Abstract
Background
To explore the time-dependent effects of acupuncture on mRNA levels of the apoptotic factors BCL-2 and BAX in a rat cerebral hemorrhage model, slow injection of autologous blood to the caudate nucleus was used to generate the cerebral hemorrhage model.
Material/Methods
A sham surgery control group, groups with acupuncture applied 3, 9, 24, and 48 hours after model induction, and time-matched model-only control groups were used. In situ hybridization was used to detect BCL-2 and BAX mRNA expression, and semi-quantitative RT-PCR was used to measure the expression.
Results
The number of BCL-2 and BAX mRNA-positive cells significantly increased during the acute phase of cerebral hemorrhage. BCL-2 mRNA was significantly upregulated in acupuncture groups compared to other groups, whereas BAX mRNA levels in the acupuncture groups were lower in the other groups, except for the sham surgery group. Additionally, earlier acupuncture intervention was associated with a lower ratio of expression between the two genes. Changes in BCL-2 and BAX mRNA expression were consistent with changes in the number of cells positive for BCL-2 and BAX mRNA; however, the change in the expression ratio was consistent with the change in the number of cells positive for BCL-2 mRNA, but opposite to the change in the number of cells positive for BAX mRNA.
Conclusions
Acupuncture ameliorated changes in expression of apoptotic factors in the brain induced by acute cerebral hemorrhage and may thus protect the brain, with greater efficacy when the delay before acupuncture was minimized.
MeSH Keywords: Acupuncture, bcl-2-Associated X Protein, Cerebral Hemorrhage
Background
BCL-2 (B-cell lymphoma-2) is a major anti-apoptotic gene with a length of 230 kb that is located at 18q21. It encodes a 26 kDa integral-membrane protein that localizes in the mitochondria, endoplasmic reticulum, and nuclear membrane and plays an important role in the long-term survival of nerve cells, hematopoietic stem/progenitor cells, and immune memory cells. Under normal physiological conditions, BCL-2 protein inhibits apoptosis and increases cell survival but does not affect the cell cycle or differentiation [1,2]. In the BCL-2 family, BAX was the first identified pro-apoptotic factor. The BAX protein consists of 192 amino acids and has a high degree of amino acid homology (21%) with BCL-2, as they both have two common conserved domains, BH I and BH II, located at the hydrophobic C-terminus. BAX and BCL-2 can both form homodimers, and can also form heterodimers with each other. The ratio between BCL-2 and BAX determines the apoptotic process; when the ratio increases, apoptosis is suppressed, whereas when the ratio decreases, apoptosis is induced. Acupuncture therapy can influence the ratio between BCL-2 and BAX, suppress apoptosis, and exert a neuroprotective function in perihematomal brain tissue upon acute cerebral hemorrhage.
In the acute phase of cerebral hemorrhage, neural cells enter the initial stage of apoptosis by both intrinsic and extrinsic pathways [3]. It is generally believed that the extrinsic pathway, also known as the extrinsic death receptor pathway, is induced by binding of tumor necrosis factor (TNF) receptor family and ligands. The intrinsic pathway, also known as intrinsic mitochondrial pathway, is induced by tumor suppressor genes such as p53 which can be activated by DNA damage. The expression of p53 then regulates the expression of BCL-2 family members (e.g., it increases the expression of BAX and decreases the expression of BCL-2) which exert their effects before apoptosis. Together, the BCL-2 family members induce the release of cytochrome C located between the inner and outer mitochondrial membrane into the cytoplasm [4]. Subsequently, the apoptotic complex composed of cytochrome C, apoptotic protease-activating factor-1 (Apaf-1), and procaspase-9 (i.e., activated caspase-9) is formed in the cytoplasm and induces caspase cascade activation [5]. In the later phase of apoptosis, proteases, such as caspase-3, are major inducers induced by caspase-8 and caspase-9, which were already activated in the initial phase, eventually leading to cell death through processes including the degradation of cytoskeleton and DNA fragments [6].
Many studies have shown that acupuncture is effective in inhibiting apoptosis [7]. Feng et al. reviewed the neuroprotective effect of electroacupuncture (EA) on cerebral ischemic strokes, and clarified that EA could reduce the brain damage caused by cerebral ischemic strokes and induce cerebral ischemic tolerance before the occurrence of cerebral stroke. The positive effects are achieved by promoting angiogenesis, reducing inflammation, regulating the blood brain barrier (BBB), and inhibiting apoptosis. Hou et al. [8] explored the impact of acupuncture on the Baihui and Dazhui acupoints on heroin relapse from the perspective of neural cell apoptosis in an animal model. The results showed that after acupuncture intervention, pathological damage in the hippocampus and frontal lobe was significantly reduced, whereas BCL-2 expression was increased and BAX expression was decreased, indicating that acupuncture exerts an effect similar to that of the western medicine methadone.
