Abstract
How species-specific developmental timing is controlled is largely unknown. By following human embryonic stem (ES) cell and mouse epiblast stem (EpiS) cell differentiation through detailed RNA-sequencing time courses, here we show that pluripotent stem cells closely retain in vivo species-specific developmental timing in vitro. In identical neural differentiation conditions in vitro, gene expression profiles are accelerated in mouse EpiS cells compared to human ES cells, with relative rates of differentiation closely reflecting the rates of progression through the Carnegie stages in utero. Dynamic Time Warping analysis identified 3,389 genes that were regulated more quickly in mouse EpiS cells, and identified none that were regulated more quickly in human ES cells. Interestingly, we also find that human ES cells differentiated in teratomas maintain the same rate of differentiation observed in vitro, in spite of being grown in a mouse host. These results suggest the existence of a cell autonomous, species-specific developmental clock that pluripotent stem cells maintain even out of context of an intact embryo.
Keywords: developmental time, differentiation, embryonic stem cells, brain development
1. INTRODUCTION
A central challenge for the human embryonic stem (ES) cell and induced pluripotent stem (iPS) cell field is that differentiation rates reflect a species with a nine month gestation period, with protocols often requiring several months (Shi et al., 2012b, Ebert et al., 2009, Espuny-Camacho et al., 2013, Krencik et al., 2011, Kriks et al., 2011). The extended time required for producing specific cell types and the lack of physiological maturity are substantial obstacles to the clinical use of human pluripotent stem cells (Saha and Jaenisch, 2009, Broccoli et al., 2014). Understanding what controls species-specific rates of differentiation is essential for determining if and how these rates can be altered.
Although it is known that human pluripotent stem cell differentiation generally takes longer than mouse pluripotent stem cell differentiation, a detailed examination of these species-specific differentiation rates is lacking. In this study, we examined the differences in species-specific developmental timing by measuring rates of human and mouse pluripotent stem cell differentiation under identical anterior neural differentiation conditions in vitro through comprehensive RNA-sequencing (RNA-seq) time courses. When compared to Carnegie stage progression in utero, we found a remarkable similarity between in utero and in vitro rates of differentiation. Furthermore, we tested the autonomy of this timing by following human ES cell differentiation in teratomas by RNA-seq to assess if mouse host factors were capable of accelerating rates of development. We found that human timing was maintained in teratomas, despite being exposed to murine host factors. These results provide evidence for an autonomous, species-specific developmental clock.
2. RESULTS
2.1 Species-Specific Timing is Maintained During Neural Differentiation In Vitro
We first examined if species-specific developmental timing is maintained outside of the embryo (ex utero) by differentiating human and mouse pluripotent stem cells towards neural lineages under identical conditions in vitro. Mouse EpiS cells and human ES cells were compared because they represent closely related developmental stages and thus respond similarly to growth factors (Brons et al., 2007, Greber et al., 2010, Tesar et al., 2007, Rossant and Tam, 2017). A single defined neural differentiation medium was used throughout the time course to differentiate cells to the early forebrain and neocortex (see materials and methods) (Espuny-Camacho et al., 2013, Levine and Brivanlou, 2007, Chambers et al., 2009). Enhanced green fluorescent protein (EGFP)-positive cell lines were used to remain consistent with the teratoma tracking studies later in this report. Mouse EpiS cells and human ES cells cultured in this defined neural differentiation medium induced similar homogeneous expression of neural marker PAX6 (Zhang et al., 2010), reaching maximal expression at day 3 in mouse and day 5 for human cells (Fig. S1).
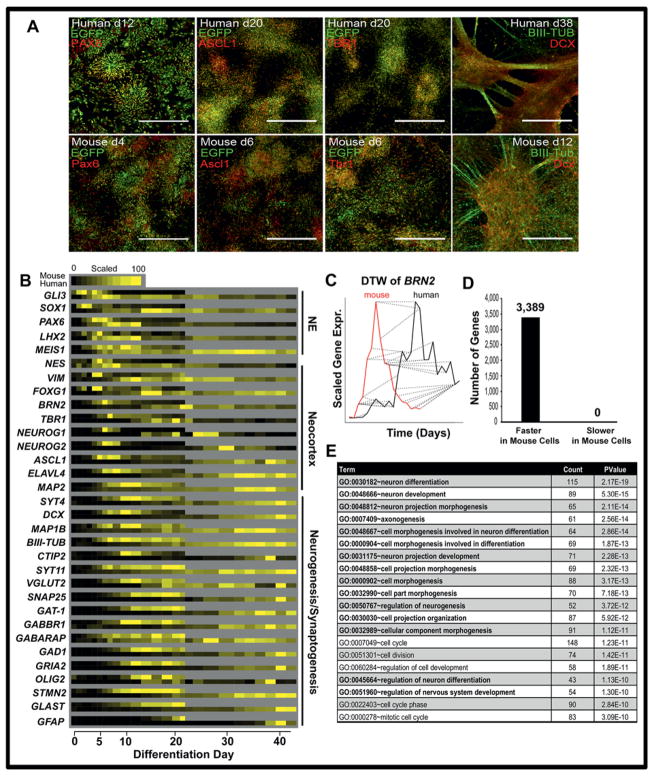
Mouse and human cell differentiation were followed for 3 and 6 weeks, respectively, and RNA-seq was performed on samples taken every day for the first 8 days, then every other day for the remainder of the time course. PAX6-positive neural rosettes were detected by immunofluorescence at days 4 and 12 for mouse and human samples, respectively (Fig. 1A). Mouse cells expressed neocortex markers Ascl1 and Tbr1 after 6 days of differentiation, while human cells required 20 days to achieve similar marker expression and cell morphology (Fig. 1A). After 12 days for mouse and 38 days for human cells, neurons appeared elongated with bundles of cable-like projections characterized with axonogenic proteins BIII-TUB and DCX.
Figure 1. In vitro neural differentiation occurs more quickly in mouse EpiS cells compared to human ES cells.
(A) EGFP-H1 and EGFP–mouse EpiS cells were exposed to identical differentiation conditions on day 0 and were fixed and stained with the indicated antibodies at various time points. Samples were imaged on a Nikon confocal A1R microscope (scale bars= 250 μm). Other samples were lysed at regular time intervals and subjected to RNAseq (B). Expression of gene TPMs were scaled from their minimum (0) to maximum (100) values to compare dynamic ranges between mouse and human samples. Classical gene markers of embryonic neuroectoderm (NE), forebrain, neocortex, neurogenesis, and synapse formation are shown. (C) BRN2 expression is shown as an example of a gene identified as accelerated in mouse (red) compared to human cells (black) using DTW analysis by identifying and warping similarly patterned regions (dotted lines). Global DTW was applied to all genes to identify significantly faster genes (p<0.01) in mouse compared to human cells during in vitro neural differentiation (D). (E) The 2,000 most significantly accelerated genes were screened for enriched functional GO terms using the DAVID functional annotation tool. The top 20 terms are shown, and neural cell differentiation-related terms are in bold.
