supplementation could be as effective as the addition of a protease inhibitor to dual therapy.31–34 Another study concluded that vitamin D supplementation has no significant impact on SVR in HCV genotype 4 patients.35
The mean 25-OH vitamin D concentration in our population was very low (13.4 ng/ml) compared to other studies.10,15 Indeed, mean vitamin D level was 31.6 ng/ml in the Kitson et al study.15 This may be due to differences in the measurement technique used. The technique used in our study is reliable and is recommended by the French authorities.36 The proportion of HCV-positive patients with severe deficiency is high in all the published studies regardless of geographic origin. It is not clear if chronic hepatitis C exposes patients to vitamin D deficiency compared to the general population. However, in the Lange et al. study, including 468 patients infected with all genotypes, baseline 25-OH-vitamin D levels were lower in the chronic hepatitis C group compared to a control group.8
Moreover, there is a higher prevalence of osteoporosis and osteopenia in patients with chronic hepatitis C. Among 43 non-cirrhotic patients, 30 infected with HCV and 13 with hepatitis B virus, there was a significantly reduced bone mineral density.37 Viral clearance could reduce that risk. In 420 post-menopausal women infected with HCV and treated with IFN monotherapy, virus clearance caused a reduction by two-thirds in the risk of bone fracture after cessation of IFN therapy.38 Cirrhotic patients secondary to hepatitis C infection have an early increase of bone resorption, leading to osteoporosis.39 The severity of metabolic osteopathy is related to the alteration in liver function.39 Treatment of chronic hepatitis C, especially ribavirin, has been reported as inducing bone loss.40,41 In 32 male patients with chronic hepatitis C treated for 12 months with either IFN alone or IFN plus ribavirin, bone mineral density was significantly lower in the dual-therapy group.40 In vitro, ribavirin, but not IFN alfa-2b, has been associated with impaired osteoblast proliferation and differentiation.41 But other conflicting results have been reported.41,42 Antiviral therapy with IFN-alfa and ribavirin led to an on-treatment increase of bone mineral density in 30 patients with genotype 1 infection without cirrhosis.43
Our study strengths are that it is the largest to evaluate a correlation between serum 25-OH vitamin D level and virologic response in naïve genotype 1 patients and that the liver histology was reassessed by a single pathologist. But it also suffers some limits. It is a retrospective study and we were not able to assess the impact of 25-OH vitamin D concentration according to predictors of SVR like insulin resistance44 and IL-28B polymorphism.45
In conclusion, in our study, 25-OH vitamin D level before antiviral therapy has no impact on the efficacy of antiviral therapy in naïve genotype 1 HCV-infected patients. Moreover, no significant association could be found with fibrosis progression.
Supplementary Material
Acknowledgements
Author contributions include: AB and EG, draft of the manuscript; MBA, data collection and critical review of the article; SR, statistical analysis; LPB; drafting of the article and critical review of the article; JMP, GC and AL critical review of the article; and JPB, study design and supervision.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest
None declared.
References
- 1.Lavanchy D. Evolving epidemiology of hepatitis C virus. Clin Microbiol Infect 2011; 17: 107–115. [DOI] [PubMed] [Google Scholar]
- 2.European Association for Study of Liver. EASL Clinical Practice Guidelines: Management of hepatitis C virus infection. J Hepatol 2014; 60: 392–420. [DOI] [PubMed] [Google Scholar]
- 3.Pawlotsky JM. New hepatitis C therapies: The toolbox, strategies, and challenges. Gastroenterology 2014; 146: 1176–1192. [DOI] [PubMed] [Google Scholar]
- 4.Lawitz E, Mangia A, Wyles D, et al. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med 2013; 368: 1878–1887. [DOI] [PubMed] [Google Scholar]
- 5.Lin R, White JH. The pleiotropic actions of vitamin D. Bioessays 2004; 26: 21–28. [DOI] [PubMed] [Google Scholar]
- 6.Hewison M. Vitamin D and the immune system: New perspectives on an old theme. Endocrinol Metab Clin North Am 2010; 39: 365–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ladero JM, Torrejón MJ, Sánchez-Pobre P, et al. Vitamin D deficiency and vitamin D therapy in chronic hepatitis C. Ann Hepatol 2013; 12: 199–204. [PubMed] [Google Scholar]
