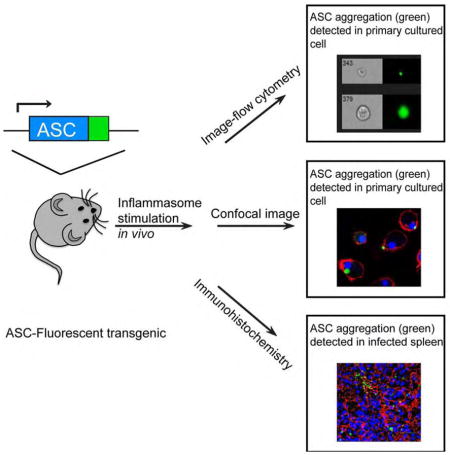
Summary
Inflammasome activation is associated with numerous diseases. However, in vivo detection of the activated inflammasome complex has been limited by a dearth of tools. We developed transgenic mice that ectopically express the fluorescent adaptor protein, ASC (apoptosis-associated speck-like protein containing a caspase recruitment domain), and characterized the formation of assembled inflammasome complexes (“specks”) in primary cells and tissues. In addition to hematopoietic cells, we found that a stromal population in the lung tissues forms specks during the early phase of influenza infection whereas myeloid cells showed speck formation after two days. In a peritonitis and Group B streptococcus infection models, a higher percentage of neutrophils formed specks at early phases of infection, while dendritic cells formed specks at later time points. Furthermore, speck-forming cells underwent pyroptosis, and extensive release of specks to the extracellular milieu in vivo. These data underscore the importance of free specks during inflammatory processes in vivo.
In Brief
In vivo detection of the activated inflammasome complex has been limited by a dearth of tools. Here, Tzeng et al. have developed a reporter mouse model expressing ASC fluorescent protein (ASC-citrine). Mice treated with inflammasome activators showed increased ASC aggregation, indicating the activation of inflammasome pathways.
Introduction
Inflammasomes are key signaling platforms that detect pathogenic microorganisms and sterile stressors and then control the caspase-1 dependent maturation of the highly pro-inflammatory cytokines interleukin-1β (IL-1β) and IL-18. Both IL-1β and IL-18 have numerous functions, including activation of feed-forward pathways that result in the production of more cytokines, such as TNF-α. Hence, the inflammasome is a central mediator of inflammation. Deregulated inflammasome activity has been linked to several sterile inflammatory diseases, including cryopyrin-associated periodic syndromes (Baroja-Mazo et al., 2014; Hoffman et al., 2001), gout (Martinon et al., 2006), Alzheimer’s disease (Halle et al., 2008; Heneka et al., 2013) and atherosclerosis (Duewell et al., 2010; Samstad et al., 2014; Sheedy et al., 2013). Inflammasome activation has also been shown to play a critical role in the clearance of pathogens, such as influenza A/PR8, Group B streptococcus (GBS) and Listeria (Costa et al., 2012; Ichinohe et al., 2009; Ichinohe et al., 2010; Rathinam et al., 2010).
Inflammasomes are multiprotein complexes that assemble in response to microbial triggers leading to activation of caspase-1 and or caspase-11 that in turn process the immature proinflammatory cytokines, pro-IL-1β and pro-IL-18, to their mature forms. In general, inflammasomes consist of three proteins: (i) a receptor that recognizes danger signals, (ii) the adapter protein ASC (apoptosis-associated speck-like protein containing a caspase recruitment domain), and (iii) the enzyme caspase-1 (Latz, 2010). After the receptor molecule has sensed a danger signal and undergoes structural rearrangements, the signal transduction domains (PYD domain) induce rapid polymerization of ASC into a protein helix forming large filaments. Filamentous ASC nucleates pro-caspase-1 via a CARD/CARD domain interaction, leading to proximity-induced activation of caspase-1 (Fernandes-Alnemri et al., 2007; Lu et al., 2014). The autocatalytically activated form of caspase-1 then converts pro-IL-1β and pro-IL-18 into the corresponding mature active cytokines.
Many inflammasomes require the assembly of the adaptor protein ASC for activation. ASC aggregation was first described by Masumoto et al., in which ASC molecules form a large aggregate that is often called a “speckle” or “speck” during the process of cell death (Masumoto et al., 1999). Because the hallmark of inflammasome activation is speck formation, many tools have been used for examining inflammasome activation using speck formation as a readout. It is relatively common, for example, to stimulate engineered macrophage cell lines that overexpress fluorescent ASC in order to look for specks (Fernandes-Alnemri et al., 2007; Sheedy et al., 2013). Alternatively, one can detect ASC oligomerization by western blot (Ataide et al., 2014) or with anti-ASC antibodies (Franklin et al., 2014).
Although we have several tools to test inflammasome activation in vitro, there is a paucity of in vivo tools to visualize or measure activation of the inflammasome directly. Recently, Bousso et al. visualized ASC polymerization and the subsequent release of ASC specks by transferring hematopoietic cells that had been retrovirally transduced with ASC-GFP (Sagoo et al., 2016). While the findings were a novel demonstration of the fate of oligomerized ASC complexes in an in vivo setting, the approach required irradiation of recipient mice in order to transplant hematopoietic reporter cells, raising the possibility of radiation-induced inflammation and artifact. Furthermore, assembled inflammasomes in non hematopoietic lineages were not detectable by this technique. Thus, we generated and exploited a transgenic mouse expressing a mouse ASC-citrine fusion protein in the Rosa26 locus to ask questions concerning inflammasome activation in native tissues during the inflammatory process without these limitations. The vector that we used contains a knock-in of the ASC-citrine chimera and a proximal floxed stop site, which allows us to conditionally express ASC-citrine in a lineage specific manner. Bone marrow-derived macrophages (BMDM) or bone marrow-derived dendritic cells (BMDC) from this reporter mouse showed ASC speck formation after inflammasome stimulation. Speck formation was detectable in the tissues of infected mice, both intracellularly and extracellularly. Both hematopoietic cells and non-hematopoietic cells are capable of expressing specks and stimulating the downstream signal pathway when activated.
Results
Generation of ASC-citrine mice
Speck formation is a widely used readout for detecting inflammasome activation (Stutz et al., 2013). However, due to the lack of genetic tools, the detection of inflammasome activation in vivo has thus far been limited. We generated a transgenic mouse expressing mouse ASC-citrine fusion protein in the Rosa26 locus. This system contains a knock-in of the ASC-citrine gene and a proximal loxP-flanked stop site, which allows us to conditionally express ASC-citrine in a lineage-specific manner (Supplemental Fig. 1A). The ES cell clones that harbored the expected ASC-citrine fragment were confirmed by Southern blot analysis (Supplemental Fig. 1B), and the targeted ES cell clones were injected into blastocysts to generate chimeric mice. Germline transmission of the target gene yielded heterozygous ASC-citrine mice, which were crossed to Cre-expressing mice to generate ASC-citrine/Cre+ expressing mice. We first bred the ASC-citrine mice with ZP3-Cre mice, in which Cre expression is controlled by the regulatory sequences from the mouse zona pellucida 3 (Zp3) gene (de Vries et al., 2000). This promoter normally directs expression exclusively in the growing oocyte prior to the completion of the first meiotic division, thus we obtained the first generation offspring with deletion of the floxed sequence in the female germ line. These females were then crossed with wild-type males to generate progeny that carry the deleted-floxed allele. Citrine is a variant of yellow fluorescent protein and is very bright when excited with the blue laser (488 nM). The expression of ASC-citrine could be detected by flow cytometry using blood samples from ASC-citrine/Zp3-Cre+ (hereafter referred to as ASC-citrine/Cre+) mice (Supplemental Fig. 1C). ASC-citrine/Cre+ mice showed normal behavior, with no significant difference in survival and no signs of inflammation.
