ABSTRACT
During development of the nervous system, radial glial cells perform self-renewing asymmetric divisions and give rise to intermediate progenitor cells (IPC) and neurons. The neuronally committed IPC subsequently undergo multiple rounds of transient amplification and migrate outwards to form cortical layers as they continue to differentiate into mature neurons. Maturing neurons extend protrusions on their cell surface to form neurites, a process called neuritogenesis. Neurite formation results in the establishment of dendrites and axons for synapse formation, which is essential for sensory and motor functions and even higher-level functioning including memory formation and cognitive function, as well as shaping of behavior and emotion. Morphological adaptation during various stages of neural development requires active participation of actin cytoskeleton remodeling. In this review, we aim to discuss current understanding of the Arp2/3 complex branching nucleator in various neural cell types during development and maturation.
KEYWORDS: actin cytoskeleton, Arp2/3, neuritogenesis, neurogenesis, neuronal migration, radial glia
Introduction
The development of the nervous system requires orchestration of various cell types to perform cell division, migration, and morphogenesis in a timely manner. The dynamics of actin filament through rapid polymerization and depolymerization plays multiple crucial roles during these processes. The actin polymers branch from the existing filaments through the Arp2/3 complex branching nucleator, creating potentials for cellular asymmetry and directionality. The Arp2/3 complex is an evolutionarily conserved stable and stoichiometric assembly of 7 polypeptide subunits, including Arp2, Arp3, and Arpc1 to 5.1 While Arp2 and Arp3 bind directly to the incoming actin monomer, the rest of the subunits function together to form a branching point on the existing filament to allow the formation of a new filament at an angle of roughly 78 degrees.2 The function of the Arp2/3 complex is highly regulated by the Wiskott-Aldrich Syndrome (WAS) family of nucleation promoting factors, which is further regulated by Rho-GTPases such as cdc42. In this review, we will discuss recent advances in the understanding of the roles of the Arp2/3 complex during neocortical neural development and neuritogenesis.
The Arp2/3 complex in neocortical neurogenesis and neuronal migration
Knowledge on mammalian cerebral cortex formation has grown exponentially thanks to the advance in in vivo cell-labeling techniques and the imaging tools during fetal development of the rodents. Recent studies comparing human and rodent brains have identified measurable differences in terms of proliferative potential of the neural stem and progenitor cells as well as layer distributions.3-5 This is easily conceivable as human brains are much bigger in size and contain much more neuronal cells and sophisticated neural circuits. Nonetheless, the ability to manipulate genes and the availability of techniques to label radial glial cells and other cell types during brain development have made the rodent brains the most widely used model system in the field of cortical development research.
The prevailing view of neocortical neurogenesis suggests that radial glial cells (RGC) undergo various rounds of symmetric and asymmetric cell division to produce neuronally committed intermediate progenitor cells (IPC) or neurons. The IPC undergo transient amplification before they relocate to the respective destinations in the cortical layers where they complete their differentiation process. Various modes of neuronal migration have been described in different species.6 RGC membranous processes provide scaffolds and guide for the migrating neurons. Scaffolding, migration, and cell division all require a dynamic cytoskeletal network, which involves the actin cytoskeleton and its regulators. In the following section, we will discuss recent advances in the understanding of how the Arp2/3 complex is involved in these processes.
Neurogenetic and scaffolding functions of the radial glial cells
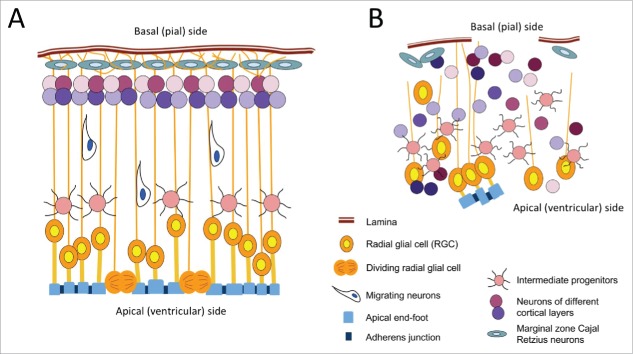
RGC are a group of specialized neuroepithelial cells with a distinct morphology (Fig. 1A). The unique establishment of the apico-basal polarity in RGC is crucial for their functions. On the apical (ventricular) side, adherens junctions (AJ) hold the apical end-feet together along the ventricular surface; on the basal (pial) side, the processes is a dynamic structure that extend from RGC cell bodies and span across the cortex to the pial surface.7,8
Figure 1.
