ABSTRACT
Astrocytes, a major type of glial cells in the mammalian central nervous system (CNS), have a wide variety of physiological functions, including formation of the blood brain barrier, and modulation of synaptic transmission and information processing, and maintenance of CNS homeostasis. The signaling pathway initiated by bone morphogenetic protein (BMP) is critical for astrogliogenesis. However, exactly how this pathway regulates astrogliogenesis remains poorly understood. We have recently provided in vitro and in vivo evidence for neogenin's function in neural stem cells (NSCs) to promote neocortical astrogliogenesis. Neogenin in NSCs as well as astrocytes is required for BMP2 activation of RhoA that promotes YAP (yes-associated protein) nuclear translocation, consequently, YAP interaction with nuclear p-Smad1/5/8, and stabilization of Smad1/5/8 signaling. We have also provided evidence that YAP in NSCs is necessary for neocortical astrogliogenesis, and expression of YAP in neogenin deficient NSCs diminishes the astrogliogenesis deficit. These recent findings identify an unrecognized function of neogenin in promoting neocortical astrogliogenesis, and reveal a pathway of BMP2-neogenin-YAP-Smad1 underlying astrogliogenesis in developing mouse neocortex.
KEYWORDS: astrocyte, BMPs, differentiation, Neogenin, YAP
Nearly 50% of the cells in the adult human brain are glial cells.1 Among which, astrocytes are the most abundant glial cell type in the mammalian brain, which play a wide variety of roles in brain development and functions, such as regulating the cerebral blood flow, forming and maintaining blood brain barrier, supporting the central nervous system (CNS) metabolism, clearing the neurotransmitter between synapse, and specific effects on synaptogenesis and synaptic plasticity.2-5 In addition, astrocytes also play critical roles in pathological CNS such as spinal cord injury and stroke.2,6,7 Defects in astrocyte generation during development contribute to dysfunctions of synaptic plasticity, neuropsychological disorders, and brain tumors.6,8 Thus, it is of considerable interest to investigate how astrocytes are generated. During mammalian brain development, astrocytes are derived from neural stem cells (NSCs) in the ventricular and sub-ventricular zone. Rodent cortico-cerebral astrogliogenesis mainly takes place at the late embryonic stages and the first 3 postnatal weeks, following neurogenesis,4,9,10 which is consisted of 2 concurrent regulatory processes: astrocyte differentiation from NSCs and the local proliferation of astrocytes.9,11 Pioneer studies have shown that rodent cortico-cerebral astrogliogenesis is controlled by both intrinsic factors12 and extracellular factors,13 which induce astrocytic gene transcription such as GFAP and S100beta. Nowadays, although studies from culture system and mouse model have demonstrated that bone morphogenetic protein (BMP)-Smads signaling,9,10,14 Notch signaling,9,10,15 and Janus kinase-signal transducer and activator of transcription (JAK-STAT) signaling pathways control the appropriate timing of astrogliogenesis,10,16,17 exactly how these pathways regulate astrogliogenesis remains poorly understood. Here, we mainly focus on recent molecular insights into the role of BMP signaling in neocortical astrogliogenesis in the developing mouse brain.
BMPs are members of the transforming growth factor β (TGFβ) superfamily of signaling ligands.18 BMPs mediate a highly conserved signal transduction cascade through the type-I and type-II receptors and intracellular Smad proteins, which regulate a wide variety of cellular processes, including cell fate specification, cell proliferation, cell migration and cell death during development.19 BMPs play dynamic roles in neurogenesis and astrogliogenesis.9,10,14,18 During late embryonic and early postnatal periods, BMP signaling promotes astroglial differentiation.9,14,20 Combination treatment of BMP2 with LIF accelerates the induction of astrocytes from the cultured E14.5 mouse telencephalic precursors,21 and such capability of BMP2 and LIF to synergically promote astrogliogenesis was further confirmed in E16.5 cortico-cerebral precursors kept under thyroid hormones.22 Mechanistically, BMP signaling is mediated by heteroterameric serine/threonine kinase receptors and their downstream transcription factors Smad1/5/8. In the nuclei, Smads form a complex with STAT3 that is bridged by the transcriptional co-activators p300/CBP,9,10,23 and participate in the induction of astrocytic gene expression. Knockout LIF24 and its receptors LIFRβ25 and gp13026 or downstream of STAT327 all result into the impairment of astrocytic differentiation, indicating that the JAK-STAT3 pathway is essential for astrogliogenesis in the developing brain. Interestingly, treatment of BMP2 alone to gp130−/− cultures, elicits a moderate but reproducible activation of the GFAP promoter,21 suggesting that pSmads might also directly activate GFAP promoter, independently of JAK-pSTAT3 pathway. In our recent studies, in the receptor and downstream levels, we provide new insights on the BMPs signaling for the neocortical astrogliogenesis.
