Abstract
Background
Continuous delivery to the jejunum of levodopa-carbidopa is a promising therapy in patients with advanced Parkinson’s disease, as it reduces motor fluctuation. Percutaneous endoscopic gastrostomy and jejunal tube (PEG-J) placement is a suitable option for this. However, studies focused in PEG-J management are lacking.
Objectives
We report our experience regarding this technique, including technical success, adverse events and outcomes, in patients with advanced Parkinson’s disease.
Methods
Twenty-seven advanced Parkinson’s disease patients (17 men, median age: 64 years, median disease duration: 11 years) were included in a retrospective study from June 2007 to April 2015. The median follow-up period was 48 months (1–96).
Results
No adverse events were noted during and after nasojejunal tube insertion (to assess treatment efficacy). After a good therapeutic response, a PEG-J was placed successfully in all patients. The PEG tube was inserted according to Ponsky’s method. The jejunal extension was inserted during the same procedure in all patients. Twelve patients (44%) experienced severe adverse events related to the PEG-J insertion, which occurred after a median follow-up of 15.5 months. Endoscopy was the main treatment modality. Patients who experienced severe adverse events had a higher comorbidity score (p = 0.011) but were not older (p = 0.941) than patients who did not.
Conclusions
While all patients responded well to levodopa-carbidopa regarding neurological outcomes, gastro-intestinal severe adverse events were frequent and related to comorbidities. Endoscopic treatment is the cornerstone for management of PEG-J related events. In conclusion, clinicians and endoscopists, as well as patients, should be fully informed of procedure-related adverse events and patients should be followed in centres experienced in their management.
Keywords: PEG, PEG-J, percutaneous endoscopic gastrostomy and jejunal tube, levodopa, duodopa, Parkinson’s disease, adverse events
Introduction
Parkinson’s disease is a neurodegenerative disorder resulting from the death of dopamine neurons in the substantia nigra pars compacta with resultant depletion of striatal dopamine leading to motor, behavioural and/or cognitive symptoms.1
Levodopa, the precursor of dopamine, is the gold standard treatment. However, chronic oral levodopa is associated with complications such as motor fluctuations (on- and off-time), including dyskinesia during the on-time.1 This is due to fluctuating levodopa plasma concentrations resulting from levodopa’s short half-life, delayed gastric emptying and diet-related competition for jejunal uptake mechanisms.2 Today, continuous delivery of levodopa with carbidopa (a decarboxylase inhibitor) into the jejunum represents one of the best alternatives for advanced Parkinson’s disease patients as it avoids motor fluctuations (on- and off-time) and dyskinesia.1 Enteral access with percutaneous endoscopic gastrostomy and jejunal tube (PEG-J) placement is the most commonly performed method to uninterruptedly deliver levodopa-carbidopa.1,3
Considering the effectiveness and success of this novel therapeutic modality, PEG-J placement for this indication will soon be routine for many endoscopists.1 Thus, PEG-J insertion techniques, occurrence and management of adverse events should be studied in detail, regarding this particular patient group.
Currently available data about adverse events experienced by patients receiving enteral levodopa-carbidopa do not focus on modalities regarding PEG-J insertion but, nevertheless, report that the majority of adverse events (63%) are related to the infusion system.3,4 In fact, series focused on insertion, adverse events and management of the PEG-J, from an endoscopist point of view, for this particular indication, are scarce.5 Up to now, modalities of PEG-J placement and PEG-J related management of adverse events rely mainly on expert opinion.6
The Belgian levodopa multicentric prospective study assessed the intestinal levodopa-carbidopa efficacy, from a neurologist point of view.7 During this trial, we also collected data concerning PEG-J management in our academic institution. We conducted a retrospective study in which insertion technical modalities, short- and long-term clinical outcomes and adverse event occurrence and management were analysed and put in line with a comorbidity scoring to assess a potential impact of overall health on PEG-J related outcomes.
Materials and methods
Patients
An observational retrospective study assessing effectiveness and safety of PEG-J insertion for continuous levodopa-carbidopa intestinal gel (LCIG, Duodopa, AbbVie, Wavre, Belgium) infusion was conducted as part of the Belgian levodopa multicentric study7 between June 2007 and April 2015. Patients recruited from our centre were considered for the present analysis (Figure 1). Inclusion required meeting the levodopa-carbidopa Belgian reimbursement criteria7 regarding Parkinson’s disease, including neurological improvement after a test dose administered through a nasojejunal tube. The protocol was submitted to and approved by the Erasme University Hospital review board and ethics committee. Patients or their legal representatives gave written informed consent for participation in the trial.