The RNA-seq time course revealed that key neural regulatory genes were upregulated in mouse cells before their human orthologs (Fig. 1B). Neuroectoderm gene expression (e.g., PAX6, SOX1) preceded neurogenic and embryonic neocortex markers (e.g. FOXG1, BRN2, TBR1, NEUROG2, ASCL1), which is consistent with previous reports (Chambers et al., 2009, Espuny-Camacho et al., 2013, Gaspard et al., 2008, Shen et al., 2006, Shi et al., 2012b, van de Leemput et al., 2014). Genes involved in axonogenesis (MAP2, DCX, BIII-TUB), neural stem cell expansion (e.g. GLAST, OLIG2, GFAP), and neural maturation and synapse formation (e.g. SYT4, SYT11, SNAP25) were all temporally upregulated in mouse EpiS cells before human ES cells (Fig. 1B). GABAergic and Glutamatergic-specific neural gene expression (e.g. GABBR1, GAD1, VGLUT2, and GRIA2), markers of the maturing synapses in the embryonic neocortex, were also expressed earlier in mouse cells. Thus, classic neural markers were expressed more quickly in mouse than human cells.
2.2 Temporal Differences in Gene Expression Across Species Identified by Dynamic Time Warping (DTW) and Pearson correlation analyses
Next, we broadly identified genes which were regulated significantly (p<0.01) more quickly in one species compared to the other. Originally developed for speech pattern recognition (Sakoe, 1978), DTW is a powerful tool for comparing two independent time series that share similar patterns operating at different speeds. By calculating pattern matches, the algorithm locally compresses or stretches the time frame of one series to best fit a similar pattern in another and statistically assess differences in pattern velocity (Fig. 1C). DTW has previously been adapted to identify changes in gene expression in time series data from a single species (Aach and Church, 2001, Wexler et al., 2011), but here we employed the approach in a novel fashion to compare temporal expression data across two species during in vitro differentiation (see materials and methods). After screening for genes that: (1) shared homologs in both humans and mice, (2) exhibited significant expression thresholds and dynamic ranges, and (3) demonstrated significant correlations of pattern similarities, we applied a DTW algorithm package (Giorgino, 2009) to the in vitro RNA-seq data. During neural differentiation under identical conditions, 3,389 dynamic genes were identified as regulated more quickly in mouse than in human cells, and none were identified as slower in mouse cells than in human cells (Fig. 1D). The top 2,000 most significantly accelerated genes were screened for functional enrichment using DAVID Gene Ontology (GO) terms to elucidate the types of genes identified (Huang da et al., 2009b, Huang da et al., 2009a). Several neural categories were significantly enriched, including the top 13 categories (Fig. 1E), illustrating that genes driving neural development are expressed more quickly in mouse than human cells during neocortex differentiation in vitro.
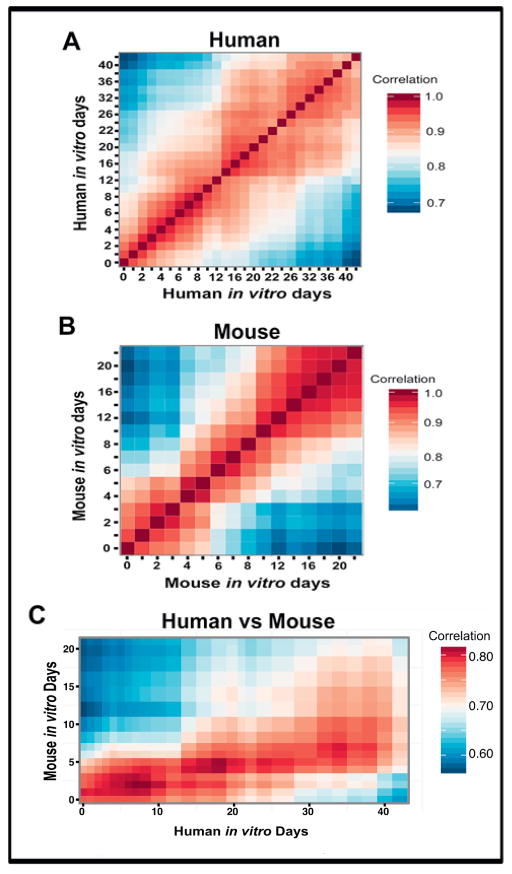
We also compared global rates of neural differentiation between mouse and human cells using sample correlation analyses of neural gene expression over time. Pearson correlation analyses were carried out using a list of 3,061 neural genes accumulated from combining three separate neural GO gene lists (see materials and methods). As expected, human in vitro samples correlated most strongly with other adjacent human in vitro time points (Fig. 2A), as did the mouse samples compared to other mouse time points (Fig. 2B). However, when human cells were correlated to mouse cells over time, a rather striking change in slope emerged (Fig. 2C). Mouse time points correlated most significantly with progressively later human samples; this distorted the stepwise slope expected if rates of differentiation were equivalent between species (Fig. 2C). For example, in vitro day 3 of mouse differentiation correlated most strongly with day 7 human, day 5 mouse with day 18 human, and day 7 mouse with day 34 human, indicating that mouse cells are differentiating more quickly than their human counterparts on a global level.
Figure 2. Sample correlations across species reveal that global changes in neural gene expression occur more quickly in mouse compared to human cell differentiation.
Pearson correlations of 3,061 neural genes were applied to either human to human samples (A), mouse to mouse samples (B), or human to mouse RNA-seq samples (C).
2.3 The rate of differentiation of human ES cells is maintained in teratomas
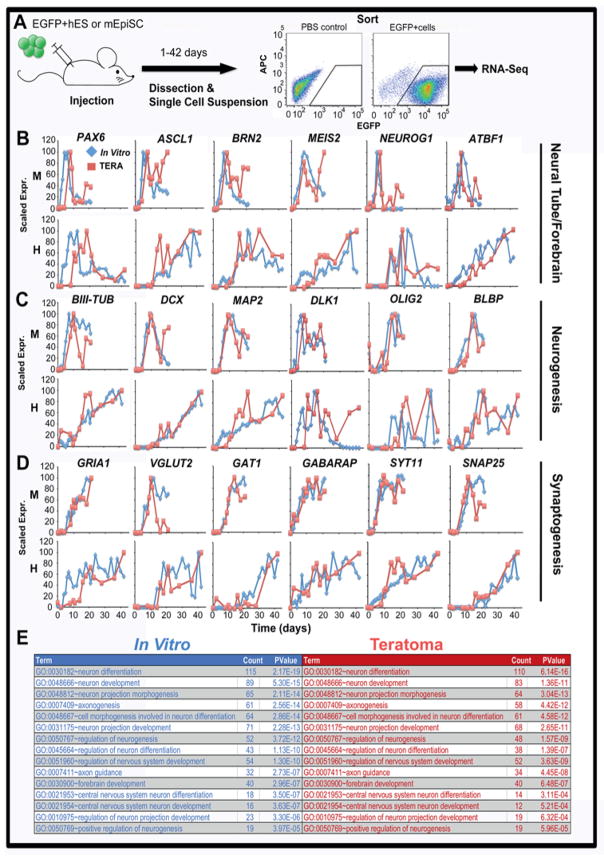
We next examined whether a mouse host would influence the timing of neural or other lineage differentiation of human ES cells in teratomas. EGFP-human ES (H1) cells or EGFP-mouse EpiS cells were injected intramuscularly into immunocompromised mice at day 0 (Fig. 3A). Mice were sacrificed at days 2, 8, 10, 11, 14, 16, 18, 21, 23, 28, 36, and 42 for human ES cells and at days 1, 2, 4, 8, 9, 10, 11, 14, 16, 18, and 21 for mouse EpiS cell-derived teratomas. Teratomas were dissected from host tissues, digested into single cell suspensions, sorted for EGFP expression by fluorescence-activated cell sorting (FACS), and analyzed by RNA-seq (Fig. 3A).
