Abstract
Background
Autoimmune gastritis (AIG) and adenocarcinoma-associated chronic atrophic gastritis (CAG) are both associated with oxyntic atrophy, but AIG patients demonstrate an increased risk of carcinoid tumors rather than the elevated risk of adenocarcinoma observed with CAG. We therefore sought to compare the characteristics of the metaplastic mucosa in AIG and CAG patients.
Methods
We examined markers for metaplasia (spasmolytic polypeptide expressing metaplasia (SPEM) and intestinal metaplasia) as well as proliferation (Ki67) and immune cell populations (neutrophils, macrophages, and eosinophils) in gastric sections from 16 female patients with autoimmune thyroiditis and AIG and 17 patients with CAG associated with gastric adenocarcinoma.
Results
Both AIG and CAG patients demonstrated prominent SPEM and intestinal metaplasia. However, AIG patients displayed significantly lower numbers of infiltrating macrophages and significantly reduced mucosal cell proliferation as compared to CAG patients.
Conclusions
These findings indicate that, while both AIG and CAG patients display prominent oxyntic atrophy and metaplasia, the AIG patients do not show proliferative metaplastic lineages that would predispose to adenocarcinoma.
Keywords: Spasmolytic polypeptide-expressing metaplasia, intestinal metaplasia, gastritis, CD44 variant 9, DMBT1, macrophage, neutrophil
Introduction
Chronic atrophic gastritis (CAG) associated with Helicobacter pylori (H. pylori) infection leads to the induction of metaplastic cell lineages, spasmolytic polypeptide expressing metaplasia (SPEM)1 and intestinal metaplasia,2 which are considered key neoplastic precursors for the development of gastric adenocarcinoma.3 Recent investigations in mice have demonstrated that the loss of parietal cells triggers transdifferentiation of chief cells into SPEM.4 In the absence of inflammation, as in the case of administration of the parietal cell protonophore DMP-777, SPEM does not progress to dysplasia.5,6 In contrast, loss of parietal cells in association with inflammation can lead to development of a more proliferative metaplasia that can lead to emergence of dysplasia and cancer.7 In humans, chronic inflammation also appears to promote further evolution of SPEM into intestinal metaplasia, which is usually highly proliferative.3 Our recent studies, and those of others, have now implicated M2-macrophages as the driving force for progression to more proliferative metaplasias and dysplasia in both mouse models and humans.7,8 Thus induction of a proliferative metaplastic response in the setting of chronic inflammation is critical for the development of gastric adenocarcinoma.
Autoimmune gastritis (AIG) is characterized by CD4 T-cell-mediated destruction of gastric parietal cells and production of autoantibodies against parietal cell H+, K+-ATPase and gastric intrinsic factor.9 In humans, AIG is often accompanied by other autoimmune maladies including autoimmune thyroiditis, type 1 diabetes, vitiligo, and Addison’s disease.9 While AIG is also associated with prominent oxyntic atrophy in the gastric body, patients with AIG without concurrent H. pylori infection do not show a prominent increased risk for adenocarcinoma.10 Rather, AIG patients display an increased propensity for developing carcinoid tumors in the stomach.11–13 Previous investigations have noted a prominent association of “pyloric metaplasia” in AIG patients, which can often obscure the delineation of the boundaries of the corpus and antrum.14 Pyloric metaplasia, or pseudopyloric metaplasia as described by Whitehead and others,15 appears to be synonymous with the lineage described more recently as SPEM16 Interestingly, a recent investigation has indicated that AIG in rodents is associated with the induction of SPEM.17
Thus, while oxyntic atrophy (parietal cell loss) is the pathological finding most highly associated with the development of gastric adenocarcinoma,18 the relatively low incidence of gastric adenocarcinoma in AIG patients, despite similar amounts of parietal cell loss, leads to a clinical conundrum: Why do AIG patients fail to show the 10–15-fold increased risk of gastric adenocarcinoma seen in patients with CAG? To address this question, in this study, we have investigated the differences in the atrophic gastric mucosa between patients with AIG and CAG to understand how these conditions may lead to different gastric cancer risks. The results demonstrate that while metaplastic lineages are present in both groups of patients, the AIG patient mucosa shows far lower levels of proliferation and less prominent macrophage infiltrates. These findings are consistent with the concept that metaplasias may not progress to adenocarcinoma in the absence of inflammatory drivers from macrophages.
