Abstract
Introduction
In patients with gastroesophageal reflux disease (GORD), co-existence of functional dyspepsia (FD) is known to be associated with poor response to proton pump inhibitors (PPIs), but the contribution of specific dyspepsia symptoms has not yet been systematically investigated.
Objective
We aimed to characterize the impact of dyspepsia symptoms on response to PPIs in patients with GORD.
Methods
The enrolled subjects were consecutive patients with a diagnosis of GORD. All patients underwent a 24 hour pH–impedance test, while on PPI therapy. Patients were divided into two groups, refractory and responders, according to the persistence of GORD symptoms. A standardized questionnaire for FD was also administered to assess presence of dyspepsia symptoms.
Results
In the subgroup of refractory patients FD was more prevalent than in responders, with post-prandial fullness, nausea, vomiting, early satiation and epigastric pain being significantly prevalent in refractory GORD patients. In the multivariate analysis only early satiation and vomiting were significantly associated with poor response to PPIs.
Conclusion
Co-existence of FD is associated with refractory GORD. We showed that only early satiation and vomiting are risk factors for poor response to therapy with PPIs. Our findings suggest that symptoms of early satiation and vomiting would help to identify the subset of PPI-refractory GORD patients.
Keywords: Refractory GERD, functional dyspepsia, early satiation, vomiting, pH–impedance monitoring, proton pump inhibitors
Introduction
Gastro-oesophageal reflux disease (GORD) is one of the most prevalent upper gastrointestinal (GI) disorders, and is characterized by the recurrence of heartburn and/or regurgitation.1 Acid suppression with proton pump inhibitors (PPIs) is the mainstay of therapy for GORD, with a good rate of response.2 However, about 30% of treated patients are referred for persistent GORD symptoms while on PPI therapy.3 The failure of acid suppression represents a challenge for the gastroenterologist; in fact, to date, there is no standardized treatment for refractory GORD, and the underlying mechanisms remain unclear.4
Several mechanisms have been hypothesized to explain refractory GORD, in particular reflux of non-acid gastroduodenal contents, defective mucosal barrier function, hypersensitivity to normal acid exposure, poor peristalsis or delayed gastric emptying and functional heartburn/chest pain syndrome.5 Some of these mechanisms seem to be also involved in functional dyspepsia (FD),6–8 and this common pathophysiological background may explain the high degree of overlap between these diseases. Depending on the study, the rate of overlap ranges from 20% to up to 50%, and the role of dyspeptic symptoms in response to PPIs is still unclear.9–11
Tack et al. showed that epigastric pain is the more frequent dyspeptic symptom reported by PPI-responder GORD patients.10 This finding likely reflects the use of acid suppressive therapy in the subtype of FD with ‘epigastric pain syndrome’ (EPS).12–14 However, PPI therapy is less efficacious in dyspeptic patients suffering from post-prandial distress syndrome (PDS), characterized by early satiation and post-prandial fullness.12–15
In a recent review, FD has been found to be more prevalent in GORD patients who had the worst response to PPIs, likely suggesting that coexisting dyspepsia is a risk factor for refractory GORD.3 However, the impact of specific dyspepsia symptoms in the subset of refractory GORD patients has not yet been systematically reported.
The aim of our study is thus to evaluate the impact of specific dyspeptic symptoms on response to PPIs in patients with GORD.
Material and methods
Subjects
For this study, 132 consecutive patients with typical GORD symptoms (heartburn and acid regurgitation) were screened. Patients underwent careful history taking, clinical examination, routine biochemistry, upper GI endoscopy, and oesophageal manometry test. During endoscopy, biopsies were taken from the antrum and corpus for routine histology and to check for the presence of Helicobacter pylori. Inclusion criteria were the presence of heartburn and/or regurgitation lasting for more than 6 months and occurring at least three times weekly. Exclusion criteria were the presence of gastric atrophy, H. pylori infection, major abdominal surgery, peptic ulcer, severe oesophageal motility disorders, eosinophilic oesophagitis, Barrett’s oesophagus, underlying psychiatric illness, and use of non-steroidal anti-inflammatory drugs (NSAIDs). All subjects received and signed an informed consent and the University Ethics Committee approved all procedures.