Ectopic expressed ASC-citrine is functional
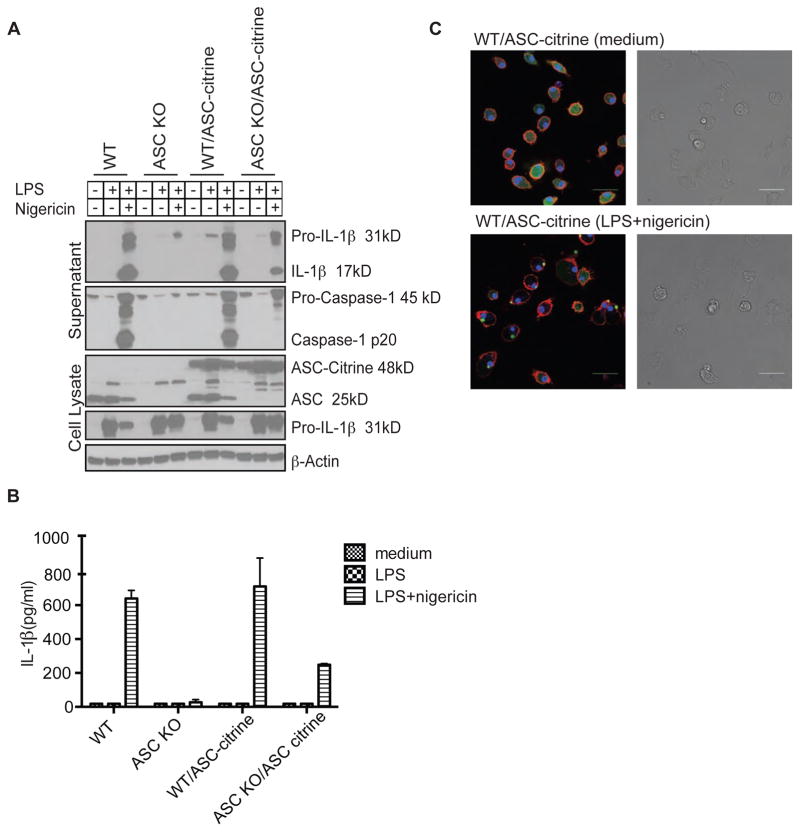
To test whether the ASC-citrine molecule is functional, we bred ASC-citrine/Cre+ mice to ASC knock out (KO) mice, to generate mice that only express ectopic ASC-citrine but lack endogenous ASC. BMDMs were cultured from ASC-citrine/Cre+ mice on a wild type (WT) and ASC KO background as well as the control littermates that are ASC-citrine negative. BMDMs that had been cultured for eight days were stimulated with LPS alone or LPS plus nigericin, a well-known NLRP3 inflammasome stimulator. The expression of ASC-citrine molecules was comparable in both the WT and ASC KO background (Fig. 1A). Cleaved IL-1β and activated caspase-1 p20 were detected in WT BMDMs with or without ectopic expression of ASC-citrine (Fig. 1A). In contrast, the activation of IL-1β and caspase-1 was absent in ASC KO mice. Ectopic expression of ASC-citrine in the ASC KO background was able to restore the activation of caspase-1 and IL-1β; however, the level of activated molecules was lower compared to that in WT macrophages (Fig. 1A). The result was similar when the level of IL-1β was measured by ELISA in the culture supernatant (Fig. 1B).
Figure 1. ASC-citrine can induce the maturation of IL-1β upon activation.
BMDMs were cultured from WT, ASC KO, ASC-citrine/Cre+, or ASC KO/ASC-citrine/Cre+ mice. Cells were primed with 100 ng/mL of ultrapure LPS (lipoprotein-free) for 2 hr followed by stimulation with nigericin for 1 hr. (A) Concentrated culture supernatants and cell lysates were subjected to western blot with antibodies against mouse IL-1β, mouse caspase-1, and ASC. Beta-Actin was used as a loading control. (B) Concentrations of IL-1β in the culture supernatants were determined by ELISA. Data are presented as mean ± SD of triplicates and are representative of three independent experiments. (C) BMDMs from ASC-citrine mice were primed with 100 ng/mL LPS for 2 hr followed by stimulation with nigericin for 1 hr. Living cells were stained with Hoechst nuclear dye (blue) and CellMask Deep Red plasma membrane dye (red). ASC-citrine fusion proteins are shown in green. Speck formation was visualized by confocal microscopy. Scale bars represent 30 μm.
Several studies have demonstrated the formation of specks in macrophage reporter cells stably expressing fluorescent ASC proteins when stimulated with nigericin, ATP, or pathogens (Fernandes-Alnemri et al., 2007; Mariathasan et al., 2006; Masumoto et al., 2001; Silva et al., 2013). The ASC-citrine/Cre+ mice enabled the examination of speck formation in primary cells. Confocal images showed increased speck formation in primary BMDMs from ASC-citrine/Cre+ mice when stimulated with LPS plus nigericin (Fig. 1C).
Structural studies of ASC have demonstrated that inflammasome assembly begins with the polymerization of ASC into fibrils by NOD-like receptors (NLRs) or by the HIN 200 family member proteins such as AIM2 (absent in melanoma 2). These fibrils of flexibly linked ASC serve as a platform for caspase-1, leading to caspase-1 activation (Lu et al., 2014) and the processing of IL-1β and IL-18. We hypothesized that the formation of ASC specks does not require downstream molecules such as caspase-1. BMDMs from ASC-citrine mice or ASC-citrine mice deficient in caspase-1 expression were stimulated with LPS and nigericin. Confocal images show that even without caspase-1, ASC-citrine was still able to form specks (Supplemental Fig. 2).
Quantitative method to detect inflammasome activation
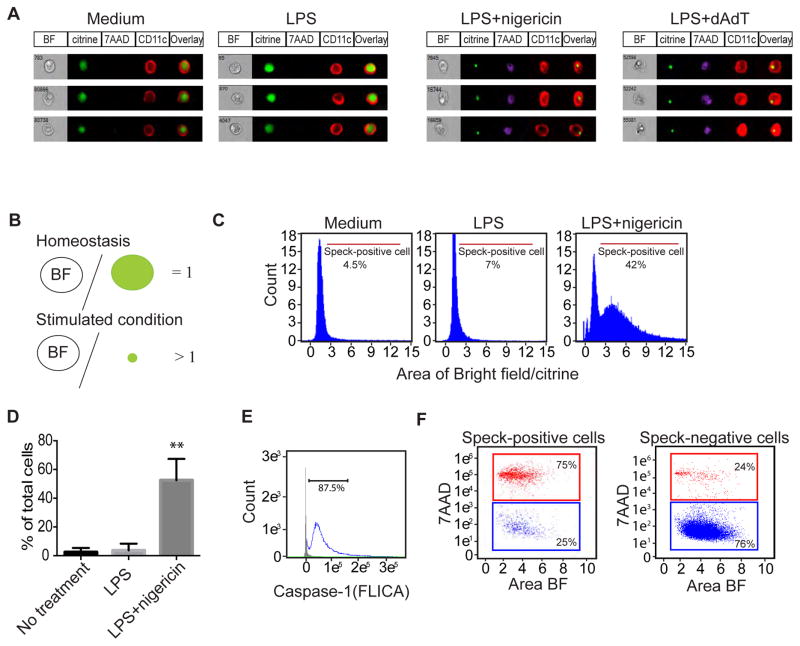
We used image-based flow cytometry to characterize the speck-positive cells in a quantitative method. The experiment shown in Fig. 2 was performed using BMDCs, and demonstrates the technique of quantitation that we developed. Note how in this experiment, speck formation was apparent in BMDCs treated with LPS plus nigericin or LPS plus poly (dA:dT) (Fig. 2A). In order to quantify the speck-positive cells, we created a matrix feature to define speck-positive cells using the IDEAS software. Under homeostatic conditions, the distribution of ASC-citrine molecules in the nucleus and cytosol is uniform; indeed, the cell is uniformly fluorescent. Thus, the total area of citrine positivity (represented by a green circle in Fig. 2B) in unstimulated cells is similar to the area of the entire cell (i.e., “Bright Field”, represented by a circle with the letters “BF” in Fig. 2B) as measured by bright field microscopy. Hence, unstimulated cells have a ratio of BF:area of citrine expression (“citrine”) that is ~ equal to 1.00. Upon stimulation, ASC-citrine molecules form compact specks, thereby dramatically increasing the ratio of BF: citrine; under these conditions, the ratio of BF:citrine is larger than 1. In this context, we used the ratio between BF:citrine as an indicator of speck formation (Fig. 2B). The ratio of BF:citrine is between 1 and 3 during homeostasis and increases 10-fold with LPS plus nigericin treatment. The shift of the FACS histogram upon stimulation allows us to gate on speck-positive cells for purposes of quantification. As seen in Figs. 2C and 2D, nearly 50% of BMDCs formed specks when stimulated with LPS plus nigericin.