Representation of normal cortical lamination during cortical development (A) and following Arpc2 depletion (B). (A) On the apical side, short apical processes form end-feet that are attached to the ventricular surface. The end-feet between radial glial cells (RGC) are anchored to each other via cadherin-containing adherens junctions (AJ).The cell bodies of the RGC are normally located close to the ventricular surface. An elongated basal process extends radially from the cell body in the opposite direction of the apical end-foot and spans the entire progressively thickening cortex until it is in contact with the basal surface. Of note, the basal process is a dynamic structure. Cell division occurs by the apical surface. The Arp2/3 complex is present throughout the RGC, with enrichment in the apical end-feet and the growth cone-like structure at the tip of the basal process. (B) Following depletion of Arpc2, multiple defects in RGC end-feet and basal processes, as well as in neuronal migration, altogether lead to disorganized positioning of the RGC, the intermediate progenitors, and the maturing neurons throughout the cortex.
Adherens junctions in association with RGC integrity and cell fate decision
N-Cadherin is a major component of the AJ. It has been shown that decreased N-Cadherin availability by RNA interference and by blocking antibody leads to drastically reduced β-catenin transcriptional activity, resulting in premature cell cycle exit and neuronal differentiation.9 The signaling cascade involved is the Akt pathway which, upon N-Cadherin loss, leads to dephosphorylation of the Ser552 residue and inactivation of β-catenin. The Arp2/3 complex is involved in the establishment and maintenance of the AJ.10 Using a tissue-specific Cre-recombinase knockout approach, we found that loss of Arp2/3 in the embryonic RGC results in similar phenotypes.11 Specifically, we showed an increase in Tuj1-positive neuronally committed cells and a decrease in Ki67-positive cells in E14.5 mouse embryo following Arpc2 knockout. We also observed a reduction in the density of Pax6-positive RGC along the VZ. In addition, while Tbr2-positive IPCs normally localize to the subventricular zone (SVZ), the Tbr2-positive cells in Arpc2-null embryos are present throughout the entire span of the cortex, with the highest density in the VZ. These findings are consistent with the phenotypes observed in the cdc42-deficient cortex which also described gradual loss of AJ and substantial reduction in self-renewing RGC in the VZ.12 Furthermore, a recent study showed that Numb and Numbl double knockout in mouse RGC leads to AJ disruption and loss of RGC polarity, leading to progenitor dispersion, ectopic rosette formation, and disorganized cortical lamination.7 Numb and Numbl are localized at the apical end-feet where they interact with the cadherin-catenin adhesion complex in the AJ.13 These findings are in line with our observation of ectopic neuroblastic rosettes and disorganized cortical lamination in the Arpc2-null embryonic brain. Together, these studies emphasize a role of the actin branching network in maintaining the morphology of the RGC and their anchorage to the apical surface through AJ formation, loss of which leads to premature neural lineage differentiation of the RGC.
It remains unclear how loss of AJ and polarity leads to a reduction in Akt signal in RGC, although it is possible that the cues from the microenvironment that are crucial for promoting a RGC self-renewing cell fate is stored in the endocytosed vesicles, which requires an intact actin branching network for excision and release into the cytoplasm. Another possibility is that the receptors that are responsible for transmitting signaling cascades to promote RGC self-renewal require an intact actin branching network to allow their expression on the membrane surface for ligand binding. The roles of Arp2/3-mediated exocytosis and endocytosis in RGC have not been explored in the mammalian system. In Drosophila, a recent study showed that Arp2/3 mediates endocytosis of Delta, a ligand for Notch signaling activation, in sensory organ precursors to guide cell fate decision.14 Notch signaling has also implicated in mPar3-regulated asymmetric cell division in RGC. It is possible that Notch signaling plays a mediator role between Arp2/3 and the RGC cell fate.15
Taken together, we propose that Arp2/3-mediated branching network of the actin cytoskeleton is upstream of N-Cadherin and Numb/Numbl in the apical end-feet in the regulation of the integrity of the AJ. By maintaining AJ and allowing RGC to anchor to the apical surface, Arp2/3 facilitates interaction between the RGC and the microenvironment to promote self-renewal and prevent premature differentiation and exhaustion.
RGC basal process dynamics during cortical development
In our Arpc2 tissue-specific knockout model, we observed a disorganized cortex without columnar formation of the processes extending radially to the basal surface.11 The laminin-enriched pial basement membrane was also thin and in discontinuity. Examining the RGC basal process directly, we found that the Arp2/3 complex is distributed throughout the entire RGC, and is enriched in the apical end-feet and the basal growth cone-like structure. Arp2/3 disruption resulted in the loss of the basal growth cone-like structure, and instead, the tip of the basal process assumed a pointed club-like structure. Furthermore, in wild-type fetal brain slice explant, we observed steady and smooth extension of the basal processes. On the contrary, the process extension in the Arpc2-null brain slice had an average speed increase of 33%, yet the final average length is much shorter due to frequent and sudden retractions. The phenomenon is reminiscent to uncontrolled microtubule polymerization followed by sudden collapse in the absence of actin cytoskeleton brakes. The interaction between microtubules and the actin cytoskeleton has been well-described in the axonal growth cone and other models, and may explain our findings in the RGC basal processes.16-18
Similar phenotypes were observed in the conditional cdc42 knockout study.19 First, Yokota et al. showed for the first time using live imaging that the basal processes and the growth-cone like structures are dynamic. This dynamic nature of the basal process suggests that constant remodeling and target-seeking activities are essential for their elongation as the cerebral cortex thickens. Secondly, cdc42 is also enriched in the apical end-feet as well as in the basal growth cone-like structures. Interestingly, cdc42 depletion in the RGC leads to excessive branching of the growth cone-like structures at the basal end, as opposed to Arpc2 disruption, which leads to a club-like structure. In either case, the processes are shorter, and are incapable of providing intact scaffolding and guide for the migrating neuronal cells.