Figure 3. Neural gene expression patterns in teratomas closely mirrors those observed in vitro in a species-dependent manner.
(A) EGFP-positive human ES or mouse EpiS cells were intramuscularly-injected into immunocompromised mice, and teratoma differentiation was followed by isolating EGFP-positive cells by FACS and sequencing transcriptomes. In vitro scaled gene expression trends (blue) were compared with those observed during teratoma formation for mouse (M) and human (H) cells (B–D). Representative genes of the neural tube early forebrain development (B), neurogenesis and neural precursor expansion (C), and GABAergic and Glutamatergic neuron identity and synapse function are shown (D). (E) The top 2,000 DTW-identified accelerated genes in mouse compared to human pluripotent stem cells during in vitro neural differentiation and teratoma formation were enriched for GO terms using the DAVID functional annotation tool. The top 15 neural development-related terms identified during in vitro neural differentiation (blue) were also identified as significantly enriched (p<0.001) in genes accelerated in mouse compared to human teratomas (red).
Neurogenic genes were examined in order to compare rates of neural differentiation in teratomas with those observed in vitro. Despite the presence of mesoderm and endoderm lineages in the teratomas (which also peaked more quickly in mouse than human teratomas, Fig. S2), neural gene expression timing was remarkably similar to the timing observed in vitro (Fig. 3). When graphed together, temporal regulation of representative genes of early neural tube and forebrain development including PAX6, ASCL1, BRN2, MEIS2, NEUROG1, and ATBF1 in teratomas paralleled those observed in vitro in a species-dependent manner (Fig. 3B). Subsequently, genes involved in both axono-/neurogenesis (BIII-TUB, DCX, MAP2, DLK1) and neural stem cell identity (OLIG2, BLBP) in teratomas also closely mirrored the temporal dynamics observed in vitro (Fig. 3C). Finally, genes involved in glutamatergic and GABAergic neuron identity and maturation as well as synapse function also followed similar expression timelines in teratomas and in vitro in a species-dependent fashion (Fig. 3D). Thus, the human-specific developmental timing observed in vitro was largely maintained in teratomas despite the presence of other germ layer lineages, and despite being formed in a mouse host.
DTW analysis identified 3,248 temporally regulated genes significantly accelerated (p<0.01) in mouse teratomas compared to human teratomas, and zero that were slower (Fig. S3A). When the 2,000 most significantly accelerated genes in teratomas were screened for function by the DAVID GO functional annotation tool, the list of terms involved in neurogenesis was enriched in the top 20 terms (Fig. S3B). Indeed, 15 out of the top 15 neural-specific GO terms enriched for genes accelerated in vitro were also significantly enriched for accelerated genes in teratomas (p<0.001, Fig. 3E), indicating that similar species-specific differences in temporal gene expression occurred in vitro and in teratomas.
Furthermore, we applied Pearson correlation analyses between samples to more globally compare rates of differentiation in teratomas to rates in vitro. Human teratoma sample differentiation progression corresponded to human in vitro progression, but with a delay during the initial onset of differentiation, confirming that the rate of differentiation in human teratomas was not accelerated by the mouse environment (Fig. S4A). Similarly, mouse teratoma samples correlated well with mouse in vitro day equivalents (Fig. S4B), supporting the notion that in vitro rates of species-specific developmental timing were maintained in teratomas.
2.4 Rates of Neural Differentiation Ex Utero Mirror Those of Carnegie Stage Progression In Utero In a Species-Specific Manner
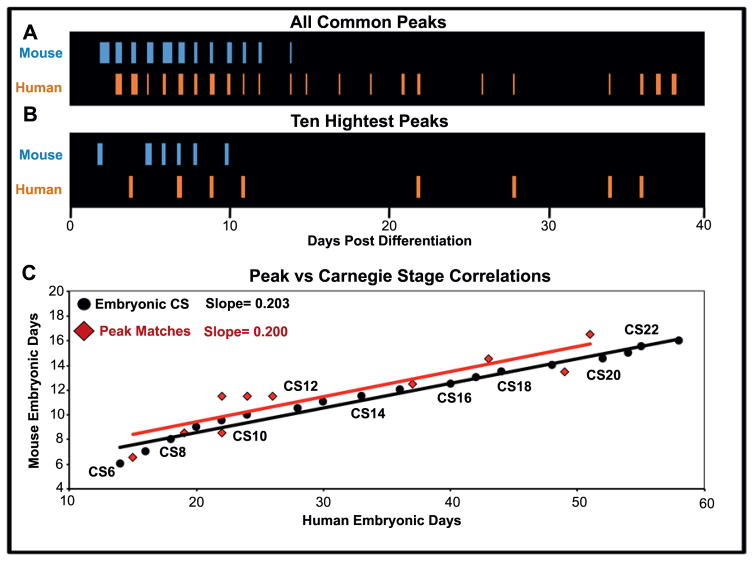
In order to compare rates of differentiation ex utero directly with those in utero, we compared the timing of neural gene peaks in mouse relative to human cells in vitro with Carnegie stage progression during gestation. First, peaks in gene expression were identified in mouse and human differentiation time courses by segmentation regression analysis (see materials and methods). A minimum of three days was used to identify individual segments, and segment breakpoints constituting an increasing and subsequently decreasing segment were identified as peaks. Overall, 187 genes were identified as having peaks in both mouse and human time courses (Fig. 4A). Using the 10 neural genes identified as having the highest amplitude in human samples over time (Fig. 4B; see materials and methods), we graphed genes based on when they shared their peak in both human and mouse time courses (Fig. 4C, red).
Figure 4. Correlation of mouse and human gene expression peaks identified by segmentation regression analysis closely mirror CS progression.
(A) Gene expression profiles with peaks identified by segmentation regression analysis are indicated over time post differentiation for mouse (blue) and human (orange) cells, and the 10 peaks with the highest amplitudes in human samples are shown in (B) with their mouse counterparts. (C) The gene peaks from B found in human and mouse samples were plotted over time (after adding 6.5 days to mouse samples and 15 days to human samples to transpose to embryonic day equivalents), and were overlaid with in utero Carnegie stages (CS, in black). The linear trendlines and their slopes are shown in their respective colors.
Next, we transposed in vitro differentiation days into embryonic day equivalents. Because a recent study suggested that mouse EpiS cells most closely reflect an embryonic age of 6.5–7.0 days post fertilization (E6.5–7.0) in the mouse embryo (Kojima et al., 2014), we assigned “day 0” mouse EpiS cells to be the equivalent of in vivo E6.5. As both human ES cells and mouse EpiS cells are believed to represent a similar phase in development (Brons et al., 2007, Mascetti and Pedersen, 2016, Tesar et al., 2007, Rossant and Tam, 2017), we also set day 0 human ES cells to represent 15 days post fertilization in the human embryo, a roughly equivalent stage to the mouse at E6.5.
After adjusting in vitro differentiation days to embryonic time by adding 6.5 days to mouse peak times and 15 days to human peak times, Carnegie stage progression during gestation was overlaid with our sample peak correlations to compare rates of differentiation of pluripotent stem cells within and without the embryo (Fig. 4C). Trendlines were calculated in Excel using a best-fit linear straight line function to identify progression rates for both Carnegie stages in utero (black) and in vitro gene expression peaks (red). Strikingly, superimposed trendlines from the matched peaks (red) and Carnegie stages (black) exhibited nearly identical slopes (Fig. 4C), indicating that differentiation rates are remarkably similar in utero and ex utero, and reinforcing the idea that a cell autonomous clock is driving differentiation rates ex utero in a species-specific manner.