Figure 2. Analysis of speck formation in BMDCs by image-based flow cytometry.
BMDCs were primed with 100 ng/mL LPS for 2 hr followed by stimulation with nigericin for 1hr or poly (dAdT) transfection for 24 hr. Cells were harvested after stimulation for image-based flow cytometry analysis. (A) Representative images from medium, LPS-, LPS+nigericin- and LPS+poly(dAdT)-treated BMDCs stained for the expression of CD11c, and the vital dye 7AAD. BF, brightfield. (B) Schematic showing the analysis rationale for the histogram plots shown in (C). Under conditions of homeostasis, the intracellular distribution of ASC-citrine molecules was throughout the entire cell, and thus the ratio of the area of the cell as determined under bright field (BF) microscopy to the area that is fluorescent is approximately 1. Upon stimulation (“Stimulated condition”), ASC-citrine molecules form a compact aggregate that leads to a decrease in the area that expresses citrine. This results in a ratio of area BF/citrine that is larger than 1. (C) The three histograms demonstrate the percentage of speck-positive dendritic cells by assuming that any cell with a BF/citrine ratio of >2.5 has formed a speck. (D) Quantitative results showing the percentage of speck-positive cells. Data are presented as mean ± SD of triplicates. **p<0.01 by one way ANOVA. (E) Image-based flow cytometric analysis of caspase-1 activation by detection of FLICA. (F) Cell death of speck-positive cells and speck-negative cells were analyzed by 7AAD staining. Data are representative of three independent experiments.
Aggregation of ASC molecules promotes the cleavage of pro-caspase-1 to activated caspase-1, which in turn processes pro-IL-1β and pro-IL-18 to active cytokines. To test whether speck-forming cells were indeed expressing activated caspase-1, we measure activated caspase-1 with a fluorescent cell-permeable probe that selectively and covalently binds to the catalytic portion of caspase-1 (Amstad et al., 2001). This reagent did not lead to any visible staining in caspase-1-deficient cells (data not shown). Overlay histogram analysis of speck-positive cells and speck-negative cells showed a robust increase in catalytically active caspase-1-positive cells in speck-positive cells, whereas speck-negative cells showed no such response (Fig. 2E).
Prolonged inflammasome activation results in a specific type of cell death called ‘pyroptosis’, characterized by loss of plasma membrane integrity and the late release of intracellular proteins (Bergsbaken et al., 2009). To test whether the presence of specks invariably was associated with cell death, we stained activated cells with 7-amino-actinomycin D (7AAD), a vital dye with a high affinity for DNA that does not pass through the intact cell membrane. As seen in the histograms plotted in Figs. 2A and 2F, 75% of speck-positive cells were 7AAD positive, suggesting that these cells are undergoing pyroptosis while forming specks, whereas 76% of speck-negative cells were alive as defined by the absence of staining with 7AAD.
To confirm that the speck formation was not due to the consequences of necrotic cell death or represented an artifact, BMDMs from ASC-citrine/Cre+ mice were stimulated with TNFα plus SMAC mimetic in the presence of the caspase inhibitor zVAD, a cocktail that is known to induce cell necrosis. We detected that 25% of cells were 7AAD+ after 24 hours of stimulation, however, no assembled inflammasome specks were detected (Supplemental Fig 3 A). In contrast, 80% of the 7AAD+ cells with LPS plus nigericin, which activates the NLRP3 inflammasome, formed specks. This data indicates that speck formation was indeed due to canonical inflammasome activation (Supplemental Fig 3 A–B).
Inflammasome activation in vivo
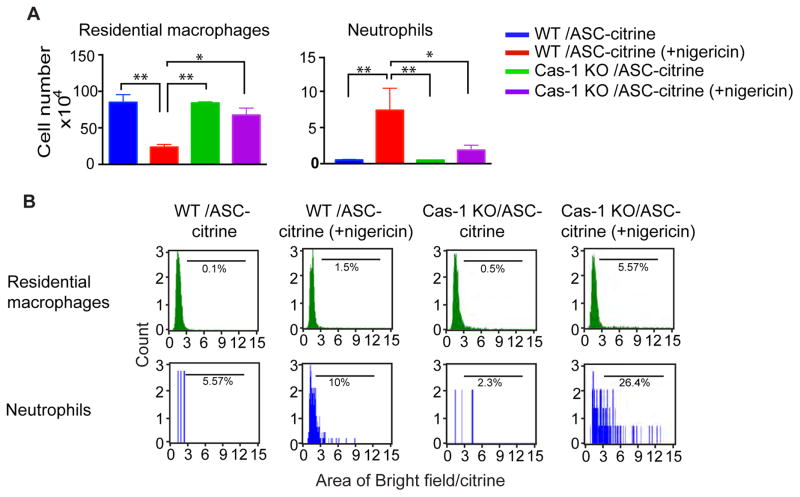
To test whether speck formation can be detected in vivo using ASC-citrine/Cre+ mice, we induced peritonitis chemically with a potent activator of the NLRP3 inflammasome, nigericin. Mice were injected intraperitoneally and peritoneal exudates were collected 90 minutes later. Nigericin induced a considerable increase in the recruitment of neutrophils (7-4high/Ly6Ghigh) to the peritoneal cavity in ASC-citrine/Cre+ mice (Fig. 3A and Supplemental Fig. 4A). In contrast, resident macrophages (F4/80high/CD11bhigh) were decreased dramatically. Using an image-based flow machine, we gated different populations of cells and analyzed the formation of specks. Unexpectedly, there were only a small percentage of speck-positive cells detected in the ASC-citrine/Cre+ mice (Fig. 3B), despite the known potency of nigericin as an activator of Inflammasomes.
Figure 3. Nigericin induces the formation of specks in vivo in a caspase-1 dependent manner.
Mice were injected intraperitoneally with 3mg/kg of nigericin for 90 minutes and the peritoneal exudates were subsequently collected. (A) Numbers of different populations of cells in the exudates. Comparison of the numbers of residential macrophages (F4/80high/CD11bhigh) vs. neutrophils (7-4pos/Ly6Ghigh). (B) The percentage of speck-positive cells in different populations is shown by FACS histogram. Bars represents average of five samples. *p < 0.05, **p < 0.01 (student’s t test).
Recent publications have demonstrated that inflammasome-activated cells undergo pyroptosis, which is accompanied by the release of ASC specks into the extracellular space. Such extracellular ASC specks retained the ability to activate pro-caspase-1 and process pro-IL-1β and pro-IL-18 into mature cytokines (Baroja-Mazo et al., 2014; Franklin et al., 2014). To test the hypothesis that ASC specks were released from the cell, we used ASC-citrine/Cre+ mice that were bred onto a caspase-1 null background. As caspase-1 activation is required for nigericin-induced pyroptosis, the release of specks would be prevented. Indeed, there was a six-fold increase of speck-positive cells in ASC-citrine/Cre+ mice on the caspase-1 KO background (Fig. 3B), suggesting that the lack of specks in nigericin-stimulated cells was due to pyroptosis in WT mice. We further analyzed the ability of different cell types to form specks and found that the percentage of speck-positive cells was mainly in the neutrophil population which was two- to three-fold higher than in residential macrophages. This was especially evident in the caspase-1 KO background, where 26% of neutrophils contained specks (Fig. 3B and Supplemental Fig. 4B).
