Abstract
Background
After liver transplantation (LT), there are liver-related, infectious and cardiovascular complications that contribute to reduced graft survival. These conditions are associated with an increase in the Von Willebrand factor antigen (VWF-Ag), which was previously correlated with survival in cirrhotic patients.
Objective
Evaluate VWF-Ag as a predictive marker of re-transplantation-free survival in patients after LT.
Methods
We measured VWF-Ag in patients after first LT and then followed them prospectively with regard to the primary endpoint, namely re-transplantation-free survival.
Results
There were 6 out of 80 patients who died or received re-LT during follow-up. In these patients, the median VWF-Ag was 510.6%, which was significantly higher (p = 0.001) than in the patients who were alive at the end of follow-up (with a median VWF-Ag = 186.8%). At a cut-off of 286.8%, VWF-Ag was significantly correlated with re-transplantation-free survival (p < 0.001). VWF-Ag was independently associated with re-transplantation-free survival in a multivariate analysis; as was alkaline phosphatase (ALP), but not the model of end-stage liver disease (MELD) score, donor age, nor cold ischemia time. A score combining VWF-Ag and ALP showed an impressive capability in the receiver operating characteristic (ROC) analysis (with area under the curve (AUC) = 0.958) to distinguish between patients with regard to the primary endpoint.
Conclusions
VWF-Ag is a non-invasive marker that can predict outcome in patients after LT. Its diagnostic performance increased when combined with ALP in a newly developed scoring system.
Keywords: Alkaline phosphatase, diagnostic tests, liver transplantation, markers, outcome prediction, scoring system, survival, transplantation, Von Willebrand factor
Introduction
Liver transplantation (LT) is a successful treatment option for end-stage liver disease (ESLD) of various etiologies. Graft survival rates reach approximately 85% at 1 year and 70% at 5 years post-transplantation.1 Despite the availability of outcome-predicting scores such as the donor risk index, or the product of donor age and the preoperative model of end-stage liver disease (D-MELD),2,3 which were both designed to assess the risk and outcome of transplantation at the time of organ transplantation, no validated score exists to predict outcome in stable liver transplant recipients. Such a score could be of interest to identify the patients at risk of graft failure and to optimize follow-up in these patients.
Mortality after LT is mainly due to liver-related causes, infections, cardiovascular diseases and malignancy.4 In all of these conditions, increased levels of Von Willebrand factor (VWF) have been described.5–7 VWF is released from endothelial cells and is an established marker of endothelial cell dysfunction.8 It is commonly elevated in ESLD9 and might not only compensate for reduced platelet counts during primary hemostasis in patients with cirrhosis,10,11 but also predict the outcome of these patients.6 The reasons underlying VWF elevation in ESLD are not yet fully understood and several mechanisms might contribute:
First, cirrhosis and especially portal hypertension are accompanied by endothelial dysfunction, resulting in VWF increase;9
Second, increased VWF synthesis within the cirrhotic liver was previously reported;12
Besides this, VWF is a marker of arteriosclerosis, and it is elevated in the presence of various cardiovascular risk factors. Consequently, it acts as a clinical marker for prognosis in cardiovascular diseases.7
Based on the aforementioned data, we hypothesized that increased levels of VWF after LT are associated with poorer outcomes; therefore, we conducted this study to prospectively test our hypothesis in patients after their first LT.
Materials and methods
Study protocol including inclusion and exclusion criteria
Patients who were seen after LT in the outpatient clinic of the Department of Internal Medicine IV at the University Hospital Heidelberg in Heidelberg, Germany, between November 2012 and August 2013 were screened for inclusion in the primary study cohort. To be eligible, patients had to be at least 18 years of age at time of inclusion, and only the patients after first LT were eligible, while those who had already undergone re-LT were excluded.
At the time of inclusion into the study, the level of VWF-Ag was measured. Therefore, citrated blood was collected and then analyzed in the central laboratory of the Heidelberg University Hospital. The VWF-Ag levels were determined by a turbidimetric assay on a Siemens BCS XP system (Siemens Healthcare, Germany) by applying appropriate reagents (VWF-Ag Kit, Siemens Healthcare, Munich, Germany).