3. DISCUSSION
In this study, we measured rates of human and mouse pluripotent stem cell neural differentiation in vitro and in teratomas, and compared them with rates of development in utero. We found that pluripotent stem cells maintained species-specific rates of differentiation in both conditions ex utero, and identified thousands of genes with species-specific differences in temporal gene expression in mouse cells compared to human cells. Although in this study we selected a simple single-inhibitor protocol that functions for both human and mouse anterior neural cell differentiation, other protocols using a variety of pathway inhibitor combinations, as well as both 2D and 3D culture systems, all result in similar neural marker expression timing (Chambers et al., 2009, Espuny-Camacho et al., 2013, Gaspard et al., 2008, Maroof et al., 2013, Shi et al., 2012a, van de Leemput et al., 2014, Kelava and Lancaster, 2016). Interestingly, when differentiated in teratomas in a mouse host, human ES cells maintained their own rather than their host species developmental timing, demonstrating that host factors failed to accelerate the donor developmental clock. Although it is generally recognized that differentiation procedures can take longer for human ES/iPS cells than for mouse ES/iPS cells, our results highlight how very closely species-specific developmental timing is maintained by pluripotent stem cells ex utero, and suggest that developmental timing involves a remarkable degree of cell autonomy.
Species-specific autonomy in size regulation or timing has been demonstrated in other biological systems at either the cell or rudiment level. Classical amphibian transplantation experiments have shown that when either eye or limb bud rudiments are transplanted between species of different sizes, the species source of the rudiment, and not the host, largely determined final structure size (Harrison, 1924, Twitty, 1931). More recently, chick limb transplants between chicks at different developmental stages also exhibited retention of donor stage-specific intrinsic time (Saiz-Lopez et al., 2015). Experiments in mice and rats have also demonstrated retention of cell autonomy in the timing of neural and glial precursor differentiation, fate choices, and apoptosis in transplants and ex vivo cultures (Watanabe and Raff, 1990, Southwell et al., 2012, Shen et al., 2006, Raff, 2007, Gao et al., 1997). During neural differentiation in vitro, regulation of cell cycle and post-mitotic states of maturation are also retained by human and non-human primate pluripotent cells according to their species of origin (Otani et al., 2016, Mora-Bermudez et al., 2016).
Mammalian development is highly regulative, with key developmental decisions controlled by inductive interactions between different tissues. Thus although our results suggest the existence of cell autonomous, species-specific developmental timing, this doesn’t mean that external signals cannot influence that timing. While in this study we found that developmental timing is maintained during anterior neural differentiation, we cannot necessarily extrapolate that species-specific timing is as robustly maintained during the differentiation of all other organ systems. Some reports have suggested that developmental timing can be at least modestly accelerated (Buchholz et al., 2013, Chan et al., 2013, Gaur et al., 2010, Parchem et al., 2015, Sasaki et al., 2010, Zhu et al., 2013). For example, by modifying culture conditions, the time required to derive different types of neural cells from human ES cells has been reduced (Chambers et al., 2012, Sasaki et al., 2010, Amoroso et al., 2013). Similarly, injecting human ES cells into the lateral ventricles of E14 mouse embryos was reported to accelerate production of neural cells (Muotri et al., 2005). In another model, differentiation of pancreatic beta cells was also sped up compared to all other previous in vitro protocols (Pagliuca et al., 2014). Because the timing of many embryonic events during human development is often not known accurately due to the lack of embryonic material, it is often unclear from these studies whether developmental timing was altered per se, or if the apparent acceleration was due to improving conditions such that the rate of differentiation better approximated the actual in vivo rate (Chen et al., 2014, Ozeki et al., 2012, Tadeu et al., 2015). Whether exposing human pluripotent stem cell to a mouse embryonic environment can more dramatically accelerate differentiation is currently unclear. Recent studies creating chimeras between human pluripotent stem cells and mouse embryos have demonstrated survival and integration of the human cells, but the differentiation status of the integrated human cells was poorly characterized (Gafni et al., 2013, Mascetti and Pedersen, 2016).
The retention of species-specific developmental timing by pluripotent stem cells presents a practical challenge for regenerative medicine, but also represents an opportunity, as pluripotent stem cells offer a new tissue culture model to study the relationship between developmental timing and scale in mammals. The extreme range of body sizes in mammals, from the ~1.8g Etruscan shrew (Jurgens, 2002) to the ~180 metric ton blue whale (Mackintosh, 1929), has fascinated developmental biologist for years (Bonner, 2006). It has long been appreciated that larger bodies generally take longer to develop to maturity than smaller ones, yet remarkably little is known about how body size is regulated between species. Because conserved developmental milestones stretch out in time as body size increases, one key element to understanding the control of body size is an understanding of the control of developmental timing. Since species-specific developmental timing is maintained by pluripotent stem cells, iPS cells offer a new opportunity to study the control of developmental timing across the entire range of mammalian body sizes. For although it was previously impossible to perform experimental embryology in a blue whale, iPS cells could effectively bring whale embryology into the laboratory.
4. MATERIALS AND METHODS
4.1 Derivation and Culture of EGFP-H1 Cells
H1 human ES cells were cultured and passed as previously described (Chen et al., 2011). Cells were maintained in E8 medium (Thermo Fisher Scientific, USA), on matrigel-coated tissue culture plates and split with EDTA. EGFP-positive cells were derived by electroporation of a pUC plasmid encoding the EGFP gene driven by the EF1α promoter. EGFP-expressing clones were generated by sorting single cells into 96-well plates by FACS on a BD Aria III (Becton Dickinson, USA). Clones resistant to gene silencing during differentiation and karyotypically normal (WiCell, WI, USA) were selected for experiments going forward.
4.2 Derivation and Culture of EGFP-mouse EpiS cells
Male and female C57BL/6-Tg(CAG-EGFP)1Osb/J (JAX Stock No. 003291) mice were intercrossed for one overnight period (0.5-day; dark-cycle). The next morning the female mice were examined for the presence of a copulatory plug. The uterine tract of timed pregnant female C57BL/6-Tg(CAG-EGFP)1Osb/J mice, at 5.5 dpc, were dissected and transferred to a 60-mm petri dish filled with FHM media (Millipore, MA, USA; MR-025-D). The embryos were dissected and grown as previously described (Brons et al., 2007, Tesar et al., 2007). Cells were maintained on P1–P3 murine embryonic fibroblasts (MEFs) in growth medium containing 20% knockout serum replacement, 0.18mM B-mercaptoethanol, 1Xnon-essential amino acids, 2mM L-glutamine, 7.5ng/mL activin A, and 5ng/mL bFGF in DMEM/F12. Cells were split by TrypLE-mediated dissociation (Thermo Fisher Scientific, USA) and replated onto MEFs with 10μM Y27632 ROCK inhibitor overnight to increase cell survival (R&D Systems, MN, USA). EGFP-negative cells were removed by cell sorting and the resulting clones maintained 100% EGFP-positive cells for the duration of the experiments. Cells were karyotyped to confirm the absence of genetic abnormalities (WiCell, USA).