Materials and methods
Study population
Both CAG and AIG patients were assessed in this study. In the CAG group, 12 tissue specimens were obtained from CAG patients through preoperative endoscopic biopsy and five tissue specimens were obtained through surgical resection specimens in gastric adenocarcinoma patients at the Second University of Naples in Italy between January 2009–December 2011. All studies were performed under approved Institutional Review Board protocols at both the Second University of Naples in Italy and Vanderbilt University School of Medicine. All 16 AIG patients had autoimmune thyroiditis, and they were diagnosed with AIG during the workup of autoimmune thyroiditis. All AIG patients were diagnosed with extensive atrophic gastritis in the fundus and body by upper endoscopy. The serum levels of gastrin, chromogranin, and anti-parietal cell antibodies were determined in AIG patients and H. pylori infection was assessed in all patients by Giemsa and Warthin-Starry staining histology. Intestinal metaplasia (IM) was classified as type I (complete) or type II (incomplete) according to standard criteria.19,20
Immunofluorescence staining
Human stomach tissue sections were deparaffinized and subjected to antigen retrieval in a pressure cooker (high pressure, 15 min) using the target retrieval solution (Dako North America Inc., Carpinteria, California, USA). Primary antibody labeling of Trefoil Factor 2 (TFF2) (Abcam, ab49536, 1: 1000), Mucin 2 (MUC2) (Santa Cruz, sc-15334, 1: 500), CD68 (Thermo Scientific, MS-397-P0, 1: 500), and myeloperoxidase (MPO) (Dako, A-0398, 1: 1000) was performed by overnight incubation at 4℃, and secondary antibodies and Alexa Fluor© 647 Conjugate-Griffonia simplicifolia II (GSII)-lectin (Molecular Probes, L3245, 1:1:2,000) were incubated for one hour at room temperature. All fluorescent samples were counter stained with 4′,6-diamidino-2-phenylindole (DAPI) and mounted in Prolong (Molecular Probes).
Immunohistochemistry
Deparaffinization and antigen retrieval was carried out as for immunofluorescence staining. Primary antibody labeling of chromogranin A (AbD Serotec, MCA845, 1: 500)CD44 variant 9 (CD44v) (Cosmo Bio Co., LKG-M001, 1: 5000) and DMBT1 (Abbiotec, 250390, 1: 2000) was performed by overnight incubation at 4℃. Labeling of Ki67 (Dako, M-7240, 1: 100) was performed by a one-hour incubation at room temperature. The secondary antibody labeling (Dako labeled polymer-HRP (horseradish peroxidase) anti-mouse, K4007) and diaminobenzidine (DAB) development of DMBT1 and Ki67 was performed using the Dako EnVision system (Dako North America Inc., Carpinteria, California, USA), according to the manufacturer’s instructions. CD44v immunochemistry was performed using the VECTASTAIN ABC-HRP KIT (Vector laboratories, Inc., Burlingame, California, USA) according to the manufacturer’s instructions.
Quantitation of immune cells and Ki67 staining
Eosinophils were quantitated in hematoxylin and eosin stained sections for 10 high power fields from at least three biopsy specimens per patient. Slides labeled for the macrophage marker CD68 and the neutrophil marker MPO were scanned using the Ariol SL-200 automated slide scanner (Leica Microsystems, Buffalo Grove, Ilinois, USA) at 20× magnification (Vanderbilt Digital Histology Shared Resource). For immunofluorescent slides, 5–10 images per patient sample were exported and analyzed using CellProfiler.21 Regions for quantitation were selected for presentation of full-length glands. Images were quantified for the number of cells labeled with either CD68 or MPO alone, or cells labeled with both markers. The positive cell number was calculated as a percentage of total cells with nuclei, and a two-tailed Student’s t-test was used to compare the mean values in CAG and AIG samples (p < 0.05). All counted cells contained nuclei.