- 8.Lange CM, Bojunga J, Ramos-Lopez E, et al. Vitamin D deficiency and a CYP27B1-1260 promoter polymorphism are associated with chronic hepatitis C and poor response to interferon-alfa based therapy. J Hepatol 2011; 54: 887–893. [DOI] [PubMed] [Google Scholar]
- 9.Arteh J, Narra S, Nair S. Prevalence of vitamin D deficiency in chronic liver disease. Dig Dis Sci 2010; 55: 2624–2628. [DOI] [PubMed] [Google Scholar]
- 10.Petta S, Cammà C, Scazzone C, et al. Low vitamin D serum level is related to severe fibrosis and low responsiveness to interferon-based therapy in genotype 1 chronic hepatitis C. Hepatology 2010; 51: 1158–1167. [DOI] [PubMed] [Google Scholar]
- 11.Bitetto D, Fabris C, Fornasiere E, et al. Vitamin D supplementation improves response to antiviral treatment for recurrent hepatitis C. Transpl Int 2011; 24: 43–50. [DOI] [PubMed] [Google Scholar]
- 12.Bitetto D, Fattovich G, Fabris C, et al. Complementary role of vitamin D deficiency and the interleukin-28B rs12979860 C/T polymorphism in predicting antiviral response in chronic hepatitis C. Hepatology 2011; 53: 1118–1126. [DOI] [PubMed] [Google Scholar]
- 13.Falleti E, Bitetto D, Fabris C, et al. Vitamin D binding protein gene polymorphisms and baseline vitamin D levels as predictors of antiviral response in chronic hepatitis C. Hepatology 2012; 56: 1641–1650. [DOI] [PubMed] [Google Scholar]
- 14.Kitson MT, Dore GJ, Button G, et al. Vitamin D status is associated with complete EVR but not SVR in chronic hepatitis C genotype 1 infection: Analysis of the Australasian Chariot study cohort. J Hepatol 2012; 56(Suppl 2): S443–S444. [Google Scholar]
- 15.Kitson MT, Dore GJ, George J, et al. Vitamin D status does not predict sustained virologic response or fibrosis stage in chronic hepatitis C genotype 1 infection. J Hepatol 2013; 58: 467–472. [DOI] [PubMed] [Google Scholar]
- 16.Abramovitch S, Dahan-Bachar L, Sharvit E, et al. Vitamin D inhibits proliferation and profibrotic marker expression in hepatic stellate cells and decreases thioacetamide-induced liver fibrosis in rats. Gut 2011; 60: 1728–1737. [DOI] [PubMed] [Google Scholar]
- 17.Cammà C, Scazzone C, Tripodo C, et al. Low vitamin D serum level is related to severe fibrosis and low responsiveness to interferon-based therapy in genotype 1 chronic hepatitis C. Hepatology 2010; 51: 1158–1167. [DOI] [PubMed] [Google Scholar]
- 18.Bronowicki JP, Ouzan D, Asselah T, et al. Effect of ribavirin in genotype 1 patients with hepatitis C responding to pegylated interferon alfa-2a plus ribavirin. Gastroenterology 2006; 131: 1040–1048. [DOI] [PubMed] [Google Scholar]
- 19.Gal-Tanamy M, Bachmetov L, Ravid A, et al. Vitamin D: an innate antiviral agent suppressing hepatitis C virus in human hepatocytes. Hepatology 2011; 54: 1570–1579. [DOI] [PubMed] [Google Scholar]
- 20.Shen L. Vitamin D controls T cell activation: Implication for causal association between vitamin D deficiency and fibrosis in chronic hepatitis C. Hepatology 2010; 52: 1864–1864. [DOI] [PubMed] [Google Scholar]
- 21.Grammatikos G, Lange C, Susser S, et al. Vitamin D levels vary during antiviral treatment but are unable to predict treatment outcome in HCV genotype 1 infected patients. PLoS One 2014; 9: e87974–e87974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petta S, Ferraro D, Cammà C, et al. Vitamin D levels and IL28B polymorphisms are related to rapid virological response to standard of care in genotype 1 chronic hepatitis C. Antivir Ther 2012; 17: 823–831. [DOI] [PubMed] [Google Scholar]
- 23.Abu-Mouch S, Fireman Z, Jarchovsky J, et al. Vitamin D supplementation improves sustained virologic response in chronic hepatitis C (genotype 1)-naïve patients. World J Gastroenterol 2011; 17: 5184–5190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.García-Álvarez M, Pineda-Tenor D, Jiménez-Sousa MA, et al. Relationship of vitamin D status with advanced liver fibrosis and response to hepatitis C virus therapy: A meta-analysis. Hepatology 2014; 60: 1541–1550. [DOI] [PubMed] [Google Scholar]
- 25.Kitson MT, Sarrazin C, Toniutto P, et al. Vitamin D level and sustained virologic response to interferon-based antiviral therapy in chronic hepatitis C: A systematic review and meta-analysis. J Hepatol 2014; 61: 1247–1252. [DOI] [PubMed] [Google Scholar]
- 26.Petta S, Grimaudo S, Marco VD, et al. Association of vitamin D serum levels and its common genetic determinants, with severity of liver fibrosis in genotype 1 chronic hepatitis C patients. J Viral Hepat 2013; 20: 486–493. [DOI] [PubMed] [Google Scholar]