Symptom questionnaires
At baseline, all patients were given a modified six-item questionnaire assessing presence of typical and atypical GORD symptoms (heartburn, regurgitation, dysphagia, food regurgitation, odynophagia and chest pain). The severity (0–3; 0 = absent, 1 = mild, 2 = relevant and 3 = severe, interfering with daily activities) of all GORD symptoms was recorded using a previously validated questionnaire.11
All patients were then put on double-dose PPI therapy (Pantoprazole 20 mg b.i.d.) for 12 weeks. At the end of the therapy, the GORD symptoms questionnaire was again administered to all subjects, and patients with persistent heartburn and/or regurgitation, at least once a week, were considered as having refractory GORD.3
Chronic dyspeptic symptoms were defined as the presence of epigastric pain/burning, nausea, vomiting, early satiation, post-prandial fullness, belching and bloating for the last 3 months with onset of symptoms at least 6 months before clinical evaluation; symptoms’ severity was also evaluated using previously validated questionnaire (0–3; interfering with daily life).11
Ambulatory pH–impedance monitoring
Patients underwent ambulatory multichannel intraluminal impedance–pH monitoring while on PPI therapy. The system included a portable data logger and a catheter with two antimony pH electrodes at 5 cm above and 10 cm below the lower oesophageal sphincter (LOS) and six impedance electrodes at 3, 5, 7, 9, 15 and 17 cm above the LOS (ZepHr Recorder, Sandhill Scientific, Colorado, USA). Data stored on a compact-flash card were analysed using specific diagnostic software (BioVIEW, Sandhill Scientific). Recorded pH–impedance data were used to determine the number of reflux events, oesophageal acid exposure, intragastric pH, percentage of time that gastric pH was less than 4 and to classify the type of refluxate (acid reflux: pH < 4, weakly acid reflux: 4 < pH < 7 and weakly alkaline reflux: pH > 7). Symptom association probability (SAP) and symptom index (SI) were also computed. Intragastric pH was evaluated in all subjects, and only patients with intragastric pH > 4 for more than 75% of the time were included.2,3
Data analysis
Age, body mass index (BMI) and all pH–impedance parameters were compared by Student’s t-test; the overall prevalence of dyspepsia and of specific dyspeptic symptoms was analysed by Fisher exact test. Stepwise multiple logistic regression analysis was used to identify the association between the risk of refractory GORD and the presence of dyspeptic symptoms and demographic features. P-values of 0.05 and 0.01 were chosen as cut-off points to respectively enter and exit the stepwise procedure. Odds ratios with 95% confidence interval (CI) were computed. Differences were considered to be significant at the 5% level. All data are given as mean ± SD. Statistical evaluations were performed using specialized software (SPSS, IBM Corporation).
Results
For this study, 132 consecutive GORD patients were screened; among them eight patients were excluded (four for peptic ulcer and concomitant H. pylori infection, three for concurrent use of NSAIDs, of whom one had also a diagnosis of Barrett’s oesophagus, and one for oesophageal motility disorder) and four patients refused to participate in the study. Twenty-two patients had previous evidence of H. pylori infection that was successfully eradicated.
In total, 120 patients (67 females, age 56 ± 15 years, GORD symptoms’ score: 3.2 ± 1.8) were included. After the pH–impedance monitoring, 20 patients were excluded because of poor acid suppression.
Demographic features and pH–impedance parameters do not affect PPIs response in GORD patients
We identified 67 responders (38 females, age 54 ± 12 years; GORD symptoms score: 0.5 ± 0.6) and 33 refractory patients (21 females, age 58 ± 15, GORD symptoms score: 2.3 ± 1.3). No significant differences in terms of sex, age or BMI were found between these two groups. Data are shown in Table 1. The analysis of the pH–impedance parameters are shown in Table 2 and in Supplementary Information, and revealed no significant differences between the two groups.