The mechanisms by which brain hemorrhage induces apoptosis are unclear, but many studies have demonstrated that the occurrence of oxidative stress could generate a large amount of superoxide radicals, which are the main inducers of apoptosis. BCL-2 overexpression was previously shown to prevent peroxidation of lipid membranes of brain cells and prevent oxidative damage through suppression of the generation of superoxide radicals [9]. Additionally, Ca2+ overload can also lead to apoptosis. It has been shown that the imbalance of Ca2+ influx and outflow is an initiation signal for Ca2+ overload and ischemic neuronal or myocardial cell death. Critically, Ca2+ can directly activate hydrolases such as proteases, phospholipase, and nuclease, thus immediately triggering cell death and tissue damage [10]. The main storage organelle for Ca2+ within cells is the endoplasmic reticulum, which is also one of the locations of BCL-2 protein. Studies have demonstrated [11] that inhibition of cell apoptosis by BCL-2 might be closely associated with the effects of high BCL-2 expression in the endoplasmic reticulum on Ca2+ release through different mechanisms. Furthermore, BCL-2 is involved in signal transduction pathways for apoptosis. In particular, is has been reported that substitution mutations of specific amino acids in the BH I and BH II domains of BCL-2 lead to a complete loss of its apoptotic inhibitory function. As a result of these mutations, although BCL-2 itself could still form homodimers, it is unable to generate heterodimers with BAX. This finding indicates that BCL-2 exerts its apoptotic inhibitory function only through BCL-2-BAX heterodimers [12]. Finally, the molecular structure of BAX determines its function in promoting apoptosis. Gavathiotis et al. [13] found that there is a unique groove or trigger site, i.e., BH III death domain (α-helix structure), in the BAX protein that triggers BAX activation under certain circumstances (such as upon activation of the cellular stress response). After being activated, BAX moves from the cytoplasm to the outer membrane of mitochondria, resulting in mitochondrial damage and the release of cytochrome C into the cytoplasm, triggering a series of reactions that ultimately lead to cell fragmentation and apoptosis.
Studies have shown that acupuncture significantly ameliorates pathological changes in BCL-2 mRNA, BAX mRNA, BCL-2 protein, BAX protein, and the BCL-2/BAX ratio. He et al. [14] examined the effects of acupuncture at the Zusanli and Waiguan acupoints on the expression of BCL-2 and BAX protein in a rat model of acute cerebral infarction. They concluded that early acupuncture treatment for acute cerebral infarction inhibited apoptosis by affecting the expression of BCL-2 and BAX, thereby protecting neural cells. Liu et al. [15] studied the effect of acupuncture on the expression of BCL-2 and BAX mRNA and protein in the hippocampus of rats with multi-infarct dementia (MID). They found that acupuncture promoted BCL-2 expression in the hippocampus of rats with MID and inhibited the expression of BAX, thus protecting the brain. In addition, the effect showed acupoint specificity.
This study explored the effects of acupuncture on apoptosis in perihematomal brain tissues at different times after the formation of hematoma in a rat cerebral hemorrhage model by evaluating the expression of BCL-2 and BAX mRNA and determining the BCL-2/BAX mRNA ratio. The study investigated the theoretical basis for the application of acupuncture therapy in the clinical treatment of acute cerebral hemorrhage.
Material and Methods
Animal selection, grouping, and treatments
In total, 135 healthy adult male Wistar rats (7 weeks, weighting 215–245 g, SPF grade) were provided by the Chinese Academy of Medical Sciences and Peking Union Medical College, Institute of Biomedical Engineering. The rats were maintained on standard diet and drinking water in multi-layer flow racks in the Animal Experiment Center (23±2 °C, 50±10% humidity) of the Institute of Biomedical Engineering, Chinese Academy of Medical Sciences and Peking Union Medical College. The rats were randomly assigned to a sham surgery group, model group, or acupuncture group according to a random number table. The acupuncture group was then further randomly divided into four subgroups, with acupuncture treatment at 3, 9, 24, or 48 hours after model induction, with 15 rats in each subgroup, for a total of 60 rats. The model group was also further divided into four subgroups with the same time points as the four acupuncture subgroups (but with only similar handling and no acupuncture), with 15 rats in each subgroup, for a total of 60 rats. The sham surgery group contained 15 rats; a pilot experiment showed no significant difference when different time points were used in the sham surgery subgroups; therefore, only one time point (i.e., 3 hours after model induction) was used for the sham surgery group. All rats were labeled with picric acid. The acupuncture groups received the first acupuncture treatment 3, 9, 24, or 48 hours after successful model induction, twice a day at intervals of six hours, and the rats were sacrificed after five consecutive days of acupuncture. The sham surgical group underwent handling without acupuncture twice a day starting three hours after the surgery, and the animals were sacrificed after five consecutive days of handling without acupuncture. The rats in each group were decapitated and the brains were removed immediately after death. All methods were conducted in accordance with relevant guidelines for animal health and welfare, and the ethical review board of Tianjin Nankai Hospital approved the study protocols.
Generation of the experimental animal model
Slow injection of autologous blood to the caudate nucleus was used to generate the cerebral hemorrhage model in Wistar rats. Animals were anesthetized with intraperitoneal injection of 10% chloral hydrate (300 mg/kg). After skin preparation, rats were fixed in the prone position on a single-arm brain stereotaxic instrument. The injection location was determined according to The Rat Brain in Stereotaxic Coordinates [3]. A longitudinal incision was made in the middle of the head to expose the bregma and coronal suture. The skull was opened manually, and a 25-μL microinjection needle was inserted 6 mm into the brain to the caudate nucleus. Then 25 μL of autologous whole blood obtained from the tail vein was slowly injected (over 5 minutes), and the needle was maintained in position for 5 minutes before withdrawal. Finally, the burr hole was closed with sterile bone wax, the cut tissue was sutured closed, and iodine solution was used to disinfect the surgical wound. When the rats recovered from anesthesia, the generation of a cerebral hemorrhage model was considered to be successful based on pilot experiments in which the Bederson [4] and Longa scores [5] were used to assess behavioral changes. For the Bederson and Longa scores, rats with a score ≥1 were considered to have successful model induction. Animals in the model group and acupuncture group underwent the cerebral hemorrhage model surgery, and the same volume of saline was injected into the caudate nucleus of the sham surgery group to mimic the surgical injury.
Acupuncture point selection and quantitative analysis
The locations of acupoints were targeted based on the Animal Acupuncture Points Atlas drafted by the Experimental Acupuncture Institute of China Association for Acupuncture and Moxibustion. In the acupuncture group, Neiguan and Renzhong acupoints were used for both sides. A No. 31 3.81 cm stainless steel acupuncture needle was selected. The penetration needling method was used for the Neiguan acupuncture point and EA was applied with a continuous wave with the frequency set on “4” and the intensity output set on “4”. The needle was maintained on site for 1 minute. For the Renzhong acupuncture point, bird-pecking needling was applied to stimulate the point 10 times, and the needle was not maintained on the site.
