ABSTRACT
Adult spinal cord neurogenesis occurs at low, constant rate under normal conditions and can be amplified by pathologic conditions such as injury or disease. The immature neurons produced through adult neurogenesis have increased excitability and migrate preferentially to the superficial dorsal horn layers responsible for nociceptive signaling. Under normal conditions, this process may be responsible for maintaining a steady-state, but adaptable level of nociceptive sensitivity, thus representing an experience-dependent mechanism of regulation similar to other neurogenic niches. Under pathologic conditions, adult spinal cord neurogenesis is greatly amplified and may therefore account for the observed changes in general spinal cord excitability and nociceptive sensitivity. This mechanism also explains many types of chronic pain present in the absence of injury or disease, which may be the result of impaired neuronal differentiation due to a variety of genetic variations. This suggests the possibility of using promoters of neuronal differentiation for the long-term treatment of the causes of chronic pain, unlike current medication which is palliative and effective only for the duration of treatment. The presence of this spinal cord neurogenic niche may also lead to new approaches in spinal cord regeneration.
KEYWORDS: adult neurogenesis, chronic pain, neuronal differentiation, nociception, Notch3, spinal cord
Introduction
Over the past half century, increasing evidence has been uncovered of adult neurogenesis in the mammalian central nervous system, under normal conditions.1-9 Adult neural stem cells are generally thought to be concentrated in 2 regions (“niches”) of the brain, the subgranular zone (SGZ) of the dentate gyrus, in the hippocampus, and the subventricular zone (SVZ) of the lateral ventricles, which sends neuron progenitors to the olfactory bulb.10,11 Humans appear to be an exception from other mammals, as olfactory bulb neurogenesis is negligible, but striatal neurogenesis is present instead.12 Initially, adult stem cells were considered to perform a repair function, as a safety mechanism to replace multiple cell types that may become damaged in the central nervous system (CNS). However, it is now widely accepted that these 2 niches perform physiological functions related to learning and memory or to the olfactory memory,13 respectively. In general, adult neurogenesis seems to be a mechanism that regulates experience-dependent changes,14 at a level that supersedes the limited changes in individual neuronal connectivity through synaptic plasticity. Although adult stem cells have been also found in other regions of the CNS,4,15-17 the presence of active neurogenesis outside of the DG and the SVZ has been disputed.18 These neural progenitors have been usually considered to be quiescent stem cells,19 ready to be activated and induced to proliferate as a repair mechanism after injury. This idea has been reinforced by the observation that the proliferation of these adult stem cells is enhanced after injury or disease.20-24 However, recent observations suggest that, in addition to the SGZ and the SVZ, adult neurogenesis is normally present on a large scale in other parts of the rodent CNS as well, for example in the spinal cord25,26 and the hindbrain.27
Adult neurogenesis in the spinal cord
Previous studies have revealed the presence of neuron progenitor cells in the rodent spinal cord,4,17 however the final conversion of these progenitors into functionally integrated neurons was only assumed, but not demonstrated, due to a lack of adequate imaging methods.28 This difficulty was circumvented using a recent method for labeling proliferating cells with EdU,29 and by analyzing the overlap of EdU staining with immunostaining for markers associated with a neuronal phenotype.25,26 In contrast to BrdU labeling, which requires high temperature, damaging the spinal cord tissue and making subsequent immunostaining very difficult, EdU labeling is performed at room temperature, subsequently allowing efficient immunostaining. Thus, it was demonstrated that adult-generated spinal cord neural stem cells transit sequential stages of differentiation, characterized by specific markers Skp2, nestin, Mash1, Ngn2, Notch3, doublecortin (DCX) and calretinin (CR), eventually becoming NeuN-expressing mature neurons (Fig. 1).26 Neural stem cells generated in the spinal cord ependymal layer26,30,31 migrate along lamina IV, at the medial edge of the dorsal horn, toward the superficial dorsal horn layers. During this migration, which may require up to one month,26,32,33 they gradually transit all the differentiation stages from neural stem cell to mature neuron. This massive migration of neural progenitor cells is significantly increased after disease24 or injury.26,32 The conversion of these progenitor cells into mature functional neurons was demonstrated both implicitly and explicitly. Explicitly, the stream of neuron progenitors generated after experimental unilateral sciatic nerve injury (CCI) in rats is concentrated on the side ipsilateral to injury, and is followed 6 weeks later (approximately the time required for their migration to the dorsal horn) by an approximately 20% excess of ipsilateral lamina I/II neurons relative to the contralateral side.26 This surplus of neurons is noticeable for at least 3 months after injury, which suggests their functional integration; otherwise the survival time of these neurons would be much shorter. Implicitly, the functional integration of these neurons is supported by the correlated timing of increased nociceptive sensitivity (Fig. 2).26
Figure 1.