Neogenin, a member of the DCC family transmembrane protein, serves as a receptor for the axon guidance cue netrins, the repulsive guidance molecules (RGMs) and BMPs.28,29 By taking advantage of X-gal reporter in neogenin mutant mice and antibodies, we have demonstrated neogenin's expression in embryonic NSCs in vitro and in vivo,30 in consistent with the previous reports.31-34 Neogenin deficient mice or NSCs showed normal self-renewal or proliferation of NSCs or neurogenesis,30 but impaired neocortical astrogliogenesis. Note that neogenin is reported to regulate adult neurogenesis by promoting neuroblast migration and cell cycle exit,35 suggesting that neogenin may play an age-dependent function during neurogenesis. Although neogenin is not required for neural differentiation in cultured NSCs and in neonatal age, multiple neogenin mutant mice, including neogenin hypomorphic allele, neogeninNestin-CKO, and neogeninGFAP-CKO, show an impairment in neocortical, but not hippocampal, astrogliogenesis.30 In addition, neogenin depletion in E15.5 cortical NSCs by in utero electroporation also results in impaired astrogliogenesis, providing additional evidence for neogenin's function in promoting neocortical astrogliogenesis. It remains unclear regarding the mechanisms underlying neogenin's selective regulation of neocortical astrogliogenesis. We propose the following potential possibilities. First, the temporal and spatial expression patterns of BMPs and neogenin may be different between cortex and hippocampus. Second, neogenin may have different signaling and functions in NSCs between cortex and hippocampus. These possibilities require further investigations in future.
Neogenin appears to be a co-receptor for BMPs signaling. It regulates iron homeostasis by regulating BMP induction of hepcidin.36 It also promotes chondrogenesis and endochondral bone formation by enhancing and sustaining BMP-Smad1/5/8 signaling in chondrocytes.37 In consistent, our recent study supports a role for neogenin in BMP-2-induced astrocyte differentiation.30 Exactly how neogenin regulates BMP signaling remains poorly understood. Several mechanisms may underlie neogenin regulation of BMP signaling. First, in the ligand level, neogenin may play a role in process and secretion of soluble HJV, an inhibitor of BMP signaling.36 Second, at the receptor level, upon BMP2 treatment, both neogenin and BMP receptors are recruited to the lipid raft microdomains, and neogenin is required for the recruitment or stabilization of BMP receptors in lipid rafts.37 Interestingly, recent structure studies have shown that neogenin ligands, RGMs, serve as a bridge between neogenin and BMPs.29,38 Third, in the downstream signaling level, our recent findings suggest that neogenin in NSCs or astrocytes is required for BMP2 activation of RhoA, which promotes YAP (yes-associated protein) nuclear translocation, consequently, YAP interaction with nuclear p-Smad1/5/830. This event is critical for stabilization of nuclear pSmad1/5/8 signaling and neocortical astrogliogenesis.30 YAP knockout in NSCs also results in a similar cortical astrogliogenesis deficit in vitro and in vivo.30,39 Overexpression of YAP in neogenin deficit NSCs diminishes the astrocytic differentiation deficit.30 These observations suggest that neogenin/YAP pathway is essential for cortical astrogliogenesis in the developing brain.