4.3 In Vitro Neural Differentiation
H1 and mouse EpiS cells were dissociated at d0 (EDTA for H1 and TrypLE for mouse EpiS cells) and seeded onto matrigel-coated 6-well plates (for RNA sequencing and FACS analysis) or 12-well plates (for immunofluorescence). Neural differentiation medium consisted of DFS3 (DMEM/F12, 64 mg/L L-ascorbic acid phosphate Mg salt, 0.014 mg/L sodium selenite, 1,743 mg/L sodium bicarbonate; Thermo Fisher Scientific, USA) complemented with N2 and B27 supplements (Thermo Fisher Scientific, USA) and 100 ng/mL Noggin (R&D Systems, MN, USA). Since human cells only became post-mitotic at much later time points than mouse cells, cells were split 2–3 more times throughout the experiment to day 42. Cells were fed every day throughout the time course.
At collection time points for RNA sequencing, mouse and human cells were washed once in phosphate buffered saline (PBS), lysed in 350μL RLT-PLUS buffer (Qiagen, CA, USA), and stored at −80°C until further processing (see sample processing below). For protein expression analysis by immunofluorescence, cells were washed once in PBS and incubated at room temperature with 2% paraformaldehyde in PBS for 15 mins. Cells were washed again with PBS and were stored in fresh PBS at 4°C until further processing.
4.4 Flow Cytometry
At the indicated time points, 350μL of TrypLE was added to each 6-well and incubated at 37°C until cells were singularized (1–5 mins) and transferred to 1.5mL non-stick microfuge tubes (Ambion). 1mL FACS buffer consisting of PBS with 5% fetal bovine serum was added to each tube and cells were spun at 400Xg for 3 mins. Cell pellets were resuspended in 500μL PBS and mixed with 500μL of 1% paraformaldehyde in PBS (0.5% working concentration), and incubated at 37°C for 10–15 mins. Cells were again washed with 1mL FACS buffer, and resuspended in 90% methanol in PBS pre-chilled to −20°C. Samples were kept at −20°C for a minimum of 18h.
For labeling, samples were spun down at 500Xg for 3 mins, washed once in 1mL FACS buffer, then incubated with PAX6 antibody (DSHB, Table S1) diluted 1:100 in FACS buffer at 4°C overnight. The next day cells were washed with 1mL FACS buffer and incubated for 30 mins in the dark at room temperature with a 1:500 dilution of Alexa647-conjugated Goat anti-mouse secondary antibody in FACS buffer. Cells were washed once more, run on a BD FACSCanto II, and analyzed using FlowJo 9.3 software (FlowJo, LLC).
4.5 Immunofluorescence Sample Preparation, Acquisition, and Image Processing
Samples were permeabilized and blocked in incubation buffer (0.25 % Triton X-100 and 1% bovine serum albumin in PBS) for 60 minutes. Samples were then treated with primary antibodies (see Table S1) in incubation buffer either at 4°C overnight or four hours at room temperature. Samples were rinsed with wash buffer (0.05% Triton X-100 in PBS; 2 x 60 minutes) and treated with incubation buffer at least 60 additional minutes at room temperature. Samples were treated with 1:200 secondary antibodies (Alexa-conjugated, Thermo Fisher Scientific, USA) and 5μg/mL DAPI (4′6-Diamidino-2-Phenylindole Dihydrochloride, MP Biomedicals, 157574) in incubation buffer overnight at 4°C or at least two hours at room temperature. Samples were mounted by adding a drop of Aqua Polymount solution (Polysciences, Inc.) to the well and placing a round glass coverslip over the top. The mounted samples were stored overnight at 4°C and then sealed around the edges with fingernail sealant until imaging.
Confocal immunofluorescence microscopy images were collected using a Nikon A1R laser scanning confocal microscope with Plan Apo 10x, Plan Fluor 20x Ph1 DLL, or Plan Apo 20x DIC M objectives. Images were processed using NIS Elements and ImageJ. Some z-stacks were aligned using the “Align Current ND Document” (NIS Elements) before creating maximum projection images.
4.6 Teratoma Formation and Harvest
5–6 week old female SCID-Beige mice (C.B-17/IcrHsd-PrkdcscidLystbg) were purchased from Envigo (USA). EGFP-H1 and EGFP-mouse EpiS cells were harvested from ~60% confluent 10-cm dishes and resuspended in 500uL of a 35%:65% mix of matrigel:growth medium spiked with 3μM Y27632 for TrypLE-lifted mEpiS cells. Each mouse was injected intramuscularly in the left thigh with 200μL of resuspended cells. 200μL of PBS was injected into opposing thighs as negative controls. From 1 day to 42 days post injection, mice were sacrificed by cervical dislocation and left thighs were surgically opened. With the aid of fluorescent goggles (BLS Ltd, Hungary), tissues containing EGFP-positive injected cells were dissected from surrounding tissues and were sliced as thin as possible with a razor blade in ice cold PBS. The resulting tissue clumps were spun down at 700Xg for 5 mins at 4°C and resuspended in 1mg/mL Collagenase/Dispase (Roche) in PBS and incubated in a 37°C water bath for 1h with vigorous pipetting every 15 mins to break up cell clumps. The cells were then washed in PBS containing 10% fetal bovine serum. Dead floating cell clumps and debris were suctioned off and cell pellets were resuspended in FACS buffer. Single cells were filtered through a 70μM cell strainer (BD Falcon) and EGFP-positive cells were sorted on a FACS Aria III into collection tubes maintained at 4°C. Sorted cells were then washed in FACS Buffer, and a maximum of 600,000 cells were resuspended in 350μL of RLT-PLUS buffer (Qiagen) and placed at −80°C until further RNA processing.
4.7 Sample Processing and Illumina RNA-Sequencing
Cell lysates were processed and sequenced as previously described (Hou et al., 2015). Briefly, total RNA was isolated using an RNeasy Plus Mini Kit (Qiagen). cDNA libraries were prepared and indexed with the Ligation Mediated Sequencing (LM-Seq) protocol. Final indexed cDNA libraries were pooled with six to eight uniquely indexed LM-Seq cDNA libraries per lane and sequenced on an Illumina HiSeq 2500 with a single 51bp read and a 10bp index read.
4.8 RNA-seq Pipeline
Reads were first trimmed of adapter sequences prior to further analysis. Average read depths were 12 +/− 2 million reads per sample. Only one sample failed our quality control due to extremely low numbers of starting cells (human ES teratoma day 1), resulting in only 3% of reads mapping to the transcriptome compared to 55%–75% for all other samples, and was therefore removed from further analyses. For each species, Expected Counts (EC) and transcripts per million (TPM) expression measures were generated by RSEM. Mitochondrial genes were removed from analysis and samples were re-scaled to nuclear gene TPMs. Technical replicates were collapsed into a single averaged sample by calculating the mean EC and TPM for each gene. When directly comparing mouse and human gene expression at the single gene level, all TPM expression levels were scaled from 0 (min) to 100 (max) to emphasize expression dynamics over time between species.
4.9 Neural Gene Correlation Analysis
For all correlation analyses between mouse and human cells, we compiled a list of neural genes including all 3,061 genes annotated as the offspring of the Gene Ontology terms: nervous system development (GO:0007399), neuron part (GO:0097458), and synapse (GO:0045202) in the Bioconductor annotation data package. Using this list, Pearson correlations of log-transformed TPMs were calculated after filtering for genes that 1) had orthologs among both mouse and human species according to the Mouse Genome Informatics (MGI) project (http://www.informatics.jax.org/), and 2) at least one time point had a TPM>0.