For Ki-67 staining quantitation, the slides were scanned using the Leica SCN400 automated slide scanner (Leica Microsystems, Buffalo Grove, Illinois, USA) at 20× magnification (Vanderbilt Digital Histology Shared Resource). Whole slide images were analyzed using the Ariol analysis software using standardized scripts. Stromal tissue was excluded in the analysis of gastric gland samples. Upper and lower thresholds for color, saturation, intensity, size, roundness, and axis length were set for brown DAB reaction products (positive cells) and blue hematoxylin only negative cells. The positive cell number was calculated per 1000 cells, and a two-tailed Student’s t-test was used to compare the mean values in CAG and AIG samples (p < 0.05).
Results
Clinical characteristics of the patient cohorts
We examined the gastric body mucosa from two groups of patients (Supplementary Material, Table 1): The CAG group included 17 patients (12 males, five females) all of whom were resected for cure for gastric adenocarcinoma (Supplementary Material, Table 2). Eight of the CAG patients had documented H. pylori infection (one patient had no record), but none of the AIG patients had detectable H. pylori infection. The AIG group included 16 female patients, all of whom also had coincident autoimmune thyroiditis (Supplementary Material, Table 3). The AIG patients displayed marked elevations in both serum gastrin (mean value: 913.3; normal <100) and serum chromogranin A (mean value: 336.2; normal <100) levels and all showed ECL cell hyperplasia (Supplementary Material, Figure 1).
Examination of metaplastic lineages in the atrophic stomach
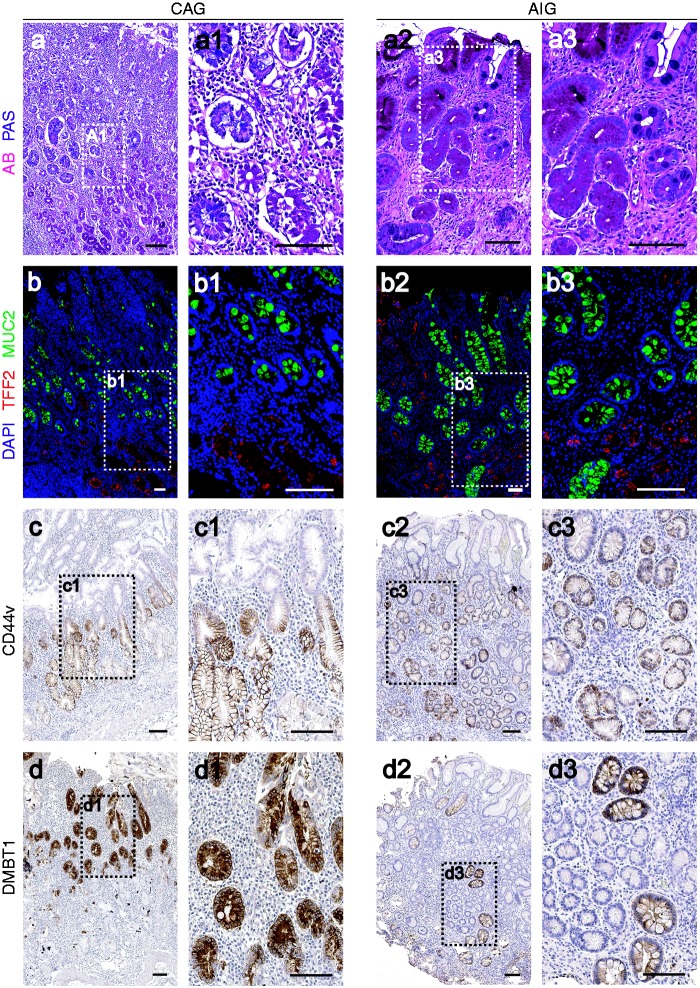
To evaluate the characteristics of metaplasias in the two patient cohorts, we investigated the presence of SPEM or intestinal metaplasia by staining with antibodies against TFF2 or MUC2, respectively. All AIG patients showed prominent parietal cell loss and TFF2-staining SPEM. Of the AIG patients, 75% (12/16) also demonstrated MUC2-positive goblet cell intestinal metaplasia (Figure 1(a)–(a3), 1(b)–(b3)). All CAG samples (17/17) showed marked parietal cell loss and both TFF2-staining SPEM and MUC2-positive intestinal metaplasia (Figure 1(a)–(a3), 1(b)–(b3)). These results suggested that, while SPEM was more prominently represented in the atrophic stomach in AIG patients, intestinal metaplasia was present in both groups.
