Abstract
Background
Early detection of liver fibrosis in thalassemia patients and rapid initiation of treatment to interfere with its progression are extremely important.
Objective
This study aimed to find a sensitive, easy-to-detect and noninvasive method other than liver biopsy for early detection of liver fibrosis in thalassemia patients.
Methods
A total of 244 Chinese Thalassemia patients with non-transfusion-dependent thalassemia (NTDT, n = 105) or thalassemia major (TM, n = 139) and 120 healthy individuals were recruited into the present study, and blood collagen type IV (C IV), precollagen type III (PIIINPC) and hyaluronic acid (HA), aspartate aminotransferase (AST), alanine aminotransferase (ALT) and ferritin were measured. Liver iron concentration was determined by MRI. The correlation of serum markers with liver iron load and liver function was evaluated.
Results
Serum C IV, PIIINPC and HA were significantly elevated in Chinese patients with NTDT and further elevated in TM patients. Moreover, C IV, PIIINPC and HA were also positively correlated to serum ferritin and liver iron concentration and further elevated during the progression to multi-organ damage in NTDT patients. Finally, serum ferritin and liver iron concentration were significantly correlated with liver dysfunction determined by AST and ALT.
Conclusion
Taken together, our results indicate that monitoring serum C IV, PIIINPC and HA is a potentially sensitive method to predict the risks for iron overload-related liver fibrosis in Chinese thalassemia patients.
Keywords: Liver fibrosis, thalassemia, markers, iron, Chinese
Introduction
Thalassemia is a genetic disease causing failure to produce red blood cells resulting in transfusion requirement from an early age in its severe form (thalassemia major) or anemia without transfusion requirement (thalassemia intermedia).1,2 As a result of hepatitis virus C (HCV) infection3,4 or iron overload,5 patients with thalassemia often develop liver fibrosis, a life-threatening complication resulting from excessive accumulation of extracellular matrix without effective treatments.6,7 Therefore, early prediction of hepatic fibrosis is critical for thalassemia patients. The invasive nature and associated serious complications of the current diagnostic gold standard,8,9 liver biopsy, have raised the demand for less-invasive methods for the evaluation of iron load in the liver. Imaging techniques such as transient elastography, real-time tissue elastography, and magnetic resonance elastography can reveal liver fibrosis, but usually at its later stages. Therefore, it is imperative to identify biomarkers for the prediction of iron overload-related liver fibrosis, which is crucial for timely treatment of liver fibrosis and prevention of its progression.
Some serum biomarkers including collagen type IV (C IV), precollagen type III (PIIINP), and hyaluronic acid (HA) have been used for the evaluation of liver fibrosis in patients with chronic hepatitis B,10 chronic HCV11–13 and alcoholic liver disease.14–16 Two small pilot studies suggest that HA and PIIINP positively correlate with iron overload in Chinese thalassemia patients.17,18 These findings raise an intriguing possibility that liver iron overload and liver function are likely correlated with serum markers of liver fibrosis. To test this hypothesis, 244 Chinese thalassemia patients without hepatitis B or C infections were recruited, and serum levels of C IV, PIIINP and HA as well as liver function (alanine aminotransferase (ALT) and aspartate aminotransferase (AST)) were determined. Additionally, the liver iron load and serum ferritin (SF) concentration were also measured in these patients. Finally, the correlation among serum biomarkers, liver iron concentration (LIC) and liver function was evaluated to determine whether these three serum biomarkers are correlated with iron overload and liver dysfunction in Chinese thalassemia patients. Our findings lay the groundwork for the potentially noninvasive early detection of iron overload-related liver fibrosis in thalassemia patients.
Materials and methods
Participants
The study protocol conformed to the Declaration of Helsinki and was approved by the Medical Ethics Committee of the First Affiliated Hospital of Guangxi Medical University. All participants, or their legal guardians if individuals were under 18, gave written informed consent.
Patients newly diagnosed and treated in our medical center for non-transfusion-dependent thalassemia (NTDT) or thalassemia major were prospectively and consecutively enrolled between November 2010 and November 2013. Patients (n = 256) were diagnosed based on hemoglobin electrophoresis and genetic and clinical analysis of thalassemia.19–21 Patients with indicators of hepatic or renal impairment, HbS variants, systemic diseases or a history of hepatitis B or C virus infection were excluded (n = 12).
The following criteria were used to determine whether TM patients were well-chelated:22,23 (i) patients received chelation therapy after the first 10–20 U of transfusions or when the SF was higher than 1000 ng/ml; (ii) patients received the treatment with chelator deferoxamine (DFO) at 20–40 mg/kg/day (children) or 50–60 mg/kg/day (adults), chelator deferiprone (DFP) at 75 mg/kg/day (adults or children) or chelator deferasirox (DFX) at 20–40 mg/kg/day (adults or children) for at least five days weekly; and (iii) patients showed ≥90% adherence to the instructions on chelation therapy provided by hematologists.