How does YAP regulate BMP2/Smad1 signaling? Our results suggest that YAP interaction with pSmad1 may be critical for maintaining pSmad1 protein stability. This view are in line with reports that YAP interacts with Smads in the nuclear to modulate BMP/Smad1 or TGF/Smad2 signaling in HEK293 cells or Eph4 cells,40-43 and that YAP-pSmad1/5/8 complex in the nuclei of HEK293 cells prevents p-Smad1/5/8 degradation by Smurf1.40,43 These reports, combine with our results, demonstrate the importance of YAP regulation of BMP2/Smad1 signaling in various cell types. Interestingly, our recent studies have shown that YAP may interact with STAT3 in the nucleus of astrocytes upon cytokine treatment.44 Thus, it is of interest to test whether YAP forms a complex with smad1 and STAT3 in the nucleus to promote BMP2-induced astrogliogenesis in future (Fig. 1, Model).
Figure 1.
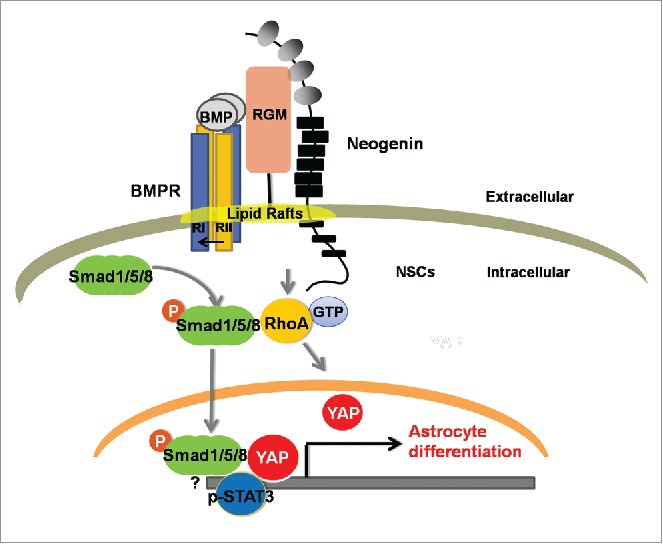
A model of neogenin and YAP in modulating BMP2-inducing astrocytic differentiation in NSCs or astrocytes. Neogenin in NSCs or astrocytes is required for BMP2 activation of RhoA, which promotes YAP nuclear translocation, consequently, YAP interaction with nuclear p-Smad1/5/8. This event is critical for stabilization of nuclear p-Smad1/5/8 signaling and neocortical astrogliogenesis. YAP is very likely form a complex with smad1 and STAT3 in the nucleus to promote BMP2-induced astrogliogenesis.
Astrocytic glioma is the most common brain tumor in adult central nervous system.45 The glioma initiating cells (GIC) are believed as be highly chemoresistant, thus they are responsible for glioma replase. One potential treatment for glioma is to induce differentiation of GICs to more benign and or/druggable cell type.46 BMP signaling (p-Smad1/5/8) is decreased in patients with glioma, compared with that of normal brain and low grade astocytomas. The expression of BMPRIB receptor is down-regulated in high grade glioma.47 Interestingly, neogenin is also reduced in these patients with glioma.48 These observations thus demonstrate a negative correlation between BMP-neogenin signaling pathway and the malignant grade of glioma. This view is supported by additional observation that BMPs inhibit proliferation and promote differentiation of GICs, thus suppressing the growth of glioma.49 Our studies suggest that neogenin/RhoA/YAP/Smad1 signaling plays a critical role in BMP2-induced astrocyte differentiation of NSCs. Although netrin-1 via DCC receptor up-regulates YAP expression, escalating YAP levels in the nucleus and promoting cancer cell proliferation and migration,50 our results showed that netrin-1 did not regulate YAP level in WT or neogenin mutant astrocytes. Further investigation is necessary to illustrate whether neogenin/RhoA/YAP/Smad1 signaling pathway is involved in BMPs-induced differentiation of GICs, which may reveal novel therapeutic targets for astroglioma.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We apologize to those whose work we could not cite due to space limitation, and thank members of Dr. Xiong and Dr. Mei's laboratories for helpful discussions and suggestions.
Funding
This study was supported in part by grants from National Institute of Aging (NIH, AG045781) and Department of Veterans Affair (BX000838), and National Natural Science Foundation of China (81371350, 31671071).