Figure 1.
Metaplasia in the gastric mucosa of autoimmune gastritis (AIG) and chronic atrophic gastritis (CAG) patients. Top row: dual Alcian blue and periodic acid-Schiff (AB/PAS) staining of gastric mucosa in CAG ((a), (a1)) and AIG ((a2), (a3)) patients. Note AB staining of intestinal metaplasia in luminal cells, and PAS-staining of spasmolytic polypeptide expressing metaplasia (SPEM) at the bases of the gland. Second row: dual immunofluorescence staining of TFF2 (Trefoil Factor 2, SPEM marker, red) and MUC2 (Mucin 2, intestinal metaplasia marker, green) in gastric mucosa from CAG ((b), (b1)) and AIG ((b2), (b3)). In all cases nuclei were labeled with 4′,6-diamidino-2-phenylindole (DAPI) (blue). Images (a1), (a3), (b1) and (b3) are higher magnification as noted by white boxed outlines. Third row: immunohistochemical staining of CD44v (SPEM marker) in CAG ((c), (c1)) and AIG ((c2), (c3)) gastric mucosa. Bottom row: immunohistochemical staining of DMBT1 (Deleted in malignant brain tumors 1, intestinal metaplasia marker) for CAG ((d), (d1)), and AIG ((d2), (d3)) mucosa. Images (c1), (c3), (d1) and (d3) are higher magnification as noted by black-boxed outlines. Bar indicates 100 µm.
To compare the metaplastic lineages further, we examined the expression of CD44 variant 9 (CD44v), which recently was demonstrated as a marker of SPEM in mice,22 and DMBT1, a marker of intestinal metaplasia in humans.23 CD44 variant was expressed in SPEM in both AIG and CAG patients, with weak or absent expression in intestinal metaplasia in both groups (Figure 1(c)–(c3)). In contrast, DMBT1 antibodies labeled IM, but not SPEM, in both groups, although the intensity of staining for DMBT1 in intestinal metaplasia was uniformly weaker in the intestinal metaplasia associated with AIG (Figure 1(d)–(d3)). All AIG patients showed type I complete IM, while among the CAG patients, 16/17 had type I, one had incomplete type II IM (Supplementary Material, Tables 2 and 3). Thus, both groups of patients displayed prominent oxyntic atrophy along with both SPEM and IM.
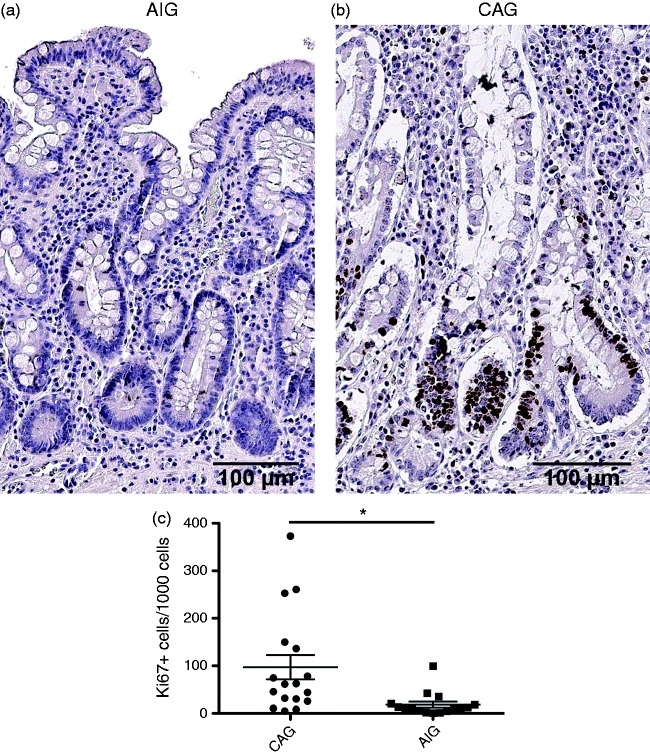
We next compared epithelial cell proliferation in the gastric mucosa in AIG and CAG patients. While both CAG and AIG patients demonstrated extensive metaplasia, the height of the mucosa appeared diminished in AIG patients. AIG patients demonstrated significantly lower rates of proliferation in mucosal cells as assessed by Ki67-immunostaining cells (18.26/1000 gland cells compared with 97.13/1000 gland cells in CAG patients) (Figure 2). These results indicated that the metaplastic glands in AIG patients demonstrated a significantly lower level of proliferation compared to the metaplasia in CAG patients.