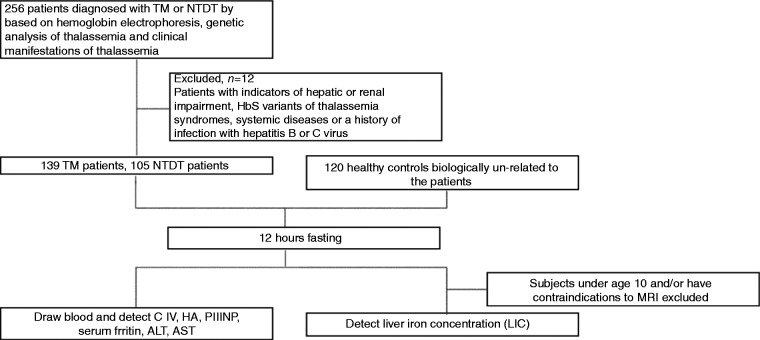
Healthy age-matched controls receiving routine physical examination (n = 120) were consecutively recruited from the outpatient center of the First Affiliated Hospital of Guangxi Medical University. Physical examinations, including routine blood test, detection of liver and kidney function and SF, and hemoglobin electrophoresis, showed normal results and they had no history of hepatitis B or C virus infection (Figure 1 and Table 1).
Figure 1.
Flowchart of participant enrollment.
TM: thalassemia major; NTDT: non-transfusion-dependent thalassemia; HA: hyaluronic acid, ng/ml; PIIINP: precollagen type III, ng/ml; C IV: collagen type IV, ng/ml; ALT: alanine aminotransferase; AST: aspartate aminotransferase; MRI: magnetic resonance imaging.
Table 1.
Demographic characteristics in healthy Chinese controls and Chinese patients with non-transfusion-dependent thalassemia (NTDT) or thalassemia major (TM)
| Parameters | NTDTa (n = 105) | TM (n = 139) | Controls (n = 120) |
|---|---|---|---|
| Age (years), median (range) | 24.0 (10–63) | 8.0 (3–49) | 21.5 (5–61) |
| Male, n (%) | 61 (58.1) | 81 (58.3) | 71 (59.2) |
| Age group (years), n (%) | |||
| <11 | 5 (4.8) | 93 (66.9) | 8 (6.7) |
| 11–20 | 39 (37.1) | 43 (31.0) | 38 (31.7) |
| 20–30 | 33 (31.4) | 2 (1.4) | 43 (35.8) |
| >30 | 28 (26.7) | 1 (0.7) | 31 (25.8) |
| Hepatomegaly, n (%) | 31 (29.5) | 42 (30.2) | – |
| Splenomegaly, n (%) | 34 (32.4) | 32 (23.0) | – |
| Splenectomized, n (%) | 47 (44.8) | 26 (18.7) | – |
| Chelation therapy, n (%) | – | ||
| DFO | 9 (8.6) | 36 (25.9) | – |
| DFP | 8 (7.6) | 24 (17.3) | – |
| DFO + DFP/DFX | 7 (6.7) | 77 (55.4) | – |
| None | 81 (77.1) | 2 (1.4) | – |
| Transfusion volume (U), median (IQR) | 1.5 (0.0–27.5) | 89.0 (12.0–456.0) | – |
| Serum ferritin (ng/ml), median (range) | 1085 (36–19704) | 4519 (121–14724) | – |
| Serum ferritin category (ng/ml), n (%) | – | ||
| <1000 | 50 (47.6) | 9 (6.5) | – |
| 1000–2500 | 41 (39.0) | 24 (17.3) | – |
| >2500 | 14 (13.4) | 106 (76.2) | – |
Of 105 patients with NTDT, 62 were diagnosed with hemoglobin H (HbH) disease, 13 with hemoglobin E (HbE)/β-thalassemia and 30 with β thalassemia intermedia. DFO: deferoxamine; DFP: deferiprone; DFX: deferasirox; IQR: interquartile range.
Measurements
Blood (5 ml) was collected from all participants after 12-hour fasting and the serum levels of C IV, PIIINP, HA, ALT, AST and SF were measured. C IV, PIIINP, and HA were determined using a commercially available time-resolved fluoroimmunoassay (Beijing North Institute of Biological Technology, Beijing, China). Serum levels of ALT and AST were measured with an automatic chemistry analyzer. SF was determined by electro-chemiluminescence immunoassay (COBAS E 601, Roche, USA). All the blood samples processed for measurement had no hemolysis.
Liver iron concentration (LIC) was measured by magnetic resonance imaging (MRI) R2 in patients older than 10 years who had no contraindications and tolerated the procedure.24–27 MRI R2 was performed using a 1.5-T MRI scanner (Siemens Magnetom Avanto, Siemens Medical Systems, Erlangen, Germany) as described previously.28 LIC was read centrally using a validated MRI R2 technique (FerriScan; Resonance Health Ltd, Claremont, WA, Australia) and the MRI staff in this study received intensive on-site training by liver MRI specialists from Resonance Health Ltd.
Statistical analysis
Statistical analysis was performed with SPSS 16.0 (IBM, USA). Data are expressed as percentages, median (range), or median (interquartile range, IQR) as appropriate. Inter-group differences in continuous variables were assessed using the Mann–Whitney U test. Since the three serum markers of liver fibrosis showed abnormal distribution, bivariate Spearman rank correlation analysis was used for the evaluation of possible correlations. A value of two-sided p less than 0.05 was considered statistically significant.