After inclusion, patients were then prospectively followed with regard to the study’s primary endpoint, namely re-transplantation-free survival. Follow-up was performed during the patients’ routine visits in our outpatient department and by gathering information on the patients’ status, either by phone or from the patients’ general practitioner. The final follow-up was performed between November 2014 and January 2015. The secondary endpoint was the re-transplantation-free survival rate after 1 year.
In addition to VWF-Ag, we assessed alanine transaminase (ALT), aspartate transaminase (AST), alkaline phosphatase (ALP), gamma-glutamyltransferase (gGT), total bilirubin, albumin, international normalized ratio (INR), C-reactive protein (CRP) and leukocyte count; and calculated the MELD score. We included information on basic demographic and health characteristics.
To validate the results obtained in the primary study cohort, we included an independent cohort of patients awaiting their first LT. A local study database was searched to identify patients awaiting first LT and a retrospective chart review was performed to identify measurements of VWF-Ag in these patients. Outcome parameters and basic demographic and health characteristics for these patients were as well obtained from the database.
All patients provided written informed consent prior to inclusion in the study. The study was previously approved by the local ethics committee of the University of Heidelberg and was conducted in accordance with the Declaration of Helsinki.
Description of the final primary study cohort
Of the 104 initially screened patients, 10 were not eligible for inclusion because they had already undergone re-transplantation and one was not eligible because he was too young. A further 13 patients were excluded because VWF-Ag was not measured. The 80 patients who were ultimately included in our study had a mean age of 56.3 years (±9.4 years) and 24 (30.0%) of them were of female gender. There were 23 patients (28.7%) with blood group O, 20 patients (25.0%) with blood group A, 13 patients (16.3%) with blood group B and 6 patients (7.5%) with blood group AB. For 18 (22.5%) patients, we were unable to retrieve information on their blood group. The etiology of liver cirrhosis prior to LT was: alcoholic in 30 patients (37.5%), viral hepatitis in 29 patients (36.3%), primary sclerosing cholangitis in 9 patients (11.3%), cryptogenic in 3 patients (3.8%) and other causes of cirrhosis in 4 patients (5.0%). Five patients (6.3%) did not have cirrhosis at the time of LT, including two patients with cystic liver disease and one each with acute liver failure, Budd-Chiari syndrome and hepatocellular carcinoma (HCC) in a non-cirrhotic liver. Overall, HCC was present in 29 patients (36.3%) prior to LT. A total of three patients (3.8%) were on the waiting list for re-transplantation at the time of vWF-Ag measurement.
Statistical analysis
Data are given as numbers with percentage for categorical data, median with interquartile range (IQR) for non-normally distributed interval-scaled data, and mean with SD for normally distributed data. We used the Chi square test or Fisher’s exact test, Mann-Whitney U test, the student t-test and Pearson’s correlation, as appropriate. The area under the curve (AUC) was calculated for a receiver operating characteristic (ROC) analysis, and Youden’s index was used to determine the optimal cut-off. Sensitivity, specificity, positive and negative predictive value, and diagnostic accuracy were calculated from a contingency table. Actuarial re-transplantation-free survival was estimated using Kaplan-Meier estimates. Differences between the actuarial estimates were tested using the log-rank test. Univariate and multivariate analyses were performed using a Cox regression model. Variables included in the model were: age, gender, VWF-Ag, MELD score, ALT, ALP, CRP; as well as the graft-related parameters of donor age, cold ischemia time and time since LT. All variables with a p value <0.05 in the univariate analysis were included in the multivariate model. We considered that p values <0.05 were statistically significant for all analyses. We performed the analyses using IBM SPSS Statistics, version 21 (IBM Corp., Armonk, NY, USA).
Results
VWF-Ag levels in patients after liver transplantation
The 80 study patients had a median VWF-Ag level of 200.6% (IQR = 141.5–287.9). VWF-Ag was measured 35 months (IQR = 8–89) after LT. Patients had a median MELD score of 8 (IQR = 7–11). Neither gender nor age influenced VWF-Ag levels (p > 0.05); however, a non-significant trend towards lower levels of VWF-Ag in blood group O (median = 194.3%; IQR = 115.4–270.9), compared to patients with blood groups that were non-O (median = 211.1%; IQR = 144.1–344.3) was found (p = 0.135). Levels of VWF-Ag correlated with CRP (r = 0.431; p < 0.001), but not with the leukocyte count (p = 0.762). In 26 of the patients (32.5%) VWF-Ag was measured within the first year after LT and their median VWF-Ag was 208.9% (IQR = 146.2–353.0%).