4.10 Dynamic Time Warping Analysis
We first required that genes be well-expressed and demonstrated sufficient variations in both species. Thus for each species, we only used genes where the average TPM over the entire time course was greater than 1 and have a dynamic range greater than 5-fold. These two criteria were applied to both species. We also filtered out genes that did not have orthologs in both humans and mice according to the MGI table (http://www.informatics.jax.org/homology.shtml). For each species, we further scaled TPMs from 0 (minimum value) to 1 (maximum value).
A DTW algorithm was then applied to measure the similarity between two temporal gene expression patterns using the established R package “DTW”. We further calculated the Spearman correlation coefficients (Rho) on aligned days. To ensure the aligned days were comparable, we required that the DTW alignments between human and mouse genes be rank correlated (Rho>0.8). Two one-sided Wilcoxon signed-rank tests were applied to assess the statistical significance of developmentally faster or slower in human compared to mouse cells, and anything with a p<0.01 was assigned as significant.
4.11 Segmentation Regression and Peak Identification Analysis
EC data were normalized using median-by-ratio normalization (Anders and Huber, 2010). A segmented regression was fit across the time course RNA-seq data for each gene using SegReg. SegReg fits a set of segmented regression models on each gene’s expression over the time course, and estimates the optimal number of breakpoints. Breakpoints are times where gene expression is changing: segments with positively sloped segments are increasing expression over time while segments with negatively sloped segments are decreasing over time. Only genes having mean expression greater than five are included in the analysis. Here, SegReg only considered a maximum of three breakpoints for each gene with each connecting segment containing at least three time points. A p-value cutoff of .2 was used to determine if a segment is increasing versus staying the same or decreasing versus stay the same. Top dynamic genes are selected as those having an adjusted R2 > .5. The peak analysis was conducted by identifying genes with an initial significant increasing segment followed by a significantly decreasing segment. The top ten human neural genes identified having the largest amplitude and used for peak correlation plots were (in order of amplitude): DLK1, ZFHX3, PAX6, LIX1, BRN2, NR2F1, LHX2, EPHA3, ACTA2, and ST18.
In vitro differentiation days were then transposed to embryonic days by adding 6.5 days to mouse EpiS cell and 15 days to human ES cell time points, and peaks were plotted for each species. In utero Carnegie stage matching of human and mouse gestation was then overlaid onto the neural gene peaks graph based on embryonic days post fertilization as summarized online at the UNSW Embryology website (Hill, 2017).
4.12 Functional Gene Ontology Group Analysis
The top 2,000 most significantly accelerated genes were selected for DAVIG GO enrichment analysis. GO enrichment for developmentally accelerated genes was performed by testing for enrichment against the default Homo sapiens background gene set using the DAVID functional annotation web tool (https://david.ncifcrf.gov). GOTERM_BP_FAT functional annotations were used for grouping genes, and categories were ranked by p-value. The top 20 unfiltered GO terms with the smallest p-values are shown in Fig. 1D and Fig. S3, and the top 15 neural GO terms in common between in vitro and teratoma differentiation are found in Fig. 3E.
Supplementary Material
HIGHLIGHTS.
Human and mouse stem cells retain species-dependent rates of development in vitro
Human ES cell differentiation is not accelerated by mouse factors in teratomas
Ex utero differentiation mirrors Carnegie stage progression in utero
Acknowledgments
The authors would like to thank Mitch Probasco, Nick Propson, and Amy Van Aartsen for experimental support, Karen Downs and Paul Tesar for their instruction pertaining to epiblast dissection, and Li-Fang Chu, David Vereide, John Maufort, and Erin Syth for critical reading and editorial assistance of the manuscript.
FUNDING
Funding for this research was provided by the Morgridge Institute for Research and U.S. National Institutes of Health grant 1UH2TR000506-01 (to J.A.T.). C.B. was supported by a CIHR Banting postdoctoral fellowship award.
Footnotes
COMPETING INTERESTS
The authors declare that they have no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- AACH J, CHURCH GM. Aligning gene expression time series with time warping algorithms. Bioinformatics. 2001;17:495–508. doi: 10.1093/bioinformatics/17.6.495. [DOI] [PubMed] [Google Scholar]
- AMOROSO MW, CROFT GF, WILLIAMS DJ, O’KEEFFE S, CARRASCO MA, DAVIS AR, ROYBON L, OAKLEY DH, MANIATIS T, HENDERSON CE, WICHTERLE H. Accelerated high-yield generation of limb-innervating motor neurons from human stem cells. J Neurosci. 2013;33:574–86. doi: 10.1523/JNEUROSCI.0906-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ANDERS S, HUBER W. Differential expression analysis for sequence count data. Genome Biol. 2010;11:R106. doi: 10.1186/gb-2010-11-10-r106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BONNER JT. Why size matters: from bacteria to blue whales. Princeton: Princeton University Press; 2006. [Google Scholar]
- BROCCOLI V, GIANNELLI SG, MAZZARA PG. Modeling physiological and pathological human neurogenesis in the dish. Front Neurosci. 2014;8:183. doi: 10.3389/fnins.2014.00183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRONS IG, SMITHERS LE, TROTTER MW, RUGG-GUNN P, SUN B, CHUVA DE SOUSA LOPES SM, HOWLETT SK, CLARKSON A, AHRLUND-RICHTER L, PEDERSEN RA, VALLIER L. Derivation of pluripotent epiblast stem cells from mammalian embryos. Nature. 2007;448:191–5. doi: 10.1038/nature05950. [DOI] [PubMed] [Google Scholar]
- BUCHHOLZ DE, PENNINGTON BO, CROZE RH, HINMAN CR, COFFEY PJ, CLEGG DO. Rapid and efficient directed differentiation of human pluripotent stem cells into retinal pigmented epithelium. Stem Cells Transl Med. 2013;2:384–93. doi: 10.5966/sctm.2012-0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAMBERS SM, FASANO CA, PAPAPETROU EP, TOMISHIMA M, SADELAIN M, STUDER L. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat Biotechnol. 2009;27:275–80. doi: 10.1038/nbt.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAMBERS SM, QI Y, MICA Y, LEE G, ZHANG XJ, NIU L, BILSLAND J, CAO L, STEVENS E, WHITING P, SHI SH, STUDER L. Combined small-molecule inhibition accelerates developmental timing and converts human pluripotent stem cells into nociceptors. Nat Biotechnol. 2012;30:715–20. doi: 10.1038/nbt.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAN LY, BIRCH WR, YIM EK, CHOO AB. Temporal application of topography to increase the rate of neural differentiation from human pluripotent stem cells. Biomaterials. 2013;34:382–92. doi: 10.1016/j.biomaterials.2012.09.033. [DOI] [PubMed] [Google Scholar]