Results
Cohort characteristics
During the study period, 256 patients were diagnosed with NTDT or TM at our medical center; 12 were excluded because of history of hepatitis B or HCV infection. The remaining 244 patients were included for final analysis. There were 105 patients with NTDT (61 males; median age, 24 years; age range, 10–63 years) and 139 patients with TM (81 males; median age, 8 years; age range, 3–49 years). The most prevalent forms of NTDT worldwide are hemoglobin H (HbH) disease, hemoglobin E (HbE)/β-thalassemia, and β thalassemia intermedia.29 In this study, there were 62 patients diagnosed with HbH disease, 13 with HbE/β-thalassemia and 30 with β thalassemia intermedia (Table 1). NTDT patients received no or only occasional blood transfusions. A small portion of patients required transfusions because of growth failure, pregnancy, infections or other specific situations. All TM patients were transfusion dependent, and 98.6% of them had a history of iron-chelation therapy. Only three patients with TM (2.2%) were well chelated based on the standard criteria in the present study.
There were 120 healthy individuals in the control group (71 males; median age, 23.5 years; age range, 5–61 years) who were not biologically related to the patients (Figure 1 and Table 1).
Serum fibrotic markers are significantly elevated in NTDT patients and further elevated in TM patients
In NTDT patients, the serum levels of C IV, HA and AST were significantly increased when compared with healthy individuals (p < 0.05), while there were no significant differences between NTDT patients and healthy controls in PIIINP or ALT levels (p > 0.05). However, the serum levels of C IV, PIIINP, HA, ALT and AST were significantly higher in TM patients as compared with NTDT patients (Table 2), implicating that serum fibrotic biomarkers are associated with the severity of liver dysfunction in TM.
Table 2.
Serum levels of liver fibrotic markers and liver functional enzymes (median, range) in healthy Chinese controls and Chinese patients with non-transfusion-dependent thalassemia (NTDT) or thalassemia major (TM)
| Groups | C IV (ng/ml) | PIIINP (ng/ml) | HA (ng/ml) | ALT (U/l) | AST (U/l) |
|---|---|---|---|---|---|
| NC (n = 120) | 30 (6–92) | 0.5 (0.0–12.5) | 11 (0–70) | 16 (6–51) | 20 (13–36) |
| NTDT (n = 105) | 60 (9–252)a | 0.9 (0.0–53.4) | 20 (0–679)a | 18 (5–120) | 28 (11–119)a |
| TM (n = 139) | 74 (52–157)b,c | 2.9 (0.0–94.7)b,c | 66 (8–864)b,c | 41 (8–282)b,c | 42 (17–177)b,c |
NC: normal control; NTDT: non-transfusion-dependent thalassemia; TM: thalassemia major. HA: hyaluronic acid; PIIINP: precollagen type III; C IV: collagen type IV. ap < 0.05 vs NC; bp < 0.05 vs NC; cp < 0.05 vs NTDT.
More NTDT and TM patients display elevated serum levels of C IV, PIIINP and HA as compared with healthy controls
The percentage of NTDT and TM patients with elevated serum levels of fibrotic biomarkers was compared. Results showed 48 of 105 (45.7%) NTDT patients and 121 of 139 (87.1%) TM patients had significantly higher serum C IV than controls did. Specifically, more TM patients had a high serum C IV than did NTDT patients (p < 0.001) (Figure 2). Moreover, 34 of 105 (32.4%) NTDT patients and 99 of 139 (71.2%) TM patients had elevated HA. Similar to C IV, more TM patients had a high serum HA than did NTDT patients (p < 0.001) (Figure 2). Additionally, 14 of 105 (13.3%) NTDT patients and 26 of 139 (18.7%) TM patients had elevated serum PIIINP, but there was no significant difference in the proportion of patients with elevated serum PIIINP between the two groups (p = 0.262) (Figure 2). Thus, our study implicates that serum C IV and HA might be more sensitive to predict the severity of thalassemia than serum PIINP. Of note, approximately 13–33% of NTDT patients and around 18–87% of TM patients had higher serum levels of circulating fibrotic biomarkers than did the controls.
Figure 2.
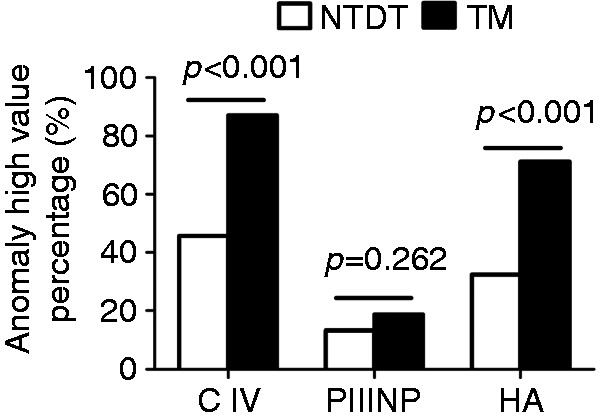
Comparison of the percentage of NTDT and TM patients with elevated levels of serum fibrotic biomarkers.
NTDT: non-transfusion-dependent thalassemia; TM: thalassemia major; HA: hyaluronic acid, ng/ml; PIIINP: precollagen type III, ng/ml; C IV: collagen type IV, ng/ml; ALT: alanine aminotransferase; AST: aspartate aminotransferase.