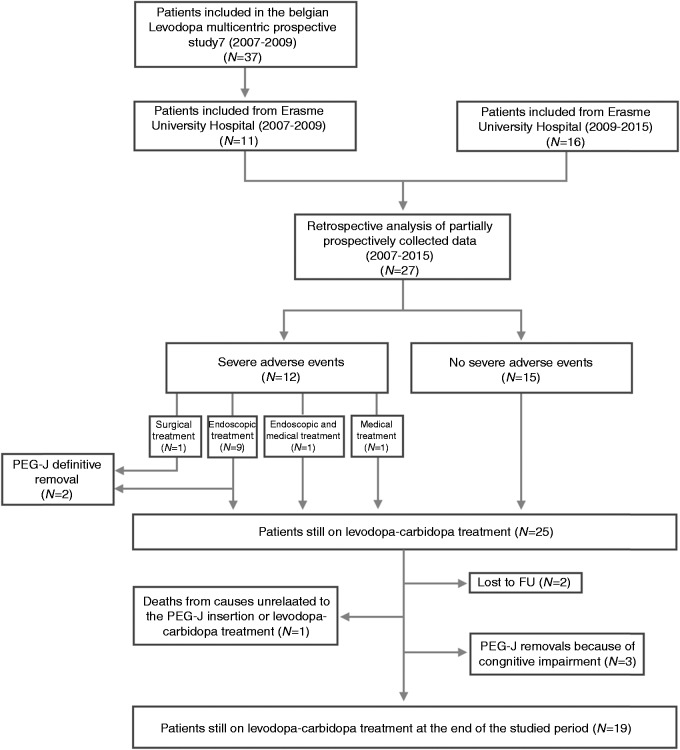
Figure 1.
Flowchart of study population.
N: number of patients; FU: follow-up; PEG-J: percutaneous endoscopic gastrostomy and jejunal tube
Endoscopic procedures and protocol
Initially, a nasojejunal tube (Freka® Endolumina Nasojejunal Feeding Tube CH8; Bad Homburg, Germany) was inserted in all patients to assess levodopa-carbidopa efficiency and dosage, for a minimal period of four days. The PEG-J was used exclusively for levodopa-carbidopa infusion. The nasojejunal tube was placed either by a transnasal route with the use of a paediatric scope (GIFXP180N, Olympus, Hamburg, Germany) or transorally with a standard gastroscope (GIF-Q160, GIF-H180, GIF-H190; Olympus, Hamburg, Germany). The PEG-J tube was placed during a second endoscopic procedure only after observation of a good response to intestinal continuous infusion of levodopa-carbidopa during clinical evaluation, which was performed by a neurologist. Both PEG and jejunal extension were placed during this second endoscopic procedure.
The PEG-J (Freka® PEG CH15, and Freka® CH9 Intestinal Tube for CH15 PEG; Bad Homburg, Germany) was placed under general anaesthesia with tracheal intubation in all patients, in order to minimize immediate adverse event occurrence.8 Antibioprophylaxis consisted in a single dose of cefazolin (2 g) or amoxicillin/clavulanic acid (2 g). The PEG tube was inserted according to the ‘Ponsky pull’ technique9 using a standard adult gastroscope (GIF-Q160, GIF-H180, GIF-H190; Olympus, Hamburg, Germany). The jejunal extension (Freka® CH9 Intestinal Tube for CH15 PEG; Bad Homburg, Germany) is a radio-opaque polyurethane jejunostomy tube designed for use with a CH15 PEG for long-term feeding. It has a distal double S-bend with an olive tip and small antenna tube. It was inserted during the same procedure in all patients, by grasping the antenna tube of the extension with a snare and dragging it up to the distal duodenum. It is suggested that the jejunal extension design will encourage anterograde progression and hinder intragastric retrograde migration. This manoeuvre was performed under fluoroscopy guidance. A 24 h fasting period followed PEG-J insertion.
PEG-J tubes were replaced after two years or earlier if required (Supplementary Material Video 1). Neurology follow-up visits were performed to optimize medication dosage and to evaluate efficacy and safety.7
Definitions
We report the results regarding endoscopic management: modality and technical success of the endoscopic procedures, adverse events, aftercare, follow-up regarding tube replacement, and outcomes. Severe adverse events (SAEs) were defined as those which required specific treatment (surgery, endoscopy) and/or additional hospitalization. Other adverse events were defined as those that could be managed in the outpatient setting and did not require additional treatment (surgery, endoscopy), nor additional hospitalization. Immediate adverse events were those occurring within 24 h after PEG-J placement or replacement.
Patients were followed up until their last visit, definitive levodopa-carbidopa therapy interruption with PEG-J retrieval, or death.
Comorbidities
To evaluate impact of comorbidities on outcomes, in this relatively homogenous population of Parkinson’s disease patients, we performed comorbidity scoring. We used a recent and simple scoring system, Comorbidity–Polypharmacy Score (CPS), which has been validated as an independent predictor of all-cause morbidity and mortality in older trauma patients.10 CPS is defined as the number of medications plus comorbidities. We also used the Charlson Comorbidity Index (CCI), which is known to provide a simple and valid method of estimating risk of death from comorbid diseases for use in longitudinal studies.11 These scores were calculated for each included patient at the time of the nasojejunal tube placement.