- CHEN G, GULBRANSON DR, HOU Z, BOLIN JM, RUOTTI V, PROBASCO MD, SMUGA-OTTO K, HOWDEN SE, DIOL NR, PROPSON NE, WAGNER R, LEE GO, ANTOSIEWICZ-BOURGET J, TENG JM, THOMSON JA. Chemically defined conditions for human iPSC derivation and culture. Nat Methods. 2011;8:424–9. doi: 10.1038/nmeth.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEN J, LI C, WANG S. Periodic heat shock accelerated the chondrogenic differentiation of human mesenchymal stem cells in pellet culture. PLoS One. 2014;9:e91561. doi: 10.1371/journal.pone.0091561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EBERT AD, YU J, ROSE FF, JR, MATTIS VB, LORSON CL, THOMSON JA, SVENDSEN CN. Induced pluripotent stem cells from a spinal muscular atrophy patient. Nature. 2009;457:277–80. doi: 10.1038/nature07677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ESPUNY-CAMACHO I, MICHELSEN KA, GALL D, LINARO D, HASCHE A, BONNEFONT J, BALI C, ORDUZ D, BILHEU A, HERPOEL A, LAMBERT N, GASPARD N, PERON S, SCHIFFMANN SN, GIUGLIANO M, GAILLARD A, VANDERHAEGHEN P. Pyramidal neurons derived from human pluripotent stem cells integrate efficiently into mouse brain circuits in vivo. Neuron. 2013;77:440–56. doi: 10.1016/j.neuron.2012.12.011. [DOI] [PubMed] [Google Scholar]
- GAFNI O, WEINBERGER L, MANSOUR AA, MANOR YS, CHOMSKY E, BEN-YOSEF D, KALMA Y, VIUKOV S, MAZA I, ZVIRAN A, RAIS Y, SHIPONY Z, MUKAMEL Z, KRUPALNIK V, ZERBIB M, GEULA S, CASPI I, SCHNEIR D, SHWARTZ T, GILAD S, AMANN-ZALCENSTEIN D, BENJAMIN S, AMIT I, TANAY A, MASSARWA R, NOVERSHTERN N, HANNA JH. Derivation of novel human ground state naive pluripotent stem cells. Nature. 2013;504:282–6. doi: 10.1038/nature12745. [DOI] [PubMed] [Google Scholar]
- GAO FB, DURAND B, RAFF M. Oligodendrocyte precursor cells count time but not cell divisions before differentiation. Curr Biol. 1997;7:152–5. doi: 10.1016/s0960-9822(06)00060-1. [DOI] [PubMed] [Google Scholar]
- GASPARD N, BOUSCHET T, HOUREZ R, DIMIDSCHSTEIN J, NAEIJE G, VAN DEN AMEELE J, ESPUNY-CAMACHO I, HERPOEL A, PASSANTE L, SCHIFFMANN SN, GAILLARD A, VANDERHAEGHEN P. An intrinsic mechanism of corticogenesis from embryonic stem cells. Nature. 2008;455:351–7. doi: 10.1038/nature07287. [DOI] [PubMed] [Google Scholar]
- GAUR M, RITNER C, SIEVERS R, PEDERSEN A, PRASAD M, BERNSTEIN HS, YEGHIAZARIANS Y. Timed inhibition of p38MAPK directs accelerated differentiation of human embryonic stem cells into cardiomyocytes. Cytotherapy. 2010;12:807–17. doi: 10.3109/14653249.2010.491821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIORGINO T. Computing and Visualizing Dynamic Time Warping Alignments in R: The dtw Package. Journal of Statistical Software. 2009;31:1–24. [Google Scholar]
- GREBER B, WU G, BERNEMANN C, JOO JY, HAN DW, KO K, TAPIA N, SABOUR D, STERNECKERT J, TESAR P, SCHOLER HR. Conserved and divergent roles of FGF signaling in mouse epiblast stem cells and human embryonic stem cells. Cell Stem Cell. 2010;6:215–26. doi: 10.1016/j.stem.2010.01.003. [DOI] [PubMed] [Google Scholar]
- HARRISON RG. Some Unexpected Results of the Heteroplastic Transplantation of Limbs. Proc Natl Acad Sci U S A. 1924;10:69–74. doi: 10.1073/pnas.10.2.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HILL MA. [Accessed January 27 2017];Embryology. 2017 [Online]. Available: https://embryology.med.unsw.edu.au/embryology/index.php/Carnegie_Stage_Comparison.
- HOU Z, JIANG P, SWANSON SA, ELWELL AL, NGUYEN BK, BOLIN JM, STEWART R, THOMSON JA. A cost-effective RNA sequencing protocol for large-scale gene expression studies. Sci Rep. 2015;5:9570. doi: 10.1038/srep09570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUANG DA W, SHERMAN BT, LEMPICKI RA. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009a;37:1–13. doi: 10.1093/nar/gkn923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUANG DA W, SHERMAN BT, LEMPICKI RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009b;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- JURGENS KD. Etruscan shrew muscle: the consequences of being small. J Exp Biol. 2002;205:2161–6. doi: 10.1242/jeb.205.15.2161. [DOI] [PubMed] [Google Scholar]
- KELAVA I, LANCASTER MA. Stem Cell Models of Human Brain Development. Cell Stem Cell. 2016;18:736–48. doi: 10.1016/j.stem.2016.05.022. [DOI] [PubMed] [Google Scholar]
- KOJIMA Y, KAUFMAN-FRANCIS K, STUDDERT JB, STEINER KA, POWER MD, LOEBEL DA, JONES V, HOR A, DE ALENCASTRO G, LOGAN GJ, TEBER ET, TAM OH, STUTZ MD, ALEXANDER IE, PICKETT HA, TAM PP. The transcriptional and functional properties of mouse epiblast stem cells resemble the anterior primitive streak. Cell Stem Cell. 2014;14:107–20. doi: 10.1016/j.stem.2013.09.014. [DOI] [PubMed] [Google Scholar]
- KRENCIK R, WEICK JP, LIU Y, ZHANG ZJ, ZHANG SC. Specification of transplantable astroglial subtypes from human pluripotent stem cells. Nat Biotechnol. 2011;29:528–34. doi: 10.1038/nbt.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRIKS S, SHIM JW, PIAO J, GANAT YM, WAKEMAN DR, XIE Z, CARRILLO-REID L, AUYEUNG G, ANTONACCI C, BUCH A, YANG L, BEAL MF, SURMEIER DJ, KORDOWER JH, TABAR V, STUDER L. Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson’s disease. Nature. 2011;480:547–51. doi: 10.1038/nature10648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVINE AJ, BRIVANLOU AH. Proposal of a model of mammalian neural induction. Dev Biol. 2007;308:247–56. doi: 10.1016/j.ydbio.2007.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACKINTOSH NA, WHEELER JFG. Southern Blue and Fin Whales. Discovery Reports. 1929;1:257–540. [Google Scholar]