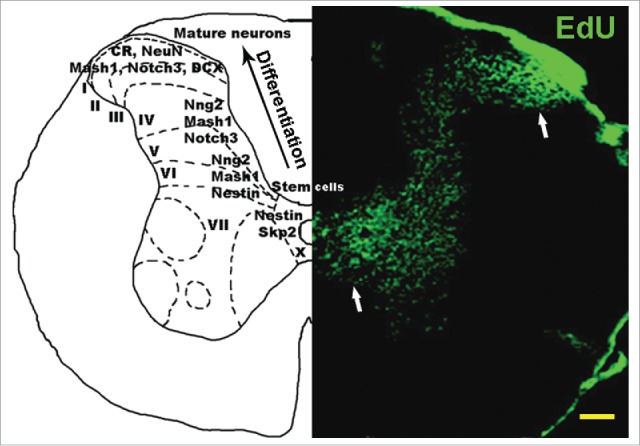
Neural stem cells are continuously generated in normal mouse spinal cord. Right panel: 3-month old mouse spinal cord stained with EdU, which labels proliferating cells. Higher density EdU staining is present around the central canal (lamina X), where neural stem cells are generated from the ependymal layer, and in laminae I/II, where these cells accumulate after migrating along the dorsal horn. Left panel: Distribution of neural progenitor markers across spinal cord layers. The ependymal cell layer stains for stem cell markers Skp2 and Notch2. As neural stem cells migrate from lamina X toward lamina I, neural markers are expressed sequentially, including nestin, Mash1, Ngn2, Notch3, DCX, CR and NeuN, showing increasing levels of neuronal differentiation toward the peripheral dorsal horn. CR = calretinin, DCX = doublecortin. Scale bar: 1 mm.
Figure 2.
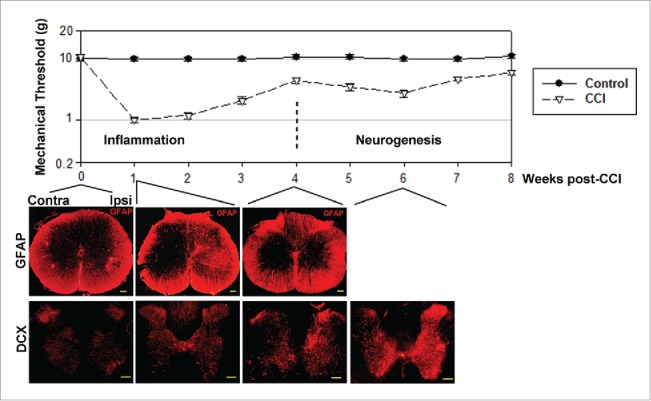
Allodynia has 2 stages, corresponding to inflammation and neurogenesis. Upper panel: Nociceptive sensitivity measured after sciatic nerve ligation in rats (the CCI model of chronic pain), shows 2 distinct stages when represented on a semi-log scale. First stage, lasting approximately 4 weeks after nerve injury, corresponds to inflammatory pain, reflected in the activation of microglia (not shown) and reactive astrocytes, identified by GFAP staining. GFAP staining ipsilateral to nerve injury (Ipsi) peaks at 1 week after nerve injury, then decays back to normal by 4 weeks, mirroring the first stage of allodynia. The second stage of allodynia, lasting from week 4 to weeks 10-12 post-CCI, corresponds to an increase in immature neuron markers such as doublecortin (DCX) and Mash1 (not shown), predominantly on the ipsilateral side. The 4-week delay is explained by the time necessary for neural progenitor cells' proliferation in lamina X, migration to laminae I/II, and partial differentiation into immature neurons. The buildup of highly excitable immature neurons in nociceptive laminae I/II generates a second peak of nociceptive sensitivity that correlates with the peak in the expression of immature neuron markers. Scale bars: 1 mm.