Statistical analysis
Data were collected and analysed using IBM SPSS statistics software for Macintosh version 24 (IBM Corp., Armonk, New York, USA). Continuous variables were expressed in median, maximal and minimal values. Categorical variables were expressed in percentage. Non-parametric tests (Mann–Whitney U test) were used for comparison. Chi-squared test was used only if each level of the categorical variable had an expected frequency count of at least 5. If necessary, the Pearson exact test was used instead. All p values reported are two-tailed and a p value < 0.05 was considered statistically significant.
Results
Study population
Thirty-seven patients were included in the multicentre trial7 and had PEG-J insertion for levodopa-carbidopa infusion. Information was gathered for the 27 patients (10 women, 17 men) who were included from our centre between June 2007 and April 2015 (Figure 1). Median age was 64 (42–80) years old and median disease duration was 11 (5–20) years. Regarding comorbidity, median CCI was 0.00 (0–4) and median CPS score was 11 (4–22) (Table 1).
Table 1.
Characteristics of study population
| Characteristics | N = 27 |
|---|---|
| Age, median (range), years | 64 (42–80) |
| Males, n (%) | 17 (63) |
| Time since PK diagnosis, median (range), months | 132 (60–240) |
| CPS score, median (range) | 11 (4–22) |
| CCI score, median (range) | 0.00 (0–4) |
PK: Parkinson’s disease; CPS: Comorbidity–Polypharmacy Score; CCI: Charlson Comorbidity Index
Endoscopic procedures
The nasojejunal tube was inserted under sedation (n = 11) or general anaesthesia (n = 16). This was performed either from the nasal route with a paediatric scope and the use of a guidewire (n = 17), or with a standard gastroscope and a biopsy forceps (n = 10). No adverse events were noted during and after nasojejunal tube insertion. PEG-J was inserted successfully in all patients after a median period of 12 weeks (0.6–35) following the nasojejunal tube placement.
Follow-up
The median follow-up duration was 48 months (1–96). At the end of the studied period, 19 (19/27, 71%) patients still had on-going levodopa-carbidopa therapy. Two patients with PEG-J were lost to follow-up. One patient died from a cause unrelated to the PEG-J insertion or levodopa-carbidopa treatment. Five patients (5/27, 19%) required PEG-J definitive removal because of progressive cognitive impairment (n = 3) and gastrointestinal adverse events (n = 2, duodenal ulcer; jejunal fistulas) (Figure 1). PEG-J withdrawal occurred after a median follow-up duration of 14 months (1–72 months).
Regarding neurological outcome, reduction of motor adverse events was significant, as were the improvements in dyskinesia and motor fluctuations.7
The median number of repeat endoscopy was 2 (0–8) (including scheduled procedures for tube replacement and procedures for adverse event management) during a median follow-up of 48 months (1–96 months) after PEG-J placement.
Adverse events
After PEG-J insertion, 11 patients (11/27, 41%) encountered immediate adverse events, including abdominal pain (n = 10), general asthenia related to anaesthesia (n = 1) and pneumoperitoneum (n = 4). There were no immediate SAEs. Pneumoperitoneum was diagnosed with an abdominal computed tomography (CT) performed because of abdominal pain. In three patients, this condition resolved spontaneously; one was treated by needle decompression with success. Hospitalization was not prolonged due to the above adverse events.
Delayed adverse events were observed in 16 patients (16/27, 59%), including 20 SAEs. Delayed minor adverse events (n = 6) were observed in six patients (6/27, 22%), including repeated but spontaneously resolved tube occlusion (n = 1), gastric ulcers (n = 2), duodenal ulcers (n = 3). Delayed SAEs (n = 20) were reported in 12 patients (12/27, 44%) (Table 2). They included J-tube migration back towards the stomach (n = 6), J-tube impaction in the jejunum (n = 1), J-tube dysfunction (n = 6: leakage (n = 1), clogging (n = 4), kinking (n = 1)), buried bumper syndrome (BBS) (n = 2), duodenal ulcer (Figure 2) (n = 2), covered duodenal perforation (Figure 3) (n = 1), infected intra-abdominal collection (n = 1) and internal bumper migration into the duodenum (D2) with jejunal fistulas due to subsequent transmural jejunal tube migration (n = 1) (Figure 4). SAEs occurred after a median follow-up of 15.5 months (0.1–74 months) (Table 3) and in a median period of 7.5 months (0.1–37) after last PEG-J (re)placement. J-tube migration occurred after a median period of 8.5 days (4–110) after PEG-J last (re)placement. This latter period (between last PEG-J (re)placement and J-tube migration) is significantly shorter than the period between the last PEG-J (re)placement and any other SAEs (p = 0.005). All SAEs were managed successfully conservatory (n = 2), by endoscopy (n = 17) or by surgery (n = 1) (Table 3).
Table 2.