Table 3.
pH–impedance parameters in patients suffering from early satiation (ES) or vomiting (VO).
| Acid exposure | Total reflux | Acid reflux | Weakly acid reflux | Gaseous acid reflux | Gaseous weakly alkaline reflux | |
|---|---|---|---|---|---|---|
| All pts without ES or VO | 0.8 ± 3 | 57.4 ± 30.7 | 7.5 ± 11.5 | 48 ± 27 | 0.6 ± 1.6 | 0.43 ± 1.4 |
| ES | 0.8 ± 2.9 | 57.7 ± 30.7 | 7 ± 11 | 48.9 ± 27.4 | 0.7 ± 1.6 | 0.64 ± 1.3 |
| p * | ns | ns | ns | ns | ns | ns |
| VO | 0.4 ± 0.5 | 44.3 ± 27.1 | 11.8 ± 19.8 | 47.1 ± 20.3 | 1.4 ± 3.7 | 2.1 ± 1.6 |
| p * | ns | ns | ns | ns | ns | ns |
p versus patients without ES and VO
Table 1.
Characteristics of patients
| Prevalence | Female | Age (years) | BMI (kg/m2) | p | |
|---|---|---|---|---|---|
| Responder | 67% | 64% | 54 ± 12 | 27 ± 4 | ns |
| Refractory | 33% | 57% | 58 ± 15 | 28 ± 5 | ns |
Table 2.
pH–impedance parameters in responder and refractory GERD patients
| Acid exposure | Total reflux | Acid reflux | Weakly acid reflux | SAP | SI | |
|---|---|---|---|---|---|---|
| Responder | 0.66 ± 1.85 | 60.5 ± 35.5 | 8.5 ± 11.2 | 49.3 ± 30 | 12.5 ± 6 | 21.2 ± 12.5 |
| Refractory | 0.67 ± 0.60 | 50.0 ± 29.5 | 7.7 ± 15.5 | 39.2 ± 24.5 | 10.6 ± 5.2 | 23 ± 15 |
| P | ns | ns | ns | ns | ns | ns |
Functional dyspepsia is prevalent in refractory GORD patients
Overall, dyspepsia symptoms were significantly more prevalent in the subgroup of refractory GORD patients than in responders (64 vs. 37%, p < 0.01). Also, the sum of intensity score of all dyspeptic symptoms was higher in refractory patients than in responders (mean score: 7.4 vs. 3.3, p < 0.01). Although when dyspepsia symptoms were grouped in EPS and PDS dyspepsia subtypes, the prevalence was similar in refractory and responder GORD patients (EPS: 3 vs. 8.9%; PDS: 9 vs. 8.9%; p all NS), the refractory GORD patients showed a significantly higher prevalence of overlap between EPS and PDS than responders (54.5 vs. 17.9%; p < 0.01).
Specific dyspeptic symptoms influence PPIs response in a different way
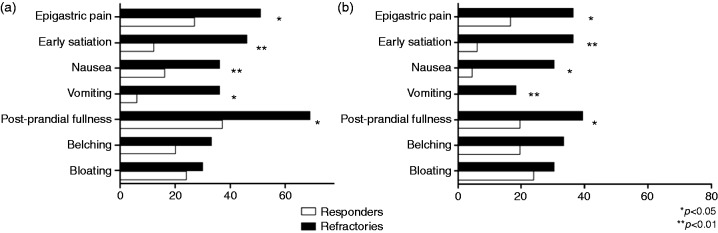
The analysis of specific dyspeptic symptoms revealed that post-prandial fullness, nausea, vomiting, early satiation and epigastric pain were all significantly more frequent in refractory than in responder patients with GORD (all p < 0.05, Figure 1(a)), while the prevalence of bloating and belching was similar (30 vs. 24 and 33 vs. 20%, respectively; p all NS). These results were also confirmed when only severe symptoms were evaluated (i.e. severity score > 2; Figure 1(b)).