Outcome of the included patients
The primary study endpoint of re-transplantation-free survival at the end of the study was reached by 74 patients (92.5%). Four patients (5.0%) underwent re-transplantation and two patients (2.5%) died during follow-up. The median time of follow-up in the 74 patients who were alive at the end of the study was 86 weeks (IQR = 82–102), and it was 42 weeks (IQR = 16–57) in the group of patients who died or underwent re-transplantation. Reasons for re-transplantation were: ischemic cholangiopathy in two patients, repeat cirrhosis due to chronic rejection in one patient and hepatitis C reinfection with decompensation of liver function during interferon therapy in one patient. The two patients died because of liver insufficiency due to chronic rejection and because of sepsis.
The median VWF-Ag was significantly lower in the 74 patients who were alive with the original transplant at the end of the study (186.8%; IQR = 139.0–272.1), compared to those six patients who underwent re-transplantation or died (median = 510.6%; IQR = 301.8–531.3; p = 0.001). A detailed comparison of patients, based upon the primary outcome parameter, is given in Table 1.
Table 1.
Patients’ characteristics. Comparison of patients based on the main primary outcome parameter of re-transplantation-free survival. Data is given as mean (±SD), median (IQR) or number (%), as appropriate
| Alive | Dead/re-LT | p | |
|---|---|---|---|
| Patients, n | 74 (92.5%) | 6 (7.5%) | |
| Gender, female | 21 (28.4%) | 3 (50.0) | 0.332 |
| Age, years | 56.6 (±9.3) | 52.7 (±10.0) | 0.362 |
| Blood group, n | 0.630 | ||
| O | 21 (36.8%) | 2 (40.0%) | |
| A | 19 (33.3%) | 1 (20.0%) | |
| B | 11 (19.3%) | 2 (40.0%) | |
| AB | 6 (10.5%) | 0 | |
| Time since LT, months | 39 (8–90) | 20 (6–78) | 0.482 |
| HCC, n | 27 (36.5%) | 2 (33.3%) | 1.000 |
| Donor age, years | 58 (44–66) | 68 (55–80) | 0.124 |
| Cold ischemia time, h | 11.0 (9.5–12.5) | 10.0 (8.5–11.5) | 0.214 |
| Awaiting re-transplantation | 2 (2.7%) | 1(16.7%) | 0.211 |
| VWF-Ag, % | 186.8 (130.0–272.1) | 510.6 (301.8–531.3) | 0.001 |
| AST, U/l | 21 (16–37) | 83 (26–361) | 0.003 |
| ALT, U/l | 25 (15–41) | 52 (13–222) | 0.204 |
| ALP, U/l | 104 (76–466) | 301 (195–533) | 0.001 |
| gGT, U/l | 39 (20–91) | 151 (62–509) | 0.028 |
| Total bilirubin, mg/dl | 0.6 (0.4–1.0) | 2.2 (1.0–19.4) | 0.006 |
| Albumin, g/l | 43.3 (40.5–45.6) | 35.7 (29.0–39.2) | 0.008 |
| INR | 1.00 (0.96–1.04) | 1.08 (1.00–1.30) | 0.074 |
| Creatinine, mg/dl | 1.06 (0.83–1.46) | 0.97 (0.74–1.20) | 0.324 |
| CRP, mg/dl | 2.2 (0.0–8.0) | 19.3 (10.4–34.6) | 0.002 |
| Leukocyte count, /nl | 5.72 (3.72–7.09) | 7.39 (5.49–8.77) | 0.093 |
ALP: alkaline phosphatase; ALT: alanine transaminase; AST: aspartate transaminase; CRP: C-reactive protein; gGT: gamma-glutamyltransferase; INR: international normalized ratio; IQR: interquartile ratio; HCC: hepatocellular carcinoma; LT: liver transplant; re-LT: repeat liver transplant; U/l: units per liter; VWF-Ag: Von Willebrand factor antigen
With regard to the secondary endpoint, namely 1-year re-transplantation-free survival, 76 patients (95.0%) were alive with the original transplant after 1 year. Three patients had undergone re-transplantation and one patient had died.