Adverse events related to PEG-J
| Type of AE | No. patients/total no. patients (%) | Median period between PEG-J placement and event (range) |
|---|---|---|
| Immediate AEs | 11/27 (41) | < 24 h |
| Severe delayed AEs | 12/27 (44) | 15.5 (0.1–74) months |
| Minor delayed AEs | 6/27 (22) | 31 (0.1–72) months |
| Delayed AEs | 16/27 (59) | 17.5 (0.1–74) months |
AE: adverse event; PEG-J: percutaneous endoscopic gastrostomy and jejunal tube
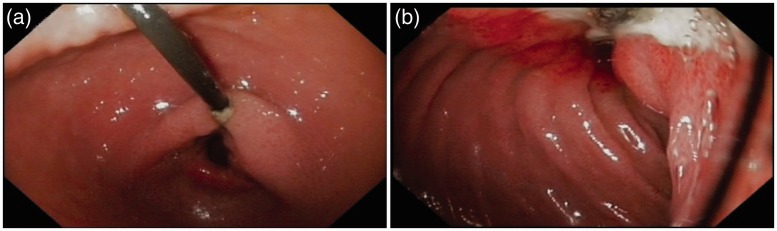
Figure 2.
Stomach (a) and duodenum (b) in a patient with a trans-pyloric duodenal linear ulcer, due to traction of the jejunal tube, who presented with abdominal pain nine months after percutaneous endoscopic gastrostomy and jejunal tube (PEG-J) insertion. This patient required PEG-J definitive removal.
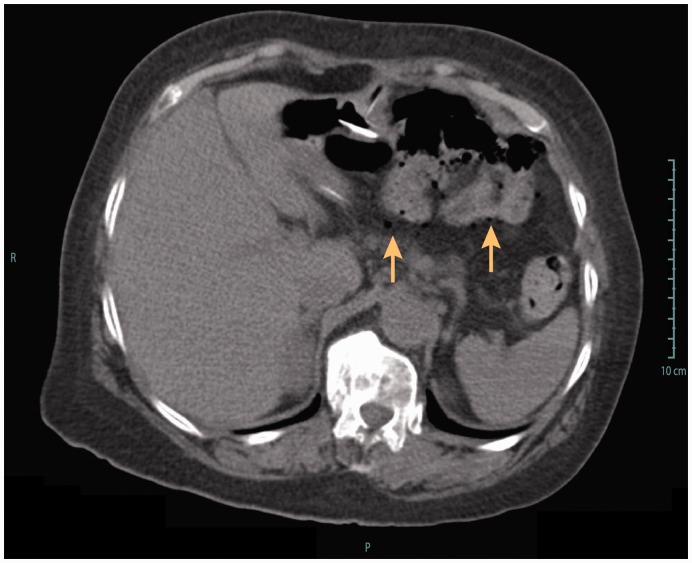
Figure 3.
Computed tomography showing extra-intestinal air bubbles (arrows), following percutaneous endoscopic gastrostomy and jejunal tube replacement (intestinal perforation). The patient presented abdominal pain and was put on antibiotics and outcome was uneventful.
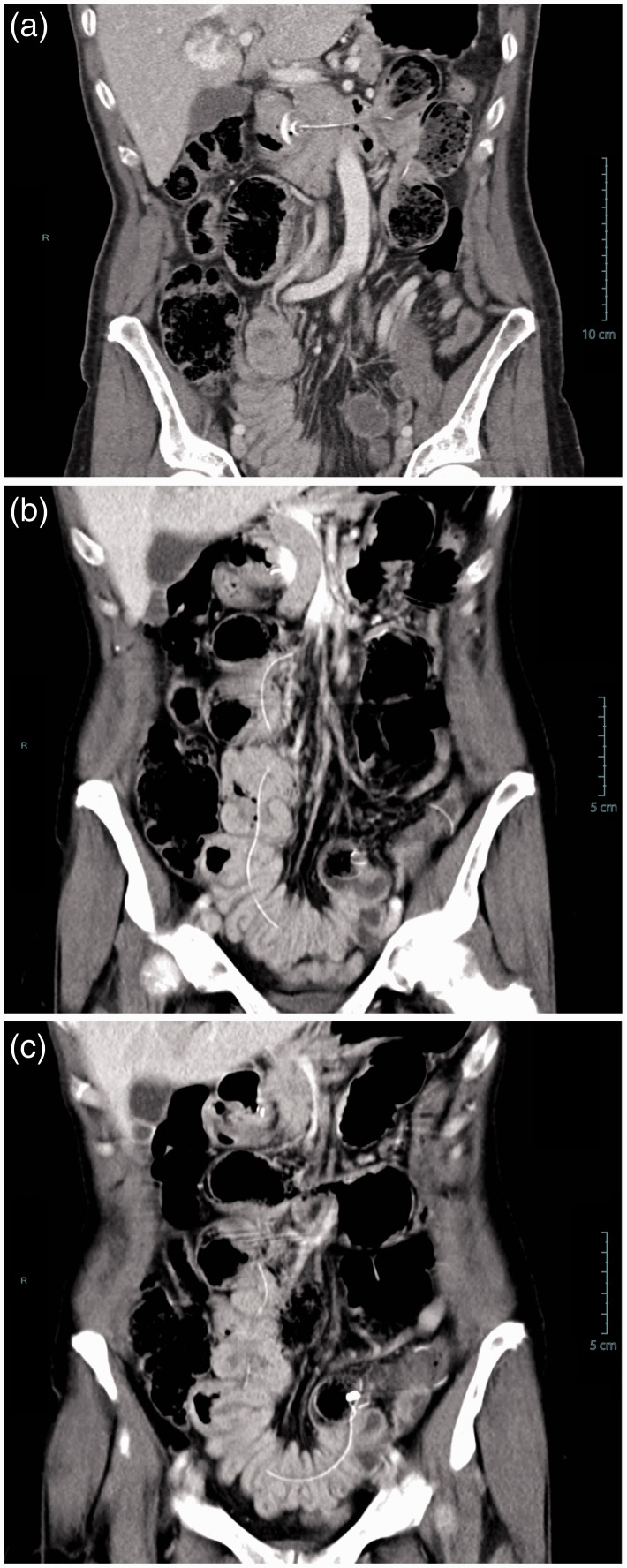
Figure 4.