Together, we, and others, showed that the Arp2/3 complex and its upstream regulators are present on both apical and basal ends of the RGC, and are critical for their morphogenesis and functions.
Neuronal migration
One interesting observation in the RGC-specific Arpc2-null embryonic brain is the disruption in cortical lamination (Fig. 1). The disorganization of the cerebral cortex can be explained by RGC scaffolding defect which leads to failure of the postmitotic neuronal cells to migrate to their pre-determined location in the cortex. Nonetheless, it is also possible that the migrating neurons lose their intrinsic migrating ability as a result of Arp2/3 depletion, as these migrating neuronal cells that are derived from the Arp2-null RGCs are also depleted of the Arp2/3 complex, and that the Arp2/3 complex is required for cell migration in general.2
To directly assess the roles of the Arp2/3 complex in the migrating neuronal cells, we first utilized a 3D organotypic brain slice/neurosphere co-culture system in our study.11 We found that the Arp2/3 complex is required for the cells to migrate out of the neurosphere on the brain slice. We further verified that, following depletion of the Arp2/3 complex by introducing dcx-cre into Arpc2 homozygous embryonic brain, neuronal cells failed to migrate in vivo. Recapitulating neurosphere cell migration on the 2D coverslip, we further showed that, as the substrate becomes more adhesive and more stiff (higher laminin concentration and higher stiffness index), the cells become less dependent on Arp2/3 for migration. These findings imply that, at least during early stages of brain development when the brain tissue is less cohesive and softer, the presence of the Arp2/3 complex allows the early-stage neuronal cells to migrate to their destinations in the cortex. Whether late-stage migrating neurons continue to depend on Arp2/3 as the brain continues to mature and becomes stiffer is not known. Nonetheless, the existing evidence supports a distinct role of Arp2/3 in the motility of the migrating neurons during cortical development. Consistent with our findings, there have been extensive studies that suggested that the Rho GTPase regulators of the Arp2/3 complex are highly involved in neuronal migration.20
The Arp2/3 complex in neuritogenesis
The structural hallmark of a mature neuron is the presence of cell membrane protrusions that possess the ability to communicate with another cell nearby or at a distance, as well as to receive extracellular cues from the microenvironment. In the axons, actin cytoskeleton is located at the far end of the microtubule-filled axonal shaft to shape the growth cone. The function of the actin cytoskeleton is in two-fold - it communicates with the intracellular microtubules and with the extracellular environment. In the dendrite, actin cytoskeleton fills the dendritic spines where it is involved in the structural plasticity of the spines for signal processing. In this section, we will discuss current understanding of the roles of the Arp2/3 complex in both axons and dendrites.
Axons
The axonal growth cone is the structure at the tip of the axonal shaft. The growth cone was first identified by Ramón y Cajal in 1890.21 The central (C) and the peripheral (P) domains are distinguishable under the microscope (Fig. 2). Between the two domains lies the transitional (T) zone.22
Figure 2.
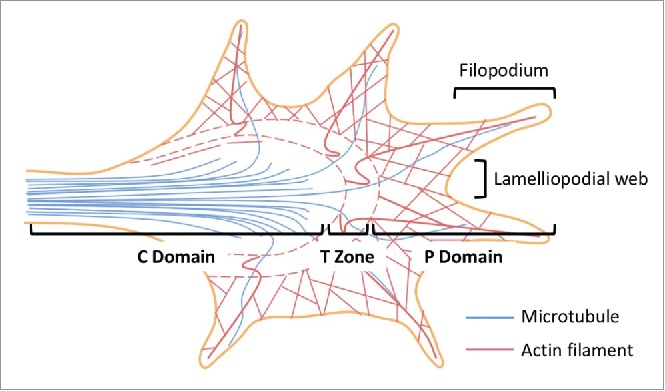
Axonal growth cone structure. Three functionally distinct regions have been identified in the growth cone - the central (C) and the peripheral (P) domains, as well as the transitional (T) zone. The C domain is at the end of the axonal shaft where the microtubules extending from the axonal shaft terminate. The C domain are also rich in numerous organelles including mitochondria and exocytotic vesicles. The primary function of the C domain is to support the P domain, which consists primarily of actin cytoskeleton and has a dynamic morphology. In between the C and the P domains lies the T zone where microtubules interact with actin filaments through acto-myosin contractile structures. Filopodia-like finger protrusions at the P domain are supported by the lamellipodia-like web structures. This figure is adapted from Box 1 in Ref. 4.