Figure 1.
A – Prevalence of post-prandial fullness, nausea, vomiting, early satiation and epigastric pain was significantly higher in refractory (black) than in responder (white) GORD patients; no significant differences were found in prevalence of belching and bloating. B – The same data were found when only moderate–severe symptoms were considered (intensity score > 2).
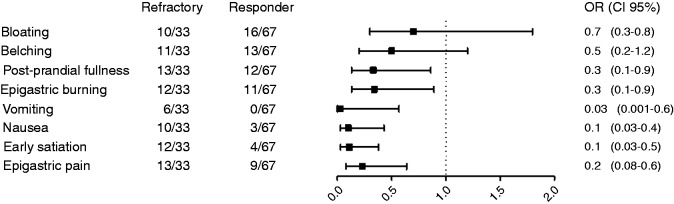
Figure 2 summarizes the risk contribution of each dyspepsia symptom on PPI response, but when data were computed in a multivariate analysis only early satiation and vomiting were confirmed to be risk factors for PPI refractoriness (OR 4, CI 1–14, p = 0.039 and OR 7, CI 1.4–34, p = 0.017; Figure 3). Also, the co-existence of several dyspeptic symptoms was associated with the worst clinical outcome in GORD patients (p < 0.05); however, specifically excluding early satiation from this analysis, no significant differences in terms of PPI response were found between patients with one or more dyspeptic symptoms.
Figure 2.
Epigastric pain, early satiation, nausea, vomiting, epigastric burning and post-prandial fullness were associated with a significantly lower response to PPIs, while bloating and belching did not interfere with PPI response in GORD patients.
Figure 3.
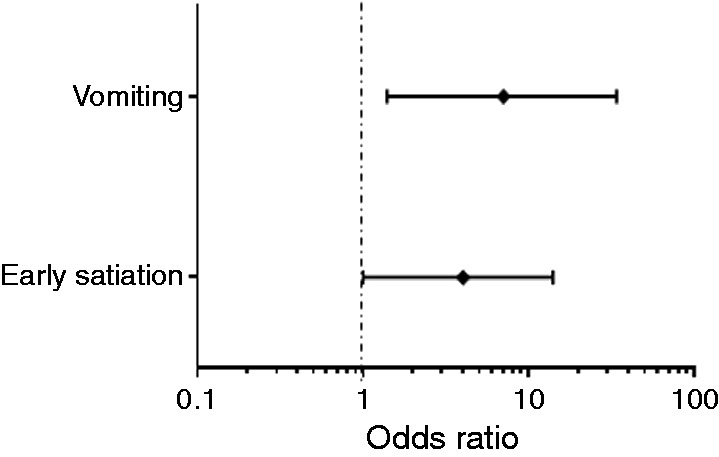
Multivariate analysis revealed that among all the dyspeptic symptoms, only vomiting and early satiation were risk factors significantly associated with poor response to PPIs in GORD patients.
Analysis of pH-II parameters in patients with prevalent early satiation and vomiting
In order to verify the role of early satiation and vomiting in the genesis of heartburn and/or regurgitation we evaluated pH-II parameters in patients referring for vomiting and early satiation. In the table 3 it is reported that no significant differences in terms of oesophageal acid exposure and number of acid or non-acid refluxes were found.