References
- [1].Azevedo FA, Carvalho LR, Grinberg LT, Farfel JM, Ferretti RE, Leite RE, Jacob Filho W, Lent R, Herculano-Houzel S. Equal numbers of neuronal and nonneuronal cells make the human brain an isometrically scaled-up primate brain. The J Comparative Neurol 2009; 513:532-41; PMID:19226510; http://dx.doi.org/ 10.1002/cne.21974 [DOI] [PubMed] [Google Scholar]
- [2].Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta Neuropathol 2010; 119:7-35; PMID:20012068; http://dx.doi.org/ 10.1007/s00401-009-0619-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Rose CR, Karus C. Two sides of the same coin: sodium homeostasis and signaling in astrocytes under physiological and pathophysiological conditions. Glia 2013; 61:1191-205; PMID:23553639; http://dx.doi.org/ 10.1002/glia.22492 [DOI] [PubMed] [Google Scholar]
- [4].Hu X, Yuan Y, Wang D, Su Z. Heterogeneous astrocytes: Active players in CNS. Brain Res Bull 2016; 125:1-18; PMID:27021168; http://dx.doi.org/ 10.1016/j.brainresbull.2016.03.017 [DOI] [PubMed] [Google Scholar]
- [5].Freeman MR, Rowitch DH. Evolving concepts of gliogenesis: a look way back and ahead to the next 25 years. Neuron 2013; 80:613-23; PMID:24183014; http://dx.doi.org/ 10.1016/j.neuron.2013.10.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Molofsky AV, Krencik R, Ullian EM, Tsai HH, Deneen B, Richardson WD, Barres BA, Rowitch DH. Astrocytes and disease: a neurodevelopmental perspective. Genes Dev 2012; 26:891-907; PMID:22549954; http://dx.doi.org/ 10.1101/gad.188326.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Burda JE, Sofroniew MV. Reactive gliosis and the multicellular response to CNS damage and disease. Neuron 2014; 81:229-48; PMID:24462092; http://dx.doi.org/ 10.1016/j.neuron.2013.12.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ullian EM, Sapperstein SK, Christopherson KS, Barres BA. Control of synapse number by glia. Science 2001; 291:657-61; PMID:11158678; http://dx.doi.org/ 10.1126/science.291.5504.657 [DOI] [PubMed] [Google Scholar]
- [9].Mallamaci A. Developmental control of cortico-cerebral astrogenesis. Int J Dev Biol 2013; 57:689-706; PMID:24307293; http://dx.doi.org/ 10.1387/ijdb.130148am [DOI] [PubMed] [Google Scholar]
- [10].Namihira M, Nakashima K. Mechanisms of astrocytogenesis in the mammalian brain. Curr Opin Neurobiol 2013; 23:921-7; PMID:23827784; http://dx.doi.org/ 10.1016/j.conb.2013.06.002 [DOI] [PubMed] [Google Scholar]
- [11].Ge WP, Miyawaki A, Gage FH, Jan YN, Jan LY. Local generation of glia is a major astrocyte source in postnatal cortex. Nature 2012; 484:376-80; PMID:22456708; http://dx.doi.org/ 10.1038/nature10959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Qian X, Shen Q, Goderie SK, He W, Capela A, Davis AA, Temple S. Timing of CNS cell generation: a programmed sequence of neuron and glial cell production from isolated murine cortical stem cells. Neuron 2000; 28:69-80; PMID:11086984; http://dx.doi.org/ 10.1016/S0896-6273(00)00086-6 [DOI] [PubMed] [Google Scholar]
- [13].Morrow T, Song MR, Ghosh A. Sequential specification of neurons and glia by developmentally regulated extracellular factors. Development 2001; 128:3585-94; PMID:11566862 [DOI] [PubMed] [Google Scholar]
- [14].Gross RE, Mehler MF, Mabie PC, Zang Z, Santschi L, Kessler JA. Bone morphogenetic proteins promote astroglial lineage commitment by mammalian subventricular zone progenitor cells. Neuron 1996; 17:595-606; PMID:8893018; http://dx.doi.org/ 10.1016/S0896-6273(00)80193-2 [DOI] [PubMed] [Google Scholar]