Survival depends on VWF-Ag levels
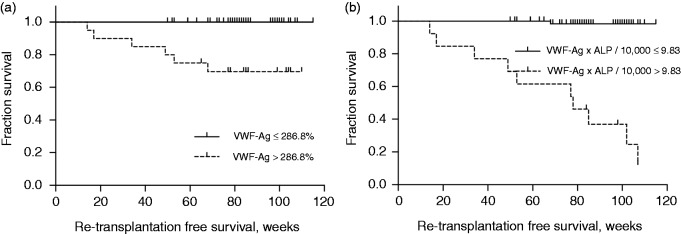
ROC analysis of VWF-Ag levels revealed an AUC for prediction of the primary endpoint of 0.914 (95% CI 0.842–0.987; p = 0.001), as shown in Figure 1. The optimal cut-off value for VWF-Ag was 286.8%. This cut-off yielded 100% sensitivity and 81.1% specificity. The positive predictive value was 30%, the negative predictive value was 100.0% and the diagnostic accuracy was 82.5%. Survival was significantly longer in patients with a VWF-Ag below this cut-off, compared to those with a higher VWF-Ag (p < 0.001), as seen in Figure 2(a)). A comparison of patients based on the optimal cut-off value is shown in Table 2.
Figure 1.
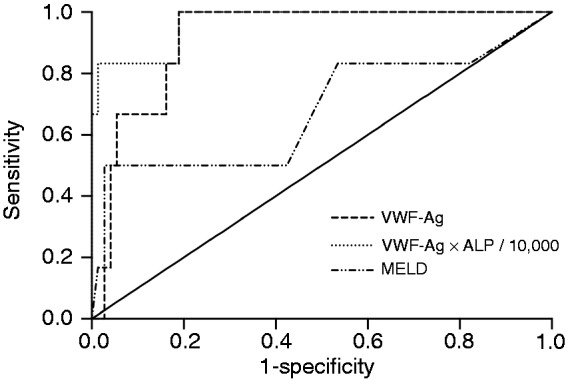
ROC analysis of VWF-Ag.
VWF-Ag levels significantly predicted survival among the study participants, with an AUC of 0.914 in the ROC analysis. VWF-Ag and ALP proved to significantly predict the outcome of the study patients in multivariate Cox regression analysis and the ROC analysis of a newly developed score (VWF-Ag × ALP/10,000) showed a high AUC as well, which was clearly superior to that of the MELD score.
ALP: alkaline phosphatase; AUC: area under the curve; MELD: model of end-stage liver disease; ROC: receiver operating characteristic; VWF-Ag: Von Willebrand factor antigen
Figure 2.
VWF-Ag and a newly developed score containing VWF-Ag and ALP predict retransplantation-free survival.
(a) The optimal cut-off for VWF-Ag as determined by Youden’s index was 286.8%, which significantly correlated with re-transplantation-free survival after the first liver transplantation, in the Kaplan-Meier analysis. (b) The newly developed score including VWF-Ag and ALP (VWF-Ag × ALP/10,000) also predicted re-transplantation-free survival in the Kaplan-Meier analysis.
ALP: alkaline phosphatase; VWF-Ag: Von Willebrand factor antigen
Table 2.