SF levels are elevated in NTDT and TM patients and positively correlated with serum liver fibrotic markers and liver function
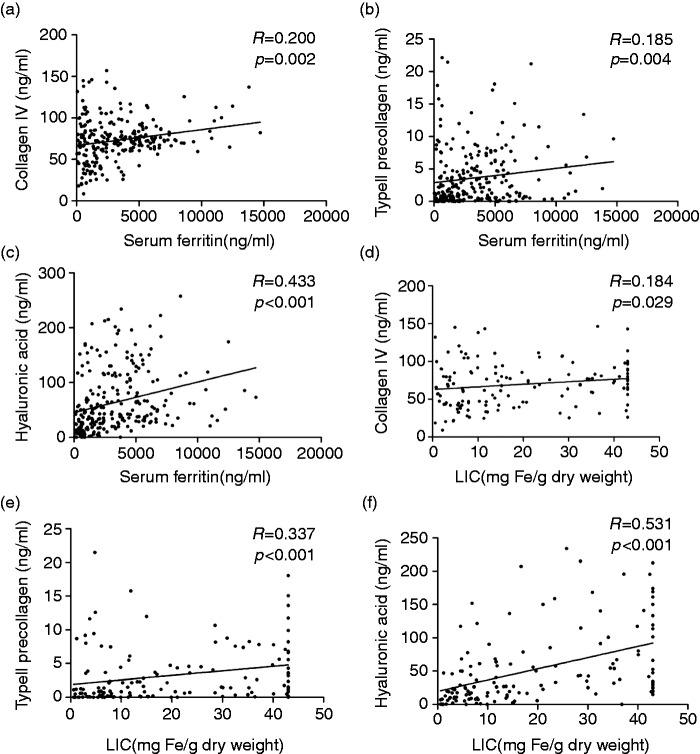
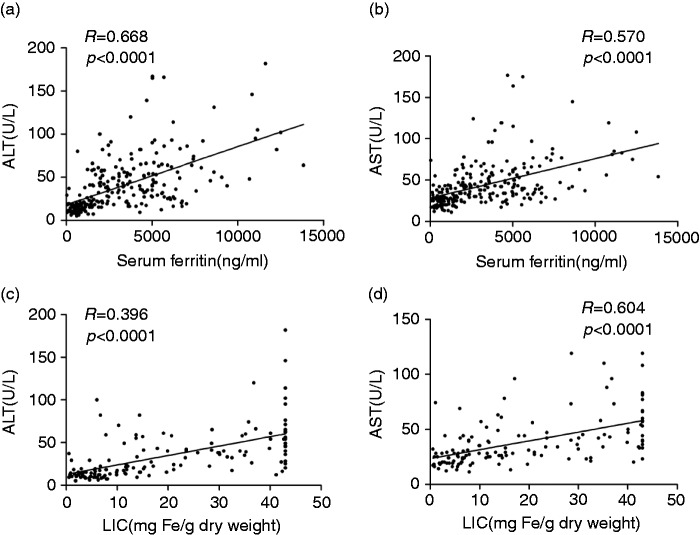
In NTDT patients, the median SF was 1085 ng/ml (range, 36–9704 ng/ml); 94 patients (89.5%) had SF greater than 300 ng/ml, while 55 (52.4%) had SF greater than 1000 ng/ml. In TM patients, the median SF was 4519 ng/ml (range, 121–14,724 ng/ml); 136 patients (97.8%) had SF greater than 300 ng/ml, while 106 (76.2%) had SF greater than 2500 ng/ml. SF showed a significantly positive correlation with serum C IV (R = 0.194, p = 0.002), PIIINP (R = 0.183, p = 0.004) and HA (R = 0.435, p < 0.001; Figures 3(a)–(c)). In addition, SF was also positively correlated with ALT (R = 0.681, p < 0.001) and AST (R = 0.565, p < 0.001; Figures 4(a) and (b)). Overall, SF was significantly elevated in NTDT patients and further elevated in TM patients as compared with the controls. Moreover, SF was positively correlated to serum fibrotic biomarkers and liver function.
Figure 3.
Serum ferritin levels and liver iron concentration was elevated and positively correlated with levels of serum liver fibrotic markers in NTDT and TM patients.
NTDT: non-transfusion-dependent thalassemia; TM: thalassemia major; LIC: liver iron concentration, mg Fe/g dry weight (dw).
Figure 4.
Liver iron concentration was elevated and positively correlated with levels of liver functional enzymes in NTDT and TM patients.
NTDT: non-transfusion-dependent thalassemia; TM: thalassemia major; ALT: alanine aminotransferase; AST: aspartate aminotransferase.
LIC was elevated and positively correlated with serum liver fibrotic markers and liver function in NTDT and TM patients
LIC was measured in 140 patients, including 105 NTDT patients and 35 (25%) TM patients. Among NTDT patients, median LIC was 9.9 mg Fe/g dry weight (range, 0.6–43 mg Fe/g dw); 60 patients (57.1%) had LIC of at least 7 mg Fe/g dw, while 31 (29.5%) had LIC greater than 15 mg Fe/g dw. Among TM patients, the median LIC was 42.2 mg Fe/g dw (range, 8.4–43 mg Fe/g dw), and all but one patient had LIC greater than 15 mg Fe/g dw. Moreover, LIC positively correlated with serum levels of C IV (R = 0.175, p = 0.039), PIIINP (R = 0.334, p < 0.001) and HA (R = 0.528, p < 0.001; Figures 3(d)–(f)). Moreover, LIC was also positively related to ALT (R = 0.693, p < 0.001) and AST (R = 0.604, p < 0.001; Figures 4(c) and (d)). Taken together, LIC is significantly elevated in NTDT and TM patients and positively correlated with serum levels of fibrotic biomarkers and liver function.