During the experiment, 10 rats were excluded because of death: 2 in the sham surgery group, 1 in the 9 hour model group, 2 in the 24 hour model group, 2 in the 48 hour model group, 1 in the 9 hour acupuncture group, and 1 in the 24 hour acupuncture group. The total number of rats in the final analysis, excluding dead rats, was 125: 14 in the sham surgery group, 13 in the 3 hour model group, 14 in the 9 hour model group, 13 in the 24 hour model group, 13 in the 48 hour model group, 15 in the 3 hour acupuncture group, 14 in the 9 hour acupuncture group, 14 in the 24 hour acupuncture group, and 15 in the 48 hour acupuncture group.
In situ hybridization (paraffin-embedded samples)
The mRNA probe sequences targeting the rat BCL-2 gene were (1) 5′ GATGA AGTAC ATCCA TTATA AGCTG TCACA 3′; (2) 5′ GCGCT CAGCC CTGTG CCACC TGTGG TCCAC 3′; and (3) 5′ GGGAG ATGTC ACCCC TGGTG GACAA CATCG 3′. The mRNA probe sequences targeting the rat BAX gene were (1) 5′ CGAGA GGTCT TCTTC CGTGT GGCAG CTGAC ATGTT 3′; (2) 5′ AACTT CAACT GGGGC CGGGT GGTTG CCCTT TTCTA 3′ and (3) 5′ CTGAT CAGAA CCATC ATGGG CTGGA CACTG GACTT 3′.
Specimens were promptly fixed after harvesting in 4% paraformaldehyde in 0.1 M PBS (pH 7.0–7.6) containing 1/1000 diethylpyrocarbonate (DEPC) for 40 minutes. Samples were routinely dehydrated and paraffin-embedded, and 4-μm sections were prepared. Slides were treated with poly-lysine and paraffin sections were routinely dewaxed and hydrated. Slides were incubated in hydrogen peroxide (30% hydrogen peroxide/distilled water: 1:10) for 10 minutes at room temperature to inactivate endogenous peroxidases, followed by three washes in distilled water.
For exposure of mRNA fragments, pepsin freshly diluted in 3% citric acid (1 mL of 3% citric acid was mixed with two drops of concentrated pepsin) was added on slides and digestion in room temperature was performed for 10 minutes, followed by three washes in PBS for five minutes each, and one wash in distilled water.
Post-fixation was performed after pepsin digestion. The fixative consisted of 1% paraformaldehyde in 0.1 M PBS (pH 7.2–7.6) and 1/1000 DEPC. Samples were post-fixed for 10 minutes at room temperature, followed by three washes in distilled water.
For preparation of the humidified hybridization chamber, 20 mL of 20% glycerol was added to the bottom of a dry hybridization box to maintain humidity. Then, 20 μL of pre-hybridization solution were added to each slice, with incubation in a 40°C incubator for 3 hour. Excess liquid was absorbed and no washing was performed.
For hybridization, 20 μL of hybridization buffer was added to each slice. The protective film was removed from in situ hybridization coverslips and coverslips were placed on each slice. Samples were left in a 40°C incubator overnight.
For washing after hybridization, the coverslip was removed and slides were first washed twice in preheated 2×SSC at 37°C for 5 minutes, followed by one wash in 0.5×SSC for 15 minutes at 37°C and a final wash in 0.2×SSC for 15 minutes at 37°C. Blocking solution was added to the slides and incubated at 37°C for 30 minutes. Excess liquid was removed and no washing was performed. Biotinylated mouse anti-digoxin was added and incubated at RT for 120 minutes followed by washing in PBS four times for 5 minutes each. Streptavidin-biotin complex (SABC) was then added and incubated at room temperature for 30 minutes followed by washing in PBS three time for 5 minutes each. Biotinylated peroxidase was added with incubation at room temperature for 30 minutes followed by washing in PBS four times for 5 minutes each.
For DAB chromogenic staining, a DAB substrate kit (Beijing Solar Technology Co., Ltd.) was used: 1 mL of distilled water was mixed with one drop of reagents A (buffer), B (substrate), and C (chromogenic agent), and the mixture was added to the specimen and incubated for 30 minutes to produce a color reaction, followed by thorough washing in water. After ethanol dehydration and xylene clearing, the slides were coverslipped.
RT-PCR
For total RNA extraction, an Ultrapure RNA extraction kit (Beijing ComWin Biotech Co., Ltd.) was used as per manufacturer’s instructions. Then 5 μL of RNA were loaded in a 1% agarose gel for electrophoresis to determine RNA integrity. A DNase I kit (Beijing ComWin Biotech Co., Ltd.) was used per the manufacturer’s instructions to digest any remaining genomic DNA in the extracted RNA.
Reverse transcription
A HiFi-MMLV cDNA first-strand synthesis kit (Beijing ComWin Biotech Co., Ltd.) was used for reverse transcription according to the manufacturer’s instructions. For the RT-PCR reaction system, DNA was amplified using the UltraSYBR Mixture (with ROX). The amplification program was 95°C for 10 minutes, followed by 45 cycles of 95°C for 15 seconds and 60°C for 60 seconds. The reaction mixture contained 10 μL of 2×UltraSYBR Mixture, 0.4 μL of 10 μM forward primer, 0.4 μL of 10 μM reverse primer, 2 μL of template, and sterilized distilled water in a total volume of 20 μL. The primer sequences of BCL-2 and BAX mRNA probes as shown in Table 1.
Table 1.