The primary function of the growth cone is to navigate in the nervous system and search for its synaptic targets. The process requires repeated cycles of protrusion, adhesion, and traction on the leading edges of the growth cone. The growth cone turns toward attractive guidance by promoting actin polymerization and/or decreasing retrograde actin flow on the membranous protrusion closest to the guidance. This results in stabilization of the filopodia and the lamellipodia in the protrusions, and facilitates outgrowth of the growth cone toward the attractive guidance; on the other hand, the growth cone turns away from the repulsive cues by preventing polymerization and/or losing the adhesion sites closest to the negative stimuli, allowing the internal retrograde flow governed by the interaction between the actin filaments and the myosin II motor units to reverse the protrusions.16
In the central nervous system, the localization of the Arp2/3 complex in the growth cone has been debated, likely due to different model systems used in the respective studies. Strass et al., used a mouse embryonic hippocampal neuron model at embryonic day E16 and reported that the Arp2/3 complex is localized primarily in the C domain. They further utilized a dominant-negative mutant of the Arp2/3 regulating protein (the N-WASP CA domain) fused with enhanced green fluorescent protein (N-WASP CA-EGFP) to demonstrate that the Arp2/3 complex is not a major determinant of growth cone actin organization and membrane protrusion, and is not required for filopodia formation in the growth cone. This is in consistency with the traditional view that Arp2/3 is specialized in lamellipodia formation but is dispensable in filopodia formation. Interestingly, they also showed that Arp2/3 inhibition enhances axonal elongation, with an associated increase in the ratio of tyrosinated to acetylated microtubules, suggesting increased microtubule polymerization. In addition, following the expression of N-WASP CA-EGFP, growth cones failed to retract in response to the negative cue (Semaphorin 3A).23 On the contrary, Korobova et al. showed that the Arp2/3 complex is present in the P domain of the growth cones in hippocampal neurons from E18–20 rat embryos. Using an RNA interference approach to knock down the Arpc2 subunit, they showed that the Arp2/3 complex is essential for the formation of both lamellipodia and filopodia.24 These latter findings were consistent with a later study that showed that Arp2/3 is required for axon guidance and filopodia initiation in C. elegans.25 The difference in the approach that was taken to disrupt Arp2/3 function in the target cells and/or the difference in the animal species used in the experiments may account for the different conclusions. Future studies to definitely investigate the roles of the Arp2/3 complex in mammalian axonal growth cones should utilize a genetic tissue-specific knockout approach.
More recent studies focused on delineating the role of the Arp2/3 complex in the peripheral nervous system. Using embryonic day 7 (E7) chicken dorsal root ganglion (DRG) cells, Spillane et al. first reported that the Arp2/3 complex contributes to the initiation of the axonal filopodia through regulating the actin patch precursors, which are a meshwork of interweaving actin filaments branching out of the axonal shaft. Interestingly, using the N-WASP CA-EGFP dominant-negative peptides described above, they found that the Arp2/3 complex is not required for further development of the actin patch into filopodia. Moreover, inhibition of Arp2/3 function by N-WASP CA-EGFP abolishes nerve growth factor (NGF)- or phosphoinositide 3-kinase (PI3K)-induced patch formation.26 In a later study using the same neuronal cell model, San Miquel-Ruiz et al. described substrate-dependent localization and function of the Arp2/3 complex in the growth cone. Specifically, when comparing L1 and laminin substrates, they first showed significant differences in growth cone morphologies, with more broad and thin veil-like structure when cultured on L1 substrate, presumably due to lamellipodia predominance, and more finger-like protrusions when cultured on the laminin substrate, likely due to filopodia predominance. They further showed that Arp2/3 is concentrated on the leading edges when on L1, and is scattering throughout the P domain when on laminin. Additionally, there were substrate-dependent differences in growth cone motility, actin retrograde flow, and total F-actin intensity following NGF stimulation. Most strikingly, axon guidance is Arp2/3-dependent on L1, but is Arp2/3-independent on laminin.27 Altogether, these studies demonstrated crucial yet substrate-dependent roles of the Arp2/3 complex in peripheral nervous system development.