Coronal multiplanar CT reconstruction showing (a) internal bumper migration into the duodenum and (b, c) multiple jejunal fistulas caused by migration of the jejunal extension. This severe adverse event occurred 14 months after percutaneous endoscopic gastrostomy and jejunal tube (PEG-J) placement. The patient presented to the emergency room with abdominal pain, nausea and vomiting. The patient required surgical treatment with intestinal resection and re-anastomosis; follow-up was favourable but PEG-J had to be definitely removed.
Table 3.
Severe adverse events and their management
| Type of SAE | No./no. SAEs (%) | No. of patients | Treatment type | Treatment modality |
|---|---|---|---|---|
| Duodenal ulcer | 2/20 (10) | 2 | Endoscopy | PPI and definitive or temporary (nine days) J tube removal |
| Buried bumper syndrome | 2/20 (10) | 2 | Endoscopy | Needle knife excision |
| J-tube impaction in the jejunum | 1/20 (5) | 1 | Endoscopy | Endoscopic release with a guidewire |
| J-tube migration | 6/20 (30) | 5 | Endoscopy | J tube replacement |
| J-tube dysfunction | 6/20 (30) | 5 | ||
| Leakage | 1/20 (5) | 1 | Endoscopy | J tube replacement |
| Kinking | 1/20 (5) | 1 | J tube replacement | |
| Clogging | 4/20 (20) | 3 | Guidewire recanalization (2/4) and replacement (2/4) | |
| Covered duodenal perforation | 1/20 (5) | 1 | Conservative | Antibiotic (amoxicillin/clavulanic acid, 2 g 3 times/day, i.v.) |
| Multiple jejunal fistulas | 1/20 (5) | 1 | Surgery | Intestinal resection |
| Infected intra-abdominal collection | 1/20 (5) | 1 | Conservative | Antibiotic (piperacillin-tazobactam, 4 g 4 times/day, i.v.) |
SAE: severe adverse event
Covered duodenal perforation (Figure 3) occurred during an endoscopic procedure for jejunal extension replacement, probably because of snare impaction into the duodenal wall, and was managed conservatory with antibiotics (amoxicillin/clavulanic acid, 2 g 3 times/day, i.v.). Jejunal fistulas presented with anterograde and transparietal migration of the jejunal tube tip (Figure 4). This latter SAE happened 14 months after PEG-J placement. The patient required surgical treatment with intestinal resection and re-anastomosis; outcome was favourable but PEG-J had to be definitely removed.
Three patients (3/27, 11%) had three SAEs, two patients (2/27, 7%) had two and seven patients (7/27, 26%) had one SAE during the follow-up. There were no deaths related to PEG-J treatment.
Comorbidity scores and outcome
Patients who encountered SAEs during the follow-up were not significantly older (p = 0.941) but had superior comorbidity scores compared with patients who did not encounter SAEs (p = 0.011). In fact, median CPS was 15 in patients who experienced SAEs compared with 10 in patients who did not. Patients who encountered immediate adverse events had higher CPS (p = 0.020) in comparison with patients who did not (Table 4). Those patients who encountered immediate adverse events experienced significantly more (delayed) SAEs (p = 0.013) in comparison with patients who did not encounter immediate adverse events. However, median CCI was 1 in patients who experienced SAEs compared with 0 in patients who did not; there was no statistical difference (p = 0.105).
Table 4.
Adverse events, age and comorbidity scoring
| Type of AE | Median CPS | p value | Median age, years | p value | |
|---|---|---|---|---|---|
| Severe AE | ≥1 | 15 | 0.011 | 64 | 0.941 |
| 0 | 10 | 63 | |||
| Immediate AE | ≥1 | 12 | 0.020 | 66 | 0.620 |
| 0 | 8.5 | 63.5 | |||
| Minor AE | ≥1 | 10.5 | 0.978 | 68 | 0.267 |
| 0 | 11 | 63.5 | |||
| Total population | 11 | 64 | |||
AE: adverse event; CPS: Comorbidity–Polypharmacy Score
Neither CCI nor CPS was significantly different between patients who encountered J-tube migration and those who did not (respectively: p = 0.525, p = 0.074). Still, patients who encountered migration were significantly more confronted by other SAEs (p = 0.048).