Hepatomegaly, splenomegaly or splenectomized (HSS) affects iron overload, serum fibrotic marker levels and liver function in NTDT patients but not in TM patients
As shown above, serum fibrotic markers were elevated in NTDT patients as compared with controls and further elevated in TM patients. Thalassemia patients may develop multiple organ damage including HSS and liver fibrosis. Then, whether three serum biomarkers, SF and LCI were further elevated in patients with HSS as compared with those without HSS (N-HSS) was further investigated (Table 3). Results showed that NTDT patients with HSS had higher levels of SF, LIC, PIIINP and HA than did N-HSS NTDT patients (p < 0.05). However, in TM patients, there were no differences in these parameters between two groups (p > 0.05). These results indicate that SF, LIC and serum fibrotic biomarkers including PIINP and HA are likely early biomarkers predicting the severity and progression of thalassemia patients with NTDT.
Table 3.
The comparison of iron overload, liver fibrosis and liver function in HSS and N-HSS (median, range)
| NTDT |
TM |
|||||
|---|---|---|---|---|---|---|
| HSS (n = 83) | N-HSS (n = 22) | p value | HSS (n = 65) | N-HSS (n = 74) | p value | |
| SF | 1283 (188–19,704) | 601 (36–1590) | p < 0.05 | 4538 (559–12,500) | 4305 (121–14,724) | p > 0.05 |
| LIC | 11.4 (0.8–43.0) | 5.8 (0.6–20.7) | p < 0.05 | 41.2 (8.4–43.0) | 42.4 (21.1–43.0) | p > 0.05 |
| C IV | 60 (9–25) | 63 (18–138) | p > 0.05 | 74 (52–126) | 73 (56–157) | p > 0.05 |
| PIIINP | 1.3 (0.0–53.4) | 0.3 (0.0–8.7) | p < 0.05 | 3.3 (0.0–41.6) | 2.9 (0.0–94.7) | p > 0.05 |
| HA | 25 (0–679) | 11 (0–137) | p < 0.05 | 64 (10–258) | 67 (8–864) | p > 0.05 |
| ALT | 19 (5–120) | 15 (9–58) | p > 0.05 | 42 (14–282) | 37 (8–232) | p > 0.05 |
| AST | 28 (11–119) | 26 (12–74) | p > 0.05 | 42 (18–177) | 42 (17–175) | p > 0.05 |
NTDT: non-transfusion-dependent thalassemia; TM: thalassemia major; SF: ferritin, ng/ml; LIC: liver iron overload, mg Fe/g dw; HA: hyaluronic acid, ng/ml; PIIINP: precollagen type III, ng/ml; C IV: collagen type IV, ng/ml; HSS: patients with history of hepatomegaly, splenomegaly or splenectomized; N-HSS: patients who without history of hepatomegaly, splenomegaly or splenectomized; ALT: alanine aminotransferase; AST: aspartate aminotransferase.
Discussion
In this large cohort study, 244 Chinese thalassemia patients and 120 controls were recruited, and results demonstrated that 1) the serum levels of liver fibrotic markers C IV, PIIINP and HA were significantly elevated in NTDT and further increased in TM as compared with controls; 2) serum levels of C IV, PIIINP and HA are positively correlated with SF, LIC and liver function. Taken together, our findings provide evidence that monitoring SF and LIC is a potentially sensitive method for the prediction of iron overload-related fibrosis in thalassemia patients.
Iron overload, which occurs in a substantial proportion of thalassemia patients because of increases in gastrointestinal iron absorption30–32 and/or repeated transfusions,33 reflects iron accumulation in the heart, liver and endocrine glands, and can lead to cardiac dysfunction, endocrine abnormalities and liver fibrosis.34–38 The gold standard for assessing liver fibrosis is liver biopsy, which is costly and may cause serious complications. Most NTDT patients in our study showed iron overload, even though the median amount of blood transfused was only 1.5 U before enrollment. The iron overload in NTDT patients is most likely due to increased intestinal absorption. Among TM patients, the median amount of blood transfused was 89.0 U prior to enrollment. The iron overload in TM patients is likely due to repeated blood transfusion and inadequate iron chelation therapy. TM patients had more severe iron overload than did NTDT patients, as evidenced by the baseline median serum ferritin level of 4519 ng/ml in TM patients and 1085 ng/ml in NTDT patients. This severe iron overload in TM patients was consistent with our previous results and supports our previous findings that Chinese TM patients have poorer iron chelation therapy than do Western patients, leading to iron overload.39,40
Liver is the major organ for iron storage and metabolism and can be greatly affected by iron-induced toxicity. Liver iron accumulation can cause lipid peroxidation, which compromises the organelle integrity, thereby contributing to hepatocyte necrosis and apoptosis, and eventually leads to hepatic fibrogenesis.41,42 Iron can also facilitate the differentiation of hepatic stellate cells into collagen-producing myofibroblasts, which directly causes fibrosis.43,44 Liver fibrosis results most often from iron overload or hepatitis B or HCV infection.6,7,13 In patients with hepatitis virus infection, several studies have confirmed the role of serum biomarkers for assessing and predicting liver fibrosis. Whether these serum markers correlate with iron overload in thalassemia patients is still poorly understood. In this study, any subject who had a history of hepatitis B or C virus infection was excluded, and C IV, PIIINP and HA were employed as serum markers of liver fibrosis. Together with YKL-40 and laminin, these macromolecules are perhaps the most extensively studied and most clinically relevant components of the extracellular matrix (ECM).3–5,14,15,45–47 C IV mainly localizes in the basement membrane of the liver, particularly in the perivascular and peribiliary regions. Serum C IV increases as the basement membrane is destroyed during fibrogenesis. PIIINP is cleaved from the amino-terminal end of procollagen III during its secretion by fibroblasts; serum PIIINP increases rapidly in the early phase of hepatic fibrosis but slowly elevates at later stages. Thus, PIIINP may be used as an early indicator of liver fibrosis. HA, a principal component of ECM, is a glycosaminoglycan produced by the hepatic stellate cells and degraded by the sinusoidal endothelial cells.48 Thus, elevated serum HA may reflect endothelial dysfunction associated with fibrosis progression. However, none of these serum markers is perfect for the evaluation of liver fibrosis. Thus, it is possible that a combination of these markers can serve as a highly sensitive indicator of liver fibrogenesis.