Patient characteristics depending on their VWF-Ag level. Comparison of patients based upon classification according to the optimal cut-off for VWF-Ag levels. Data is given as the mean (±SD), median (IQR), or number (%), as appropriate
| VWF-Ag |
|||
|---|---|---|---|
| ≤286.8% | >286.8% | p | |
| Patients, n | 60 (75.0%) | 20 (25.0%) | |
| Gender, female | 19 (31.7%) | 5 (25.0%) | 0.779 |
| Age, years | 55.7 (±9.3) | 57.9 (±9.6) | 0.371 |
| Blood group, n | 0.314 | ||
| O | 19 (42.2%) | 4 (23.5%) | |
| A | 15 (33.3%) | 5 (29.4%) | |
| B | 8 (17.8%) | 5 (29.4%) | |
| AB | 3 (6.7%) | 3 (17.6%) | |
| Time since LT, months | 42 (8–97) | 25 (7–57) | 0.383 |
| HCC, n | 18 (30.0%) | 11 (55.0%) | 0.061 |
| Donor age, years | 56 (41–68) | 64 (54–72) | 0.062 |
| Cold ischemia time, h | 12.0 (10.0–14.0) | 10,0 (9.0–11.0) | 0.006 |
| Awaiting re-transplantation | 1 (1.7%) | 2 (10.0%) | 0.153 |
| AST, U/l | 21 (16–33) | 42 (17–66) | 0.014 |
| ALT, U/l | 24 (15–42) | 31 (15–58) | 0.597 |
| ALP, U/l | 100 (73–134) | 180 (115–269) | 0.002 |
| gGT, U/l | 36 (19–88) | 67 (37–190) | 0.032 |
| Total bilirubin, mg/dl | 0.6 (0.4–1-0) | 0.9 (0.5–1.8) | 0.090 |
| Albumin, g/l | 43.5 (41.2–45.5) | 39.5 (34.9–45.7) | 0.021 |
| INR | 1.00 (0.96–1.03) | 1.03 (0.95–1.10) | 0.338 |
| Creatinine, mg/dl | 1.05 (0.84–1.35) | 1.12 (0.74–1.48) | 0.982 |
| CRP, mg/dl | 2.0 (0.0–5.4) | 13.4 (2.3–28.4) | <0.001 |
| Leukocyte count, /nl | 5.68 (3.61–6.88) | 6.99 (3.92–7.82) | 0.071 |
ALP: alkaline phosphatase; ALT: alanine transaminase; AST: aspartate transaminase; CRP: C-reactive protein; gGT: gamma-glutamyltransferase; INR: international normalized ratio; IQR: interquartile ratio; HCC: hepatocellular carcinoma; LT: liver transplant; U/l: units per liter; VWF-Ag: Von Willebrand factor antigen
The AUC for prediction of the secondary end-point of 1-year re-transplantation-free survival was 0.961 (95% CI 0.918–1.000; p = 0.002). The optimal cut-off for VWF-Ag was 478.0%, with a sensitivity of 100% and specificity of 94.7%.
If we included only the patients with VWF-Ag measurement performed within the first year after LT, VWF-Ag still significantly predicted the patients’ outcome: Of the 26 patients included for this analysis, two underwent re-LT or died during follow-up. The outcome was predicted with an AUC of 0.958 in the ROC analysis (95% CI 0.878–1.000; p = 0.034).
Univariate and multivariate analyses
Of the variables included in the univariate analysis, only MELD, VWF-Ag, ALT and ALP levels were significantly associated with patient outcome. Inclusion of these variables in the multivariate model revealed only VWF-Ag and ALP to be independently associated with re-transplantation-free survival. The hazard ratio for a 1-point increase in VWF-Ag was 1.009 (95% CI 1.001–1.017; p = 0.027) and for ALP it was 1.005 (95% CI 1.001–1.008; p = 0.004), as seen in Table 3. This is equivalent to hazard ratios of 1.092 (95% CI 1.015–1.174) per 10-point increase and of 2.404 (95% CI 1.159–4.987) per 100-point increase in VWF-Ag.
Table 3.
Univariate and multivariate analysis. Results of univariate and multivariate analyses for the identification of independent risk factors that predicted re-transplantation-free survival after the first liver transplantation
| Univariate |
Multivariate |
|||
|---|---|---|---|---|
| hazard ratio (95% CI) | p | hazard ratio (95% CI) | p | |
| Age | 0.957 (0.878–1.043) | 0.318 | ||
| Gender, male | 0.423 (0.085–2.098) | 0.292 | ||
| Time since LT, months | 0.997 (0.984–1.011) | 0.686 | ||
| Donor age, years | 1.059 (0.987–1.136) | 0.109 | ||
| Cold ischemia time, h | 0.766 (0.504–1.167) | 0.215 | ||
| HCC prior to LT | 0.898 (0.164–4.904) | 0.901 | ||
| MELD score | 1.199 (1.055–1.362) | 0.005 | 1.129 (0.823–1.547) | 0.452 |
| VWF-Ag, % | 1.007 (1.003–1.012) | <0.001 | 1.009 (1.001–1.016) | 0.018 |
| ALT, U/l | 1.019 (1.008–1.029) | 0.001 | 1.008 (0.991–1.026) | 0.344 |
| ALP, U/l | 1.003 (1.001–1.005) | 0.001 | 1.005 (1.002–1.008) | 0.002 |
| CRP, mg/dl | 1.014 (0.996–1.032) | 0.130 | ||
ALP: alkaline phosphatase; ALT: alanine transaminase; CRP: C-reactive protein; HCC: hepatocellular carcinoma; LT: liver transplant; MELD: model of end-stage liver disease; U/l: units per liter; VWF-Ag: Von Willebrand factor antigen
ROC analysis of ALP alone and of a combined score for VWF-Ag and ALP