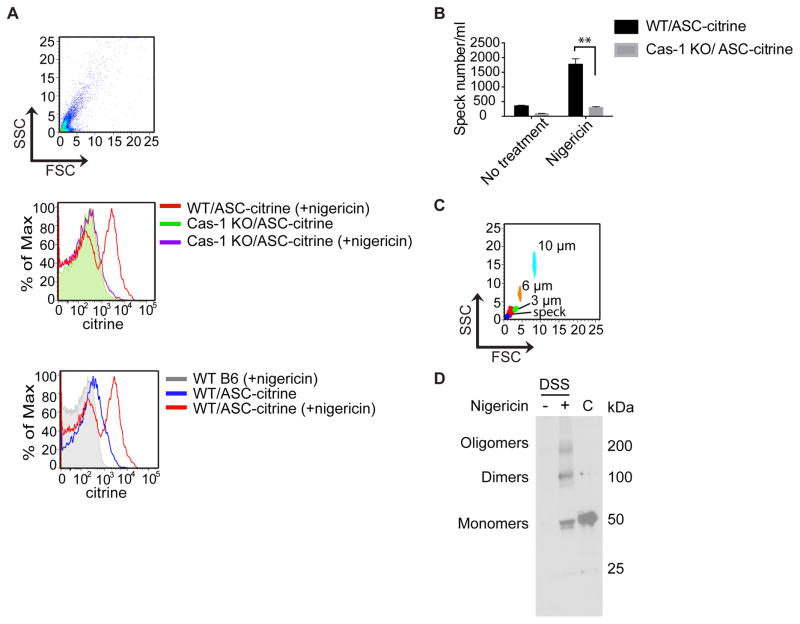
These data led us to ask if active inflammasome complexes might be released into the peritoneum of nigericin-treated mice. An analysis of the exudate supernatants that were collected by lavage detected citrine positive particles only from the ASC-citrine/Cre+ reporter mice treated with nigericin. A citrine signal was conspicuously absent from the exudates of nigericin-treated C57BL/6J (B6) mice or ASC-citrine/Cre+/caspase-1 KOs (Figs. 4A and 4B), whose cells would not be expected to undergo pyroptosis. We gated on the citrine-positive population of particles in the exudates from the ASC-citrine/Cre+ mice. This population of particles were between 1–3 μm, which is the expected size for a speck (Fig. 4C). Immunoblotting analysis confirmed the presence of oligomerized ASC after chemically crosslinking of the exudates with disuccinimidyl suberate (DSS) (Fig. 4D, middle lane). Non-crosslinked control macrophage lysates expressed abundant monomeric ASC-citrine (~50 kilodaltons in size) (Fig. 4D, right hand lane).
Figure 4. Nigericin induces the release of specks in vivo in a caspase-1 dependent manner.
(A) Cell-free supernatants of peritoneal exudates were analyzed by flow cytometry for the detection of extracellular specks. Note the large quantity of citrine-positive particles in the supernatants from ASC-citrine positive animals that were treated with nigericin, and the absence of these particles in the caspase-1 deficient mouse (top set of histograms). Citrine was not detected in the exudates from WT B6 mice, which were tested as a control group, while unstimulated ASC-citrine mice had a small quantity of peritoneal specks (bottom set of histograms). (B) The number of free specks in the exudates were quantified by flow cytometry. (C) A size comparison of the citrine-positive population shown in (A) with 1, 3, 6 and 10 μm calibrated beads. ASC specks should be approximately 1–3 μm in size. (D) Immunoblot analysis of released ASC oligomers in the cell-free supernatant of peritoneal exudates from mice that were untreated (−) or treated (+) with nigericin. Filtered exudates were subsequently crosslinked by DSS. Cell lysates from ASC-citrine BMDMs cells were shown as a positive control for the ASC-citrine band (C). Right margin, molecular size, in kilodaltons (kDa). The results show an increased number of specks per mL of supernatant in WT/ASC-citrine mice after nigericin stimulation.
Influenza virus A/PR8 induces inflammasome activation in lung stromal cells during the early phase of infection
Different strains of virus have different capabilities to induce inflammasome activation. We stimulated primary ASC-citrine/Cre+ macrophages with different RNA and DNA viruses including influenza A/PR8 (NLRP3), vesicular stomatitis virus (VSV; NLRP3 and RIG-I), mouse cytomegalovirus (mCMV; AIM2) and herpes simplex virus (HSV; IFI16 and NLRP3). There was a robust increase in speck-positive cells while stimulating cells for 12 hours, whereas a small percentage of cells formed specks after 4 hours of stimulation. The increased number of speck-positive cells was more obvious when cells were infected with RNA viruses, especially after infection with influenza strain A/PR8 (Supplemental Fig. 5A).
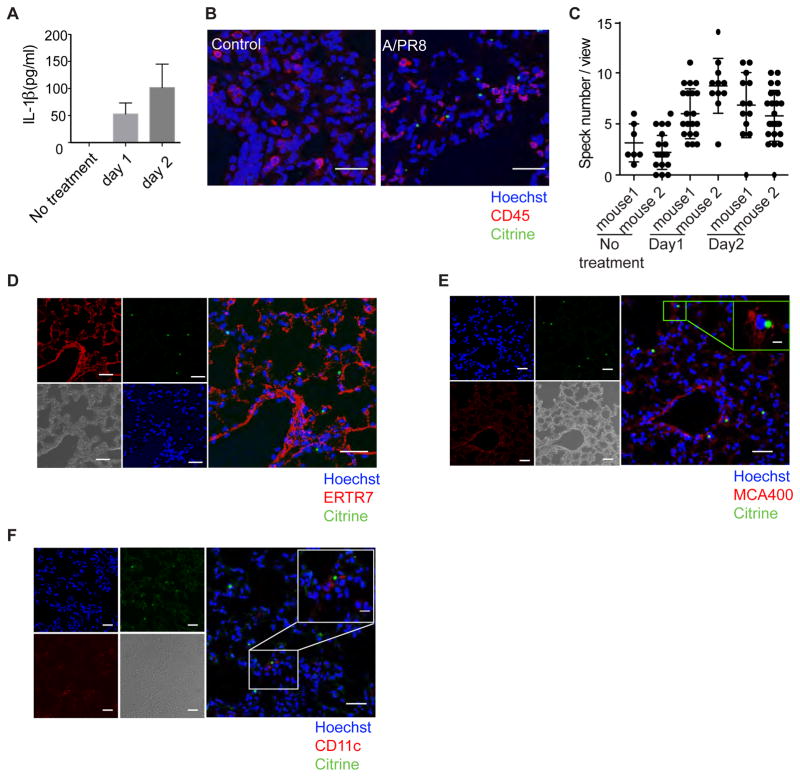
The role of the inflammasome in influenza infection has been studied in detail. Not only are inflammasomes activated during experimental influenza, but both ASC and caspase-1 play essential roles in inducing adaptive immune responses (Ichinohe et al., 2009; Ichinohe et al., 2010). To test for speck formation in vivo, mice were challenged intranasally with influenza strain A/PR8. Active IL-1β was detectable in lung tissue as early as day 1 and increased by day 2 (Fig. 5A). Confocal microscope imaging of lung tissue of day 1 infected mice showed increased speck-positive cells in every field that was viewed (Fig. 5B and 5C). The control mice were treated with PBS and showed a minimal number of specks in each field (Fig. 5B). Surprisingly, we did not see speck-positive cells co-localize with CD45 staining, indicating that after 1 day of infection, the speck-positive cells were the non-hematopoietic cells (Fig. 5B). The mAb ERTR7 recognizes antigens that are located in the cytoplasm of reticular fibroblasts and are a component of the extracellular matrix of lymphoid and non-lymphoid organs. Many speck-positive cells were either close to or inside the ERTR7-positive cells (Fig. 5D), which suggests that fibroblasts were either able to form specks or to internalize released specks. We confirmed this result using a different fibroblast marker, podoplanin (Supplemental Fig. 5B).
Figure 5. Influenza virus A/PR8-infected mice form specks in the lung parenchyma.
Mice were challenged intranasally with influenza strain A/PR8. (A) Lung homogenates were prepared in 0.5 mL PBS containing 0.1% BSA. Total IL-1β levels detected from the lung homogenates were measured at different time points by ELISA. (B) Confocal microscopic images of mouse lung tissue after one day of infection. Lung tissue was fixed and stained with CD45 (red) and Hoechst nuclear dye (blue). The white scale bar represents 30 μm. (C) The tile function was used to photograph multiple images over a defined area using confocal laser microscopy. The total number of specks were counted, and ~20 images were analyzed in each sample. The results represent the mean number of specks per tile in each of 2 mice. (D-E) Lung tissue from mice infected for 24 h was stained with mAb ERTR7 (red in D) or MCA400 (red in E), in combination with Hoechst nuclear dye (blue). (F) CD11c (red) combined with Hoechst nuclear dye (blue) stained sections at day 3 of infection demonstrate the presence of an activated dendritic cell in situ. The scale bar represents 30 μm for the lower magnified images (100X) and 10μm for the enlarged image (400X). Green color represents ASC-citrine. Data are representative of three independent experiments.