Findings in the present study confirmed our previous findings that serum liver fibrotic biomarkers were significantly elevated in TM patients.17 In addition, a correlation of iron overload with serum levels of HA and PIIINP was consistent with the finding from a previous study with small sample size.17,18 However, no positive correlation of serum HA with SF and LIC was observed in earlier reports.49,50 One of the potential explanations is that earlier studies included 20% and 68% of patients who were positive for hepatitis C viral RNA in which results demonstrated hepatitis C viral infection contributed to the increased serum fibrotic markers. Since the individuals in the present study were negative for hepatitis B and HCV, our findings indicate that liver iron overload is another major cause of liver fibrosis in thalassemia patients.
In our study, both NTDT and TM patients had higher serum levels of ALT or AST as compared with healthy controls, which is related to liver iron overload even in the absence of hepatitis. This finding was in agreement with previous results.51,52 Ineffective hematopoiesis of the bone marrow is often present in thalassemia patients and may cause extramedullary hematopoiesis, resulting in clinical hepatomegaly and splenomegaly. Under this condition, splenectomy is the treatment of choice. After splenectomy, the volume of blood transfused reduces, the half-life of red blood cells increases and the symptoms of anemia are also improved. In our study, the NTDT patients with HSS had significant higher SF, LIC and serum fibrotic markers as compared with those without HSS. In contrast, no significant difference was observed in TM patients with or without HSS. Because the disease in TM patients was much more severe than in NTDT patients, the serum levels of fibrotic biomarkers, SF and LIC in TM patients are elevated to higher levels than in NTDT patients. These biomarkers in TM patients without HSS already reached the levels shown in TM with HSS. In contrast, in NTDT patients without HSS, these biomarkers were still at low levels and thus they further increased during the progression from N-HSS to HSS in NTDT patients. Overall, these findings strongly support our conclusion that SF level and LIC are potential clinical parameters for early prediction of liver fibrosis associated with liver iron overload in thalassemia patients.
Our results demonstrate in a large cohort of patients without hepatitis virus infection that the serum levels of three well-established markers of liver fibrosis are significantly increased in NTDT patients and further increased in TM patients, and they correlate well both with SF level and LIC. Detection of serum biomarkers of liver fibrosis is less invasive and less risky than the standard liver biopsy. In addition, it may be performed repeatedly, whereas results from biopsy reflect only a static state of the tissues. Thus, this detection is crucially important for the evaluation of liver fibrosis, especially in the reversible phase, before progressing to irreversible cirrhosis.53,54
Acknowledgments
We thank the technical staff in the Laboratory Centre of Guangxi Medical University and Department of Clinical Laboratory Center in the First Affiliated Hospital of Guangxi Medical University.
Funding
The work was supported by the Guangxi Natural Science Foundation (No. 2011GXNSFD018033), Guangxi Scientific and Technological Research Foundation (No. 1140003A-5), the Scientific Research Fund of Guangxi Education Department (No. 2013YB044), and the National Natural Science Foundation of China (No. 81260090, 81360085, and 81460025). This work was also supported by National Institute of Health Grants HL113574 (to Y.X.), DK083559 (to Y.X), HL119549 (Y.X.), and American Heart Association Grant 12IRG9150001 (to Y.X.).
Conflict of interest
None declared.