ROC analysis of ALP to predict the primary outcome parameter revealed an AUC of 0.923 (95% CI 0.844–01.000; p = 0.001), and the optimal cut-off index was 142 U/l. To further enhance the diagnostic value, VWF-Ag and ALP were combined in a simple score:
| (1) |
This new score had an AUC of 0.950 (95% CI 0.879–1.000; p < 0.001) in ROC analysis and was superior to VWF-Ag, ALP or MELD alone (Figure 1). Patients who died or underwent re-transplantation had a median value of 15.69 (IQR = 9.30–21.96), compared to a median of 1.95 (IQR = 1.11–3.97) among patients who were alive at the end of the study (p < 0.001). The optimal cut-off for this score was determined to be 9.83. This cut-off yielded a sensitivity of 83.3% and a specificity of 97.3% to predict the patients’ survival. Survival significantly differed between patients with a score ≤9.83 (mean 114 weeks; 95% CI 113–116), compared to patients with a score >9.83 (mean 54 weeks; 95% CI 14–81; p < 0.001), as seen in Figure 2(b).
Validation of results in patients with ESLD on the waiting list for a first LT
Overall, 93 patients were identified as eligible for inclusion in this cohort. They were 51.5 (±9.6) years old and 60 (64.5%) were of male gender. The etiology of their liver disease was: alcoholic in 42 patients (45.2%), viral hepatitis in 25 patients (26.9%), biliary disease in 11patients (11.9%), cryptogenic in 7 patients (7.5%) and other causes of cirrhosis in 8 patients (8.6%). There were 33 patients (35.5%) who underwent LT during follow-up, 17 (18.3%) who died and 43 (46.2%) who were alive at the end of the follow-up period.
The median VWF-Ag level was 329.9% (IQR = 244.2–456.8) and MELD score was 12 (IQR = 9–17) in this cohort. Cox-regression analysis revealed that VWF-Ag was, as well, significantly associated with transplantation-free survival in this cohort and revealed a hazard ratio of 1.003 per 1-point increase in VWF-Ag (95% CI 1.001–1.004; p = 0.004). In contrast, ALP and our newly developed score (median 4.98; IQR = 2.71–8.43) were not significantly associated with transplantation-free survival in these patients (p = 0.171 and p = 0.089).
Discussion
In this prospective we demonstrated that levels of VWF-antigen are a new and non-invasive marker to predict the outcome of patients after LT. The predictive ability was proven in a second cohort and increased even further when VWF-Ag was combined with ALP in a simple score.
These novel results are in line with previous studies in patients with cirrhosis before liver transplantation. In two studies, VWF-Ag was identified as a marker of portal hypertension and predicted survival, in addition to the MELD score.5,6 It is further related to the degree of fibrosis in patient with chronic hepatitis C.13 Consequently we could confirm the results obtained in patients after LT in a second cohort of patients with ESLD. Previously, VWF-Ag was identified as a prognostic marker in cardiovascular diseases such as coronary artery disease.7 To summarize, some of the major reasons for morbidity and mortality after LT,4 namely liver-related and cardiovascular causes as well as infectious complications, are accompanied by increased VWF levels. It thus seems plausible that VWF-Ag provides an integral measurement of these important complications and causes of death after LT; however, in our study the causes for re-transplantation or death were of hepatobiliary origin, in most patients. Thus, further studies are needed to investigate whether VWF-Ag elevation is also a predictor of death due to cardiovascular or infectious causes in patients after LT.
In addition to VWF-Ag, we also found ALP to be independently associated with the primary endpoint. Interestingly, this finding is in line with results that identified ALP as an important predictor of cholestatic liver disease, such as primary biliary cirrhosis14–16 and primary sclerosing cholangitis.13,17,18 Among the hepatobiliary complications after LT, non-anastomotic biliary strictures or ischemic-type biliary lesions that occur in approximately 5–15% of patients are among the most challenging to treat and worrisome, with regard to their prognosis.19,20 Thus, it is not surprising that ALP also predicted outcome in patients after LT; and consequently, combining VWF-Ag and ALP in a simple score demonstrated excellent prediction of re-transplantation-free survival. ALP and the new score did not predict outcome in the cohort of patients with ESLD, yet only few patients had cholestatic liver disease in that cohort.