Spinal cord adult neurogenesis is a dynamic regulator of nociceptive sensitivity
The presence of spinal cord immature neurons appears to play a key role in spinal cord physiology. In contrast to mature neurons, immature neurons depolarize in response to GABA, due to a high intracellular [Cl−] concentration.34 A similar increase in [Cl−], resulting in increased excitability has been reported after peripheral nerve injury as a result of an increased expression of NKCC1 and a decrease in KCC2 expression,35,36 2 cation-chloride cotransporters which regulate [Cl−] homeostasis. The combination of these 2 separate observations suggests that the neurons with increased excitability observed in the spinal cord dorsal horn after nerve injury may be in fact immature neurons. This hypothesis was demonstrated experimentally, by showing the abundant expression of immature neuron markers in the ipsilateral dorsal horn after CCI.26 Physiologically, the time period of increased expression of these immature neuron markers correlates quite well with the period of increased nociceptive sensitivity to mechanical (allodynia) and thermal (hyperalgesia) stimulation after CCI. In contrast, the inflammation and synaptic plasticity induced by nerve injury, considered for a long time the major mechanisms in chronic pain, occur on a significantly shorter time scale (Fig. 2). The role of immature spinal cord neurons in regulating nociceptive sensitivity would also be in agreement with other characteristics of chronic pain, which cannot be easily explained by current theories. For example the motor abnormalities (dystonia) associated with chronic pain and the spontaneous pain beyond the dermatome distribution of the injured nerve could be explained by the random, radial and longitudinal migration of neuron progenitors throughout the spinal cord, away from the spinal segment corresponding to the injured nerve.
Another unexplained aspect of the chronic constriction injury model of neuropathic pain is the spontaneous and abrupt decrease in nociceptive sensitivity which follows approximately 2-3 months after nerve injury,37 resulting in a period of strong desensitization to nociception. Neither the inflammation, nor the synaptic plasticity models of chronic pain can adequately explain this observation. However, in a model of neurogenesis-dependent regulation of nociception, this period of reduced sensitivity corresponds to the timing of the maturation of the massive influx of hyper-excitable immature neurons into an excess of mature neurons. Mature neurons hyper-polarize in response to GABA, reducing overall dorsal horn excitability below the normal level. Gradually, over several weeks or months, this excess of dorsal horn neurons is eliminated, presumably through programmed cell death, restoring nociceptive sensitivity to its normal levels.
Conceivably, in normal uninjured animals the constant production and migration of neural stem cells from the ependymal cell layer to the spinal cord laminae I-II maintains a constant level of steady-state nociceptive sensitivity. The forced, accelerated maturation of these neuron precursors in normal rats, using promoters of neuronal differentiation such as TrkB agonists, correlates with a dramatic decrease in nociceptive sensitivity.26 Since inflammation and synaptic plasticity are unlikely to occur under normal conditions, a probable explanation of this observation is that TrkB agonists eliminate the presence of highly excitable immature neurons, resulting in an overall decrease in dorsal horn excitability.
As a result of these considerations, a new model of regulation of nociceptive sensitivity was proposed, which applies to both normal and pathologic conditions, such as chronic pain.26 This model is based on a continuous contribution of spinal cord adult neurogenesis, which maintains a number of highly excitable immature neurons in the superficial dorsal horn layers. The number of these neurons determines the level of nociceptive sensitivity. Such a mechanism is very similar to the experience-dependent changes regulated by adult neurogenesis in the SGZ and SVZ. Under normal conditions, adult spinal cord neurogenesis is low, only sufficient to maintain a steady-state level of nociceptive sensitivity. Pathologic conditions that elicit an increase in neurogenesis would determine an increase in the number of immature dorsal horn neurons, resulting in increased nociceptive sensitivity, in agreement with existing observations. After the injury has healed, spinal cord neurogenesis returns to normal levels, and the excess of immature neurons mature and hyperpolarize in response to GABA, increasing the nociceptive threshold. The evidence that new neurons are constantly produced in the spinal cord ependymal layer, and then migrate to laminae I-II, elicits the question of the fate of the excess neurons present in the upper dorsal horn layers. Since under normal conditions the number of laminae I-II neurons is fairly constant, it is logical to assume that neurons in these layers die through programmed cell death at a rate similar to the rate of neurogenesis. A similar renewal mechanism occurs in the olfactory bulb. The physiological significance of such a constant renewal is unclear, but it may indicate the ability to constantly readjust the threshold to pain.
It is interesting to note that nociceptive sensitivity changes with age.38 The sensitivity to mechanical stimuli increases with age, possibly indicating a reduced number of inhibitory interneurons in the nociceptive layers. At the same time, nociceptive sensitivity to other types of painful stimuli may decrease with age,39 correlating with a decreased rate of immature neuron production. The contradictory nature of these observations correlates with the dual effect of a decreased rate of neurogenesis in old age,40 and is consistent with a role of neurogenesis in regulating nociceptive threshold.