We next sought to determine if the speck-positive cells were actually infected with influenza by staining for influenza A nucleoprotein. Many speck-positive cells were positive for influenza A nucleoprotein, indicating that the speck-positive cells were primarily infected with influenza A (Fig. 5E). In contrast, we found that CD11c cells did not form specks until day 3 (Fig. 5F). These data suggested a scenario in which the inflammasomes of the non-hematopoietic cells were activated in the early phase of infection, while the inflammasome response was initiated in hematopoietic cells at a later time point.
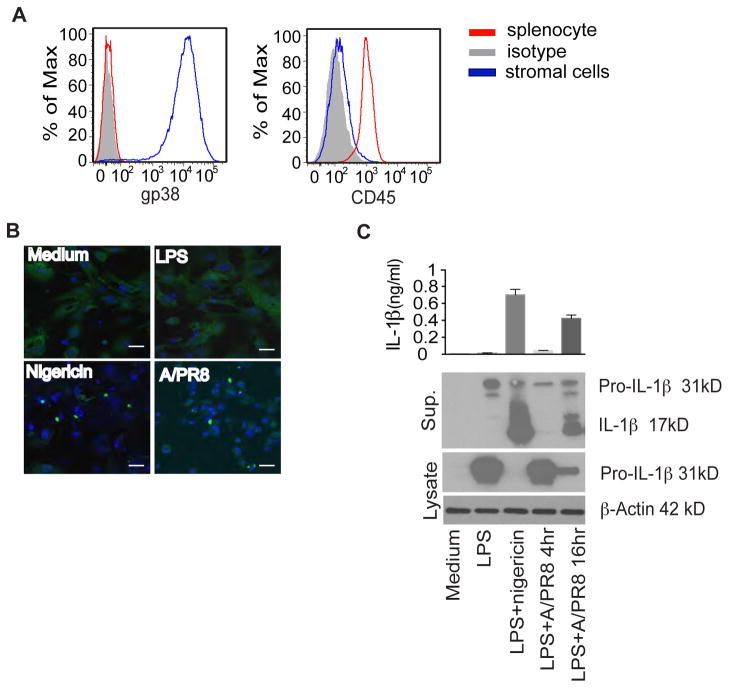
To confirm that non-hematopoietic cells from lung tissue could respond to influenza and form specks, we cultured lung primary cells from ASC-citrine/Cre+ mice as described previously (Ichinohe et al., 2009). Under these culture conditions, most of the cells are lung primary fibroblasts; indeed, 99% of the cells were podoplanin-positive and CD45-negative (Fig. 6A). When primary lung fibroblasts were stimulated with LPS plus nigericin or infected with influenza A/PR8, we observed that fibroblasts formed specks four hours after stimulation (Fig. 6B). Furthermore, there was a significant amount of IL-1β in the culture supernatant, as detected by ELISA (total IL-1β) or western blot (mature IL-1β; Fig. 6C).
Figure 6. Influenza virus infection results in the formation of inflammasome complexes in purified lung stromal cells.
(A) Primary lung stromal cells prepared from ASC-citrine mice were subjected to flow cytometry analysis using mAbs for gp38 and CD45. Splenocytes were used as a control for CD45 positive cells. (B) Primary lung stromal cells were plated and then infected with influenza A/PR8 (MOI=2) and visualized by confocal laser microscopy. (C) Primary lung stromal cells were primed with LPS for 2 hours and then treated with influenza A/PR8 (A/PR8) or nigericin as a positive control. Supernatants or cell lysates were collected at different time points after infecting primary lung stromal cells with A/PR8. LPS alone control was treated for 16 hours with LPS. Samples were subjected to both ELISA or western blot analysis. Data are representative of three independent experiments.
Group B streptococcus induces inflammasome activation as early as 4 hours after infection
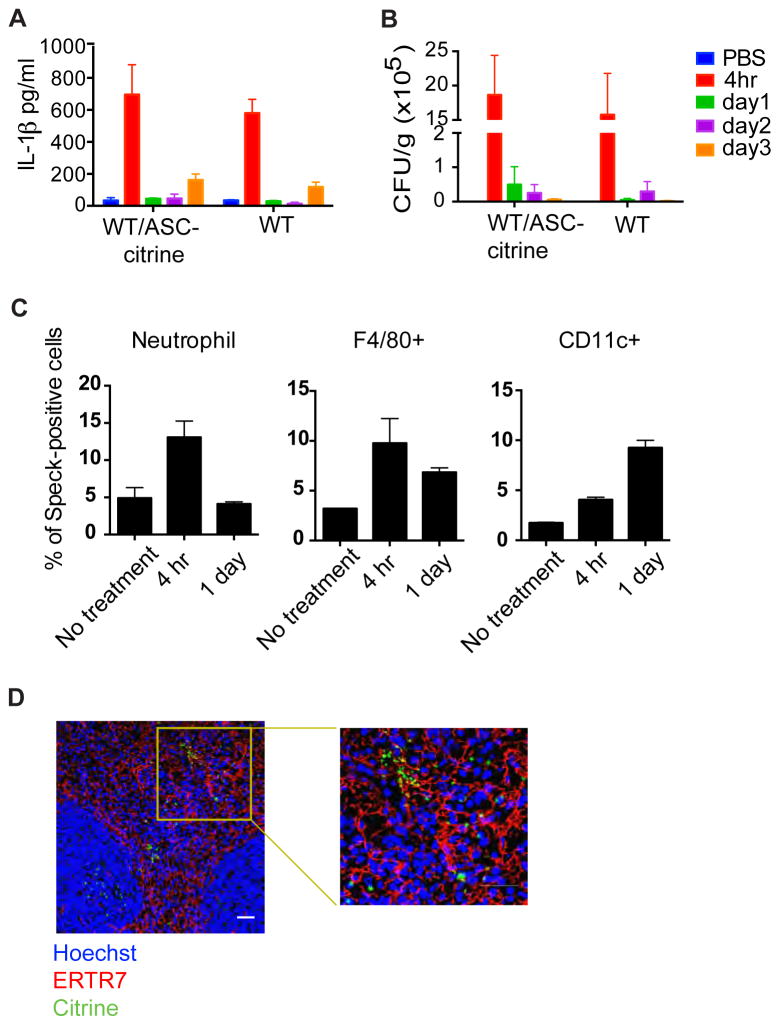
Despite advances in intrapartum antibiotic prophylaxis, GBS remains one of the most important causes of neonatal sepsis worldwide (Parks et al., 2015). GBS has been shown to be a potent NLRP3 inflammasome stimulator, and the hemolytic activity is critical for its function for inflammasome activation (Costa et al., 2012; Gupta et al., 2014). Mice were infected with GBS by intravenous injection. ASC-citrine/Cre+ mice produced similar levels of IL-1β in splenic tissue compared to WT B6 mice. The peak for IL-1β level was four hours after infection, corresponding to the peak in bacterial colony-forming units (CFUs) (Fig. 7A–B). Since IL-1β has been shown to play important roles in recruiting neutrophils to inflammatory sites (Kono et al., 2012), we hypothesize that cells with specks should be detectable as early as four hours after infection. Speck formation was analyzed by image-based flow cytometry. We observed increased speck formation in neutrophils and F4/80+ macrophages as early as four hours after infection. The percentage of speck-positive cells then decreased in the neutrophil population on day 1, whereas the percentage stayed the same in the macrophage population. In contrast, there were very few speck-positive CD11c+ cells detected at four hours; the number of CD11c+ cells increased by the beginning of day 1 (Fig. 7C). Frozen sections of 1-day infected spleens samples were evaluated for speck-forming cells. Confocal images showed that numerous specks were formed in the dense network of stromal cells in the red pulp, where many dendritic cells are located. Some of the specks are close to each other and located right next to the ERTR7+ fibroblast conduit system (Fig. 7D). Both intracellular and extracellular specks, presumably arising from pyroptotic cell death, were seen in the splenic samples.