- MAROOF AM, KEROS S, TYSON JA, YING SW, GANAT YM, MERKLE FT, LIU B, GOULBURN A, STANLEY EG, ELEFANTY AG, WIDMER HR, EGGAN K, GOLDSTEIN PA, ANDERSON SA, STUDER L. Directed differentiation and functional maturation of cortical interneurons from human embryonic stem cells. Cell Stem Cell. 2013;12:559–72. doi: 10.1016/j.stem.2013.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MASCETTI VL, PEDERSEN RA. Human-Mouse Chimerism Validates Human Stem Cell Pluripotency. Cell Stem Cell. 2016;18:67–72. doi: 10.1016/j.stem.2015.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORA-BERMUDEZ F, BADSHA F, KANTON S, CAMP JG, VERNOT B, KOHLER K, VOIGT B, OKITA K, MARICIC T, HE Z, LACHMANN R, PAABO S, TREUTLEIN B, HUTTNER WB. Differences and similarities between human and chimpanzee neural progenitors during cerebral cortex development. Elife. 2016:5. doi: 10.7554/eLife.18683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUOTRI AR, NAKASHIMA K, TONI N, SANDLER VM, GAGE FH. Development of functional human embryonic stem cell-derived neurons in mouse brain. Proc Natl Acad Sci U S A. 2005;102:18644–8. doi: 10.1073/pnas.0509315102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OTANI T, MARCHETTO MC, GAGE FH, SIMONS BD, LIVESEY FJ. 2D and 3D Stem Cell Models of Primate Cortical Development Identify Species-Specific Differences in Progenitor Behavior Contributing to Brain Size. Cell Stem Cell. 2016;18:467–80. doi: 10.1016/j.stem.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OZEKI A, SUZUKI K, SUZUKI M, OZAWA H, YAMASHITA S. Acceleration of astrocytic differentiation in neural stem cells surviving X-irradiation. Neuroreport. 2012;23:290–3. doi: 10.1097/WNR.0b013e3283509a79. [DOI] [PubMed] [Google Scholar]
- PAGLIUCA FW, MILLMAN JR, GURTLER M, SEGEL M, VAN DERVORT A, RYU JH, PETERSON QP, GREINER D, MELTON DA. Generation of functional human pancreatic beta cells in vitro. Cell. 2014;159:428–39. doi: 10.1016/j.cell.2014.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARCHEM RJ, MOORE N, FISH JL, PARCHEM JG, BRAGA TT, SHENOY A, OLDHAM MC, RUBENSTEIN JL, SCHNEIDER RA, BLELLOCH R. miR-302 Is Required for Timing of Neural Differentiation, Neural Tube Closure, and Embryonic Viability. Cell Rep. 2015;12:760–73. doi: 10.1016/j.celrep.2015.06.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAFF M. Intracellular developmental timers. Cold Spring Harb Symp Quant Biol. 2007;72:431–5. doi: 10.1101/sqb.2007.72.007. [DOI] [PubMed] [Google Scholar]
- ROSSANT J, TAM PP. New Insights into Early Human Development: Lessons for Stem Cell Derivation and Differentiation. Cell Stem Cell. 2017;20:18–28. doi: 10.1016/j.stem.2016.12.004. [DOI] [PubMed] [Google Scholar]
- SAHA K, JAENISCH R. Technical challenges in using human induced pluripotent stem cells to model disease. Cell Stem Cell. 2009;5:584–95. doi: 10.1016/j.stem.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAIZ-LOPEZ P, CHINNAIYA K, CAMPA VM, DELGADO I, ROS MA, TOWERS M. An intrinsic timer specifies distal structures of the vertebrate limb. Nat Commun. 2015;6:8108. doi: 10.1038/ncomms9108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAKOE S, CHIBA C. Dynamic Programming Algorithm Optimization for Spoken Word Recognition. IEE Transactions on Acoustics, Speech, and Signal Processing. 1978;ASSP-26:43–49. [Google Scholar]
- SASAKI N, HIRANO T, KOBAYASHI K, TOYODA M, MIYAKAWA Y, OKITA H, KIYOKAWA N, AKUTSU H, UMEZAWA A, NISHIHARA S. Chemical inhibition of sulfation accelerates neural differentiation of mouse embryonic stem cells and human induced pluripotent stem cells. Biochem Biophys Res Commun. 2010;401:480–6. doi: 10.1016/j.bbrc.2010.09.085. [DOI] [PubMed] [Google Scholar]
- SHEN Q, WANG Y, DIMOS JT, FASANO CA, PHOENIX TN, LEMISCHKA IR, IV, ANOVA NB, STIFANI S, MORRISEY EE, TEMPLE S. The timing of cortical neurogenesis is encoded within lineages of individual progenitor cells. Nat Neurosci. 2006;9:743–51. doi: 10.1038/nn1694. [DOI] [PubMed] [Google Scholar]
- SHI Y, KIRWAN P, LIVESEY FJ. Directed differentiation of human pluripotent stem cells to cerebral cortex neurons and neural networks. Nat Protoc. 2012a;7:1836–46. doi: 10.1038/nprot.2012.116. [DOI] [PubMed] [Google Scholar]
- SHI Y, KIRWAN P, SMITH J, ROBINSON HP, LIVESEY FJ. Human cerebral cortex development from pluripotent stem cells to functional excitatory synapses. Nat Neurosci. 2012b;15:477–86. S1. doi: 10.1038/nn.3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOUTHWELL DG, PAREDES MF, GALVAO RP, JONES DL, FROEMKE RC, SEBE JY, ALFARO-CERVELLO C, TANG Y, GARCIA-VERDUGO JM, RUBENSTEIN JL, BARABAN SC, ALVAREZ-BUYLLA A. Intrinsically determined cell death of developing cortical interneurons. Nature. 2012;491:109–13. doi: 10.1038/nature11523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TADEU AM, LIN S, HOU L, CHUNG L, ZHONG M, ZHAO H, HORSLEY V. Transcriptional profiling of ectoderm specification to keratinocyte fate in human embryonic stem cells. PLoS One. 2015;10:e0122493. doi: 10.1371/journal.pone.0122493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TESAR PJ, CHENOWETH JG, BROOK FA, DAVIES TJ, EVANS EP, MACK DL, GARDNER RL, MCKAY RD. New cell lines from mouse epiblast share defining features with human embryonic stem cells. Nature. 2007;448:196–9. doi: 10.1038/nature05972. [DOI] [PubMed] [Google Scholar]
- TWITTY VC, SCHWIND JL. The growth of eyes and limbs transplanted heteroplastically between two species of Amblystoma. Journal of Experimental Zoology. 1931;59:61–86. [Google Scholar]
- VAN DE LEEMPUT J, BOLES NC, KIEHL TR, CORNEO B, LEDERMAN P, MENON V, LEE C, MARTINEZ RA, LEVI BP, THOMPSON CL, YAO S, KAYKAS A, TEMPLE S, FASANO CA. CORTECON: a temporal transcriptome analysis of in vitro human cerebral cortex development from human embryonic stem cells. Neuron. 2014;83:51–68. doi: 10.1016/j.neuron.2014.05.013. [DOI] [PubMed] [Google Scholar]
- WATANABE T, RAFF MC. Rod photoreceptor development in vitro: intrinsic properties of proliferating neuroepithelial cells change as development proceeds in the rat retina. Neuron. 1990;4:461–7. doi: 10.1016/0896-6273(90)90058-n. [DOI] [PubMed] [Google Scholar]
- WEXLER EM, ROSEN E, LU D, OSBORN GE, MARTIN E, RAYBOULD H, GESCHWIND DH. Genome-wide analysis of a Wnt1-regulated transcriptional network implicates neurodegenerative pathways. Sci Signal. 2011;4:ra65. doi: 10.1126/scisignal.2002282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHANG X, HUANG CT, CHEN J, PANKRATZ MT, XI J, LI J, YANG Y, LAVAUTE TM, LI XJ, AYALA M, BONDARENKO GI, DU ZW, JIN Y, GOLOS TG, ZHANG SC. Pax6 is a human neuroectoderm cell fate determinant. Cell Stem Cell. 2010;7:90–100. doi: 10.1016/j.stem.2010.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHU Y, CARIDO M, MEINHARDT A, KURTH T, KARL MO, ADER M, TANAKA EM. Three-dimensional neuroepithelial culture from human embryonic stem cells and its use for quantitative conversion to retinal pigment epithelium. PLoS One. 2013;8:e54552. doi: 10.1371/journal.pone.0054552. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.