- [15].Morrison SJ, Perez SE, Qiao Z, Verdi JM, Hicks C, Weinmaster G, Anderson DJ. Transient Notch activation initiates an irreversible switch from neurogenesis to gliogenesis by neural crest stem cells. Cell 2000; 101:499-510; PMID:10850492; http://dx.doi.org/ 10.1016/S0092-8674(00)80860-0 [DOI] [PubMed] [Google Scholar]
- [16].He F, Ge W, Martinowich K, Becker-Catania S, Coskun V, Zhu W, Wu H, Castro D, Guillemot F, Fan G, et al.. A positive autoregulatory loop of Jak-STAT signaling controls the onset of astrogliogenesis. Nat Neurosci 2005; 8:616-25; PMID:15852015; http://dx.doi.org/ 10.1038/nn1440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Bonni A, Sun Y, Nadal-Vicens M, Bhatt A, Frank DA, Rozovsky I, Stahl N, Yancopoulos GD, Greenberg ME. Regulation of gliogenesis in the central nervous system by the JAK-STAT signaling pathway. Science 1997; 278:477-83; PMID:9334309; http://dx.doi.org/ 10.1126/science.278.5337.477 [DOI] [PubMed] [Google Scholar]
- [18].Bond AM, Bhalala OG, Kessler JA. The dynamic role of bone morphogenetic proteins in neural stem cell fate and maturation. Dev Neurobiol 2012; 72:1068-84; PMID:22489086; http://dx.doi.org/ 10.1002/dneu.22022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Wu MY, Hill CS. Tgf-beta superfamily signaling in embryonic development and homeostasis. Dev Cell 2009; 16:329-43; PMID:19289080; http://dx.doi.org/ 10.1016/j.devcel.2009.02.012 [DOI] [PubMed] [Google Scholar]
- [20].Mehler MF, Mabie PC, Zhu G, Gokhan S, Kessler JA. Developmental changes in progenitor cell responsiveness to bone morphogenetic proteins differentially modulate progressive CNS lineage fate. Dev Neurosci 2000; 22:74-85; PMID:10657700; http://dx.doi.org/ 10.1159/000017429 [DOI] [PubMed] [Google Scholar]
- [21].Nakashima K, Yanagisawa M, Arakawa H, Taga T. Astrocyte differentiation mediated by LIF in cooperation with BMP2. FEBS Lett 1999; 457:43-6; PMID:10486560; http://dx.doi.org/ 10.1016/S0014-5793(99)00997-7 [DOI] [PubMed] [Google Scholar]
- [22].Adachi T, Takanaga H, Kunimoto M, Asou H. Influence of LIF and BMP-2 on differentiation and development of glial cells in primary cultures of embryonic rat cerebral hemisphere. J Neurosci Res 2005; 79:608-15; PMID:15678513; http://dx.doi.org/ 10.1002/jnr.20373 [DOI] [PubMed] [Google Scholar]
- [23].Nakashima K, Yanagisawa M, Arakawa H, Kimura N, Hisatsune T, Kawabata M, Miyazono K, Taga T. Synergistic signaling in fetal brain by STAT3-Smad1 complex bridged by p300. Science 1999; 284:479-82; PMID:10205054; http://dx.doi.org/ 10.1126/science.284.5413.479 [DOI] [PubMed] [Google Scholar]
- [24].Bugga L, Gadient RA, Kwan K, Stewart CL, Patterson PH. Analysis of neuronal and glial phenotypes in brains of mice deficient in leukemia inhibitory factor. J Neurobiol 1998; 36:509-24; PMID:9740023; http://dx.doi.org/ 10.1002/(SICI)1097-4695(19980915)36:4%3c509::AID-NEU5%3e3.0.CO;2- [DOI] [PubMed] [Google Scholar]
- [25].Koblar SA, Turnley AM, Classon BJ, Reid KL, Ware CB, Cheema SS, Murphy M, Bartlett PF. Neural precursor differentiation into astrocytes requires signaling through the leukemia inhibitory factor receptor. Proc Natl Acad Sci U S A 1998; 95:3178-81; PMID:9501236; http://dx.doi.org/ 10.1073/pnas.95.6.3178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Nakashima K, Wiese S, Yanagisawa M, Arakawa H, Kimura N, Hisatsune T, Yoshida K, Kishimoto T, Sendtner M, Taga T. Developmental requirement of gp130 signaling in neuronal survival and astrocyte differentiation. J Neurosci 1999; 