Specific signaling in adult neurogenesis
Adult neurogenesis is likely to involve a different set of signal transduction mechanisms relative to embryonic neurogenesis, given the vastly different cellular environments. In addition, some of the same signaling molecules may play different roles in the adult versus embryo. For example, during embryonic development Notch3 is considered to play a proliferative role, similar to the other Notch receptors, and leads mostly to an astroglial cell phenotype.41 However, in neural precursor cells generated in the adult, Notch3 is co-expressed with neuronal markers, even with mature neuron marker NeuN, but not with astroglial marker GFAP.25 Therefore, in the adult, Notch3 has a divergent, even opposite function from the other Notch receptors. This difference may underlie a variation from the classical embryonic “lateral inhibition” mechanism, where Notch receptors segregate from Notch ligands expressed on adjacent cells.42,43 Unlike embryonic development, in the adult spinal cord Notch3 and Jagged2 are expressed in neuronal precursors, while Notch1, Jagged1, Delta and Delta 4 are expressed on adjacent cells,25 suggesting more individualized roles for Notch receptors and ligands, and a differentiated regulation of their expression. Other receptor families on neighboring cells may also play a role in regulating the outcome of adult neurogenesis.
Role of inflammation in adult neurogenesis
Stem cells transplanted into the spinal cord appear to preferentially differentiate into glia,44 despite numerous attempts, including human clinical trials. Therefore, a key question remains why endogenous spinal cord neurogenesis can constantly produce a large number of neurons, while transplanted exogenous stem cells cannot. Understanding this difference would be key in adapting the endogenous process for spinal cord regeneration. Several factors are likely involved. A critical factor appears to be the contribution of inflammation, through the cytokines and growth factors secreted by microglia and reactive astrocytes. This idea is supported by the fact that, while under normal conditions adult spinal cord neurogenesis is a very slow process, it is significantly amplified under inflammatory conditions such as peripheral nerve injury.26 In that regard, the second, neurogenesis-dependent stage of allodynia can be considered an effect and a continuation of the first, inflammation-dependent stage. It is interesting to note that a standard procedure in stem cell transplantation is the use of immunosuppressive therapy, to prevent transplant rejection. This treatment also has an anti-inflammatory effect, which may in fact negatively influence the proliferation of transplanted stem cells and their ability to differentiate into neurons.
Another observation is that most neural progenitor cells generated in the subependymal layer of the spinal cord migrate toward the superficial dorsal and ventral horns through a narrow path at the medial edge of the dorsal and ventral horns, respectively (Fig. 2). This path is identical with the area of highest GFAP expression, suggesting that reactive astrocytes may assist or even control the migration of neural progenitors, by creating cytokine and/or growth factor gradients, in a manner similar to the rostral migratory stream. It is possible that stem cells transplanted in other regions of the spinal cord lack this trophic/cytokine support, thereby differentiating into glia.
Genetic variations that interfere with neuronal maturation may cause chronic pain
Many clinical cases of chronic pain (e.g. fibromyalgia) cannot be linked to an injury or disease, being assumed instead to be caused by genetic factors.45,46 In fact several transgenic mouse lines have been generated, which show lifetime symptoms of constitutively increased nociceptive sensitivity.25,47,48 It is interesting to note that all the genes found so far to play a role in the regulation of nociceptive sensitivity are also coincidentally involved in the regulation of neuronal differentiation. Such genes include ALDH2,48 Shp2,46 Notch325 and c-kit,47 which are part of signal transduction pathways that stimulate neuronal differentiation. This coincidence supports the idea that genetic variations that interfere with neuronal differentiation will maintain an excess of highly excitable spinal cord immature neurons into adulthood. The resulting increased level of spinal cord excitability will determine a higher basal level of nociceptive sensitivity.
These neuronal differentiation pathways are not limited to spinal cord neurons, and may in general regulate the differentiation of many other types of neurons. This may explain the overlap of several pain syndromes including fibromyalgia, headaches, irritable bowel syndrome and chronic fatigue syndrome,49 which may arise from shared neuronal differentiation defects. The idea that nociceptive sensitivity is regulated through neuronal differentiation remains to be verified in additional transgenic mouse models with defects in other neuronal differentiation pathways. The large number of signal transduction pathways involved in the regulation of neuronal differentiation suggests the possibility of multiple mechanisms and mutations that could alter nociceptive sensitivity. Therefore the use of promoters of neuronal differentiation may provide a general treatment for chronic pain by inducing and/or accelerating the differentiation of immature, hyper-excitable spinal cord neurons. Some exceptions to this idea may arise for example when the genetic variation which interferes with neuronal differentiation is directly down-stream of the receptor subjected to pharmacological treatment. For example the pharmacological activation of TrkB receptors might not be able to overcome loss-of-function defects in down-stream MAPK or Shp2. In that case, the pharmacological activation of alternate signal transduction pathways (e.g., Notch, Wnt) may be able to bypass such defects in neuronal differentiation, and may reduce pain sensitivity.