Figure 7. Bacteremia with Group B streptococcus results in widespread activation of inflammasomes in mouse spleen.
WT or ASC-citrine mice were injected intravenously with GBS and harvested at different time points. (A) Total IL-1β or (B) bacterial colony forming units in splenic homogenates were detected over a three day period. (C) Splenocytes were stained with cell markers for neutrophils, macrophages (F4/80+) and dendritic cells (CD11c+). Cells were then analyzed by image-based flow cytometry for speck formation. The percentage of speck-positive cells is shown. (D) Spleens were fixed and stained with the mAb ERTR7 (fibroblasts, red) and Hoechst nuclear dye (blue). ASC specks are seen both intracellularly and extracellularly. The white scale bar represents 30 μm.
Discussion
Speck formation is the hallmark of inflammasome activation and can be studied in vitro using fluorescent ASC-overexpressing macrophage cell lines. However, the lack of an in vivo tool for studying ASC speck formation has limited the understanding of inflammasome activation under conditions of inflammation, especially in the context of an ongoing infection. In the present study, we generated an ASC-citrine reporter mouse that enables us to study speck formation in real time in an in vivo setting.
The primary cells from ASC-citrine mice showed robust speck formation and cleavage of caspase-1 and IL-1β. In addition, expression of ASC-citrine restored caspase-1 activation and IL-1β cleavage in ASC KO macrophages, although the amount of cleaved caspase-1 was lower compared to WT background. In part, this may have been because there was about 1/3 less total ASC in the knockout background. In addition, the efficiency of the ASC-citrine chimera to activate caspase-1 appears to be lower than the native molecule. Lu et al. showed that the CARD domain acts as a platform for caspase-1 molecules (Lu et al., 2014). In our mouse model, the citrine was fused to the C terminus of ASC, which is adjacent to the caspase-1 recruitment domain. We hypothesize that the addition of citrine impedes successful assembly of the inflammasome complex, such as the recruitment of caspase-1, and thus decreases its activation. In fact, as studies have shown fluorescent ASC PYD or fluorescent ASC CARD are capable of forming filaments in vitro, we were originally concerned that ectopic expression of fluorescent ASC would lead to autoinflammation in the mouse, and that the establishment of the reporter mouse might be difficult or even impossible. To our surprise, no signs of inflammation were observed in ASC-citrine/Cre+ mice due to the lower efficiency of ASC-citrine to activate downstream signals.
The purpose of our in vivo experiments was to learn about inflammasome activation during the course of inflammation in real time. One surprise was that in the peritonitis model (LPS + nigericin), the major speck-forming population detected was neutrophils, as the number of resident macrophages was dramatically decreased. As resident macrophages may adhere to the peritoneal membrane after stimulation, or undergo pyroptosis upon inflammasome activation (Chen et al., 2014), it is possible that activated residential macrophages were simply not collected as part of the peritoneal exudate. Therefore, we cannot rule out the possibility that resident macrophages are the first wave of speck-forming cells that recruit neutrophils (via the production of IL-1β and IL-18 and subsequent activation of autocrine pathways) and subsequently monocytes. Our results showing a high percentage of neutrophils with specks is consistent with a recent publication showing that neutrophils are resistant to pyroptosis upon inflammasome activation (Chen et al., 2014).
Recent reports have demonstrated that, after inflammasome activation, fully formed specks are released from activated cells as a result of pyroptosis (Baroja-Mazo et al., 2014; Franklin et al., 2014). These specks were reported to become internalized by neighboring cells, of both phagocytic and non-phagocytic origin, potentially changing the character of the stromal milieu into a nest of inflammation. In fact, we observed the release of large quantities of specks into the peritoneal space after inflammasome activation. Similarly, splenic tissue samples from bacterially-infected mice were also loaded with free specks in the extracellular space, as well as specks that appeared to have been internalized by non-phagocytic cells. As the specks were observed to be localized close to the ERTR7+ conduit system, one might hypothesize that ASC complexes can travel via this conduit system as a means of amplifying systemic inflammation. All of these suggest that releasing specks into extracellular space may be an efficient way of spreading inflammation in a short period of time.
All together, we have shown that ASC-citrine/Cre+ reporter mouse is a useful tool for studying inflammasome activation in vivo. The expression of ASC-citrine in the mouse is beneficial for the study of inflammasome activation in primary cells in real-time, including both hematopoietic and non-hematopoietic cells. In these studies, for example, we have observed a strong in vivo correlate of the recently reported role of extracellular specks as mediators of inflammation in vitro, underscoring the high probability that extracellular specks are far more important than previously thought.
Experimental Procedures
Mice
Mice between 6–10 weeks old were used. C57Bl/6 mice were from The Jackson Laboratory (JAX)(Bar Harbor, ME). The Rosa-26-ASC citrine floxed allele was generated following a strategy previously developed by Sasaki et al. (Sasaki et al., 2006). Namely, the Rosa-26 allele was targeted with a construct containing mouse ASC-citrine cDNA preceded by a loxP flanked STOP cassette and marked by a signaling deficient truncated version of hCD2 under the control of an internal ribosomal entry site (IRES) downstream of the inserted cDNA. Transgene transcription is controlled by a CAG promoter. ES cells containing targeting genes were injected into blastocysts to generate chimeric mice by the transgenic animal modeling core at UMASS medical school. ASC-citrine/Cre+ mice were bred with Caspase-1 KO or ASC KO to generated Caspse-1 KO/ASC-citrine or ASC KO/ASC-citrine. All mice were bred and maintained in specific pathogen-free conditions; mouse protocols were approved by the Institutional Animal Care and Use Committee at the University of Massachusetts Medical School.
Reagents
Lipofectamine 2000 was obtained from Invitrogen. Poly(dAdT) and nigericin were obtained from Sigma-Aldrich. Dulbecco’s modified Eagle’s medium (DMEM) was obtained from Cellgro, and low endotoxin FBS was obtained from Atlas Biologicals. The ELISA kit for mouse IL-1β was from R&D Systems.
Antibodies
The following antibodies were used: Anti-IL-1β (AF-401-NA) from R&D; mouse monoclonal antibody to NLRP3 (Cryo-2; AG-20B-0014-C100) and to caspase-1 p20 (casper-1; AG-20B-0042-C100) from Adipogen; anti-ERTR7 from Abcam; anti-podoplanin from Biolegend; polyclonal anti-ASC (sc-22514-R; N-15R) from Santa Cruz; and anti-ASC, anti CD11c, anti-CD3, anti-B220, anti-CD45, anti-CD11b, anti-Ly6G, anti-7-4, and anti-F4/80 from eBioscience. CellMask Plasma membrane stain, DAPI, and Hoechst were obtained from Invitrogen.
Cell culture and stimulation
BMDMs were prepared by culturing bone marrow cells in the presence of supernatants from L929 cells for 8 days. On day 8, BMDMs were harvested and plated at 2 × 105 cells/well in 96-well plates for ELISA or 2 × 106 cells/well in 12-well plates for immunoblotting. BMDCs were prepared by culturing bone marrow cells in the presence of GM/CSF (PeproTech 31503) for 7 days. On day 7, BMDCs were harvested and plated 1 × 106 cells/well in 12-well plates for image-based flow analysis. In order to examine inflammasome activity in primary lung fibroblast, lungs from mice were excised, washed in PBS, cut into small pieces, agitated, and digested enzymatically for 30 min at 37°C. The digestion buffer (10 ml/lung) was composed of 0.25% trypsin solution containing 400nM EDTA. Ice-cold 10%FBS MEM medium was added to the resulting cell suspension. After centrifugation (1500 rpm, 5 min), pellets were resuspended in complete medium, then cultured on dishes. Lung fibroblasts were used for each experiment ten days after preparation as described (Yamamoto et al., 2003).