References
- 1.Orkin SH, Nathan DG. The thalassemias. N Engl J Med 1976; 295: 710–714. [DOI] [PubMed] [Google Scholar]
- 2.Higgs DR, Engel JD, Stamatoyannopoulos G. Thalassaemia. Lancet 2012; 379: 373–383. [DOI] [PubMed] [Google Scholar]
- 3.Vento S, Cainelli F, Cesario F. Infections and thalassaemia. Lancet Infect Dis 2006; 6: 226–233. [DOI] [PubMed] [Google Scholar]
- 4.Elalfy MS, Esmat G, Matter RM, et al. Liver fibrosis in young Egyptian beta-thalassemia major patients: Relation to hepatitis C virus and compliance with chelation. Ann Hepatol 2013; 12: 54–61. [PubMed] [Google Scholar]
- 5.Li CK, Chik KW, Lam CW, et al. Liver disease in transfusion dependent thalassaemia major. Arch Dis Child 2002; 86: 344–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Friedman SL. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J Biol Chem 2000; 275: 2247–2250. [DOI] [PubMed] [Google Scholar]
- 7.Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology 2008; 134: 1655–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bravo AA, Sheth SG, Chopra S. Liver biopsy. N Engl J Med 2001; 344: 495–500. [DOI] [PubMed] [Google Scholar]
- 9.Dienstag JL. The role of liver biopsy in chronic hepatitis C. Hepatology 2002; 36(5 Suppl. 1): S152–S160. [DOI] [PubMed] [Google Scholar]
- 10.Enomoto M, Morikawa H, Tamori A, et al. Noninvasive assessment of liver fibrosis in patients with chronic hepatitis B. World J Gastroenterol 2014; 20: 12031–12038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McHutchison JG, Blatt LM, de Medina M, et al. Measurement of serum hyaluronic acid in patients with chronic hepatitis C and its relationship to liver histology. Consensus Interferon Study Group. J Gastroenterol Hepatol 2000; 15: 945–951. [DOI] [PubMed] [Google Scholar]
- 12.Wong VS, Hughes V, Trull A, et al. Serum hyaluronic acid is a useful marker of liver fibrosis in chronic hepatitis C virus infection. J Viral Hepat 1998; 5: 187–192. [DOI] [PubMed] [Google Scholar]
- 13.Schiavon Lde L, Narciso-Schiavon JL, de Carvalho-Filho RJ. Non-invasive diagnosis of liver fibrosis in chronic hepatitis C. World J Gastroenterol 2014; 20: 2854–2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hirayama C, Suzuki H, Takada A, et al. Serum type IV collagen in various liver diseases in comparison with serum 7S collagen, laminin, and type III procollagen peptide. J Gastroenterol 1996; 31: 242–248. [DOI] [PubMed] [Google Scholar]
- 15.Nøjgaard C, Johansen JS, Christensen E, et al. Serum levels of YKL-40 and PIIINP as prognostic markers in patients with alcoholic liver disease. J Hepatol 2003; 39: 179–186. [DOI] [PubMed] [Google Scholar]
- 16.Stickel F, Poeschl G, Schuppan D, et al. Serum hyaluronate correlates with histological progression in alcoholic liver disease. Eur J Gastroenterol Hepatol 2003; 15: 945–950. [DOI] [PubMed] [Google Scholar]
- 17.Xu HG, Fang JP, Huang SL, et al. Diagnostic values of serum levels of HA, PC III, C IV and LN to the liver fibrosis in children with beta-thalassemia major [article in Chinese]. Zhonghua Er Ke Za Zhi [Chinese Journal of Pediatrics] 2003; 41: 603–606. [PubMed] [Google Scholar]
- 18.Xu LH, Fang JP, Xu HG, et al. Evaluation of hepatic iron overload in Chinese children with beta-thalassemia major. Pediatr Hematol Oncol 2011; 28: 702–707. [DOI] [PubMed] [Google Scholar]
- 19.Hoffbrand AV, Taher A, Cappellini MD. How I treat transfusional iron overload. Blood 2012; 120: 3657–3669. [DOI] [PubMed] [Google Scholar]
- 20.Taher A, Vichinsky E, Musallam K, et al. CHAPTER 1 INTRODUCTION. In: Weatherall D. (eds). Guidelines for the management of non transfusion dependent thalassaemia (NTDT), Nicosia, Cyprus: Thalassaemia International Federation, 2013. [PubMed] [Google Scholar]
- 21.Galanello R, Origa R. Beta-thalassemia. Orphanet J Rare Dis 2010; 5: 11–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aessopos A, Farmakis D, Hatziliami A, et al. Cardiac status in well-treated patients with thalassemia major. Eur J Haematol 2004; 73: 359–366. [DOI] [PubMed] [Google Scholar]
- 23.Cappellini MD, Cohen A, Eleftheriou A. Guidelines for the clinical management of thalassaemia, 2nd ed Nicosia, Cyprus: Thalassaemia International Federation, 2008. [PubMed] [Google Scholar]
- 24.Wood JC. Magnetic resonance imaging measurement of iron overload. Curr Opin Hematol 2007; 14: 183–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taher A, El Rassi F, Isma’eel H, et al. Correlation of liver iron concentration determined by R2 magnetic resonance imaging with serum ferritin in patients with thalassemia intermedia. Haematologica 2008; 93: 1584–1586. [DOI] [PubMed] [Google Scholar]
- 26.Taher AT, Musallam KM, Wood JC, et al. Magnetic resonance evaluation of hepatic and myocardial iron deposition in transfusion-independent thalassemia intermedia compared to regularly transfused thalassemia major patients. Am J Hematol 2010; 85: 288–290. [DOI] [PubMed] [Google Scholar]
- 27.St Pierre TG, Clark PR, Chua-Anusorn W, et al. Noninvasive measurement and imaging of liver iron concentrations using proton magnetic resonance. Blood 2005; 105: 855–861. [DOI] [PubMed] [Google Scholar]
- 28.St Pierre TG, Clark PR, Chua-Anusorn W. Measurement and mapping of liver iron concentrations using magnetic resonance imaging. Ann N Y Acad Sci 2005; 1054: 379–385. [DOI] [PubMed] [Google Scholar]