Figure 2.
Characteristics of proliferation in metaplastic mucosa. Representative immunostaining for Ki67 in (a) a chronic atrophic gastritis (CAG) patient and (b) an autoimmune gastritis (AIG) patient. (c) Quantitation of Ki67-positive cells demonstrates a markedly lower level of proliferation in AIG patients. In CAG patients, filled circles indicate females and open circles indicate males. *p = 0.0066. Bar indicates 100 µm.
Examination of the metaplastic milieu
Recent studies have suggested that macrophages are an important driver of the evolution of metaplasia.7 In rodents, SPEM can develop after acute loss of parietal cells in the absence of inflammation.24 However, in the absence of immune cell infiltrates, especially macrophages, SPEM lesions do not progress to dysplasia.6,7
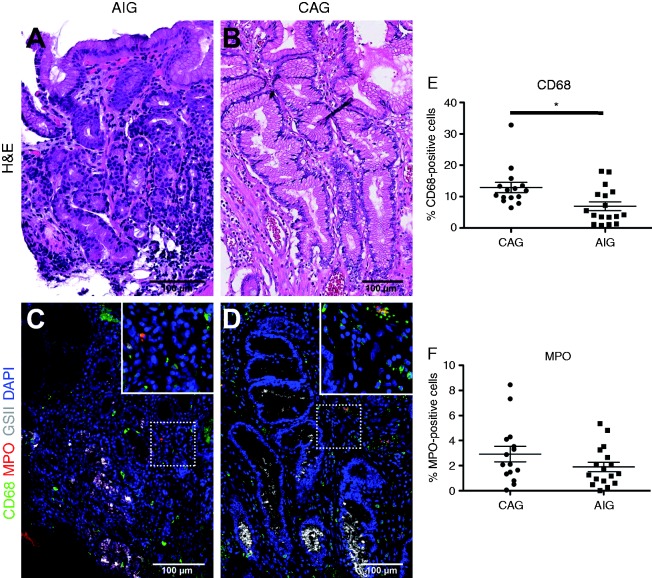
We therefore analyzed the immune infiltrates in AIG and CAG patients (Figure 3). As previously reported,7 CAG patients showed prominent infiltrates of CD68-expressing macrophages, often presenting as groups of cells clustered around metaplastic glands (Figure 3(c)). In contrast, AIG patients had significantly fewer CD68-positive macrophages as compared to CAG patients (Figure 3(d), 3 (e)). AIG patients showed similar numbers of MPO-positive neutrophils as CAG patients (Figure 3(e)). Interestingly, while a previous study suggested that AIG patients show increased numbers of eosinophils compared to normal subjects,25 we found significantly more eosinophils in CAG patients compared with AIG patients (Supplementary Material, Table 1). These results indicated that the AIG patients lacked prominent macrophage infiltrates that may drive the evolution of proliferative metaplasia.
Figure 3.
Characteristics of inflammatory infiltrates in metaplastic mucosa. Comparison of the infiltration of macrophages (CD68, green) and neutrophils (MPO, Myeloperoxidase, red) between chronic atrophic gastritis (CAG) ((a), (c)) and autoimmune gastritis (AIG) ((b), (d)) patients. Hematoxylin and eosin (H&E) staining of sections are shown from AIG (a) and CAG (b) patient stomach. In (c) and (d), spasmolytic polypeptide expressing metaplasia (SPEM) cells were stained with Griffonia simplicifolia II-lectin (white) and nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI) (blue). Insets show higher magnification images as noted by white boxed outlines. Bar indicates 100 µm. (e) Quantitation of immunostaining demonstrated the presence of significantly fewer CD68-positive macrophages in AIG patients as compared to CAG patients (*p < 0.01).