For stimulation, BMDCs, BMDMs or lung fibroblasts cells fibroblasts (1×106 cells/well in a 12-well plate) were primed with ultrapure LPS 100ng/ml (from Escherichia coli O111:B4, Invivogen) for 2 h, followed by stimulation with nigericin (10 μM) for 1 hr or influenza A/PR8 (MOI=2) for 4 or 16 hrs. Cells were transfected with poly(dA:dT) DNA through the use of Lipofectamine 2000 at a concentration of 1.5 μg/ml. IL-1β p17 and caspase-1 p20 immunoblots were conducted as described (Davaro et al., 2014) with antibodies from Adipogen (caspase-1 p20) and R&D Systems (IL-1β). The antibodies against β-actin were from Sigma. Speck formation was assessed by confocal microscopy after stimulation, and cell-free supernatant was collected after 24 hours of stimulation and analyzed for IL-1β by ELISA or western blot.
In vivo and in vitro infections
Influenza A/PR8 virus for in vivo infection was purchased from Charles River Laboratories. For intranasal infection, mice were fully anesthetized by isofluorene and then infected by intranasal application of 30 μL of virus suspension (40,000 PFU in PBS). This procedure leads to the upper and lower respiratory tract infection. For in vitro infection, Influenza A/PR8 virus was grown in Madin-Darby canine kidney (MDCK) cells. Supernatants were harvested when at least 75% of the cell monolayer exhibited a cytopathic effect and standard plaque assays were used to quantify the amount of influenza viruses in the supernatants. The other viruses used in these experiments, including VSV, MCMV and HSV, were the gifts of by Dr. Katherine Fitzgerald (University of Massachusetts Medical School, Worcester). For GBS infection, the WT GBS strain (NEM316) was cultured in BBL media (purchased from BD) overnight and 2x107 CFUs of GBS was intravenously injected. Spleens were harvested and embedded in optimal cutting temperature (OCT) medium to prepare frozen sections. Homogenates from spleens were used for bacteria counts and IL-1β measurement.
Immunoblot analysis
BMDMs or primary lung fibroblasts were harvested and lysed in lysis buffer (25mM Tris-HCL [pH 7.4], 150 mM NaCl, 1 mM EDTA, 1% NP-40, and 5% glycerol) containing protease and phosphatase inhibitor ‘cocktails’ (Roche). Protein concentrations were determined with the BCA protein assay kit (Thermo Scientific). Equal amounts of protein were loaded onto 4–20% gels (Bio-Rad) and separated by electrophoresis, then transferred to a nitrocellulose membrane. After nonspecific binding was blocked by incubation for 1 h in 5% nonfat milk, the membranes were incubated overnight with primary antibodies (as described in the antibodies section) in 1% BSA. After three washes with TBS, the membranes were incubated for 1 h with secondary antibodies. Finally, the signal was visualized with the SuperSignal West Dura Chemiluminescent Substrate (GE).
ELISA
Cell culture supernatants were assayed for IL-1β with ELISA kits from R&D Systems according to the manufacturer’s instructions. Lung or spleen tissues were homogenized with a tissuelyser II (Qiagen) for 1 min at speed 25/second. Homogenates were centrifuged at 16,000g for 10 min and supernatants were collected for detecting IL-1β.
Immunohistochemistry and confocal microscopy
Isolated tissues were fixed for 4 h in 4% (vol/vol) paraformaldehyde (PFA), followed by incubation in 30% (vol/vol) sucrose for dehydration. Tissues were then embedded in OCT medium, frozen, and cut into 7 μm thick sections. Sections were immunostained (antibodies identified above) and were imaged with a Leica SP8X laser-scanning confocal microscope.
Flow cytometry
Blood samples from ASC-citrine/Cre+ mice were treated with heparin to prevent blood clotting, and then RBC lysis for flow cytometry analysis. BMDMs or BMDCs were treated with inflammasome stimulus and stained for antibodies. Cells were resuspended at a concentration of 107/mL for running on the Amnis® brand FlowSight® Imaging Flow Cytometer (hereafter referred to as flowsight). The results were analyzed with the IDEAS software, also purchased from Amnis.
Quantification of speck-forming cells in tissue
Sections from influenza A/PR8-infected lung tissue were imaged with the Leica SP8X laser-scanning confocal microscope. The tile function of the microscope was used to scan 10–20 fields per section. The average number of specks per field of view was counted.
Quantification of free ASC specks
Peritonitis was induced in ASC-citrine/Cre+ mice in the WT or caspase-1−/− background by the i.p. injection of 3mg/kg of nigericin for 90 min. Animals were euthanized by CO2 exposure, and their peritoneal cavities were lavaged with 6 ml of RPMI with 10% FCS containing 3 mM EDTA and 10 U/ml heparin. The cells collected in these exudates were stained with mAbs CD11b APC-Cy7, Ly-6G PE and 7/4 APC for 30 minutes at ice temperature in the presence of mAb 2.4G2 (FcγRIIB/III receptor blocker). Labeled cells were analyzed by flowsight for the detection of assembled specks. Supernatants from peritoneal exudates were passed through a 5μm filter to eliminate cell and nuclear debris. The specks were concentrated from the supernatants by centrifugation at 21000g for 15 min. Pellets were then resuspended in 200 μL of FACs buffer for FACs analysis. 150 μL of samples were analyzed by FACs, and the number of specks were compared by analyzing dot plots of FSC vs SSC. For investigation of the oligomerization of extracellular ASC the pellets from the supernatants of peritoneal exudate were washed with 0.5ml CHAPS buffer and were chemically crossliked for 30min at room temperature with 2mM DSS (Pierce), prior to immunoblot analysis.
Statistics
Values are expressed as mean ± SEM. Statistics were calculated with Prism software (GraphPad). For two group comparisons, a two-tailed unpaired t-test was used. Comparisons of multiple groups were analyzed by one-way analysis of variance ANOVA with Bonferroni’s multiple-comparison test or Dunnett’s test for comparison of all groups with the control group. We found that the variance from the groups compared did not present statistically significant differences.
Supplementary Material
Highlights.
The ASC-citrine mouse is a transgenic model that reports inflammasome activation
ASC-citrine retains the function of endogenous ASC
Non-hematopoietic cells are capable of forming ASC specks upon activation
The ASC-citrine mouse recapitulates the formation of free specks in vivo
Acknowledgments
We thank Neal Silverman for helpful discussions. This work was supported by GM54060 (DTG). The authors have no conflicting financial interests.