- 29.Weatherall DJ. The definition and epidemiology of non-transfusion-dependent thalassemia. Blood Rev 2012; 26(Suppl 1): S3–S6. [DOI] [PubMed] [Google Scholar]
- 30.Pippard MJ, Callender ST, Warner GT, et al. Iron absorption and loading in beta-thalassaemia intermedia. Lancet 1979; 2: 819–821. [DOI] [PubMed] [Google Scholar]
- 31.Pootrakul P, Kitcharoen K, Yansukon P, et al. The effect of erythroid hyperplasia on iron balance. Blood 1988; 71: 1124–1129. [PubMed] [Google Scholar]
- 32.Gardenghi S, Grady RW, Rivella S. Anemia, ineffective erythropoiesis, and hepcidin: Interacting factors in abnormal iron metabolism leading to iron overload in beta-thalassemia. Hematol Oncol Clin North Am 2010; 24: 1089–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Porter JB. Pathophysiology of transfusional iron overload: Contrasting patterns in thalassemia major and sickle cell disease. Hemoglobin 2009; 33(Suppl. 1): S37–S45. [DOI] [PubMed] [Google Scholar]
- 34.Jensen CE, Tuck SM, Old J, et al. Incidence of endocrine complications and clinical disease severity related to genotype analysis and iron overload in patients with beta-thalassaemia. Eur J Haematol 1997; 59: 76–81. [DOI] [PubMed] [Google Scholar]
- 35.Lekawanvijit S, Chattipakorn N. Iron overload thalassemic cardiomyopathy: Iron status assessment and mechanisms of mechanical and electrical disturbance due to iron toxicity. Can J Cardiol 2009; 25: 213–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim MK, Lee JW, Baek KH, et al. Endocrinopathies in transfusion-associated iron overload. Clin Endocrinol (Oxf) 2013; 78: 271–277. [DOI] [PubMed] [Google Scholar]
- 37.Kew MC. Hepatic iron overload and hepatocellular carcinoma. Liver Cancer 2014; 3: 31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rossi F, Perrotta S, Bellini G, et al. Iron overload causes osteoporosis in thalassemia major patients through interaction with transient receptor potential vanilloid type 1 (TRPV1) channels. Haematologica 2014; 99: 1876–1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lai YR, Liu RR, Li CF, et al. Efficacy of Deferasirox for the treatment of iron overload in Chinese thalassaemia major patients: Results from a prospective, open-label, multicentre clinical trial. Transfus Med 2013; 23: 389–396. [DOI] [PubMed] [Google Scholar]
- 40.Yang G, Liu R, Peng P, et al. How early can myocardial iron overload occur in beta thalassemia major? PloS One 2014; 9: e85379–e85379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bacon BR, Britton RS. The pathology of hepatic iron overload: A free radical-mediated process? Hepatology 1990; 11: 127–137. [DOI] [PubMed] [Google Scholar]
- 42.Bacon BR, Tavill AS, Brittenham GM, et al. Hepatic lipid peroxidation in vivo in rats with chronic iron overload. J Clin Invest 1983; 71: 429–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yokoi Y, Namihisa T, Matsuzaki K, et al. Distribution of Ito cells in experimental hepatic fibrosis. Liver 1988; 8: 48–52. [DOI] [PubMed] [Google Scholar]
- 44.Friedman SL. Seminars in medicine of the Beth Israel Hospital, Boston. The cellular basis of hepatic fibrosis. Mechanisms and treatment strategies. N Engl J Med 1993; 328: 1828–1835. [DOI] [PubMed] [Google Scholar]
- 45.Gabrielli GB, Corrocher R. Hepatic fibrosis and its serum markers: A review. Dig Dis 1991; 9: 303–316. [DOI] [PubMed] [Google Scholar]
- 46.Maruyama K, Okazaki I, Takagi T, et al. Formation and degradation of basement membrane collagen. Alcohol Alcohol Suppl 1991; 1: 369–374. [PubMed] [Google Scholar]
- 47.Rosenberg WM, Voelker M, Thiel R, et al. Serum markers detect the presence of liver fibrosis: A cohort study. Gastroenterology 2004; 127: 1704–1713. [DOI] [PubMed] [Google Scholar]
- 48.McGary CT, Raja RH, Weigel PH. Endocytosis of hyaluronic acid by rat liver endothelial cells. Evidence for receptor recycling. Biochem J 1989; 257: 875–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Papastamataki M, Delaporta P, Premetis E, et al. Evaluation of liver fibrosis in patients with thalassemia: The important role of hyaluronic acid. Blood Cells Mol Dis 2010; 45: 215–218. [DOI] [PubMed] [Google Scholar]
- 50.El-Shabrawi MH, Zein El Abedin MY, Omar N, et al. Predictive accuracy of serum hyaluronic acid as a non-invasive marker of fibrosis in a cohort of multi-transfused Egyptian children with beta-thalassaemia major. Arab J Gastroenterol 2012; 13: 45–48. [DOI] [PubMed] [Google Scholar]
- 51.Soliman A, Yassin M, Al Yafei F, et al. Longitudinal study on liver functions in patients with thalassemia major before and after deferasirox (DFX) therapy. Mediterr J Hematology Infect Dis 2014; 6: e2014025–e2014025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kountouras D, Tsagarakis NJ, Fatourou E, et al. Liver disease in adult transfusion-dependent beta-thalassaemic patients: Investigating the role of iron overload and chronic HCV infection. Liver Int 2013; 33: 420–427. [DOI] [PubMed] [Google Scholar]
- 53.Manning DS, Afdhal NH. Diagnosis and quantitation of fibrosis. Gastroenterology 2008; 134: 1670–1681. [DOI] [PubMed] [Google Scholar]
- 54.Friedman SL. Liver fibrosis—from bench to bedside. J Hepatol 2003; 38(Suppl. 1): S38–S53. [DOI] [PubMed] [Google Scholar]