Dendrites
On the dendrites there are protrusions of various morphologies called the dendritic spines. Ramón y Cajal also first described these structures in 1888. At the time, it was proposed that these dendritic spines are the contacting sites between neurons. This was later proven to be true with the advent of electron microscopy (EM).28,29 Subsequently, both EM and protein assays demonstrated that actin filaments are highly concentrated in the dendritic spines, especially in the junctional region of the synapse called the postsynaptic densities (PSD).30,31 A later study utilizing a GFP-tagged actin overexpression system along with chemical inhibition of actin polymerization further showed that dendritic spines are the bona fide post-synaptic structure, and that the morphologies of the dendritic spines are actin-dependent and are dynamic.32 Additionally, actin was shown to play a role in anchoring NMDA and AMPA glutamate receptors to the plasma membrane in the post-synaptic sites, and is required for AMPA receptor-mediated long-term potentiation (LTP) induction and NMDA receptor-mediated long-term depression (LTD).33-35 These findings all pointed to a role of the actin cytoskeleton in postsynaptic plasticity.
Changes in dendritic spine morphology, including their size and shape, have been linked to synaptic plasticity.36-38 As a branching nucleator, the Arp2/3 complex has been proposed to play a role in spine morphology, hence the synaptic plasticity. Indeed, using quantitative immunoelectron microscopy, the Arpc2 subunit (of the Arp2/3 complex) has been found to concentrate in the PSD of the dendritic spines in the CA2 subregion of the rat hippocampus.39 Intriguingly, using a CaMKIIα promoter-driven Cre recombinase-mediated Arpc3 knockout mouse model to deplete the Arp2/3 complex in the forebrain excitatory neurons, it was found that the Arp2/3 complex is responsible only for the LTP-induced spine volume expansion but not the LTD-induced spine shrinkage, thus dissociating the previous model of a reciprocal mechanism in the regulation of the spinous morphology. Surprisingly, loss of the Arp2/3 complex in the dendritic spines led to progressive loss of normally formed axo-spinous synapses and various types of glutamate receptors on the synaptosomal membrane, as well as the corresponding progression of distinct cognitive, psychomotor, and social disturbances as the mice age.40 Intriguingly, while the number of the axo-spinous synapse is reduced following loss of Arpc3, the number of the abnormal axo-dendritic and double axonal synapses is significantly increased (Fig. 3). It is not clear whether only the post-synapse neuron loses Arpc3, or both presynaptic and postsynaptic neurons are depleted of Arpc3. This increase is accompanied by excitation of the dopaminergic neurons in a long-range (from the frontal cortex to the ventral tegmental area and substantia nigra pars compacta) circuit and an elevated dopamine level in the striatum, which explain the manifestations of psychomotor agitation and stereotypical behaviors in the Arpc3-depleted mice.41 Taken together, these findings suggest that the Arp2/3 complex is required for dendritic spine dynamics, and its loss progressively lead to behavioral abnormalities in mice. In line with animal findings, abnormalities in dendritic spines have been associated with multiple human neuropsychiatric disorders, including Alzheimer disease, schizophrenia, and autism spectrum disorder.42 It merits further investigation to determine whether a causal role exists for the Arp2/3 complex in human neuropsychiatric disorders.
Figure 3.
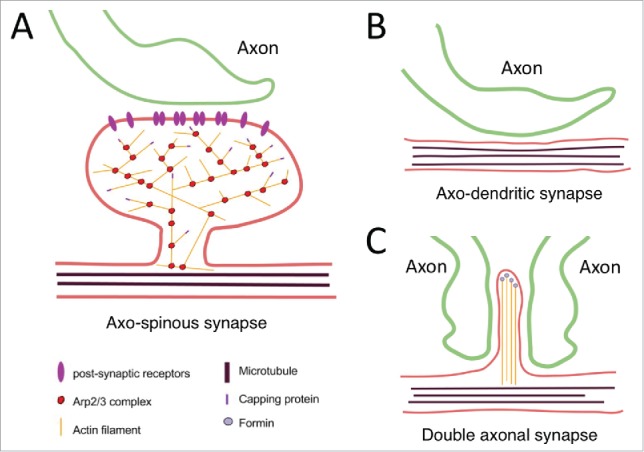
Synapse formation between dendrites and axons in the presence (A) and absence of Arp2/3 (B, C). (A) Dendritic spine with a mature-type morphology supported by actin cytoskeleton meshwork. Presynaptic axon is in contact with the dendritic spine. Postsynaptic receptors are supported by actin cytoskeleton on the plasma membrane. (B) Lack of dendritic spines in the absence of Arp2/3 complex. Presynaptic axon is in direct contact with the dendritic shaft. (C) Immature dendritic spines supported by filopodia-like protrusions with non-branching actin filaments in the absence of Arp2/3 complex lead to anomalous formation of double-axonal synapses. This figure is modified from Figure 4 in Ref. 24.