Discussion
In this study, PEG-J was successfully inserted in all patients, confirming the technical efficiency of the PEG-J placement method.12–14 However, the major message of our analysis is that approximately half of patients (44%) treated with this modality encountered at least one SAE during a median total follow-up of 48 months. A high rate of adverse events has already been reported in studies regarding outcome of PEG-J inserted for enteral nutrition in malnourished patients to prevent aspiration.13,15–18 Follow-up in published studies was shorter than the present cohort (median follow-up of four months). It is expected that SAE rate (including migration and dysfunction) will progressively increase with a longer follow-up. Nevertheless, our adverse event rate, adapted to our longer mean follow-up period (49 months), was lower (Table 513,15–19).
Table 5.
Reported data of SAEs related to PEG-J analysis of the literature
| Reference | Author, year | Subject | No. of patients encountering a SAE/total no. patients (%) | Mean follow-up duration with PEG-J in place | SAE rate, %/month |
|---|---|---|---|---|---|
| 15 | DiSario et al., 1990 | PEG-J | 19/20 (95) | 1.5 months | 63 |
| 13 | DeLegge et al., 1995 | PEG-J | 1/17 (5.8)a | 2 months | 2.9 |
| 16 | Simon and Fink, 2000 | PEG-J | 7/13 (53.8)b | Not specified | Unknown |
| 17 | Fan et al., 2002 | PEG-J | 19/49 (38.8) | 6 months | 6.5 |
| 18 | Doede et al., 2002 | PEG-J | 11/12 (91.6)b,c | 36 months | 25 |
| Our study, 2015 | PEG-J | 12/27 (44.4) | 49 months | 0.9 | |
| 19 | Figueiredo et al., 2007 | PEG | 4/168 (2.4)d | 5 months | 0.5 |
Data deduced from the available text study. DeLegge et al. reported an overall adverse event rate of 35% (6/17 patients). Only one adverse event (1/17) should be considered major as it required endoscopy (5.8%).
Severity (severe or minor) was not specified or was unclear.
Studies conducted with a paediatric population.
SAEs were defined as those requiring surgery, causing permanent adverse sequelae, or resulting in death.
SAE: severe adverse event; PEG-J: percutaneous endoscopic gastrostomy and jejunal tube
Among these SAEs, BBS is defined as progressive migration of the inner bumper of the PEG tube until it becomes embedded in the stomach wall.20,21 BBS occurred in two patients (7%) aged 60 and 68 years old after a follow-up after PEG-J placement of 17 and 34 months, respectively. Endoscopic needle knife excision21–23 was successfully performed (n = 2). Adequate nurse care applied after PEG insertion and continued during the follow-up could result in a lower prevalence of BBS.21 Such prophylactic attitudes are not yet described to prevent the other SAEs (migration, ulcers, enteral fistulas, etc.). In this regard, we observed that patients encountering SAEs had higher comorbidities, suggesting that more fragile patients would be at higher risk of SAEs. Recently, retrospective studies comparing younger and older patients demonstrated that PEG procedure-related adverse events and mortality were similar in both groups.24,25 In line with this, we did not observe a statistical difference in terms of median age between patients encountering adverse events and those who did not. Our results suggest that age is not a good indicator of vulnerability in this Parkinson’s disease population. However, comorbidity score could be a surrogate of adverse event risk. Indeed, we observed that patients who encountered SAEs had a significantly higher CPS. The same trend was observed using the CCI, without being statistically significant. This could be explained by a lower sensitivity in Parkinson’s disease patients for the CCI in comparison with the CPS.26 Even if we demonstrated that CPS could be used as a determinant risk factor, we still observed a low median CPS of 11. Thus, we think that more accurate comorbidity scores should be validated to characterize this particular population. Furthermore, in light of these results, comorbidity scores could be used during the patient’s selection process. As well, patients who encountered immediate adverse events following PEG-J placement should be more closely followed as it seems, from our results, that they are more vulnerable to delayed SAEs.
In a recent study evaluating outcome and adverse events of PEG utilization for long-term enteral feeding, a SAE rate of 2.4% (during a mean follow-up period of five months) has been reported.19 In fact, our rate per month of SAEs related to PEG-J is nearly twice as high in comparison with this reported rate per month related to PEG (Table 5). This could be largely attributed to the jejunal tube. Moreover, we think that jejunal tube structure played a role in adverse event incidence. We observed an increased rigidity of jejunal tubes in place during longer periods of time and in patients developing a SAE. Rigid jejunal extension could potentially cause intestinal wall erosion and progressive transmural migration. Rigidity development could be linked with the long-term infusion of the levodopa-carbidopa medication through the jejunal tube, which might deserve improvements. Hence, an earlier systematic replacement of the PEG-J could be proposed.