Although we cannot provide a precise pathophysiological explanation for our findings (which would have been far beyond the scope of this study) several mechanisms seem possible. First, VWF is a well-established marker of endothelial dysfunction8 and it is increased in cases of cardiovascular or inflammatory pathology.7 The relationship to portal pressure might be due to endothelial dysfunction as well, and explain the good predictive capacity of VWF-Ag in ESLD. Because most of the patients in our study died or underwent re-LT because of hepatic causes, we assume that elevation of VWF-Ag in these patients is based on the same mechanisms as in cirrhotic patients prior to LT.
In contrast to scoring systems for outcome prediction after LT, such as the donor risk index or D-MELD,2,3 donor age and cold ischemia time were not associated with graft survival in our cohort. In contrast to the two aforementioned scores, which were shown to predict outcome in the months and first years after LT, our study was conducted in outpatients a median of 35 months after liver transplantation. This difference could explain the diminished influence of donor age and cold ischemia time. Of interest in future studies should be to investigate VWF-Ag at fixed time points (e.g. 6 months) after LT, as this could further enhance the clinical application of VWF-Ag measurement after LT.
This prospective study is the first to investigate VWF-Ag as a marker of survival in patients after LT and it confirmed results from pre-LT patients. Nevertheless, some limitations have to be noted: Patients with an increased VWF-Ag as well showed worse liver function, compared to patients with lower VWF-Ag. Thus, it cannot be excluded that VWF-Ag is only a surrogate marker of liver function. We aimed to address this in the multivariate analysis, which revealed that in contrast to VWF-Ag, the MELD score and ALT were not associated with patient outcome. VWF-Ag is further elevated in an acute-phase reaction; thus, the correlation with CRP was not surprising. CRP levels were further increased in patients who died or underwent re-LT, compared to those alive at the end of the study; however, CRP was as well not associated with the patients’ outcome in the multivariate analysis. We thus concluded that there is additional value derived from VWF measurement, in addition to their MELD score or CRP. Since patients were screened during their regular follow-up in our outpatient clinic, the time point of VWF-Ag measurement after LT was heterogeneous. We thus separately analyzed those patients with VWF-Ag measurement performed within the first year after LT, which confirmed the results. Additionally, in six patients we were able to identify further VWF-Ag measurements during follow-up. In four patients, VWF-Ag was below the threshold of 286.8% in both measurements, while it was above the threshold in both measurements in one patient. The last patient developed portal hypertension due to stenosis of the venous piggyback anastomosis and the VWF-Ag consequently rose from 184.3% to 335.8%. This further supported the idea of regular VWF-Ag assessment during the follow-up after LT. Despite the above-mentioned minor limitations, our study, being the initial study to address VWF-Ag after LT, profits from its prospective design and the validation of results in a second cohort.
To summarize, we demonstrated for the first time that VWF-Ag is a marker of prognosis after LT. Our findings should promote further investigation of VWF-Ag in patients after LT and possibly the future implementation of VWF-Ag measurement in clinical practice, to identify patients who might benefit from closer follow-up or require consideration for re-transplantation.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This work was supported by the University of Heidelberg (medical faculty grant to Andreas Wannhoff).