Primer sequences.
| Gene | Primer | Primer sequence (5′ to 3′) | Product size (bp) |
|---|---|---|---|
| BCL-2 | Forward primer | GAGTACCTGAACCGGCATCT | 172 |
| Reverse primer | GGTATGCACCCAGAGTGATG | ||
| BAX | Forward primer | CAGGATGCGTCCACCAAGAA | 193 |
| Reverse primer | AGTAGAAGAGGGCAACCACG | ||
| GAPDH | Forward primer | AAGAGGGATGCTGCCCTTAC | 119 |
| Reverse primer | TACGGCCAAATCCGTTCACA |
For PCR, after each sample cDNA was diluted 10-fold, 2 μL of the diluted cDNA was used as the template, and the target and reference genes were amplified. Melting curve analyses were performed at 60°C to 95°C (each sample was amplified in triplicate).
Relative quantitative analysis
The 2−ΔΔct relative quantitation formula was used for quantification:
Using this formula, the target genes’ expression for each sample was calculated relative to the levels in the control samples.
Statistical analysis
All data were processed using SPSS 17.0 software. Quantitative data were analyzed by single factor variance analysis (one-way ANOVA) and grade-evaluation data were analyzed by Wilcoxon’s rank sum test. Data are presented as the mean ± standard deviation (χ̄±s) and p<0.05 was considered statistically significant.
Results
In situ hybridization
Comparison of the number of cells positive for BCL-2 mRNA between groups
As shown in Table 2 and Figure 1, among all groups, the rate of cells positive for BCL-2 mRNA was significantly higher in the model and acupuncture groups than in the sham surgery groups at the same time point (p<0.05). The increase in the acupuncture groups was significantly higher than that in the model groups at the same time point (with the exception of the 9 hour acupuncture group, in which the rate was not significantly different from that in the 9 hour model group) (p<0.05). When comparing results within the groups, the rate of cells positive for BCL-2 mRNA was lower in the 3 hour model group than in the other subgroups, and no significant differences were observed among the rest of the subgroups, i.e., the 9 hour model group, 24 hour model group, and 48 hour model group (p<0.05). Among the subgroups of the acupuncture group, the rate of cells positive for BCL-2 mRNA was significantly increased in the 3 hour group and 24 hour group compared to the 9 hour and 48 hour groups (p<0.05), and no significant differences were observed between the 3 hour and 24 hour groups or between the 9 hour and 48 hour groups.
Table 2.
Comparisons of rates of BCL-2 mRNA and BAX mRNA positive cells (χ̄±s).
| Groups | n | BCL-2 mRNA positive cell rate (%) | BAX mRNA positive cell rate (%) |
|---|---|---|---|
| 3-h sham surgery group | 14 | 10.50±2.98 | 9.71±3.36 |
| 9-h sham surgery group | 14 | 10.50±2.98 | 9.71±3.36 |
| 24-h sham surgery group | 14 | 10.50±2.98 | 9.71±3.36 |
| 48-h sham surgery group | 14 | 10.50±2.98 | 9.71±3.36 |
| 3-h model group | 13 | 16.77±4.40*#@& | 30.38±5.35* |
| 9-h model group | 14 | 26.93±5.03* | 30.57±4.93* |
| 24-h model group | 13 | 25.54±3.57* | 29.31±3.28* |
| 48-h model group | 13 | 25.38±3.86* | 26.00±4.97* |
| 3-h acupuncture group | 15 | 34.73±4.18*#@& | 14.13±4.09*$#@ |
| 9-h acupuncture group | 14 | 29.71±4.89*@ | 15.50±4.75*$#@ |
| 24-h acupuncture group | 14 | 34.71±5.55*$# | 19.36±5.12*$ |
| 48-h acupuncture group | 15 | 28.80±4.11*$ | 21.20±4.31*$ |
Between groups: compared with the sham surgery group
P<0.05, compared with the same-time point model group
P<0.05; within the Group: compared with the 48-h group
P<0.05, compared with the 24-h group
P<0.05, compared with the 9-h group
P<0.05.
Figure 1.
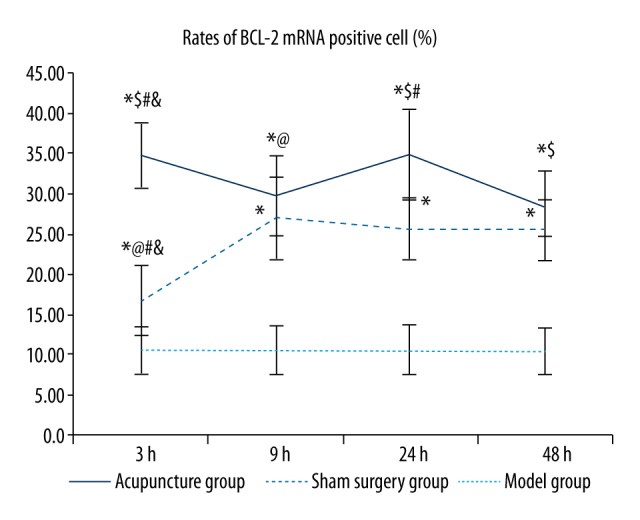
Comparisons of the rates of BCL-2 mRNA-positive cells between groups. Between groups: Compared with the sham surgery group * p<0.05, compared with the same time-point model group $ p<0.05; within the group: compared with the 48 hour group # p<0.05, compared with the 24 hour group @ p<0.05, compared with the 9 hour group & p<0.05. (BCL-2 in situ hybridization, 400×).