Discussion
PPIs are widely used across the world, due to the high prevalence of acid-related GI disorders, PPI effectiveness and safety.15,16 They are the most potent inhibitors of acid secretion available, and represent the mainstay of therapy for GORD with a good rate of response.2 However, according to literature, 30% of patients refer for persistent heartburn and regurgitation despite the therapy.3 As expected, the prevalence of refractory GORD in our population was 33%, confirming the current literature data.3
Recent findings have shown that FD is a risk factor for poor response to acid suppression and, given the high prevalence of both the diseases, it is well known that these entities frequently overlap.1,3,9–12 In keeping with previous studies,9–12 in our population of patients with GORD the prevalence of FD was at least 50%. As expected, the occurrence of FD symptoms was significantly higher in the refractory group than in responders. This evidence was also sustained by the higher intensity of FD symptoms in patients with persistent typical GORD symptoms. Our data thus confirm the high degree of overlap and, in agreement with Sifrim and Zerbib, support the role of FD in refractory GORD.3
When meal-related dyspeptic symptoms were analysed they were found to be more prevalent in refractory GORD patients, but when the analysis was run considering the subgroups of PDS and EPS no significant difference in prevalence was found between refractory and responder groups of GORD patients. However, this result likely reflects the contribution of patients overlapping epigastric pain and post-prandial distress syndromes; in fact, the prevalence of overlap between EPS and PDS was significantly higher in refractory GORD patients. These data highlight the role of specific symptoms rather than dyspepsia subtypes in response to PPIs.
In keeping with this, a systematic analysis revealed that post-prandial fullness, nausea, vomiting, early satiation and epigastric pain were more significantly prevalent and they were associated with a higher risk of refractory GORD. However, from the multivariate analysis only early satiation and vomiting were confirmed to be risk factors for poor response to PPI therapy.
Moreover, according to our data, the number of dyspeptic symptoms also plays a role in the outcome for GORD patients; in fact, here we report that the co-existence of several dyspeptic symptoms was associated with a worse clinical outcome. However, we observed that among patients suffering from several FD symptoms, the prevalence of refractory GORD was significantly higher only in the cluster of patients with early satiation. This result supports the hypothesis that the type, rather than the co-existence of multiple dyspepsia symptoms is likely to affect the outcome in patients with GORD. Our results confirm that these diseases influence each other, and suggest the presence of one or more pathophysiological backgrounds likely associated with both refractory GORD and specific FD symptoms.
In fact, although acid reflux is considered the main cause of GORD symptoms, the mechanisms involved in the genesis of heartburn and regurgitation, especially in refractory patients, seem to be more complicated and are still unclear.5 Mechanical stimulation of oesophagus, mucosal hypersensitivity and impairment of centrally acting pain modulators are postulated to underlie heartburn in patients with persistent symptoms despite acid suppression.4 Similarly, an altered perception of mechanical and chemical stimuli seems to play an important role also in the pathophysiology of FD.11,16–18 Moreover, motility disorders commonly associated with FD, such as delayed gastric emptying and impaired accommodation, may facilitate both acid and non-acid oesophageal reflux, reducing the rate of response to PPIs,19–22 although in this study we did not observe any significant difference in terms of number of refluxes in patients suffering from early satiation and vomiting compared with other GORD patients.
In conclusion, our study confirms the close relationship between FD and GORD, highlighting that specific dyspeptic symptoms influence the rate of response to PPIs. These findings may guide further pathophysiological studies and help clinicians in the management of refractory GORD.
Supplementary information
Prevalence of normal and abnormal pH–impedance monitoring was similar in responder and refractory GORD patients (p = 0.35; Fisher’s exact test).
| Responder (%) | Refractory (%) | |
|---|---|---|
| Normal pH–impedance | 46 | 26 |
| Abnormal pH–impedance | 21 | 7 |
| Total | 67 | 33 |
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Declaration of conflicting interests
None declared.