Conclusion and future directions
The Arp2/3 complex, as a downstream effector, is required at various stages of development in the nervous system. Although generally thought to be ubiquitously expressed, the protein levels of the 7 subunits must be tightly regulated in order for the complex to assemble and disassemble in accordance with the biological needs of the cells. Dysregulation of one or more subunits have been speculated in human diseases, such as in Down syndrome and in schizophrenia.42,43 Future studies may explore the mechanisms underlying the accumulation and degradation of each subunit, and how the expression of the 7 subunits are coordinated for different biological functions. In addition, although the involvement of the Arp2/3 complex in cellular functions is diverse, some static (adherens junction formation and polarity establishment), and others dynamic (cell division and migration) at the tissue level, the fundamental event that occurs at the molecular level is the dynamic placement and removal of the actin monomers along the actin filaments and the Arp2/3 branching nucleators. Even in seemingly static structures like the RGC end-feet, constant recycling of the components of the AJ and the membranous receptors through the work of the Arp2/3 complex plays a vital part in maintaining the structures in association with the microenvironmental cues. How exactly the external cues impact on the upstream regulators of Arp2/3 and its assembly is another interesting topic for future studies. In conclusion, the Arp2/3 complex as an effector of cellular morphogenesis is essential for multiple cellular and subcellular processes including the development of the nervous system.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors would like to thank Connie Kang at University of California, Riverside for her contribution to the figures.
Funding
This work is funded by Children's Mercy Children's Research Institute.
References
- [1].Rouiller I, Xu XP, Amann KJ, Egile C, Nickell S, Nicastro D, Li R, Pollard TD, Volkmann N, Hanein D. The structural basis of actin filament branching by the Arp2/3 complex. J Cell Biol 2008; 180:887-95; PMID:18316411; http://dx.doi.org/ 10.1083/jcb.200709092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Swaney KF, Li R. Function and regulation of the Arp2/3 complex during cell migration in diverse environments. Curr Opin Cell Biol 2016; 42:63-72; PMID:27164504; http://dx.doi.org/ 10.1016/j.ceb.2016.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Hansen DV, Lui JH, Parker PR, Kriegstein AR. Neurogenic radial glia in the outer subventricular zone of human neocortex. Nature 2010; 464:554-61; PMID:20154730; http://dx.doi.org/ 10.1038/nature08845 [DOI] [PubMed] [Google Scholar]
- [4].Fietz SA, Kelava I, Vogt J, Wilsch-Bräuninger M, Stenzel D, Fish JL, Corbeil D, Riehn A, Distler W, Nitsch R, et al.. OSVZ progenitors of human and ferret neocortex are epithelial-like and expand by integrin signaling. Nat Neurosci 2010; 13:690-9; PMID:20436478; http://dx.doi.org/ 10.1038/nn.2553 [DOI] [PubMed] [Google Scholar]
- [5].Lui JH, Hansen DV, Kriegstein AR. Development and evolution of the human neocortex. Cell 2011; 146:18-36; PMID:21729779; http://dx.doi.org/ 10.1016/j.cell.2011.06.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Rakic P. Developmental and evolutionary adaptations of cortical radial glia. Cereb Cortex 2003; 13:541-9; PMID:12764027; http://dx.doi.org/ 10.1093/cercor/13.6.541 [DOI] [PubMed] [Google Scholar]
- [7].Rašin MR, Gazula VR, Breunig JJ, Kwan KY, Johnson MB, Liu-Chen S, Li HS, Jan LY, Jan YN, Rakic P, et al, Numb and Numbl are required for maintenance of cadherin-based adhesion and polarity of neural progenitors. Nat Neurosci 2007; 10:819-27; http://dx.doi.org/ 10.1038/nn1924 [DOI] [PubMed] [Google Scholar]