Clinical applications of spinal cord adult neurogenesis
An obvious application of this neurogenesis-based model of regulating nociceptive sensitivity is the development of new treatments for chronic pain. Current pain treatments based on the activation of inhibitory receptors such as GABA or opioid receptors, or on the inhibition of Na-channel activation are inherently palliative solutions that treat only the symptoms, but not the cause of chronic pain. Such treatments require constant administration, and their effects dissipate as soon as the treatment ceases. Such long-term treatments almost invariably lead to receptor up-regulation or desensitization, requiring an increased dose of antagonist or agonist, respectively, thus resulting in addiction to analgesics. In contrast, treatments based on inducers of neuronal differentiation result in a long-term analgesic effect after only a short treatment,26 by reducing the duration of the immature stage of neurons. Of course, treatments with inducers of neuronal differentiation may also affect many other physiological processes in the central nervous system that rely on the presence of adult neurogenesis, and will therefore require a thorough investigation.
Another application of adult spinal cord neurogenesis is its potential utilization in spinal cord repair after injury or disease. While inflammation may promote adult neurogenesis, it may also promote neuronal death.50,51 Therefore, understanding this process and adapting it for spinal cord repair will be a complex endeavor. For example, stem cells generated in the ependymal layer may be “primed” by surrounding cells in the subependymal layer, possibly by reactive astrocytes, to differentiate into neurons through a Notch receptor-ligand lateral inhibition type of interaction. Such priming may be absent or ambiguous in the rest of the spinal cord, which may explain the limited success with stem cell transplantation. A possibility to circumvent that problem is to “prime” stem cells for neuronal differentiation before transplantation, by inducing them, or transfecting them to express appropriate signaling molecules.
Conclusion
The presence of spinal cord adult neurogenesis and the accumulation of newly generated immature neurons in the upper dorsal horn layers responsible for nociceptive signaling suggest a new mechanism of nociceptive regulation through adult neurogenesis. This mechanism is moderately active under normal conditions and is significantly amplified under pathologic conditions. Such a mechanism can explain many features of chronic pain which cannot be explained by existing models. However, a neurogenesis-based mechanism of pain would function in correlation with existing models such as inflammation and synaptic plasticity. In addition, an adult neurogenesis-based mechanism of regulating nociceptive sensitivity would be similar to other experience-dependent adaptation mechanisms that occur in well-established neurogenic niches in the brain. These findings establish a new neurogenic niche in the spinal cord, and open new opportunities in chronic pain treatment and spinal cord regeneration.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- [1].Graziadei PP, Graziadei GA. Neurogenesis and neuron regeneration in the olfactory system of mammals. I. Morphological aspects of differentiation and structural organization of the olfactory sensory neurons. J Neurocytol 1979; 8:1-18; PMID:438867; http://dx.doi.org/ 10.1007/BF01206454 [DOI] [PubMed] [Google Scholar]
- [2].Kaplan MS, Hinds JW. Neurogenesis in the adult rat: electron microscopic analysis of light radioautographs. Science 1977; 197:1092-4; http://dx.doi.org/ 10.1126/science.887941 [DOI] [PubMed] [Google Scholar]
- [3].Gage FH. Mammalian neural stem cells. Science 2000; 287:1433-8; http://dx.doi.org/ 10.1126/science.287.5457.1433 [DOI] [PubMed] [Google Scholar]