Discussion
Oxyntic atrophy and intestinal metaplasia are considered as critical precursors in the development of intestinal-type gastric adenocarcinoma.2 We have reported that mature chief cells transdifferentiate into SPEM in setting of oxyntic atrophy, and that SPEM can progress to intestinal metaplasia in humans.3,4 Intestinal metaplasia can be categorized as either complete or incomplete.26 Incomplete or Type II intestinal metaplasia has a 4–11-fold higher cancer risk.27 In contrast, complete intestinal metaplasia is associated with a much lower risk of gastric adenocarcinoma. A previous study reported that 21% (21/99) of CAG patients showed incomplete IM, while only 4% (1/24) of AIG patients demonstrated incomplete intestinal metaplasia.27 In the present study, all AIG patients presented with complete or type I intestinal metaplasia, but only one CAG patient showed type II incomplete intestinal metaplasia. The phenotype of more “benign” metaplasia observed in this report is more consistent with a significantly lower proliferative rate. Previous investigations have noted that the Ki-67 expression level is increased in intestinal metaplasia compared with normal gastric mucosa.28 In addition, patients with type I intestinal metaplasia show lower Ki-67 expression in the gastric mucosa compared with Type II intestinal metaplasia and gastric cancer.29 The low proliferative rate we have observed in metaplastic lineages in AIG patients suggests that the metaplasia in these patients may lack the proliferative drive necessary for evolution of adenocarcinoma.
As compared with H. pylori-associated CAG, AIG patients have greater basal lymphoid aggregation and lymphocyte infiltration of crypt epithelium, but lower neutrophil cryptitis.25 Previous studies reported an increase in eosinophils compared with normal gastric mucosa,25 but we observed greater numbers of eosinophils in the CAG cohorts in the present study. We and others have recently noted the importance of macrophages in the progression of metaplasias towards neoplasia in both mice and humans.7 Oshima et al. reported accumulation of macrophages in the pre-neoplastic lesions of GAN mice (K19-Wnt1/C2mE mice).30 We have found here that AIG patients have significantly lower macrophage infiltration compared with CAG patients. Thus, AIG patients appear to have fewer immune cell drivers that are necessary for promotion of the transitions to cancer. It should be noted, however, that while all of samples examined in CAG patients were from areas of the stomach without dysplasia, we have not directly examined whether CAG patients with lower macrophage infiltrates are less likely to progress to cancer. Nevertheless, our previous investigations did indicate that CAG patients with intestinal metaplasia and/or SPEM have significantly elevated numbers of intramucosal macrophages.7
While both CAG and AIG patients display similar amounts of parietal cell loss and demonstrate extensive metaplastic lineages, AIG patients appear to possess a more benign metaplastic phenotype. The CAG patient group examined in this study showed similar amounts of intestinal metaplasia and inflammation as previously described in a number of studies.18,31 Overall, in AIG patients the metaplastic response was dominated by SPEM rather than IM. We have previously noted that, in rodents, SPEM develops as a response to parietal cell loss, but the extension of metaplasia to a more proliferative and intestinalized phenotype requires the additional influence of inflammatory components, especially M2-polarized macrophages.5,7 In the absence of inflammation, SPEM does not progress to dysplastic lesions.6 In the absence of coincident H. pylori infection, the AIG patients therefore appear to demonstrate this more stable metaplastic phenotype, which resembles more closely the scenario displayed in Barrett’s epithelium without dysplasia. Nevertheless, this metaplastic mucosa is not completely benign, since AIG patients with chronic atrophy remain at higher risk for the development of endocrine carcinoid tumors from transformation of ECL cells. It remains uncertain what forces in turn promote enteroendocrine cell neoplasia in AIG patients.
In summary
What is known
Autoantibodies against parietal cells in patients with autoimmune gastritis lead to gastric atrophy.
Patients with autoimmune gastritis without H. pylori infection have less of an increased risk for gastric adenocarcinoma compared to patients with chronic atrophic gastritis.
Patients with autoimmune gastritis are at increased risk for development of gastric carcinoid tumors.
New findings
Patients with either autoimmune gastritis or chronic atrophic gastritis associated with adenocarcinoma showed parietal cell loss and metaplasia.
Patients with autoimmune gastritis showed a significantly lower level of mucosal cell proliferation.
Patients with autoimmune gastritis had significantly less macrophage infiltration than patients with chronic atrophic gastritis.