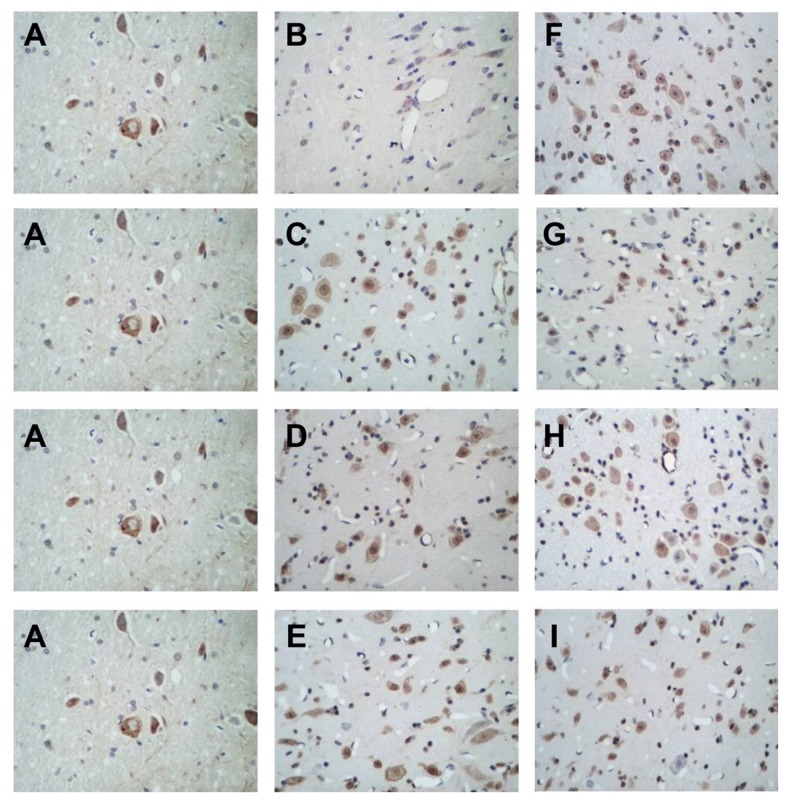
BCL-2 mRNA was expressed at different levels in the cytoplasm in perihematomal brain tissues of rats with acute cerebral hemorrhage, and cytoplasm positive for BCL-2 mRNA exhibited a brown or tan color (Figure 2). Cells positive for BCL-2 mRNA were unevenly and sporadically distributed among normal cells, with varied shapes and sizes. In the sham surgery group, positive cells were rarely seen, whereas in the acupuncture subgroups, the number of positive cells increased significantly, especially in the 3 hour and 24 hour acupuncture groups. In subgroups of the model group, the number of positive cells increased compared with the sham surgery group, but significantly decreased compared with the acupuncture groups at the corresponding time points (with the exception of the 9 hour model group, in which the rate of positive cells was similar to that in the 9 hour acupuncture group). Among the subgroups of the model group, the 3 hour model group showed the fewest positive cells.
Figure 2.
In situ hybridization images showing BCL-2 expression, 400×. (A) Sham operation group, (B) 3 hour model group, (C) 9 hour model group, (D) 24 hour model group, (E) 48 hour model group, (F) 3 hour acupuncture group, (G) 9 hour acupuncture group, (H) 24 hour acupuncture group, (I) 48 hour acupuncture group.
Comparison of the rate of cells positive for BAX mRNA between groups
As shown in Table 2 and Figure 3, the rate of cells positive for BAX mRNA was significantly higher in the model and acupuncture groups than in the sham surgery groups at the same time points, and the increase was greater in the model groups than in the acupuncture groups. The differences between any two of the sham operation group, model group, and acupuncture group were statistically significant (p<0.05) at all time points. No significant differences were found in the rate of cells positive for BAX mRNA between any two subgroups among the 3, 9, 24, and 48 hour model groups. In the acupuncture group, the rates of cells positive for BAX mRNA were higher in the 24 hour and 48 hour acupuncture groups than in the 3 hour and 9 hour acupuncture groups (p<0.05), and no significant differences were observed between the 24 hour and 48 hour groups or between the 3 hour and 9 hour groups.
Figure 3.
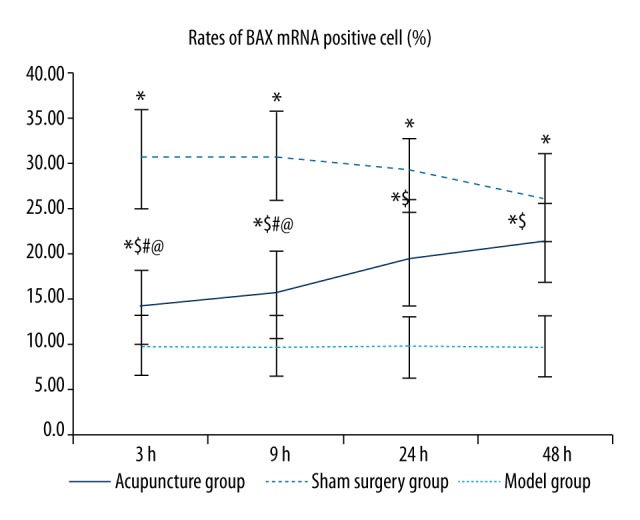
Comparisons of rates of BAX mRNA-positive cells between groups. Between groups: compared with the sham surgery group * p<0.05, compared with the same time point model group $ p<0.05; within the group: compared with the 48 hour group # p<0.05, compared with the 24 hour group @ p<0.05, compared with the 9 hour group & p<0.05. (BAX situ hybridization, 400×).