- [4].Horner PJ, Power AE, Kempermann G, Kuhn HG, Palmer TD, Winkler J, Thal LJ, Gage FH. Proliferation and differentiation of progenitor cells throughout the intact adult rat spinal cord. J Neurosci 2000; 20:2218-28; PMID:10704497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Lois C, Alvarez-Buylla A. Proliferating subventricular zone cells in the adult mammalian forebrain can differentiate into neurons and glia. Proc Natl Acad Sci U S A 1993; 90:2074-7; PMID:8446631; http://dx.doi.org/ 10.1073/pnas.90.5.2074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Richards LJ, Kilpatrick TJ, Bartlett PF. De novo generation of neuronal cells from the adult mouse brain. Proc Natl Acad Sci U S A 1992; 89:8591-5; PMID:1528866; http://dx.doi.org/ 10.1073/pnas.89.18.8591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science 1992; 255:1707-10; PMID:1553558; http://dx.doi.org/ 10.1126/science.1553558 [DOI] [PubMed] [Google Scholar]
- [8].Johansson CB, Momma S, Clarke DL, Risling M, Lendahl U, Frisen J. Identification of a neural stem cell in the adult mammalian central nervous system. Cell 1999; 96:25-34; PMID:9989494; http://dx.doi.org/ 10.1016/S0092-8674(00)80956-3 [DOI] [PubMed] [Google Scholar]
- [9].Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol 1965; 124:319-35; PMID:5861717; http://dx.doi.org/ 10.1002/cne.901240303 [DOI] [PubMed] [Google Scholar]
- [10].Suh H, Deng W, Gage FH. Signaling in adult neurogenesis. Annu Rev Cell Dev Biol 2009; 25:253-75; PMID:19575663; http://dx.doi.org/ 10.1146/annurev.cellbio.042308.113256 [DOI] [PubMed] [Google Scholar]
- [11].Ming GL, Song H. Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron 2011; 70:687-702; PMID:21609825; http://dx.doi.org/ 10.1016/j.neuron.2011.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Bergmann O, Spalding KL, Frisen J. Adult Neurogenesis in Humans. Cold Spring Harb Perspect Biol 2015; 7:a018994; PMID:26134318; http://dx.doi.org/ 10.1101/cshperspect.a018994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Breton-Provencher V, Lemasson M, Peralta MR III, Saghatelyan A. Interneurons produced in adulthood are required for the normal functioning of the olfactory bulb network and for the execution of selected olfactory behaviors. J Neurosci 2009; 29:15245-57; http://dx.doi.org/ 10.1523/JNEUROSCI.3606-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Opendak M, Gould E. Adult neurogenesis: a substrate for experience-dependent change. Trends Cogn Sci 2015; 19:151-61; PMID:25715908; http://dx.doi.org/ 10.1016/j.tics.2015.01.001 [DOI] [PubMed] [Google Scholar]
- [15].Weiss S, Dunne C, Hewson J, Wohl C, Wheatley M, Peterson AC, Reynolds BA. Multipotent CNS stem cells are present in the adult mammalian spinal cord and ventricular neuroaxis. J Neurosci 1996; 16:7599-609; PMID:8922416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kaplan MS. Neurogenesis in the 3-month-old rat visual cortex. J Comp Neurol 1991; 195:323-38; PMID:7251929; http://dx.doi.org/ 10.1002/cne.901950211 [DOI] [PubMed] [Google Scholar]
- [17].Shechter R, Ziv Y, Schwartz M. New GABAergic interneurons supported by myelin-specific T cells are formed in intact adult spinal cord. Stem Cells 2007; 25:2277-82; PMID:17540856; http://dx.doi.org/ 10.1634/stemcells.2006-0705 [DOI] [PubMed] [Google Scholar]
- [18].Kornack DR, Rakic P. Cell proliferation without neurogenesis in adult primate neocortex. Science 2001; 294:2127-30; PMID:11739948; http://dx.doi.org/ 10.1126/science.1065467 [DOI] [PubMed] [Google Scholar]
- [19].Morshead CM, Reynolds BA, Craig CG, McBurney MW, Staines WA, Morassutti D, Weiss S, van der Kooy D. Neural stem cells in the adult mammalian forebrain: a relatively quiescent subpopulation of subependymal cells. Neuron 1994; 13:1071-82; http://dx.doi.org/ 10.1016/0896-6273(94)90046-9 [DOI] [PubMed] [Google Scholar]
- [20].Magavi SS, Leavitt BR, Macklis JD. Induction of neurogenesis in the neocortex of adult mice. Nature 2000; 405:951-5; http://dx.doi.org/ 10.1038/35016083 [DOI] [PubMed] [Google Scholar]