Footnotes
Author Contributions:
T.J.T. designed and performed experiments and analyzed data; S.S. and A.C. did influenza A/PR8 and GBS injections and helped with some experiments. B.M. performed experiments and provided critical suggestions and discussion. D.W. designed the reporter constructs and provided critical suggestions and discussion throughout the study. K.F. provided critical suggestions. D.T.G. and E.L. designed the study. T.J.T. and D.T.G wrote the paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amstad PA, Yu G, Johnson GL, Lee BW, Dhawan S, Phelps DJ. Detection of caspase activation in situ by fluorochrome-labeled caspase inhibitors. BioTechniques. 2001;31:608–610. 612, 614. doi: 10.2144/01313pf01. passim. [DOI] [PubMed] [Google Scholar]
- Ataide MA, Andrade WA, Zamboni DS, Wang D, do Souza MC, Franklin BS, Elian S, Martins FS, Pereira D, Reed G, et al. Malaria-induced NLRP12/NLRP3-dependent caspase-1 activation mediates inflammation and hypersensitivity to bacterial superinfection. PLoS pathogens. 2014;10:e1003885. doi: 10.1371/journal.ppat.1003885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baroja-Mazo A, Martin-Sanchez F, Gomez AI, Martinez CM, Amores-Iniesta J, Compan V, Barbera-Cremades M, Yague J, Ruiz-Ortiz E, Anton J, et al. The NLRP3 inflammasome is released as a particulate danger signal that amplifies the inflammatory response. Nature immunology. 2014;15:738–748. doi: 10.1038/ni.2919. [DOI] [PubMed] [Google Scholar]
- Bergsbaken T, Fink SL, Cookson BT. Pyroptosis: host cell death and inflammation. Nature reviews Microbiology. 2009;7:99–109. doi: 10.1038/nrmicro2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen KW, Gross CJ, Sotomayor FV, Stacey KJ, Tschopp J, Sweet MJ, Schroder K. The neutrophil NLRC4 inflammasome selectively promotes IL-1beta maturation without pyroptosis during acute Salmonella challenge. Cell reports. 2014;8:570–582. doi: 10.1016/j.celrep.2014.06.028. [DOI] [PubMed] [Google Scholar]
- Costa A, Gupta R, Signorino G, Malara A, Cardile F, Biondo C, Midiri A, Galbo R, Trieu-Cuot P, Papasergi S, et al. Activation of the NLRP3 inflammasome by group B streptococci. Journal of immunology. 2012;188:1953–1960. doi: 10.4049/jimmunol.1102543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davaro F, Forde SD, Garfield M, Jiang Z, Halmen K, Tamburro ND, Kurt-Jones E, Fitzgerald KA, Golenbock DT, Wang D. 3-Hydroxyl-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitor (statin)-induced 28-kDa interleukin-1beta interferes with mature IL-1beta signaling. The Journal of biological chemistry. 2014;289:16214–16222. doi: 10.1074/jbc.M114.571505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries WN, Binns LT, Fancher KS, Dean J, Moore R, Kemler R, Knowles BB. Expression of Cre recombinase in mouse oocytes: a means to study maternal effect genes. Genesis. 2000;26:110–112. [PubMed] [Google Scholar]
- Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, Abela GS, Franchi L, Nunez G, Schnurr M, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–1361. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes-Alnemri T, Wu J, Yu JW, Datta P, Miller B, Jankowski W, Rosenberg S, Zhang J, Alnemri ES. The pyroptosome: a supramolecular assembly of ASC dimers mediating inflammatory cell death via caspase-1 activation. Cell death and differentiation. 2007;14:1590–1604. doi: 10.1038/sj.cdd.4402194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin BS, Bossaller L, De Nardo D, Ratter JM, Stutz A, Engels G, Brenker C, Nordhoff M, Mirandola SR, Al-Amoudi A, et al. The adaptor ASC has extracellular and ‘prionoid’ activities that propagate inflammation. Nature immunology. 2014;15:727–737. doi: 10.1038/ni.2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R, Ghosh S, Monks B, DeOliveira RB, Tzeng TC, Kalantari P, Nandy A, Bhattacharjee B, Chan J, Ferreira F, et al. RNA and beta-hemolysin of group B Streptococcus induce interleukin-1beta (IL-1beta) by activating NLRP3 inflammasomes in mouse macrophages. The Journal of biological chemistry. 2014;289:13701–13705. doi: 10.1074/jbc.C114.548982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halle A, Hornung V, Petzold GC, Stewart CR, Monks BG, Reinheckel T, Fitzgerald KA, Latz E, Moore KJ, Golenbock DT. The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nature immunology. 2008;9:857–865. doi: 10.1038/ni.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heneka MT, Kummer MP, Stutz A, Delekate A, Schwartz S, Vieira-Saecker A, Griep A, Axt D, Remus A, Tzeng TC, et al. NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature. 2013;493:674–678. doi: 10.1038/nature11729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman HM, Mueller JL, Broide DH, Wanderer AA, Kolodner RD. Mutation of a new gene encoding a putative pyrin-like protein causes familial cold autoinflammatory syndrome and Muckle-Wells syndrome. Nature genetics. 2001;29:301–305. doi: 10.1038/ng756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichinohe T, Lee HK, Ogura Y, Flavell R, Iwasaki A. Inflammasome recognition of influenza virus is essential for adaptive immune responses. The Journal of experimental medicine. 2009;206:79–87. doi: 10.1084/jem.20081667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichinohe T, Pang IK, Iwasaki A. Influenza virus activates inflammasomes via its intracellular M2 ion channel. Nature immunology. 2010;11:404–410. doi: 10.1038/ni.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kono H, Orlowski GM, Patel Z, Rock KL. The IL-1-dependent sterile inflammatory response has a substantial caspase-1-independent component that requires cathepsin C. Journal of immunology. 2012;189:3734–3740. doi: 10.4049/jimmunol.1200136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latz E. The inflammasomes: mechanisms of activation and function. Current opinion in immunology. 2010;22:28–33. doi: 10.1016/j.coi.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu A, Magupalli VG, Ruan J, Yin Q, Atianand MK, Vos MR, Schroder GF, Fitzgerald KA, Wu H, Egelman EH. Unified polymerization mechanism for the assembly of ASC-dependent inflammasomes. Cell. 2014;156:1193–1206. doi: 10.1016/j.cell.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariathasan S, Weiss DS, Newton K, McBride J, O’Rourke K, Roose-Girma M, Lee WP, Weinrauch Y, Monack DM, Dixit VM. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228–232. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- Masumoto J, Taniguchi S, Ayukawa K, Sarvotham H, Kishino T, Niikawa N, Hidaka E, Katsuyama T, Higuchi T, Sagara J. ASC, a novel 22-kDa protein, aggregates during apoptosis of human promyelocytic leukemia HL-60 cells. The Journal of biological chemistry. 1999;274:33835–33838. doi: 10.1074/jbc.274.48.33835. [DOI] [PubMed] [Google Scholar]
- Masumoto J, Taniguchi S, Nakayama J, Shiohara M, Hidaka E, Katsuyama T, Murase S, Sagara J. Expression of apoptosis-associated speck-like protein containing a caspase recruitment domain, a pyrin N-terminal homology domain-containing protein, in normal human tissues. The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society. 2001;49:1269–1275. doi: 10.1177/002215540104901009. [DOI] [PubMed] [Google Scholar]
- Parks T, Barrett L, Jones N. Invasive streptococcal disease: a review for clinicians. British medical bulletin. 2015;115:77–89. doi: 10.1093/bmb/ldv027. [DOI] [PubMed] [Google Scholar]
- Rathinam VA, Jiang Z, Waggoner SN, Sharma S, Cole LE, Waggoner L, Vanaja SK, Monks BG, Ganesan S, Latz E, et al. The AIM2 inflammasome is essential for host defense against cytosolic bacteria and DNA viruses. Nature immunology. 2010;11:395–402. doi: 10.1038/ni.1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagoo P, Garcia Z, Breart B, Lemaitre F, Michonneau D, Albert ML, Levy Y, Bousso P. In vivo imaging of inflammasome activation reveals a subcapsular macrophage burst response that mobilizes innate and adaptive immunity. Nature medicine. 2016;22:64–71. doi: 10.1038/nm.4016. [DOI] [PubMed] [Google Scholar]
- Samstad EO, Niyonzima N, Nymo S, Aune MH, Ryan L, Bakke SS, Lappegard KT, Brekke OL, Lambris JD, Damas JK, et al. Cholesterol crystals induce complement-dependent inflammasome activation and cytokine release. Journal of immunology. 2014;192:2837–2845. doi: 10.4049/jimmunol.1302484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki Y, Derudder E, Hobeika E, Pelanda R, Reth M, Rajewsky K, Schmidt-Supprian M. Canonical NF-kappaB activity, dispensable for B cell development, replaces BAFF-receptor signals and promotes B cell proliferation upon activation. Immunity. 2006;24:729–739. doi: 10.1016/j.immuni.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Sheedy FJ, Grebe A, Rayner KJ, Kalantari P, Ramkhelawon B, Carpenter SB, Becker CE, Ediriweera HN, Mullick AE, Golenbock DT, et al. CD36 coordinates NLRP3 inflammasome activation by facilitating intracellular nucleation of soluble ligands into particulate ligands in sterile inflammation. Nature immunology. 2013;14:812–820. doi: 10.1038/ni.2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva GK, Costa RS, Silveira TN, Caetano BC, Horta CV, Gutierrez FR, Guedes PM, Andrade WA, De Niz M, Gazzinelli RT, et al. Apoptosis-associated speck-like protein containing a caspase recruitment domain inflammasomes mediate IL-1beta response and host resistance to Trypanosoma cruzi infection. Journal of immunology. 2013;191:3373–3383. doi: 10.4049/jimmunol.1203293. [DOI] [PubMed] [Google Scholar]
- Stutz A, Horvath GL, Monks BG, Latz E. ASC speck formation as a readout for inflammasome activation. Methods in molecular biology. 2013;1040:91–101. doi: 10.1007/978-1-62703-523-1_8. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Sato S, Hemmi H, Hoshino K, Kaisho T, Sanjo H, Takeuchi O, Sugiyama M, Okabe M, Takeda K, Akira S. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science. 2003;301:640–643. doi: 10.1126/science.1087262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.