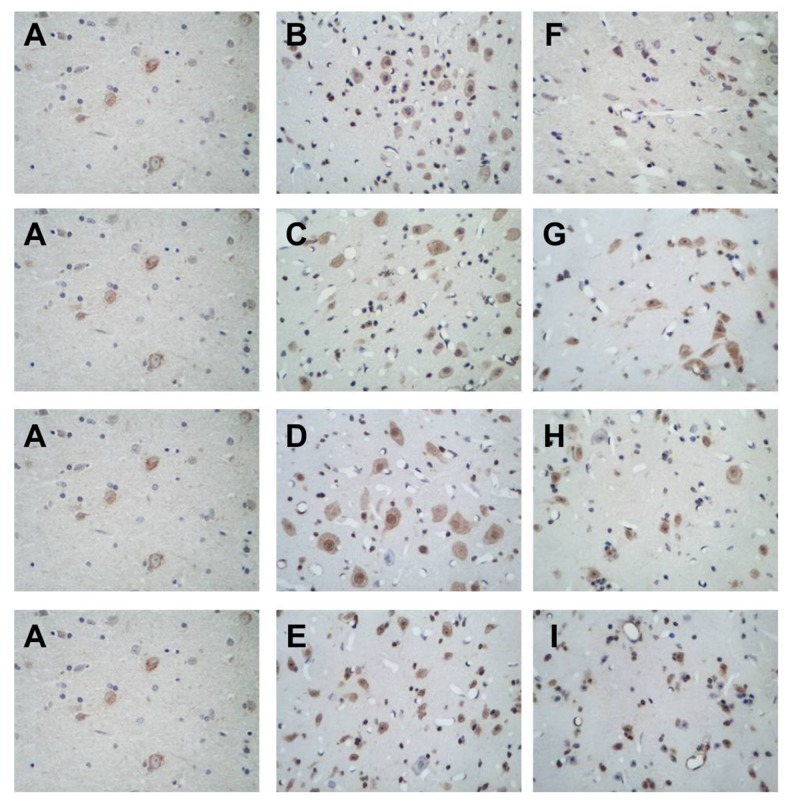
As shown in Figure 4, BAX mRNA was expressed at different levels in the cytoplasm in perihematomal brain tissues after acute cerebral hemorrhage, and the cytoplasm was stained brown or tan. Cells positive for BAX mRNA had varied shapes and sizes and were unevenly and sporadically distributed among normal cells. Positive cells were rarely seen in the sham surgery group. The rate of positive cells significantly increased in all model subgroups but was similar among the 3, 9, 24, and 48 hour model groups. The number of positive cells in the acupuncture subgroups was significantly higher than in the sham surgery group, but significantly lower than in the model subgroups at the same time point, with the 3 hour and 9 hour acupuncture groups exhibiting the fewest positive cells, followed by the 24 hour and 48 hour acupuncture groups.
Figure 4.
(A–I) Sham surgery group; 3, 9, 24, and 48 hour model groups; 3, 9, 24, and 48 hour acupuncture groups, respectively.
RT-PCR results
Comparison of BCL-2 mRNA levels among groups
BCL-2 mRNA levels were lower in the sham operation group than in the model group, except in the 24 hour and 48 hour model groups, in which the expression of BCL-2 was decreased significantly compared to the sham group (p<0.05, Table 3 and Figure 5). Additionally, the expression was significantly increased in the 3 hour and 9 hour model groups compared to the sham operation group (p<0.05). Compared to the sham operation and model groups, BCL-2 mRNA levels in the acupuncture groups at the same time points were significantly increased (p<0.05). Additionally, BCL-2 mRNA levels in the 3 hour and 9 hour model groups were significantly higher than those in the 24 hour and 48 hour model groups (p<0.05). However, the differences between the 3 hour and 9 hour model groups and those between the 24 hour and 48 hour model groups were not significant. Among the acupuncture subgroups, the BCL-2 mRNA levels were higher (p<0.05) in the 9, 24, and 48 hour groups than in the 3 hour group, but no significant differences were observed among the 9, 24, and 48 hour groups.
Table 3.
Comparisons of BCL-2 mRNA and BAX mRNA levels and BCL-2/BAX mRNA ratios between groups (χ̄±s).
| Groups | n | BCL-2 mRNA expression levels | BAX mRNA expression levels | Ratio of BCL-2 mRNA expression/BAX mRNA expression |
|---|---|---|---|---|
| 3-h sham surgery group | 14 | 1.03±0.04 | 1.06±0.04 | 0.98±0.05 |
| 9-h sham surgery group | 14 | 1.03±0.04 | 1.06±0.04 | 0.98±0.05 |
| 24-h sham surgery group | 14 | 1.03±0.04 | 1.06±0.04 | 0.98±0.05 |
| 48-h sham surgery group | 14 | 1.03±0.04 | 1.06±0.04 | 0.98±0.05 |
| 3-h model group | 13 | 1.07±0.04*#@ | 2.23±0.06*#@& | 0.48±0.02*#@ |
| 9-h model group | 14 | 1.11±0.03*#@ | 2.38±0.06*#@ | 0.47±0.02*#@ |
| 24-h model group | 13 | 0.98±0.09* | 3.05±0.04*# | 0.32±0.03* |
| 48-h model group | 13 | 0.97±0.13* | 3.17±0.05* | 0.30±0.04* |
| 3-h acupuncture group | 15 | 2.11±0.06*$#@& | 1.74±0.04*$#@& | 1.21±0.05*$#@ |
| 9-h acupuncture group | 14 | 2.24±0.05*$ | 1.87±0.04*$#@ | 1.20±0.04*$#@ |
| 24-h acupuncture group | 14 | 2.28±0.04*$ | 2.08±0.05*$# | 1.10±0.03*$# |
| 48-h acupuncture group | 15 | 2.28±0.04*$ | 62.19±0.04*$ | 1.04±0.02*$ |
Between groups: compared with the sham surgery group
P<0.05, compared with the same-time point model group
P<0.05; within the Group: compared with the 48-h group
P<0.05, compared with the 24-h group
P<0.05, compared with the 9-h group.
Figure 5.