References
- 1.Kim WR, Smith JM, Skeans MA, et al. OPTN/SRTR 2012 Annual data report: Liver. Am J Transplant 2014; 14: 69–96. [DOI] [PubMed] [Google Scholar]
- 2.Feng S, Goodrich NP, Bragg-Gresham JL, et al. Characteristics associated with liver graft failure: The concept of a donor risk index. Am J Transplant 2006; 6: 783–790. [DOI] [PubMed] [Google Scholar]
- 3.Halldorson JB, Bakthavatsalam R, Fix O, et al. D-MELD, a simple predictor of post-liver transplant mortality for optimization of donor/recipient matching. Am J Transplant 2009; 9: 318–326. [DOI] [PubMed] [Google Scholar]
- 4.Lucey MR, Terrault N, Ojo L, et al. Long-term management of the successful adult liver transplant: 2012 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Liver Transpl 2013; 19: 3–26. [DOI] [PubMed] [Google Scholar]
- 5.Ferlitsch M, Reiberger T, Hoke M, et al. Von Willebrand factor as new noninvasive predictor of portal hypertension, decompensation and mortality in patients with liver cirrhosis. Hepatology 2012; 56: 1439–1447. [DOI] [PubMed] [Google Scholar]
- 6.La Mura V, Reverter JC, Flores-Arroyo A, et al. Von Willebrand factor levels predict clinical outcome in patients with cirrhosis and portal hypertension. Gut 2011; 60: 1133–1138. [DOI] [PubMed] [Google Scholar]
- 7.Spiel AO, Gilbert JC, Jilma B. Von Willebrand factor in cardiovascular disease: Focus on acute coronary syndromes. Circulation 2008; 117: 1449–1459. [DOI] [PubMed] [Google Scholar]
- 8.Van Mourik JA, Boertjes R, Huisveld IA, et al. Von Willebrand factor propeptide in vascular disorders: A tool to distinguish between acute and chronic endothelial cell perturbation. Blood 1999; 94: 179–185. [PubMed] [Google Scholar]
- 9.Ferro D, Quintarelli C, Lattuada A, et al. High plasma levels of Von Willebrand factor as a marker of endothelial perturbation in cirrhosis: Relationship to endotoxemia. Hepatology 1996; 23: 1377–1383. [DOI] [PubMed] [Google Scholar]
- 10.Lisman T, Bongers TN, Adelmeijer J, et al. Elevated levels of Von Willebrand Factor in cirrhosis support platelet adhesion despite reduced functional capacity. Hepatology 2006; 44: 53–61. [DOI] [PubMed] [Google Scholar]
- 11.Wannhoff A, Muller OJ, Friedrich K, et al. Effects of increased Von Willebrand factor levels on primary hemostasis in thrombocytopenic patients with liver cirrhosis. PLoS One 2014; 9: e112583–e112583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hollestelle MJ, Geertzen HG, Straatsburg IH, et al. Factor VIII expression in liver disease. Thromb Haemost 2004; 91: 267–275. [DOI] [PubMed] [Google Scholar]
- 13.Maieron A, Salzl P, Peck-Radosavljevic M, et al. Von Willebrand factor as a new marker for non-invasive assessment of liver fibrosis and cirrhosis in patients with chronic hepatitis C. Aliment Pharmacol Ther 2014; 39: 331–338. [DOI] [PubMed] [Google Scholar]
- 14.Pares A, Caballeria L, Rodes J. Excellent long-term survival in patients with primary biliary cirrhosis and biochemical response to ursodeoxycholic acid. Gastroenterology 2006; 130: 715–720. [DOI] [PubMed] [Google Scholar]
- 15.Corpechot C, Abenavoli L, Rabahi N, et al. Biochemical response to ursodeoxycholic acid and long-term prognosis in primary biliary cirrhosis. Hepatology 2008; 48: 871–877. [DOI] [PubMed] [Google Scholar]
- 16.Kuiper EM, Hansen BE, De Vries RA, et al. Improved prognosis of patients with primary biliary cirrhosis that have a biochemical response to ursodeoxycholic acid. Gastroenterology 2009; 136: 1281–1287. [DOI] [PubMed] [Google Scholar]
- 17.Al Mamari S, Djordjevic J, Halliday JS, et al. Improvement of serum alkaline phosphatase to <1.5 upper limit of normal predicts better outcome and reduced risk of cholangiocarcinoma in primary sclerosing cholangitis. J Hepatol 2013; 58: 329–334. [DOI] [PubMed] [Google Scholar]
- 18.Rupp C, Rossler A, Halibasic E, et al. Reduction in alkaline phosphatase is associated with longer survival in primary sclerosing cholangitis, independent of dominant stenosis. Aliment Pharmacol Ther 2014; 40: 1292–1301. [DOI] [PubMed] [Google Scholar]
- 19.Sanchez-Urdazpal L, Gores GJ, Ward EM, et al. Ischemic-type biliary complications after orthotopic liver transplantation. Hepatology 1992; 16: 49–53. [DOI] [PubMed] [Google Scholar]
- 20.Sharma S, Gurakar A, Jabbour N. Biliary strictures following liver transplantation: Past, present and preventive strategies. Liver Transpl 2008; 14: 759–769. [DOI] [PubMed] [Google Scholar]