- [21].Tighilet B, Brezun JM, Sylvie GD, Gaubert C, Lacour M. New neurons in the vestibular nuclei complex after unilateral vestibular neurectomy in the adult cat. Eur J Neurosci 2007; 25:47-58; http://dx.doi.org/ 10.1111/j.1460-9568.2006.05267.x [DOI] [PubMed] [Google Scholar]
- [22].Danilov AI, Covacu R, Moe MC, Langmoen IA, Johansson CB, Olsson T, Brundin L. Neurogenesis in the adult spinal cord in an experimental model of multiple sclerosis. Eur J Neurosci 2006; 23:394-400; http://dx.doi.org/ 10.1111/j.1460-9568.2005.04563.x [DOI] [PubMed] [Google Scholar]
- [23].Carlen M, Meletis K, Goritz C, Darsalia V, Evergren E, Tanigaki K, Amendola M, Barnabe-Heider F, Yeung MS, Naldini L, et al.. Forebrain ependymal cells are Notch-dependent and generate neuroblasts and astrocytes after stroke. Nat Neurosci 2009; 12:259-67; PMID:19234458; http://dx.doi.org/ 10.1038/nn.2268 [DOI] [PubMed] [Google Scholar]
- [24].Chi L, Ke Y, Luo C, Li B, Gozal D, Kalyanaraman B, Liu R. Motor neuron degeneration promotes neural progenitor cell proliferation, migration, and neurogenesis in the spinal cords of amyotrophic lateral sclerosis mice. Stem Cells 2006; 24:34-43; PMID:16099995; http://dx.doi.org/ 10.1634/stemcells.2005-0076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Rusanescu G, Mao J. Notch3 is necessary for neuronal differentiation and maturation in the adult spinal cord. J Cell Mol Med 2014; 18:2103-16; PMID:25164209; http://dx.doi.org/ 10.1111/jcmm.12362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Rusanescu G, Mao J. Immature spinal cord neurons are dynamic regulators of adult nociceptive sensitivity. J Cell Mol Med 2015; 19:2352-64; PMID:26223362; http://dx.doi.org/ 10.1111/jcmm.12648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Rusanescu G, Mao J. Peripheral nerve injury induces adult brain neurogenesis and remodeling. J Cell Mol Med 2016; 20; http://dx.doi.org/ 10.1111/jcmm.12965. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Gould E. How widespread is adult neurogenesis in mammals? Nat Rev Neurosci 2007; 8:481-8; PMID:17514200; http://dx.doi.org/ 10.1038/nrn2147 [DOI] [PubMed] [Google Scholar]
- [29].Salic A, Mitchison TJ. A chemical method for fast and sensitive detection of DNA synthesis in vivo. Proc Natl Acad Sci U S A 2008; 105:2415-20; PMID:18272492; http://dx.doi.org/ 10.1073/pnas.0712168105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Meletis K, Barnabe-Heider F, Carlen M, Evergren E, Tomilin N, Shupliakov O, Frisen J. Spinal cord injury reveals multilineage differentiation of ependymal cells. PLoS Biol 2008; 6:e182; PMID:18651793; http://dx.doi.org/ 10.1371/journal.pbio.0060182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Barnabe-Heider F, Goritz C, Sabelstrom H, Takebayashi H, Pfrieger FW, Meletis K, Frisen J. Origin of new glial cells in intact and injured adult spinal cord. Cell Stem Cell 2010; 7:470-82; PMID:20887953; http://dx.doi.org/ 10.1016/j.stem.2010.07.014 [DOI] [PubMed] [Google Scholar]
- [32].Vessal M, Aycock A, Garton MT, Ciferri M, Darian-Smith C. Adult neurogenesis in primate and rodent spinal cord: comparing a cervical dorsal rhizotomy with a dorsal column transection. Eur J Neurosci 2007; 26:2777-94; http://dx.doi.org/ 10.1111/j.1460-9568.2007.05871.x [DOI] [PubMed] [Google Scholar]
- [33].Vessal M, Darian-Smith C. Adult neurogenesis occurs in primate sensorimotor cortex following cervical dorsal rhizotomy. J Neurosci 2010; 30:8613-23; PMID:20573907; http://dx.doi.org/ 10.1523/JNEUROSCI.5272-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].LoTurco JJ, Owens DF, Heath MJ, Davis MB, Kriegstein AR. GABA and glutamate depolarize cortical progenitor cells and inhibit DNA synthesis. Neuron 1995; 15:1287-98; PMID:8845153; http://dx.doi.org/ 10.1016/0896-6273(95)90008-X [DOI] [PubMed] [Google Scholar]