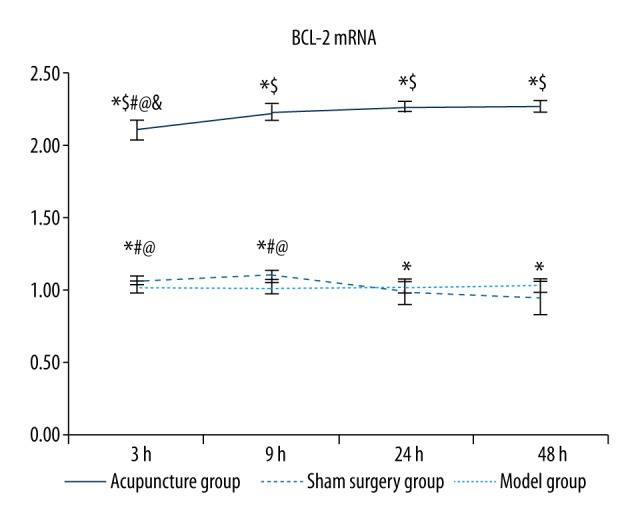
Comparisons of BCL-2 mRNA levels between groups. Between groups: compared with the sham surgery group * p<0.05, compared with the same time point model group $ p<0.05; within the group: compared with the 48 hour group # p<0.05, compared with the 24 hour group @ p<0.05, compared with the 9 hour group & p<0.05.
Comparison of BAX mRNA levels between groups
The BAX mRNA levels were significantly higher in the model groups and the acupuncture groups than in the sham groups at the same time point (p<0.05, Table 3 and Figure 6), and the increase in the model groups was more significant than that in the acupuncture groups (p<0.05). The 48 hour model group had the highest BAX mRNA levels among all model subgroups, followed in order by the 24, 9, and 3 hour model groups, and the differences between any two of these groups were significant (p<0.05). Among all acupuncture subgroups, the 48 hour acupuncture group also showed the highest BAX mRNA levels, followed in order by the 24, 9, and 3 hour groups, and the differences between any two of these groups were significant (p<0.05).
Figure 6.
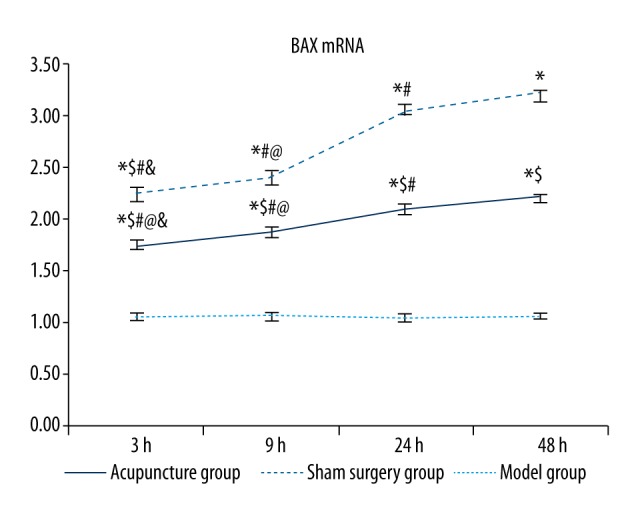
Comparisons of BAX mRNA levels between groups. Between groups: compared with the sham surgery group * p<0.05, compared with the same time point model group $ p<0.05; within the group: compared with the 48 hour group # p<0.05, compared with the 24 hour group @ p<0.05, compared with the 9 hour group & p<0.05.
Comparison of ratios of BCL-2 and BAX mRNA levels between groups
As shown in Table 3 and Figure 7, the ratio of the BCL-2 mRNA level to the BAX mRNA level was significantly higher in the sham surgery and acupuncture groups than in the model groups at the same time point (p<0.05), with the highest ratio in the acupuncture groups. The ratio of BCL-2 mRNA level to BAX mRNA level was significantly higher in the 3 hour and 9 hour model groups compared to the 24 hour and 48 hour model groups (p<0.05), and no significant differences were found between the 3 hour and 9 hour model groups or between the 24 hour and 48 hour model groups. The ratio of BCL-2 mRNA level to BAX mRNA level was also significantly higher in the 3 hour and 9 hour acupuncture groups compared to the 24 hour and 48 hour acupuncture groups (p<0.05), and significantly higher in the 24 hour acupuncture group than in the 48 hour group (p<0.05).
Figure 7.
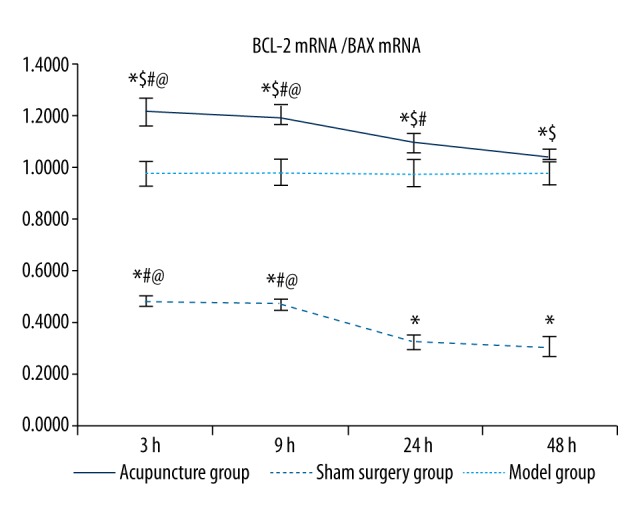
Comparisons of BCL-2/BAX mRNA ratios between groups. Between groups: compared with the sham surgery group * p<0.05, compared with the same time point model group $ p<0.05; within the group: compared with the 48 hour group # p<0.05, compared with the 24 hour group @ p<0.05, compared with the 9 hour group & p<0.05.