19:5429-34; PMID:10377352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].He F, Ge W, Martinowich K, Becker-Catania S, Coskun V, Zhu W, Wu H, Castro D, Guillemot F, Fan G, et al.. A positive autoregulatory loop of Jak-STAT signaling controls the onset of astrogliogenesis. Nat Neurosci 2005; 8:616-25; PMID:15852015; http://dx.doi.org/ 10.1038/nn1440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].De Vries M, Cooper HM. Emerging roles for neogenin and its ligands in CNS development. J Neurochem 2008; 106:1483-92; PMID:18485097; http://dx.doi.org/ 10.1111/j.1471-4159.2008.05485.x [DOI] [PubMed] [Google Scholar]
- [29].Tian C, Liu J. Repulsive guidance molecules (RGMs) and neogenin in bone morphogenetic protein (BMP) signaling. Mol Reprod Dev 2013; 80:700-17; PMID:23740870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Huang Z, Sun D, Hu JX, Tang FL, Lee DH, Wang Y, Hu G, Zhu XJ, Zhou J, Mei L, et al.. Neogenin Promotes BMP2 Activation of YAP and Smad1 and Enhances Astrocytic Differentiation in Developing Mouse Neocortex. J Neurosci 2016; 36:5833-49; PMID:27225772; http://dx.doi.org/ 10.1523/JNEUROSCI.4487-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Fitzgerald DP, Bradford D, Cooper HM. Neogenin is expressed on neurogenic and gliogenic progenitors in the embryonic and adult central nervous system. Gene expression patterns : GEP 2007; 7:784-92; PMID:17604699; http://dx.doi.org/ 10.1016/j.modgep.2007.05.004 [DOI] [PubMed] [Google Scholar]
- [32].van den Heuvel DM, Hellemons AJ, Pasterkamp RJ. Spatiotemporal expression of repulsive guidance molecules (RGMs) and their receptor neogenin in the mouse brain. PLoS One 2013; 8:e55828; PMID:23457482; http://dx.doi.org/ 10.1371/journal.pone.0055828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Bradford D, Faull RL, Curtis MA, Cooper HM. Characterization of the netrin/RGMa receptor neogenin in neurogenic regions of the mouse and human adult forebrain. J Comparative Neurol 2010; 518:3237-53; PMID:20575069; http://dx.doi.org/ 10.1002/cne.22397 [DOI] [PubMed] [Google Scholar]
- [34].Gad JM, Keeling SL, Wilks AF, Tan SS, Cooper HM. The expression patterns of guidance receptors, DCC and Neogenin, are spatially and temporally distinct throughout mouse embryogenesis. Dev Biol 1997; 192:258-73; PMID:9441666; http://dx.doi.org/ 10.1006/dbio.1997.8756 [DOI] [PubMed] [Google Scholar]
- [35].O'Leary CJ, Bradford D, Chen M, White A, Blackmore DG, Cooper HM. The Netrin/RGM receptor, Neogenin, controls adult neurogenesis by promoting neuroblast migration and cell cycle exit. Stem Cells 2015; 33:503-14; PMID:25308084; http://dx.doi.org/ 10.1002/stem.1861 [DOI] [PubMed] [Google Scholar]
- [36].Lee DH, Zhou LJ, Zhou Z, Xie JX, Jung JU, Liu Y, Xi CX, Mei L, Xiong WC. Neogenin inhibits HJV secretion and regulates BMP-induced hepcidin expression and iron homeostasis. Blood 2010; 115:3136-45; PMID:20065295; http://dx.doi.org/ 10.1182/blood-2009-11-251199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Zhou Z, Xie J, Lee D, Liu Y, Jung J, Zhou L, Xiong S, Mei L, Xiong WC. Neogenin regulation of BMP-induced canonical Smad signaling and endochondral bone formation. Dev Cell 2010; 19:90-102; PMID:20643353; http://dx.doi.org/ 10.1016/j.devcel.2010.