References
- 1.Boeckxstaens G, El-Serag HB, Smout AJ, et al. Symptomatic reflux disease: the present, the past and the future. Gut 2014; 63: 1185–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vela MF. Medical treatments of GERD: The old and new. Gastroenterol Clin North Am 2014; 43: 121–133. [DOI] [PubMed] [Google Scholar]
- 3.Sifrim D, Zerbib F. Diagnosis and management of patients with reflux symptoms refractory to proton pump inhibitors. Gut 2012; 61: 1340–1354. [DOI] [PubMed] [Google Scholar]
- 4.Subramanian CR, Triadafilopoulos G. Refractory gastroesophageal reflux disease. Gastroenterol Rep (Oxf) 2014; 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ang D, Sifrim D and Tack J. Mechanisms of heartburn. 2008; Nat Clin Pract Gastroenterol Hepatol 5: 383–392. [DOI] [PubMed]
- 6.Mimidis K, Tack J. Pathogenesis of dyspepsia. Dig Dis 2008; 26: 194–202. [DOI] [PubMed] [Google Scholar]
- 7.Tack J, Bisschops R, Sarnelli G. Pathophysiology and treatment of functional dyspepsia. Gastroenterology 2004; 127: 1239–1255. [DOI] [PubMed] [Google Scholar]
- 8.Savarino E, Pohl D, Zentilin P, et al. Functional heartburn has more in common with functional dyspepsia than with non-erosive reflux disease. Gut 2009; 58: 1185–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gerson LB, Kahrilas PJ, Fass R. Insights into gastroesophageal reflux disease-associated dyspeptic symptoms. Clin Gastroenterol Hepatol 2011; 9: 824–833. [DOI] [PubMed] [Google Scholar]
- 10.Tack J, Caenepeel P, Arts J, et al. Prevalence of acid reflux in functional dyspepsia and its association with symptom profile. Gut 2005; 54: 1370–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sarnelli G, De Giorgi F, Efficie E, et al. Correlation between oesophageal acid exposure and dyspeptic symptoms in patients with nonerosive reflux disease. Eur J Gastroenterol Hepatol 2008; 20: 264–268. [DOI] [PubMed] [Google Scholar]
- 12.Camilleri M, Stanghellini V. Current management strategies and emerging treatment for functional dyspepsia. Nat Rev Gastroenterol Hepatol 2013; 10: 320–320. [DOI] [PubMed] [Google Scholar]
- 13.Tack J, Talley NJ. Functional dyspepsia – symptoms, definitions and validity of the Rome III criteria. Nat Rev Gastroenterol Hepatol 2013; 10: 134–141. [DOI] [PubMed] [Google Scholar]
- 14.Miwa H, Kusano M, Arisawa T, et al. Evidence-based clinical practice guidelines for functional dyspepsia. J Gastroenterol 2015; 50: 125–139. [DOI] [PubMed] [Google Scholar]
- 15.Heidelbaugh JJ, Goldberg KL, Inadomi JM. Overutilization of proton pump inhibitors: A review of cost-effectiveness and risk. Am J Gastroenterol 2009; 104(Suppl 2): S27–S32. [DOI] [PubMed] [Google Scholar]
- 16.Chubineh S, Birk J. Proton pump inhibitors: The good, the bad, and the unwanted. South Med J 2012; 105: 613–618. [DOI] [PubMed] [Google Scholar]
- 17.Quigley EM, Keohane J. Dyspepsia. Curr Opin Gastroenterol 2008; 24: 692–697. [DOI] [PubMed] [Google Scholar]
- 18.Vanheel H1and Farré R. Changes in gastrointestinal tract function and structure in functional dyspepsia. Nat Rev Gastroenterol Hepatol 2013; 10: 142–149. [DOI] [PubMed] [Google Scholar]
- 19.Tack J, Bisschops R, Sarnelli G. Pathophysiology and treatment of functional dyspepsia. Gastroenterology 2004; 127: 1239–1255. [DOI] [PubMed] [Google Scholar]
- 20.Samsom M, Verhagen MA, van Berge Henegouwen GP, et al. Abnormal clearance of exogenous acid and increased acid sensitivity of the proximal duodenum in dyspeptic patients. Gastroenterology 1999; 116: 515–520. [DOI] [PubMed] [Google Scholar]
- 21.Schwarz MP, Samsom M, Smout AJPM. Chemospecific alterations in duodenal perception and motor response in functional dyspepsia. Am J Gastroenterol 2001; 96: 2596–2602. [DOI] [PubMed] [Google Scholar]
- 22.Sarnelli G, Caenepeel P, Geypens B, et al. Symptoms associated with impaired gastric emptying of solids and liquids in functional dyspepsia. Am J Gastroenterol 2003; 98: 783–788. [DOI] [PubMed] [Google Scholar]