Discussion
The results of the present study showed that the number of cells positive for BCL-2 and BAX mRNA was significantly increased after acute cerebral hemorrhage, and that acupuncture resulted in a significant increase in the levels of BCL-2 mRNA and a decrease in the levels of BAX mRNA, even when compared to the sham surgery group. In addition, earlier acupuncture intervention was associated with lower numbers of cells positive for BCL-2 and BAX mRNA. The changes in the expression of BCL-2 and BAX mRNA were consistent with the changes in the rate of cells positive for BCL-2 and BAX mRNA. The change in the expression ratio of BCL-2 and BAX mRNA was consistent with the change in the number of cells positive for BCL-2 mRNA but inversely correlated with the change in the rate of cells positive for BAX mRNA. Acupuncture can improve the ratio of BCL-2/BAX mRNA and thus inhibit apoptosis. Apoptosis might be an important mechanism of delayed injury in neurocytes surrounding the hematoma, and it has a close relationship to the inflammatory reaction of hemorrhagic tissue after intracerebral hemorrhage. Therefore, this finding is of great significance to the early treatment of intracerebral hemorrhage by acupuncture.
Conclusions
This study indicates that by ameliorating the changes in the mRNA expression of the apoptotic factors BCL-2 and BAX caused by acute cerebral hemorrhage, acupuncture therapy exerts a neuroprotective function in perihematomal brain tissue upon acute cerebral hemorrhage. The most significant effects were observed when treatment was started 3 hours or 9 hours after cerebral hemorrhage, i.e., efficacy was highest when the interval between cerebral hemorrhage and acupuncture therapy was minimized.
Footnotes
Conflict of interest
None.
Source of support: Tianjin Science and Technology Commission (05YFSZSF02600)
References
- 1.Zhao W, Shou H, Yan F. Apoptosis. Henan: Henan Medical University Press; 1997. [Google Scholar]
- 2.Vaux DL, Cory S, Adams JM. Bcl-2 gene promotes haemopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature. 1988;335:440–42. doi: 10.1038/335440a0. [DOI] [PubMed] [Google Scholar]
- 3.Hasegawa Y, Suzuki H, Sozen T, et al. Apoptotic mechanisms for neuronal cells in early brain injury after subarachnoid hemorrhage. Acta Neurochir Suppl. 2011;110:43–48. doi: 10.1007/978-3-7091-0353-1_8. [DOI] [PubMed] [Google Scholar]
- 4.Xie MJ, Ma YH, Miao L, et al. Emodin-provoked oxidative stress induces apoptosis in human colon cancer HCT116 cells through a p53-mitochondrial apoptotic pathway. Asian Pac J Cancer Prev. 2014;15:5201–5. doi: 10.7314/apjcp.2014.15.13.5201. [DOI] [PubMed] [Google Scholar]
- 5.Marek Ł. The role of the apoptosome in the activation of procaspase-9. Postepy Hig Med Dosw (Online) 2013;67:54–64. doi: 10.5604/17322693.1032333. [DOI] [PubMed] [Google Scholar]
- 6.Tian HY, Li ZX, Li HY, et al. Effects of 14 single herbs on the induction of caspase-3 in tumor cells: a brief review. Chin J Integr Med. 2013;19:636–40. doi: 10.1007/s11655-013-1539-y. [DOI] [PubMed] [Google Scholar]
- 7.Feng R, Zhang F. The neuroprotective effect of electro-acupuncture against ischemic stroke in animal model: A review. Afr J Tradit Complement Altern Med. 2014;11:25–29. doi: 10.4314/ajtcam.v11i3.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hou X, Zhang R, Lv H, et al. Acupuncture at Baihui and Dazhui reduces brain cell apoptosis in heroin readdicts. Neural Regen Res. 2014;9:164–70. doi: 10.4103/1673-5374.125345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee AL, Campbell LB, Sapolsky RM. Neighbor effects of neurons bearing protective transgenes. Brain Res. 2010;1339:70–75. doi: 10.1016/j.brainres.2010.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berliocchi L, Bano D, Nicotera P. Ca2+ signals and death programmes in neurons. Philos Trans R Soc Lond B Biol Sci. 2005;360:2255–58. doi: 10.1098/rstb.2005.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lewis A, Hayashi T, Su TP, Betenbaugh MJ. Bcl-2 family in inter-organelle modulation of calcium signaling; Roles in bioenergetics and cell survival. J Bioenerg Biomembr. 2014;46:1–15. doi: 10.1007/s10863-013-9527-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yin XM, Oltvai ZN, Korsmeyer SJ. BH1 and BH2 domains of Bcl-2 are required for inhibition of apoptosis and heterodimerization with Bax. Nature. 1994;369:321–23. doi: 10.1038/369321a0. [DOI] [PubMed] [Google Scholar]
- 13.Gavathiotis E, Reyna DE, Davis ML, et al. BH3-triggered structural reorganization drives the activation of proapoptotic. Mol Cell. 2010;40:481–92. doi: 10.1016/j.molcel.2010.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He N, Zhu C, He X. Research of neuron protection mechanism in treating brain infarction by acupuncture. Contemporary Medicine. 2012;18:3–4. [Google Scholar]
- 15.Liu J, Fan X, Li P. Acupuncture effect on rat hippocampus BCL-2 and BAX in multiple infarct dementia model. Traditional Chinese Medicinal Research. 2006;19:11–14. [Google Scholar]
- 16.Bao X, Shu S. The rat brain in stereotaxic coordinates. Beijing: People’s Medical Publishing House; 1991. [Google Scholar]
- 17.Berderson JB, Pitts LH, Tsuji M, et al. Rat middle cerebral artery occlusion: Evaluation of the model and development of a neurologic examination. Stroke. 1986;17:472–76. doi: 10.1161/01.str.17.3.472. [DOI] [PubMed] [Google Scholar]
- 18.Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniotomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]