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Healey EG, Bishop B, Elegheert J, Bell CH, Padilla-Parra S, Siebold C. Repulsive guidance molecule is a structural bridge between neogenin and bone morphogenetic protein. Nat Struct Mol Biol 2015; 22:458-65; PMID:25938661; http://dx.doi.org/ 10.1038/nsmb.3016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Huang Z, Hu J, Pan J, Wang Y, Hu G, Zhou J, Mei L, Xiong WC. YAP stabilizes SMAD1 and promotes BMP2-induced neocortical astrocytic differentiation. Development 2016; 143:2398-409; PMID:27381227; http://dx.doi.org/ 10.1242/dev.130658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Aragon E, Goerner N, Zaromytidou AI, Xi Q, Escobedo A, Massague J, Macias MJ. A Smad action turnover switch operated by WW domain readers of a phosphoserine code. Genes Dev 2011; 25:1275-88; PMID:21685363; http://dx.doi.org/ 10.1101/gad.2060811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Narimatsu M, Samavarchi-Tehrani P, Varelas X, Wrana JL. Distinct polarity cues direct Taz/Yap and TGFbeta receptor localization to differentially control TGFbeta-induced Smad signaling. Dev Cell 2015; 32:652-6; PMID:25758863; http://dx.doi.org/ 10.1016/j.devcel.2015.02.019 [DOI] [PubMed] [Google Scholar]
- [42].Nallet-Staub F, Yin X, Gilbert C, Marsaud V, Ben Mimoun S, Javelaud D, Leof EB, Mauviel A. Cell density sensing alters TGF-beta signaling in a cell-type-specific manner, independent from Hippo pathway activation. Dev Cell 2015; 32:640-51; PMID:25758862; http://dx.doi.org/ 10.1016/j.devcel.2015.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Alarcon C, Zaromytidou AI, Xi Q, Gao S, Yu J, Fujisawa S, Barlas A, Miller AN, Manova-Todorova K, Macias MJ, et al.. Nuclear CDKs drive Smad transcriptional activation and turnover in BMP and TGF-beta pathways. Cell 2009; 139:757-69; PMID:19914168; http://dx.doi.org/ 10.1016/j.cell.2009.09.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Huang Z, Wang Y, Hu G, Zhou J, Mei L, Xiong WC. YAP Is a Critical Inducer of SOCS3, Preventing Reactive Astrogliosis. Cereb Cortex 2016; 26:2299-310; PMID:26679195; http://dx.doi.org/ 10.1093/cercor/bhv292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Schwartzbaum JA, Fisher JL, Aldape KD, Wrensch M. Epidemiology and molecular pathology of glioma. Nat Clin Pract Neurol 2006; 2:494-503; quiz 1 p following 16; PMID:16932614; http://dx.doi.org/ 10.1038/ncpneuro0289 [DOI] [PubMed] [Google Scholar]
- [46].Jordan CT, Guzman ML, Noble M. Cancer stem cells. N Engl J Med 2006; 355:1253-61; PMID:16990388; http://dx.doi.org/ 10.1056/NEJMra061808 [DOI] [PubMed] [Google Scholar]
- [47].Gonzalez-Gomez P, Anselmo NP, Mira H. BMPs as therapeutic targets and biomarkers in astrocytic glioma. Biomed Res Int 2014; 2014:549742; PMID:24877113; http://dx.doi.org/ 10.1155/2014/549742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Wu X, Li Y, Wan X, Kayira TM, Cao R, Ju X, Zhu X, Zhao G. Down-regulation of neogenin accelerated glioma progression through promoter Methylation and its overexpression in SHG-44 Induced Apoptosis. PLoS One 2012; 7:e38074; PMID:22666451; http://dx.doi.org/ 10.1371/journal.pone.0038074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Piccirillo SG, Reynolds BA, Zanetti N, Lamorte G, Binda E, Broggi G, Brem H, Olivi A, Dimeco F, Vescovi AL. Bone morphogenetic proteins inhibit the tumorigenic potential of human brain tumour-initiating cells. Nature 2006; 444:761-5; PMID:17151667; http://dx.doi.org/ 10.1038/nature05349 [DOI] [PubMed] [Google Scholar]
- [50].Qi Q, Li DY, Luo HR, Guan KL, Ye K. Netrin-1 exerts oncogenic activities through enhancing Yes-associated protein stability. Proc Natl Acad Sci U S A 2015; 112:7255-60; PMID:26039999; http://dx.doi.org/ 10.1073/pnas.1505917112 [DOI] [PMC free article] [PubMed] [Google Scholar]