Impact on clinical practice
Despite similar amounts of parietal cell loss and metaplasia, compared with chronic atrophic gastritis patients, without macrophage infiltrates to drive proliferation, autoimmune gastritis patients may be at a lower risk for developing adenocarcinoma.
Supplementary Material
Acknowledgements
Author roles included the following: S Jeong designed and performed studies, analyzed data, drafted manuscript; E Choi designed and performed studies, analyzed data, drafted manuscript; JT Roland designed and performed studies, analyzed data, revised manuscript; CP Petersen performed studies, revised manuscript; A Frederico designed studies, interpreted pathology, edited manuscript; R Ippolito designed studies, interpreted pathology, edited manuscript; FP D'Armiento designed studies, interpreted pathology, edited manuscript; G Nardone designed studies, interpreted pathology, edited manuscript; O Nagano designed studies, edited manuscript; H Saya designed studies, edited manuscript; M Romano designed studies, analyzed data, drafted manuscript; JR Goldenring designed studies, analyzed data, drafted manuscript.
Funding
These studies were supported by grants to JRG from the National Institutes of Health RO1 DK071590, RO1 DK101332 and American Recovery and Reinvestment Act Supplemental Funding from RO1 DK071590-S1, from a Department of Veterans Affairs Merit Review I01BX000930 and from a grant from the TJ Martell Foundation. MR was in part supported by grants from CIRANAD. and from the Department of Medicine of the Second University of Naples. This work utilized resources of the Vanderbilt Digital Histology Shared Resource. This work was supported by core resources of the Vanderbilt Digestive Disease Center (P30 DK058404), and the Vanderbilt-Ingram Cancer Center through NCI (National Cancer Institute) Cancer Center Support Grant P30 CA068485 utilizing the Translational Pathology Shared Resource.
Conflict of interest
None of the authors has any conflicts of interest to declare.
References
- 1.Nomura S, Baxter S, Yamaguchi T, et al. Spasmolytic polypeptide expressing metaplasia (SPEM) to pre-neoplasia in H. felis-infected mice. Gastroenterology 2004; 127: 582–594. [DOI] [PubMed] [Google Scholar]
- 2.Correa P. A human model of gastric carcinogenesis. Cancer Res 1988; 48: 3554–3560. [PubMed] [Google Scholar]
- 3.Goldenring JR, Nam KT, Wang TC, et al. Spasmolytic polypeptide-expressing metaplasia and intestinal metaplasia: Time for reevaluation of metaplasias and the origins of gastric cancer. Gastroenterology 2010; 138: 2207–2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nam KT, Lee H-J, Sousa JF, et al. Mature chief cells are cryptic progenitors for metaplasia in the stomach. Gastroenterology 2010; 139: 2028–2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weis VG, Sousa JF, LaFleur BJ, et al. Heterogeneity in mouse SPEM lineages identifies markers of metaplastic progression. Gut 2013; 62: 1270–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldenring JR, Ray GS, Coffey RJ, et al. Reversible drug-induced oxyntic atrophy in rats. Gastroenterology 2000; 118: 1080–1093. [DOI] [PubMed] [Google Scholar]
- 7.Petersen CP, Weis VG, Nam KT, et al. Macrophages promote progression of spasmolytic polypeptide-expressing metaplasia following acute loss of parietal cells. Gastroenterology 2014; 146: 1727–1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oshima H, Hioki K, Popivanova BK, et al. Prostaglandin E signaling and bacterial infection recruit tumor-promoting macrophages to mouse gastric tumors. Gastroenterology 2011; 140: 596–607 e7. [DOI] [PubMed] [Google Scholar]
- 9.Neumann WL, Coss E, Rugge M, et al. Autoimmune atrophic gastritis–pathogenesis, pathology and management. Nat Rev Gastroenterol Hepatol 2013; 10: 529–541. [DOI] [PubMed] [Google Scholar]
- 10.Rugge M, Fassan M, Pizzi M, et al. Autoimmune gastritis: Histology phenotype and OLGA staging. Aliment Pharmacol Ther 2012; 35: 1460–1466. [DOI] [PubMed] [Google Scholar]
- 11.Toh BH. Diagnosis and classification of autoimmune gastritis. Autoimmun Rev 2014; 13: 459–462. [DOI] [PubMed] [Google Scholar]