The small number of patients who withdrew from therapy is encouraging, as demonstrated by 71% of patients still on treatment on 1 April 2015. This could be explained by a close follow-up and by the fact that Belgian reimbursement criteria for levodopa-carbidopa are restrictive, as an objective therapeutic effect needs to be demonstrated. This results in a selection of patients who are living at home with a significant degree of autonomy and have no or only mild cognitive impairment, making them less susceptible for discontinuation.
Many options have been proposed to deliver medication and/or nutrition directly to the jejunum including PEG-J, direct percutaneous endoscopic jejunostomy (DPEJ), and (open and laparoscopic) surgical jejunostomies.14 Yet, no comparative study has been performed to reveal which technique would have the best outcome in fragile patients. Nevertheless, endoscopic techniques are favoured over surgery.14 PEG-J is the most commonly performed method despite the fact that it presents frequent J-tube migration and dysfunction.27,28 DPEJ could have been proposed bearing in mind that it is a more stable jejunal access which requires fewer interventions than PEG-J.17 However, in two recent retrospective studies, the relatively low placement success rate of DPEJ was confirmed, mainly due to failure to identify the puncture site.28,29 Currently PEG-J is considered the enteral access of choice for continuous levodopa-carbidopa delivery.
Our data confirm, however, the high incidence of SAEs along the course of this treatment, related to the PEG-J itself. While subject to all the limitations of a retrospective case series, this report with a long follow-up of four years is helpful in delineating the risk–benefit ratio of PEG-J placement for levodopa-carbidopa infusion in patients with Parkinson’s disease and provides useful information to clinicians and endoscopists. It is, to our knowledge, the largest study which analysed global PEG-J management for levodopa-carbidopa infusion from the endoscopist point of view.
In conclusion, placement of a PEG-J tube for levodopa-carbidopa jejunal treatment in patients with advanced Parkinson’s disease is an effective technique but which carries a high risk of adverse events, probably linked to the overall fragility of these patients and to devices which would still benefit from improvements. Patients should be fully informed of procedure-related adverse events and should be followed up accordingly in referral centres.
Acknowledgement
This work has been presented at the Belgian Week of Gastroenterology (BWGE) and at the United European Gastroenterology (UEG), P0738, 2015, Barcelona, Spain.
Declaration of conflicting interests
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Olanow CW, Kieburtz K, Odin P, et al. Continuous intrajejunal infusion of levodopa-carbidopa intestinal gel for patients with advanced Parkinson’s disease: A randomised, controlled, double-blind, double-dummy study. Lancet Neurol 2014; 13: 141–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hardoff R, Sula M, Tamir A, et al. Gastric emptying time and gastric motility in patients with Parkinson’s disease. Mov Disord 2001; 16: 1041–1047. [DOI] [PubMed] [Google Scholar]
- 3.Nyholm D. Duodopa® treatment for advanced Parkinson’s disease: A review of efficacy and safety. Parkinsonism Relat Disord 2012; 18: 916–929. [DOI] [PubMed] [Google Scholar]
- 4.Clarke CE, Worth P, Grosset D, et al. Systematic review of apomorphine infusion, levodopa infusion and deep brain stimulation in advanced Parkinson’s disease. Parkinsonism Relat Disord 2009; 15: 728–741. [DOI] [PubMed] [Google Scholar]
- 5.Negreanu L, Popescu BO, Babiuc RD, et al. Duodopa infusion treatment: A point of view from the gastroenterologist. J Gastrointestin Liver Disord 2011; 20: 325–327. [PubMed] [Google Scholar]
- 6.Dam-Larsen S, Darkahi B, Glad A, et al. Best practice in placement of percutaneous endoscopic gastrostomy with jejunal extension tube for continuous infusion of levodopa carbidopa intestinal gel in the treatment of selected patients with Parkinson’s disease in the Nordic region. Scand J Gastroenterol 2015; 50: 1500–1507. [DOI] [PubMed] [Google Scholar]
- 7.Pickut BA, van der Linden C, Dethy S, et al. Intestinal levodopa infusion: The Belgian experience. Neurol Sci 2014; 35: 861–866. [DOI] [PubMed] [Google Scholar]
- 8.Verschoore T, Vandecandelaere S, Vandecandelaere P, et al. Risk factors for complications and mortality related to endoscopic procedures in adults. Acta Gastroenterol Belg 2016; 79: 39–46. [PubMed] [Google Scholar]
- 9.Gauderer MW, Ponsky JL, Izant RJ. Gastrostomy without laparotomy: A percutaneous endoscopic technique. J Pediatr Surg 1980; 15: 872–875. [DOI] [PubMed] [Google Scholar]
- 10.Evans DC, Gerlach AT, Christy JM, et al. Pre-injury polypharmacy as a predictor of outcomes in trauma patients. Int J Crit Illn Inj Sci 2011; 1: 104–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis 1987; 40: 373–383. [DOI] [PubMed] [Google Scholar]
- 12.Silas AM, Pearce LF, Lestina LS, et al. Percutaneous radiologic gastrostomy versus percutaneous endoscopic gastrostomy: A comparison of indications, complications and outcomes in 370 patients. Eur J Radiol 2005; 56: 84–90. [DOI] [PubMed] [Google Scholar]
- 13.DeLegge MH, Duckworth PF, McHenry L, et al. Percutaneous endoscopic gastrojejunostomy: A dual center safety and efficacy trial. JPEN J Parenter Enteral Nutr 1995; 19: 239–243. [DOI] [PubMed] [Google Scholar]
- 14.Itkin M, DeLegge MH, Fang JC, et al. Multidisciplinary practical guidelines for gastrointestinal access for enteral nutrition and decompression from the Society of Interventional Radiology and American Gastroenterological Association (AGA) Institute, with endorsement by Canadian Interventional Radiological Association (CIRA) and Cardiovascular and Interventional Radiological Society of Europe (CIRSE). Gastroenterology 2011; 141: 742–765. [DOI] [PubMed] [Google Scholar]
- 15.DiSario JA, Foutch PG, Sanowski RA. Poor results with percutaneous endoscopic jejunostomy. Gastrointest Endosc 1990; 36: 257–260. [DOI] [PubMed] [Google Scholar]
- 16.Simon T, Fink AS. Recent experience with percutaneous endoscopic gastrostomy/jejunostomy (PEG/J) for enteral nutrition. Surg Endosc 2000; 14: 436–438. [DOI] [PubMed] [Google Scholar]
- 17.Fan AC, Baron TH, Rumalla A, et al. Comparison of direct percutaneous endoscopic jejunostomy and PEG with jejunal extension. Gastrointest Endosc 2002; 56: 890–894. [DOI] [PubMed] [Google Scholar]
- 18.Doede T, Faiss S, Schier F. Jejunal feeding tubes via gastrostomy in children. Endoscopy 2002; 34: 539–542. [DOI] [PubMed] [Google Scholar]
- 19.Figueiredo FA, da Costa MC, Pelosi AD, et al. Predicting outcomes and complications of percutaneous endoscopic gastrostomy. Endoscopy 2007; 39: 333–338. [DOI] [PubMed] [Google Scholar]
- 20.Klein S, Heare BR, Soloway RD. The “buried bumper syndrome”: A complication of percutaneous endoscopic gastrostomy. Am J Gastroenterol 1990; 85: 448–451. [PubMed] [Google Scholar]
- 21.El AZ, Arvanitakis M, Ballarin A, et al. Buried bumper syndrome: Low incidence and safe endoscopic management. Acta Gastroenterol Belg 2011; 74: 312–316. [PubMed] [Google Scholar]
- 22.Braden B, Brandstaetter M, Caspary WF, et al. Buried bumper syndrome: Treatment guided by catheter probe US. Gastrointest Endosc 2003; 57: 747–751. [DOI] [PubMed] [Google Scholar]
- 23.Ma MM, Semlacher EA, Fedorak RN, et al. The buried gastrostomy bumper syndrome: Prevention and endoscopic approaches to removal. Gastrointest Endosc 1995; 41: 505–508. [DOI] [PubMed] [Google Scholar]
- 24.Oh DJ, Kim B, Lee JK, et al. Can percutaneous endoscopic gastrostomy be carried out safely in the elderly? Geriatr Gerontol Int 2016; 16(4): 481–5. Epub 23 Apr 2015. doi: 10.1111/ggi.12495. [DOI] [PubMed]
- 25.Cagin YF, Atayan Y, Erdoğan MA, et al. Relationship of percutaneous endoscopic gastrostomy-related mortality and morbidity rates and effectiveness with advancing age. Acta Gastroenterol Belg 2015; 78: 292–298. [PubMed] [Google Scholar]
- 26.Phillips AR, Genever RW. The impact of Parkinson’s disease as a comorbid diagnosis. Age Ageing 2011; 40: 294–296. [DOI] [PubMed] [Google Scholar]
- 27.DiSario JA, Baskin WN, Brown RD, et al. Endoscopic approaches to enteral nutritional support. Gastrointest Endosc 2002; 55: 901–908. [DOI] [PubMed] [Google Scholar]
- 28.Toussaint E, Van Gossum A, Ballarin A, et al. Percutaneous endoscopic jejunostomy in patients with gastroparesis following lung transplantation: Feasibility and clinical outcome. Endoscopy 2012; 44: 772–775. [DOI] [PubMed] [Google Scholar]
- 29.Maple JT, Petersen BT, Baron TH, et al. Direct percutaneous endoscopic jejunostomy: Outcomes in 307 consecutive attempts. Am J Gastroenterol 2005; 100: 2681–2688. [DOI] [PubMed] [Google Scholar]