- [35].Price TJ, Cervero F, de Koninck Y. Role of cation-chloride-cotransporters (CCC) in pain and hyperalgesia. Curr Top Med Chem 2005; 5:547-55; PMID:16022677; http://dx.doi.org/ 10.2174/1568026054367629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Lu Y, Zheng J, Xiong L, Zimmermann M, Yang J. Spinal cord injury-induced attenuation of GABAergic inhibition in spinal dorsal horn circuits is associated with down-regulation of the chloride transporter KCC2 in rat. J Physiol 2008; 586:5701-15; PMID:18845615; http://dx.doi.org/ 10.1113/jphysiol.2008.152348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain 1988; 33:87-107; http://dx.doi.org/ 10.1016/0304-3959(88)90209-6 [DOI] [PubMed] [Google Scholar]
- [38].Lautenbacher S, Kunz M, Strate P, Nielsen J, Arendt-Nielsen L. Age effects on pain thresholds, temporal summation and spatial summation of heat and pressure pain. Pain 2005; 115:410-18; PMID:15876494; http://dx.doi.org/ 10.1016/j.pain.2005.03.025 [DOI] [PubMed] [Google Scholar]
- [39].Gibson SJ, Helme RD. Age-related differences in pain perception and report. Clin Geriatr Med 2001; 17:433-4vi; http://dx.doi.org/ 10.1016/S0749-0690(05)70079-3 [DOI] [PubMed] [Google Scholar]
- [40].Galvan V, Jin K. Neurogenesis in the aging brain. Clin Interv Aging 2007; 2:605-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Tanigaki K, Nogaki F, Takahashi J, Tashiro K, Kurooka H, Honjo T. Notch1 and Notch3 instructively restrict bFGF-responsive multipotent neural progenitor cells to an astroglial fate. Neuron 2001; 29:45-55; http://dx.doi.org/ 10.1016/S0896-6273(01)00179-9 [DOI] [PubMed] [Google Scholar]
- [42].Campos-Ortega JA. Mechanisms of early neurogenesis in Drosophila melanogaster. J Neurobiol 1993; 24:1305-27; PMID:8228961; http://dx.doi.org/ 10.1002/neu.480241005 [DOI] [PubMed] [Google Scholar]
- [43].Muskavitch MA. Delta-notch signaling and Drosophila cell fate choice. Dev Biol 1994; 166:415-30; PMID:7813766; http://dx.doi.org/ 10.1006/dbio.1994.1326 [DOI] [PubMed] [Google Scholar]
- [44].Shihabuddin LS, Horner PJ, Ray J, Gage FH. Adult spinal cord stem cells generate neurons after transplantation in the adult dentate gyrus. J Neurosci 2000; 20:8727-35; PMID:11102479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Yunus MB, Khan MA, Rawlings KK, Green JR, Olson JM, Shah S. Genetic linkage analysis of multicase families with fibromyalgia syndrome. J Rheumatol 1999; 26:408-12 [PubMed] [Google Scholar]
- [46].Vegunta S, Cotugno R, Williamson A, Grebe TA. Chronic pain in Noonan syndrome: A previously unreported but common symptom. Am J Med Genet 2015; 167A:2998-3005; http://dx.doi.org/ 10.1002/ajmg.a.37337 [DOI] [PubMed] [Google Scholar]
- [47].Milenkovic N, Frahm C, Gassmann M, Griffel C, Erdmann B, Birchmeier C, Lewin GR, Garratt AN. Nociceptive tuning by stem cell factor/c-Kit signaling. Neuron 2007; 56:893-906; PMID:18054864; http://dx.doi.org/ 10.1016/j.neuron.2007.10.040 [DOI] [PubMed] [Google Scholar]
- [48].Zambelli VO, Gross ER, Chen CH, Gutierrez VP, Cury Y, Mochly-Rosen D. Aldehyde dehydrogenase-2 regulates nociception in rodent models of acute inflammatory pain. Sci Transl Med 2014; 6:251ra118; PMID:25163478; http://dx.doi.org/ 10.1126/scitranslmed.3009539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Yunus MB. Fibromyalgia and overlapping disorders: the unifying concept of central sensitivity syndromes. Semin Arthritis Rheum 2007; 36:339-56; PMID:17350675; http://dx.doi.org/ 10.1016/j.semarthrit.2006.12.009 [DOI] [PubMed] [Google Scholar]
- [50].Bamberger ME, Landreth GE. Inflammation, apoptosis, and Alzheimer's disease. Neuroscientist 2002; 8:276-83; PMID:12061507 [DOI] [PubMed] [Google Scholar]
- [51].Beattie MS. Inflammation and apoptosis: linked therapeutic targets in spinal cord injury. Trends Mol Med 2004; 10:580-3; PMID:15567326; http://dx.doi.org/ 10.1016/j.molmed.2004.10.006 [DOI] [PubMed] [Google Scholar]