- 27.Terrier B, Carrat F, Geri G, et al. Low 25-OH vitamin D serum levels correlate with severe fibrosis in HIV-HCV co-infected patients with chronic hepatitis. J Hepatol 2011; 55: 756–761. [DOI] [PubMed] [Google Scholar]
- 28.Ho AS, Cheng CC, Lee SC, et al. Novel biomarkers predict liver fibrosis in hepatitis C patients: Alpha 2 macroglobulin, vitamin D binding protein and apolipoprotein AI. J Biomed Sci 2010; 17: 58–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baur K, Mertens JC, Schmitt J, et al. Combined effect of 25-OH vitamin D plasma levels and genetic vitamin D receptor (NR 1I1) variants on fibrosis progression rate in HCV patients. Liver Int 2012; 32: 635–643. [DOI] [PubMed] [Google Scholar]
- 30.Kondo Y, Kato T, Kimura O, et al. 1(OH) Vitamin D3 supplementation improves the sensitivity of the immune-response during Peg-IFN/RBV therapy in chronic hepatitis C patients – case controlled trial. PLoS One 2013; 8: e63672–e63672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jacobson IM, McHutchison JG, Dusheiko G, et al. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med 2011; 364: 2405–2416. [DOI] [PubMed] [Google Scholar]
- 32.Zeuzem S, Andreone P, Pol S, et al. Telaprevir for retreatment of HCV infection. N Engl J Med 2011; 364: 2417–2428. [DOI] [PubMed] [Google Scholar]
- 33.Kwo PY, Lawitz EJ, McCone J, et al. Efficacy of boceprevir, an NS3 protease inhibitor, in combination with peginterferon alfa-2b and ribavirin in treatment-naive patients with genotype 1 hepatitis C infection (SPRINT-1): An open-label, randomised, multicentre phase 2 trial. Lancet 2010; 376: 705–716. [DOI] [PubMed] [Google Scholar]
- 34.Poordad F, McCone J, Bacon BR, et al. Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med 2011; 364: 1195–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Esmat G, El Raziky M, Elsharkawy A, et al. Impact of vitamin D supplementation on sustained virological response in chronic hepatitis C genotype 4 patients treated by pegylated interferon/ribavirin. J Interferon Cytokine Res 2015; 35: 49–54. [DOI] [PubMed] [Google Scholar]
- 36.Agence française de sécurité sanitaire des produits de santé. Recommandations à destination des biologistes concernant la spécificité des dosages de vitamine D. 2009, http://www.ansm.sante.fr/var/ansm_site/storage/original/application/b8d261e1e6faae42c5423a93bc104224.pdf.
- 37.Schiefke I, Fach A, Wiedmann M, et al. Reduced bone mineral density and altered bone turnover markers in patients with non-cirrhotic chronic hepatitis B or C infection. World J Gastroenterol 2005; 11: 1843–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arase Y, Suzuki F, Suzuki Y, et al. Virus clearance reduces bone fracture in postmenopausal women with osteoporosis and chronic liver disease caused by hepatitis C virus. J Med Virol 2010; 82: 390–395. [DOI] [PubMed] [Google Scholar]
- 39.Corazza GR, Trevisani F, Di Stefano M, et al. Early increase of bone resorption in patients with liver cirrhosis secondary to viral hepatitis. Dig Dis Sci 2000; 45: 1392–1399. [DOI] [PubMed] [Google Scholar]
- 40.Solís-Herruzo JA, Castellano G, Fernández I, et al. Decreased bone mineral density after therapy with alpha interferon in combination with ribavirin for chronic hepatitis C. J Hepatol 2000; 33: 812–817. [DOI] [PubMed] [Google Scholar]
- 41.Moreira RO, Balduíno A, Martins HS, et al. Ribavirin, but not interferon alpha-2b, is associated with impaired osteoblast proliferation and differentiation in vitro. Calcif Tissue Int 2004; 75: 160–168. [DOI] [PubMed] [Google Scholar]
- 42.Trombetti A, Giostra E, Mentha G, et al. Lack of evidence for ribavirin-induced bone loss. Hepatology 2002; 36: 255–257. [DOI] [PubMed] [Google Scholar]
- 43.Hofmann WP, Kronenberger B, Bojunga J, et al. Prospective study of bone mineral density and metabolism in patients with chronic hepatitis C during pegylated interferon α and ribavirin therapy. J Viral Hepat 2008; 15: 790–796. [DOI] [PubMed] [Google Scholar]
- 44.Romero-Gómez M, Del Mar Viloria M, Andrade RJ, et al. Insulin resistance impairs sustained response rate to peginterferon plus ribavirin in chronic hepatitis C patients. Gastroenterology 2005; 128: 636–641. [DOI] [PubMed] [Google Scholar]
- 45.Ge D, Fellay J, Thompson AJ, et al. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature 2009; 461: 399–401. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials