Abstract
Background
Photodynamic therapy (PDT) is a palliative treatment for malignant biliary obstruction.
Objective
The objective of this article is to assess the feasibility and safety of this technique.
Methods
In this nationwide, retrospective study of prospectively collected clinical data, all patients treated with PDT using polyhematoporphyrin in Austria from March 2004 to May 2013 were included. Feasibility, adverse events, stent patency and mortality rates were investigated.
Results
Eighty-eight patients (54 male, 34 female, median age 69 years) underwent 150 PDT procedures at seven Austrian referral centers for biliary endoscopy. The predominant underlying disease was Klatskin tumor (79/88). All PDT procedures were feasible without technical issues. Cholangitis was the most frequent adverse event (21/88). Stent patency was 246 days (95% CI 203–289) median and was significantly longer for metal than for plastic stents (269 vs. 62 days, p < 0.01). The median survival was 12.4 months (95% CI 9.7–14.9 m) calculated from first PDT and 15.6 months (95% CI 12.3–18.7 m) calculated from initial diagnosis. In patients suffering from biliary tract cancer, Cox regression revealed the number of PDT treatment sessions as the only independent predictor of survival at a multivariate analysis (p = 0.048).
Conclusion
PDT using polyhematoporphyrin was feasible and safe in this nationwide analysis. Survival data suggest a benefit of PDT in this unselected real-life patient population. Prospective trials comparing PDT to other palliative treatments will help to define its role in the management of malignant biliary obstruction. The study is registered at ClinicalTrials.gov number: NCT02504957.
Keywords: Malignant biliary obstruction, photodynamic therapy, endoscopic retrograde cholangiopancreatography, therapeutic endoscopy, hilar cholangiocarcinoma
Introduction
Malignant biliary obstruction is a common condition in patients suffering from primary or secondary bile duct malignancies such as extrahepatic cholangiocarcinoma (CCa) of Klatskin type, pancreatic adenocarcinoma or metastases of colorectal cancer (mCRC). When presenting with symptoms of biliary obstruction, only a minority of affected patients can be treated by curative surgical resection. Thus, palliative therapies aim to prevent complications associated with the obstruction such as jaundice, cholangitis or cholangiosepsis, all of which are limiting factors for quality of life and survival in these patients.1,2
Endoscopic stenting with plastic stents or self-expanding metal stents (SEMS) is the current gold standard to restore biliary drainage in patients with unresectable tumors.3 In addition to stenting, endoscopic therapies directly affecting the local tumor mass such as biliary photodynamic therapy (PDT) or biliary radiofrequency ablation (RFA) have been developed.4,5 Growing data suggest an additional benefit of these therapies by extending stent patency and overall survival.6,7 Although there is still room for improvement in the level of evidence (most studies on PDT or RFA have a retrospective design and a very small sample size), PDT and RFA are now frequently used as a palliative treatment for malignant biliary obstruction in many referral centers for bilio-pancreatic endoscopy.8,9
The aim of the present study was to evaluate the feasibility and safety of PDT with polyhematoporphyrin, the photosensitizer most commonly used for PDT in Austria, in the context of a nationwide analysis.
Materials and methods
This retrospective study was conducted at seven Austrian referral centers for bilio-pancreatic endoscopy. The study protocol was approved by the internal review board of the Medical University of Vienna (EK 1448/2012) and registered at ClinicalTrials.gov (NCT02504957). Patients who underwent PDT with polyhematoporphyrin as therapy for malignant biliary obstruction were identified using examination report databases and cross-checked with the supplier of the PDT equipment. Examination reports and patient charts were analyzed to assess underlying diseases, oncological co-therapies, intra-procedural adverse events, details of stenting, hospital stay, adverse events within 30 days post-intervention, stent patency after the last electively performed PDT procedure in each patient as well as 30-day, 90-day, and overall mortality. All patient data had been de-identified using pseudonymization prior to any further processing. Descriptive statistics were used to present the study parameters. Stent patency and survival were assessed using Kaplan–Meier statistics and compared between the different stent materials and underlying tumor entities, respectively, using the Log Rank test. Cox regression was used to identify independent predictors of survival in biliary tract cancer patients. Significant factors at univariate analysis were entered into multivariate testing. p values less than 0.05 were considered significant. SPSS version 23.0 was used for data analysis. All co-authors had access to the study data and reviewed and approved the final manuscript.
PDT procedure
PDT uses a photosensitizer that is preferentially internalized by tumor cells and activated by laser light at the tumor site to induce tumor cell death. In this study, polyhematoporphyrin (supplied by PDT® HGesmbH, Vienna, Austria) was used as a photosensitizer at a standard dose of 2 mg/kg body weight. Polyhematoporphyrin is a hematoporphyrin derivate that is pharmacologically, immunologically and metabolically inactive but has the ability to transfer energy to oxygen molecules creating cytotoxic singlet oxygen after excitation with laser light. In this study, light excitation was performed 48 hours after intravenous injection of the photosensitizer using an energy-modulating diode laser with a 30 or 70 mm radial light applicator (PDT® HGesmbH, Vienna, Austria) that was placed fluoroscopically into the bile duct via endoscopic retrograde cholangiography (ERC) or percutaneous transhepatic cholangiography (PTC). The diode laser delivered energy-modulated red light at 650 nm with 400 mW/cm2 and 250 J/cm2 during 650 sec radiation time. Due to photosensitivity of patients treated with PDT, certain precautions were required peri-interventionally: For a time span of two weeks after infusion of the photosensitizer, patients were required to avoid direct natural or artificial light exposure. Measures for sun protection (clothing, sunglasses, sunblock) were recommended to minimize this discomfort. All PDT procedures in this study including preparation and aftercare of patients were performed according to a standard operating procedure (SOP) provided by the supplier of the PDT equipment.
Results
From March 2004 to May 2013 a total of 88 patients (54 male, 34 female, median age 69 years, range 28 to 86 years) underwent 150 PDT procedures as a treatment for histologically proven malignant biliary obstruction. Underlying diseases included 79 cases of Klatskin tumor (four tumors of Bismuth I stage, three of Bismuth II stage, 15 of Bismuth III stage, and 57 of Bismuth IV stage), four cases of mCRC, three cases of gallbladder carcinoma and two cases of distal CCa. Local nonresectability defined a palliative treatment setting at the time of PDT in all cases. In 59 patients, PDT was used without any additional anti-tumor treatment, 24 patients underwent concomitant chemotherapy, nine patients had undergone curatively intended liver surgery, two patients had been previously treated with (percutaneous) RFA and one patient each had undergone prior external radiotherapy, ethanol instillation and selective internal radiotherapy (SIRT) (Table 1).
Table 1.
Patient characteristics (summary)
| Biliary tract cancer (Klatskin tumor, gallbladder carcinoma, distal CCa) | Metastatic colorectal cancer | Total | |
|---|---|---|---|
| Number of patients | 84 | 4 | 88 |
| Gender (male:female) | 51:33 | 3:1 | 54:34 |
| Age (range) | 69 years (28–86) | 73 years (69–81) | 69 years (28–86) |
| Co-therapies | CHT = 20, S = 6, RFA = 1, EI = 1 | CHT = 4, S = 3, RFA = 1, RT = 1, SIRT = 1 | CHT = 24, S = 9, RFA = 2, RT = 1, EI = 1, SIRT = 1 |
| PDT sessions | 1 × = 58, 2 × = 11, 3 × = 5, 4 × = 5, 5 × = 3, 7 × = 2 | 1 × = 2, 2 × = 2 | 1 × = 60, 2 × = 13, 3 × = 5, 4 × = 5, 5 × = 3, 7 × = 2 |
| Adverse events within 30 days post-PDT | Cholangitis = 20, E. coli sepsis = 2, Klebsiella sepsis = 1, liver abscess = 1, non-ST-elevated myocardial infarction = 1, Clostridium difficile colitis = 1, acute renal failure = 1 | Cholangitis = 1, transfusion-dependent anemia = 1 | (as listed) |
| Survival after first PDT/initial diagnosis (95% CI) | 12.1 m/15.5 m | 16.3 m/128.1 m | 12.4 m (9.7–14.9 m) |
PDT: photodynamic therapy; CCa: cholangiocarcinoma; CHT: chemotherapy; CI: confidence interval; EI: ethanol injection; RFA: radiofrequency ablation; RT: radiotherapy (external), S: surgery; SIRT: selective internal radiotherapy; CI: confidence interval; m: months.
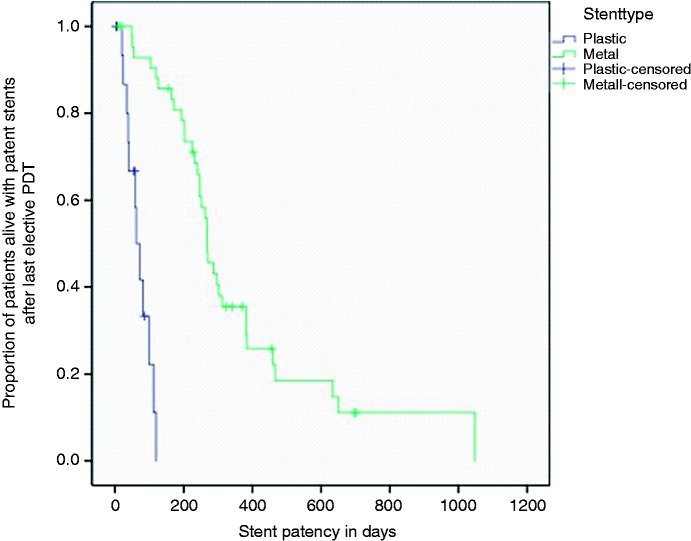
PDT was feasible without any technical issues or intra-procedural adverse events in all 150 cases (146 were performed via endoscopic retrograde cholangiopancreatography (ERCP) and four via a percutaneous approach). The majority of patients (60 of 88) underwent only one PDT procedure; repetitive PDT treatments (up to seven) were performed electively in 28 patients with a median time span of three months (m) between two PDT procedures. After the last electively performed PDT procedure, SEMS were placed in the majority of patients (58 of 88): Twenty-nine patients received plastic stents; in one patient no stenting was performed. Most patients received bilateral stenting (48 of 88). Stent patency was counted from the time point of the last electively performed PDT procedure in each patient, a median of 246 days (95% confidence interval (CI) 203–289). At log rank analysis, a significantly different stent patency was observed between metal and plastic stents (269 vs. 62 days, p < 0.01, Figure 1).
Figure 1.
Stent patency.
Calculation starts from the last electively performed photodynamic therapy (PDT) in each patient. Median stent patency was 269 days median for metal stents and 62 days median for plastic stents, p < 0.01.
The peri-interventional hospital stay of all study patients was three days median. Within 30 days after PDT the following adverse events were recorded: 21 cases of cholangitis, two cases of E. coli sepsis and one case each of Klebsiella sepsis, liver abscess, non-ST-elevated myocardial infarction, transfusion-dependent anemia, Clostridium difficile colitis and acute renal failure. Importantly, no case of phototoxicity occurred. There was neither an obvious association of adverse events to other clinical parameters nor an imbalance in occurrence among the study centers.
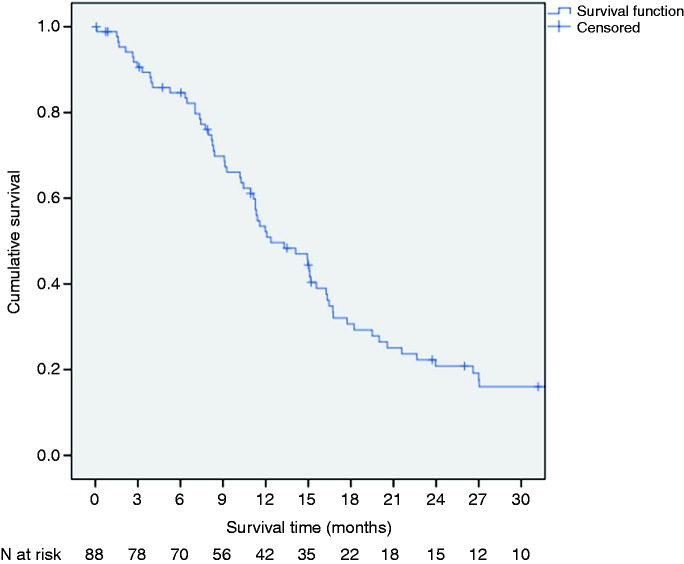
Thirty-day and 90-day mortality (referring to the first PDT procedure) were 1% (1/88) and 8% (7/88), respectively (three patients died due to tumor cachexia after 64, 79, and 81 days; two patients died from acute renal failure after 3 and 45 days; two patients died due to cholangitis after 48 and 50 days; and one patient died due to sepsis after 89 days). The median survival was 12.4 months (95% CI 9.7–14.9 m) calculated from the first PDT procedure and 15.6 months (95% CI 12.3–18.7 m) calculated from the date of initial diagnosis (Figure 2).
Figure 2.
Kaplan–Meier survival curve of the study patients.
Calculation starts on the day of the first photodynamic therapy. Median survival is 12.4 months (95% confidence interval (CI) 9.7–14.9 months).
Comparing biliary tract cancer (Klatskin tumor, gallbladder carcinoma and distal CCa) to mCRC as underlying tumor entity, there was a significant difference in the overall survival calculated from the date of initial diagnosis (15.5 vs. 128.1 m, p = 0.005) but not from the first PDT procedure (12.1 vs. 16.3 m, p =0.265). In biliary tract cancer, univariate analysis revealed concomitant chemotherapy (p = 0.042) as well as the number of PDT treatment sessions (p = 0.025) but not age (p = 0.111), stage of Klatskin tumor (p = 0.865), stent material (p = 0.134) or stent side (p = 0.635) as independent predictors of survival. At multivariate analysis only the number of PDT treatment sessions (p = 0.048) remained significant (Table 2).
Table 2.
Univariate and multivariate analysis of independent predictors of survival in biliary tract cancer patients
| N | Median survival after first PDT | p (univariate) | p (multivariate) | Hazard ratio (95% CI) | |
|---|---|---|---|---|---|
| Age | |||||
| < 60 years | 19 | 15.6 m | 0.111 | – | – |
| ≥ 60 years | 65 | 11.6 m | |||
| Klatskin typea | |||||
| I-II | 7 | 20.6 m | 0.865 | – | – |
| III-IV | 72 | 12.1 m | |||
| Stent material | |||||
| Metal | 55 | 14.1 m | 0.134 | – | – |
| Plastic | 28 | 6.3 m | |||
| Stent side | |||||
| Unilateral | 37 | 15.0 m | 0.635 | – | – |
| Bilateral | 46 | 10.3 m | |||
| Chemotherapy | |||||
| YES | 20 | 16.7 m | 0.042 | 0.137 | 1.59 (0.86–2.94) |
| NO | 64 | 11.4 m | |||
| PDT sessions | |||||
| >1 | 26 | 16.7 m | 0.025 | 0.048 | 1.70 (1.01–2.87) |
| 1 | 58 | 10.2 m |
According to Bismuth–Corlette Classification, other underlying diseases excluded.
CI: confidence interval; PDT: photodynamic therapy; m: months.
Discussion
This study reports on the largest clinical experience with biliary PDT using polyhematoporphyrin reported so far. All consecutive applications in Austria over a period of 10 years had been collected to represent a nationwide data set also including long-term follow up data, stent patency and survival. In general, PDT with polyhematoporphyrin was well tolerated and proved to be feasible and safe in this large retrospective series.
PDT has been studied for many other clinical applications such as lung cancer, tumors of the skin or age-related wet macular degeneration.10–12 In gastroenterology, it has also been used for the treatment of Barrett’s esophagus and associated dysplasia but its role in the esophagus has decreased because of the high rate of treatment-emergent adverse events, above all the development of strictures in up to one-third of applications.13 In our study, the most frequent adverse event was cholangitis, experienced by almost a quarter of our study population (21/88). This is relatively high compared to previous data on PDT14,15 and, of course, might not be associated with PDT alone but also with the underlying malignant biliary obstruction or the biliary manipulation during ERC. However, occurring without any obvious association with other clinical parameters and observed with the same frequency among all participating centers, cholangitis might be mainly attributed to local inflammation after PDT.
A median stent patency of 246 days can be seen as a surrogate of PDT efficacy in this study as it is superior to most publications on solitary stenting. Consistent with previous data, metal stents showed a significantly longer patency than plastic stents.7,16 However, this finding has to be interpreted with caution since stent selection might have been influenced by the general condition of the patients. Therefore, overall survival is an even better surrogate of efficacy. The survival rates seen in our sample are within the range of previous publications.17–19 However, our overall survival is much lower than the overwhelming survival time of 21 months reported by Zoepf et al. in one of the first randomized, controlled trials of PDT.14 An obvious reason for that is the selection of patients. Whereas prospective studies used strict inclusion criteria, a retrospective feasibility study like ours rather reflects a real-life patient population including patients in whom other therapeutic options might have already failed. Keeping in mind that our retrospective sample is very heterogeneous (including also patients treated second line for recurrence after surgical resection), an overall survival of 15.6 months (from initial diagnosis until death) is still very impressive and much more than expected with best supportive care or biliary stenting alone.1,20
Similar overall survival rates have been recently published for biliary RFA, the only other local tumor therapy that can be applied by endoscopy. Our group found a median survival of 10.6 m from first RFA treatment and 17.9 months from initial diagnosis until death in a sample of 58 patients treated with 88 RFA procedures in Austria.7 A retrospective cohort study directly comparing RFA and PDT also found median survival rates of both methods that were not significantly different (9.6 vs. 7.5 months).21 The big advantage of RFA over PDT is that it can be used without previous injection of a photosensitizer and without any precautions of phototoxicity. This makes it less cumbersome for the treating physician, more comfortable for the patient and potentially cheaper for the institution. Nevertheless, two vascular adverse events (partial liver infarction) associated with biliary RFA treatment have been documented so far,7,22 therefore RFA has to be used very carefully, especially in proximal tumor strictures. Furthermore, biliary RFA has not yet proven its efficacy in a randomized, controlled trial.
Calculated from the date of initial diagnosis but not from the date of first PDT, patients suffering from mCRC showed a better overall survival than those suffering from biliary tract cancer in our study. This finding has to be interpreted with caution, since the subgroup of mCRC was very small (n = 4). However, it somehow reflects the clinical course of mCRC, in which curatively intended treatment may result in long-term remission of the disease while (late) relapses often represent a palliative setting with very similar treatment outcomes to other underlying diseases.
In this study, concomitant chemotherapy was detected as an independent predictor of survival at a univariate analysis but failed to be significant at a multivariate analysis where only the number of PDT treatment sessions remained to be independently predictive for the outcome. This is consistent with previous data,23–26 although it might inherit a methodological bias since patients unfit for chemotherapy might have a worse clinical performance status also associated with higher mortality. The same is true for the number of PDT treatments per patient as patients who live longer can also be treated repetitively. Recently, a similar finding was reported by another retrospective study that showed that a combination of repeated PDT and chemotherapy offered a significant survival benefit in patients with nonresectable hilar CCa.27
The retrospective design may be considered as the main shortcoming of our study. A selection bias caused by different underlying tumor entities as well as the heterogeneity of additional oncological therapies (as present in 29 of 88 patients) makes it difficult to draw general conclusions from this analysis. However, the strength of this study lies in its great sample size, which is much bigger than in most papers published on PDT so far. Furthermore, our dataset contains all PDT procedures performed with polyhematoporphyrin in Austria within the study period of 10 years; therefore, this can be seen as a very complete observational study.
In summary, PDT using polyhematoporphyrin proved to be feasible and safe in this nationwide, retrospective analysis. Survival data clearly implicate a beneficial effect of PDT treatment, even in an unselected real-life patient population. Prospective trials that compare PDT to other evolving treatment options such as biliary RFA are warranted to define the best therapeutic approach for patients suffering from malignant biliary obstruction.
Acknowledgments
We would like to acknowledge the following people who substantially contributed to the data collection in the context of this study: Lukas Booz, Manfred Fleischer, Hans Peter Gröchenig, Isolde Hinterberger, Yumiko Kamogawa, Martina Ortner, Kurt Possnig, Barbara Tribl and Michael Wasilewski.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflicts of interest
None declared.
References
- 1.de Groen PC, Gores GJ, LaRusso NF, et al. Biliary tract cancers. N Engl J Med 1999; 341: 1368–1378. [DOI] [PubMed] [Google Scholar]
- 2.Vauthey JN, Blumgart LH. Recent advances in the management of cholangiocarcinomas. Semin Liver Dis 1994; 14: 109–114. [DOI] [PubMed] [Google Scholar]
- 3.Larghi A, Tringali A, Lecca PG, et al. Management of hilar biliary strictures. Am J Gastroenterol 2008; 103: 458–473. [DOI] [PubMed] [Google Scholar]
- 4.Ortner MA, Liebetruth J, Schreiber S, et al. Photodynamic therapy of nonresectable cholangiocarcinoma. Gastroenterology 1998; 114: 536–542. [DOI] [PubMed] [Google Scholar]
- 5.Steel AW, Postgate AJ, Khorsandi S, et al. Endoscopically applied radiofrequency ablation appears to be safe in the treatment of malignant biliary obstruction. Gastrointest Endosc 2011; 73: 149–153. [DOI] [PubMed] [Google Scholar]
- 6.Berr F, Wiedmann M, Tannapfel A, et al. Photodynamic therapy for advanced bile duct cancer: Evidence for improved palliation and extended survival. Hepatology 2000; 31: 291–298. [DOI] [PubMed] [Google Scholar]
- 7.Dolak W, Schreiber F, Schwaighofer H, et al. Endoscopic radiofrequency ablation for malignant biliary obstruction: A nationwide retrospective study of 84 consecutive applications. Surg Endosc 2014; 28: 854–860. [DOI] [PubMed] [Google Scholar]
- 8.Gao F, Bai Y, Ma SR, et al. Systematic review: Photodynamic therapy for unresectable cholangiocarcinoma. J Hepatobiliary Pancreat Sci 2010; 17: 125–131. [DOI] [PubMed] [Google Scholar]
- 9.Lu Y, Liu L, Wu JC, et al. Efficacy and safety of photodynamic therapy for unresectable cholangiocarcinoma: A meta-analysis. Clin Res Hepatol Gastroenterol 2015; 39: 718–724. [DOI] [PubMed] [Google Scholar]
- 10.Dougherty TJ. Hematoporphyrin derivative for detection and treatment of cancer. J Surg Oncol 1980; 15: 209–210. [DOI] [PubMed] [Google Scholar]
- 11.Morton CA, MacKie RM, Whitehurst C, et al. Photodynamic therapy for basal cell carcinoma: Effect of tumor thickness and duration of photosensitizer application on response. Arch Dermatol 1998; 134: 248–249. [DOI] [PubMed] [Google Scholar]
- 12.Arroyo JG, Michaud N, Jakobiec FA. Choroidal neovascular membranes treated with photodynamic therapy. Arch Ophthalmol 2003; 121: 898–903. [DOI] [PubMed] [Google Scholar]
- 13.Overholt BF, Wang KK, Burdick JS, et al. Five-year efficacy and safety of photodynamic therapy with Photofrin in Barrett’s high-grade dysplasia. Gastrointest Endosc 2007; 66: 460–468. [DOI] [PubMed] [Google Scholar]
- 14.Zoepf T, Jakobs R, Arnold JC, et al. Palliation of nonresectable bile duct cancer: Improved survival after photodynamic therapy. Am J Gastroenterol 2005; 100: 2426–2430. [DOI] [PubMed] [Google Scholar]
- 15.Pereira SP, Aithal GP, Ragunath K, et al. Safety and long term efficacy of porfimer sodium photodynamic therapy in locally advanced biliary tract carcinoma. Photodiagnosis Photodyn Ther 2012; 9: 287–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaassis M, Boyer J, Dumas R, et al. Plastic or metal stents for malignant stricture of the common bile duct? Results of a randomized prospective study. Gastrointest Endosc 2003; 57: 178–182. [DOI] [PubMed] [Google Scholar]
- 17.Ortner ME, Caca K, Berr F, et al. Successful photodynamic therapy for nonresectable cholangiocarcinoma: A randomized prospective study. Gastroenterology 2003; 125: 1355–1363. [DOI] [PubMed] [Google Scholar]
- 18.Harewood GC, Baron TH, Rumalla A, et al. Pilot study to assess patient outcomes following endoscopic application of photodynamic therapy for advanced cholangiocarcinoma. J Gastroenterol Hepatol 2005; 20: 415–420. [DOI] [PubMed] [Google Scholar]
- 19.Kahaleh M, Mishra R, Shami VM, et al. Unresectable cholangiocarcinoma: Comparison of survival in biliary stenting alone versus stenting with photodynamic therapy. Clin Gastroenterol Hepatol 2008; 6: 290–297. [DOI] [PubMed] [Google Scholar]
- 20.Kullman E, Frozanpor F, Soderlund C, et al. Covered versus uncovered self-expandable nitinol stents in the palliative treatment of malignant distal biliary obstruction: Results from a randomized, multicenter study. Gastrointest Endosc 2010; 72: 915–923. [DOI] [PubMed] [Google Scholar]
- 21.Strand DS, Cosgrove ND, Patrie JT, et al. ERCP-directed radiofrequency ablation and photodynamic therapy are associated with comparable survival in the treatment of unresectable cholangiocarcinoma. Gastrointest Endosc 2014; 80: 794–804. [DOI] [PubMed] [Google Scholar]
- 22.Topazian M, Levy MJ, Patel S, et al. Hepatic artery pseudoaneurysm formation following intraductal biliary radiofrequency ablation. Endoscopy 2013; 45(Suppl 2 UCTN): E161–E162. [DOI] [PubMed] [Google Scholar]
- 23.Park do H, Lee SS, Park SE, et al. Randomised phase II trial of photodynamic therapy plus oral fluoropyrimidine, S-1, versus photodynamic therapy alone for unresectable hilar cholangiocarcinoma. Eur J Cancer 2014; 50: 1259–1268. [DOI] [PubMed] [Google Scholar]
- 24.Hong MJ, Cheon YK, Lee EJ, et al. Long-term outcome of photodynamic therapy with systemic chemotherapy compared to photodynamic therapy alone in patients with advanced hilar cholangiocarcinoma. Gut Liver 2014; 8: 318–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheon YK, Lee TY, Lee SM, et al. Longterm outcome of photodynamic therapy compared with biliary stenting alone in patients with advanced hilar cholangiocarcinoma. HPB (Oxford) 2012; 14: 185–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Höblinger A, Gerhardt T, Gonzalez-Carmona MA, et al. Feasibility and safety of long-term photodynamic therapy (PDT) in the palliative treatment of patients with hilar cholangiocarcinoma. Eur J Med Res 2011; 16: 391–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wentrup R, Winkelmann N, Mitroshkin A, et al. Photodynamic therapy plus chemotherapy compared with photodynamic therapy alone in hilar nonresectable cholangiocarcinoma. Gut Liver 2016; 10: 470–475. [DOI] [PMC free article] [PubMed] [Google Scholar]