- 12.Vannella L, Lahner E, Annibale B. Risk for gastric neoplasias in patients with chronic atrophic gastritis: A critical reappraisal. World J Gastroenterol 2012; 18: 1279–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Borch K, Renvall H, Liedberg G. Gastric endocrine cell hyperplasia and carcinoid tumors in pernicious anemia. Gastroenterology 1985; 88: 638–648. [DOI] [PubMed] [Google Scholar]
- 14.Lewin KJ, Dowling F, Wright JP, et al. Gastric morphology and serum gastrin levels in pernicious anaemia. Gut 1976; 17: 551–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Whitehead R, Truelove SC, Gear MWL. The histological diagnosis of chronic gastritis in fibreoptic gastroscope biopsy specimens. J Clin Pathol 1972; 25: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmidt PH, Lee JR, Joshi V, et al. Identification of a metaplastic cell lineage associated with human gastric adenocarcinoma. Lab Invest 1999; 79: 639–646. [PMC free article] [PubMed] [Google Scholar]
- 17.Nguyen TL, Khurana SS, Bellone CJ, et al. Autoimmune gastritis mediated by CD4+ T cells promotes the development of gastric cancer. Cancer Res 2013; 7: 2117–2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.El-Zimaity HMT, Ota H, et al. Patterns of gastric atrophy in intestinal type gastric carcinoma. Cancer 2002; 94: 1428–1436. [DOI] [PubMed] [Google Scholar]
- 19.Jass JR, Filipe MI. Sulphomucins and precancerous lesions of the human stomach. Histopathology 1980; 4: 271–279. [DOI] [PubMed] [Google Scholar]
- 20.Stemmermann GN. Intestinal metaplasia of the stomach. A status report. Cancer 1994; 74: 556–564. [DOI] [PubMed] [Google Scholar]
- 21.Carpenter AE, Jones TR, Lamprecht MR, et al. CellProfiler: Image analysis software for identifying and quantifying cell phenotypes. Genome Biol 2006; 7: R100–R100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wada T, Ishimoto T, Seishima R, et al. Functional role of CD44v-xCT system in the development of spasmolytic polypeptide-expressing metaplasia. Cancer Sci 2013; 104: 1323–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sousa JF, Ham AJ, Whitwell C, et al. Proteomic profiling of paraffin-embedded samples identifies metaplasia-specific and early-stage gastric cancer biomarkers. Am J Pathol 2012; 181: 1560–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nomura S, Yamaguchi H, Ogawa M, et al. Alterations in gastric mucosal lineages induced by acute oxyntic atrophy in wild-type and gastrin-deficient mice. Am J Physiol Gastrointest Liver Physiol 2005; 288: G362–G375. [DOI] [PubMed] [Google Scholar]
- 25.Bettington M, Brown I. Autoimmune gastritis: Novel clues to histological diagnosis. Pathology 2013; 45: 145–149. [DOI] [PubMed] [Google Scholar]
- 26.Correa P, Piazuelo MB, Wilson KT. Pathology of gastric intestinal metaplasia: Clinical implications. Am J Gastroenterol 2010; 105: 493–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Petersson F, Borch K, Franzen LE. Prevalence of subtypes of intestinal metaplasia in the general population and in patients with autoimmune chronic atrophic gastritis. Scand J Gastroenterol 2002; 37: 262–266. [DOI] [PubMed] [Google Scholar]
- 28.Feng XS, Wang YF, Hao SG, et al. Expression of Das-1, Ki67 and sulfuric proteins in gastric cardia adenocarcinoma and intestinal metaplasia lesions. Exp Ther Med 2013; 5: 1555–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zheng Y, Wang L, Zhang JP, et al. Expression of p53, c-erbB-2 and Ki67 in intestinal metaplasia and gastric carcinoma. World J Gastroenterol 2010; 16: 339–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oshima H, Matsunaga A, Fujimura T, et al. Carcinogenesis in mouse stomach by simultaneous activation of the Wnt signaling and prostaglandin E2 pathway. Gastroenterology 2006; 131: 1086–1095. [DOI] [PubMed] [Google Scholar]
- 31.El-Zimaity HMT, Ramchatesingh J, Saeed MA, et al. Gastric intestinal metaplasia: Subtypes and natural history. J Clin Pathol 2001; 54: 679–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.