- [8].Kriegstein A, Alvarez-Buylla A. The glial nature of embryonic and adult neural stem cells. Annu Rev Neurosci 2009; 32:149-84; PMID:19555289; http://dx.doi.org/ 10.1146/annurev.neuro.051508.135600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Zhang J, Woodhead GJ, Swaminathan SK, Noles SR, McQuinn ER, Pisarek AJ, Stocker AM, Mutch CA, Funatsu N, Chenn A. Cortical Neural Precursors Inhibit Their Own Differentiation via N-Cadherin Maintenance of β-Catenin Signaling. Dev Cell 2010; 18:472-9; PMID:20230753; http://dx.doi.org/ 10.1016/j.devcel.2009.12.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Tang VW, Brieher WM. α-Actinin-4/FSGS1 is required for Arp2/3-dependent actin assembly at the adherens junction. J Cell Biol 2012; 196:115-30; PMID:22232703; http://dx.doi.org/ 10.1083/jcb.201103116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Wang PS, Chou FS, Ramachandran S, Xia S, Chen HY, Guo F, Suraneni P, Maher BJ, Li R. Crucial roles of the Arp2/3 complex during mammalian corticogenesis. Development 2016; 143:2741-52; PMID:27385014; http://dx.doi.org/ 10.1242/dev.130542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Cappello S, Attardo A, Wu X, Iwasato T, Itohara S, Wilsch-Bräuninger M, Eilken HM, Rieger MA, Schroeder TT, Huttner WB, et al.. The Rho-GTPase cdc42 regulates neural progenitor fate at the apical surface. Nat Neurosci 2006; 9:1099-107; PMID:16892058; http://dx.doi.org/ 10.1038/nn1744 [DOI] [PubMed] [Google Scholar]
- [13].Buchman JJ, Tsai LH. Spindle regulation in neural precursors of flies and mammals. Nat Rev Neurosci 2007; 8:89-100; PMID:17228329; http://dx.doi.org/ 10.1038/nrn2058 [DOI] [PubMed] [Google Scholar]
- [14].Rajan A, Tien AC, Haueter CM, Schulze KL, Bellen HJ. The Arp2/3 complex and WASp are required for apical trafficking of Delta into microvilli during cell fate specification of sensory organ precursors. Nat Cell Biol 2009; 11:815-24; PMID:19543274; http://dx.doi.org/ 10.1038/ncb1888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Bultje RS, Castaneda-Castellanos DR, Jan LY, Jan YN, Kriegstein AR, Shi SH. Mammalian Par3 regulates progenitor cell asymmetric division via notch signaling in the developing neocortex. 2009; Neuron 63:189-202; PMID:19640478; http://dx.doi.org/ 10.1016/j.neuron.2009.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Gomez TM, Letourneau PC. Actin dynamics in growth cone motility and navigation. J Neurochem 2014; 129:221-34; PMID:24164353; http://dx.doi.org/ 10.1111/jnc.12506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Dent EW, Gupton SL, Gertler FB. The growth cone cytoskeleton in axon outgrowth and guidance. Cold Spring Harb Perspect Biol 2011; 3:a001800; PMID:21106647; http://dx.doi.org/ 10.1101/cshperspect.a001800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Rodriguez OC, Schaefer AW, Mandato CA, Forscher P, Bement WM, Waterman-Storer CM. Conserved microtubule-actin interactions in cell movement and morphogenesis. Nat Cell Biol 2003; 5:599-609; PMID:12833063; http://dx.doi.org/ 10.1038/ncb0703-599 [DOI] [PubMed] [Google Scholar]
- [19].Yokota Y, Eom TY, Stanco A, Kim WY, Rao S, Snider WD, Anton ES. Cdc42 and Gsk3 modulate the dynamics of radial glial growth, inter-radial glial interactions and polarity in the developing cerebral cortex. Development 2010; 137:4101-10; PMID:21062867; http://dx.doi.org/ 10.1242/dev.048637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Govek EE, Hatten ME, Van Aelst L. The role of Rho GTPase proteins in CNS neuronal migration. Dev Neurobiol 2011; 71:528-53; PMID:21557504; http://dx.doi.org/ 10.1002/dneu.20850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ramón y Cajal S. A quelle epoque apparaissent les expansions des cellule nerveuses de la moelle epinere du poulet. Anat Anzerger 1890; 5:609–613. [Google Scholar]
- [22].Lowery LA, Van Vactor D. The trip of the tip: understanding the growth cone machinery. Nat Rev Mol Cell Biol 2009; 10:332-43; PMID:19373241; http://dx.doi.org/ 10.1038/nrm2679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Strasser GA, Rahim NA, VanderWaal KE, Gertler FB, Lanier LM. Arp2/3 is a negative regulator of growth cone translocation. Neuron 2004; 43:81-94; PMID:15233919; http://dx.doi.org/ 10.1016/j.neuron.2004.05.015 [DOI] [PubMed] [Google Scholar]
- [24].Korobova F, Svitkina T. Arp2/3 complex is important for filopodia formation, growth cone motility, and neuritogenesis in neuronal cells. Mol Biol Cell 2008; 19:1561-74; PMID:18256280; http://dx.doi.org/ 10.1091/mbc.E07-09-0964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Norris AD, Dyer JO, Lundquist EA. The Arp2/3 complex, UNC-115/abLIM, and UNC-34/Enabled regulate axon guidance and growth cone filopodia formation in Caenorhabditis elegans. Neural Dev 2009; 4:38; PMID:19799769; http://dx.doi.org/ 10.1186/1749-8104-4-38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Spillane M, Ketschek A, Jones SL, Korobova F, Marsick B, Lanier L, Svitkina T, Gallo G. The actin nucleating Arp2/3 complex contributes to the formation of axonal filopodia and branches through the regulation of actin patch precursors to filopodia. Dev Neurobiol 2011; 71:747-58; PMID:21557512; http://dx.doi.org/ 10.1002/dneu.20907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].San Miguel-Ruiz JE, Letourneau PC. The role of Arp2/3 in growth cone actin dynamics and guidance is substrate dependent. J Neurosci 2014; 34:5895-908; PMID:24760849; http://dx.doi.org/ 10.1523/JNEUROSCI.0672-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Ramón y Cajal S. Estructura de los centros nerviosos de las aves. Rev Trim Histol Norm Pat 1888; 1:1–10. [Google Scholar]
- [29].Gray EG. Electron microscopy of synaptic contacts on dendrite spines of the cerebral cortex. Nature 1959; 183:1592-3; PMID:13666826; http://dx.doi.org/ 10.1038/1831592a0 [DOI] [PubMed] [Google Scholar]
- [30].Matus A, Ackermann M, Pehling G, Byers HR, Fujiwara K. High actin concentrations in brain dendritic spines and postsynaptic densities. Proc Natl Acad Sci U. S. A 1982; 79:7590-94; PMID:6760199; http://dx.doi.org/ 10.1073/pnas.79.23.7590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Kelly PT, Cotman CW. Synaptic proteins. Characterization of tubulin and actin and identification of a distinct postsynaptic density polypeptide. J Cell Biol 1978; 79:173-83; PMID:568145; http://dx.doi.org/ 10.1083/jcb.79.1.173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Fischer M, Kaech S, Knutti D, Matus A. Rapid actin-based plasticity in dendritic spines. Neuron 1998; 20:847-54; PMID:9620690; http://dx.doi.org/ 10.1016/S0896-6273(00)80467-5 [DOI] [PubMed] [Google Scholar]
- [33].Allison DW, Gelfand VI, Spector I, Craig AM. Role of actin in anchoring postsynaptic receptors in cultured hippocampal neurons: differential attachment of NMDA versus AMPA receptors. J Neurosci 1998; 18:2423-36; PMID:9502803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Fukazawa Y, Saitoh Y, Ozawa F, Ohta Y, Mizuno K, Inokuchi K. Hippocampal LTP is accompanied by enhanced F-actin content within the dendritic spine that is essential for late LTP maintenance in vivo. Neuron 2003; 38:447-60; PMID:12741991; http://dx.doi.org/ 10.1016/S0896-6273(03)00206-X [DOI] [PubMed] [Google Scholar]
- [35].Zhou Q, Homma KJ, Poo M-M. Shrinkage of dendritic spines associated with long-term depression of hippocampal synapses. Neuron 2004; 44:749-57; PMID:15572107; http://dx.doi.org/ 10.1016/j.neuron.2004.11.011 [DOI] [PubMed] [Google Scholar]
- [36].Matus A. Actin-based plasticity in dendritic spines. Science 2000; 290:754-8; PMID:11052932; http://dx.doi.org/ 10.1126/science.290.5492.754 [DOI] [PubMed] [Google Scholar]
- [37].Knott GW, Holtmaat A, Wilbrecht L, Welker E, Svoboda K. Spine growth precedes synapse formation in the adult neocortex in vivo. Nat Neurosci 2006; 9:1117-24; PMID:16892056; http://dx.doi.org/ 10.1038/nn1747 [DOI] [PubMed] [Google Scholar]
- [38].Chen LY, Rex CS, Casale MS, Gall CM, Lynch G. Changes in synaptic morphology accompany actin signaling during LTP. J Neurosci 2007; 27:5363-72; PMID:17507558; http://dx.doi.org/ 10.1523/JNEUROSCI.0164-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Racz B, Weinberg RJ. Organization of the Arp2/3 Complex in Hippocampal Spines. J Neurosci 2008; 28:5654-9; PMID:18509026; http://dx.doi.org/ 10.1523/JNEUROSCI.0756-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Kim IH, Racz B, Wang H, Burianek L, Weinberg R, Yasuda R, Wetsel WC, Soderling SH. Disruption of Arp2/3 results in asymmetric structural plasticity of dendritic spines and progressive synaptic and behavioral abnormalities. J. Neurosci 2013; 33:6081-92; PMID:23554489; http://dx.doi.org/ 10.1523/JNEUROSCI.0035-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Kim IH, Rossi MA, Aryal DK, Racz B, Kim N, Uezu A, Wang F, Wetsel WC, Weinberg RJ, Yin H, et al.. Spine pruning drives antipsychotic-sensitive locomotion via circuit control of striatal dopamine. Nat Neurosci 2015; 18:883-91; PMID:25938885; http://dx.doi.org/ 10.1038/nn.4015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Penzes P, Cahill ME, Jones KA, VanLeeuwen J-E, Woolfrey KM. Dendritic spine pathology in neuropsychiatric disorders. Nat Neurosci 2011; 14:285-93; PMID:21346746; http://dx.doi.org/ 10.1038/nn.2741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Weitzdoerfer R, Fountoulakis M, Lubec G. Reduction of actin-related protein complex 2/3 in fetal Down syndrome brain. Biochem Biophys Res Commun 2002; 293:836-41; PMID:12054546; http://dx.doi.org/ 10.1016/S0006-291X(02)00291-7 [DOI] [PubMed] [Google Scholar]