Abstract
Background
Prevalence of atrial fibrillation (AF) is over 10% at age ≥80 years, but the impact of population ageing on rates of AF-related ischaemic events is uncertain.
Methods and Results
We studied age-specific incidence, outcome and cost of all AF-related incident strokes and systemic emboli from 2002-2012 in the Oxford Vascular Study (OXVASC). We determined trends in incidence of AF-related stroke since a sister study in 1981-1986, extrapolated numbers to the UK population and projected future numbers. Of 3096 acute cerebral or peripheral vascular events in the 92,728 study population, 383 incident ischaemic strokes and 71 systemic emboli were related to AF, of which 272 (59.9%) occurred at ≥80 years. Of 597 fatal or disabling incident ischaemic strokes, 262 (43.9%) were AF related. Numbers of AF-related ischaemic strokes at age ≥80 increased nearly three-fold from 1981-1986 to 2002-2012 (extrapolated to the UK: 6621 to 18,176), due partly to increased age-specific incidence (RR 1.52, 96%CI 1.31-1.77, p=0.001), with potentially preventable AF-related events at age≥80 costing the UK £374 million/year. At current incidence rates, numbers of AF-related embolic events at age ≥80 will treble again by 2050 (72,974/year), with 83.5% of all events occurring in this age group.
Conclusions
Numbers of AF-related incident ischaemic strokes at age ≥80 years have trebled over the last 25 years, despite the introduction of anticoagulants, and are projected to treble again by 2050, along with the numbers of systemic emboli. Improved prevention in older people with AF should be a major public health priority.
Keywords: atrial flutter, atrial fibrillation, stroke, embolism, population studies
Introduction
The increase in life-expectancy, due partly to prevention of premature vascular deaths, and the consequent increase in the elderly population have implications for the effective targeting of preventive medicine and healthcare costs. A key issue for prevention of vascular disease and for future burden is the extent to which incidence of non-fatal events is also moving to older ages. If disease incidence moves to older ages at the same rate as life-expectancy increases, such that age-specific incidence falls, then some aspects of screening and prevention will also need to be targeted towards older age groups, but overall disease burden may not increase (depending on how outcome is related to age). Such a “right-shift” of incidence to older ages is evident for some conditions, such as acute coronary events.1 If, however, disease incidence does not move to older ages and age-specific incidence remains constant then the absolute number of elderly individuals affected will increase. If, in addition, disease incidence at older ages also increases, due for example to a greater susceptibility in individuals who would previously have had a premature vascular death, then the absolute number of older individuals affected could increase dramatically.
Stroke is a major cause of death and disability,1, 2 leaving five million people permanently disabled in Europe each year at a cost of 38 billion euros in 2006.3 Atrial fibrillation (AF) is one of the most common preventable causes of stroke. AF-related ischaemic strokes tend to be severe and to incur high mean costs,4 and non-cerebral systemic embolism secondary to AF is also a major clinical burden.5, 6 With 2.3 million people estimated to have AF in the USA,7 with prevalence increasing from 0.5% at 50-59 years to 10% at ≥80 years, and with age-specific prevalence possibly also increasing,7–9 the burden and cost of AF-related stroke and systemic embolism could increase dramatically. However, anticoagulation with warfarin is highly effective in primary prevention of AF-related embolic events,10, 11 and several new oral anticoagulants may be of at least equivalent clinical benefit,12 albeit with some disadvantages.13,14 Yet, there is widespread under-use of warfarin for AF,15–17 particularly in the elderly.18–20 Although there are few published data on the safety of newer anticoagulants at age ≥80 years,12,21 there is good evidence that warfarin is more effective than aspirin in primary prevention in high-risk elderly patients with AF.22
We could therefore be facing a substantial increase in potentially preventable AF-related embolic events at older ages due to the multiplicative effects of increased life-expectancy, the strongly age-related prevalence of AF with no evidence of right-shift to older ages and possibly increasing age-specific prevalence, and low-rates of use of anticoagulation in older people with AF with little evidence of any recent improvement.15,20 One major barrier to action at public health policy level is that in contrast to the numerous studies documenting low rates of anticoagulation (web-tables IA-C), there are few published data on the consequences and costs of such under-treatment in terms of potentially preventable embolic events at the population level and no data on projected future burden. We therefore did a prospective population-based study of all stroke and systemic embolism associated with AF in Oxfordshire, UK, during 2002-2012, to determine age and sex-specific incidence, rates of prior AF, pre-morbid treatment in relation to age, sex, risk scores and contraindications, pre-morbid disability, clinical outcome and cost. By comparison with a sister study in the same population in 1981-86, we also determined the change in numbers of AF-related ischaemic strokes, reflecting the combined impact of trends in age-specific incidence of AF, ageing of the population and the impact of anticoagulation. Finally, we projected future numbers of AF-related thromboembolic events in older people if prevention is not improved.
Methods
OXVASC is a population-based study of the incidence and outcome of all acute vascular events in a mixed urban/rural population of Oxfordshire, UK. Methods and definitions of events (supplemental methods) have been reported previously.2, 23 Briefly, the study population comprises 92,728 individuals registered with nine general practices (about 100 family doctors) that refer patients to the main Oxford Hospitals. The OXVASC population was 94% white, 3.1% Asian, 1.5% Chinese and 1.4% Afro-Caribbean.24 Based on the Index of Multiple Deprivation (IMD),25 the electoral wards covering our population were less deprived than the rest of England (mean IMD score: 8.69 vs 16.98, t-test - p<0.001), but had a broad range of deprivation with 22% of wards ranking in the lower third nationally. Ascertainment of acute vascular events started in 1st April 2002 and is on-going. Case ascertainment uses multiple overlapping methods of hot and cold pursuit (supplemental methods) and has been shown to be near-complete.23,26 For this paper, only incident strokes and systemic emboli ascertained up to 31/03/12 were included. OXVASC has local research ethics committee approval.
All patients gave informed consent to participate, or assent was gained from a relative. Patients were seen by study physicians as soon as possible after presentation (supplemental methods: details of assessment and investigation). Clinical study reports of all strokes were reviewed by the senior study neurologist and reports of all peripheral vascular events were reviewed by a vascular surgeon. We obtained additional premorbid baseline clinical characteristics, lipid profile, BP measurements and medications by interviewing patients and relatives and by review of primary care and hospital records.
Stroke was defined as an event with appropriate symptoms lasting longer than 24 hours.23,27 Systemic emboli included all cases of presumed-embolic acute limb ischaemia or acute visceral ischaemia (including aortic, renal, splenic, hepatic and intestinal). AF-related events were defined as those associated with paroxysmal, persistent or permanent AF28,29 (defined on the basis of an ECG showing either absent p waves or atrial flutter with an irregular ventricular response) documented before the event, at the time of assessment, or within one month after the event.30 Patients were subdivided according to whether AF had been documented prior to the acute event (“known prior AF”), with confirmation from primary care or hospital records.
In all patients with known prior AF, we used pre-morbid clinical characteristics to calculate the CHADS2 score31 and CHA2DS2VASc score32 for risk of embolic ischaemic events and the HAS-BLED score33 for the risk of bleeding on anticoagulation. In those patients not on anticoagulation at the time of the event, we reviewed primary care and hospital records to identify any written explanation as to why anticoagulation was not used and identified any reasons for previous discontinuation. Aetiologic subtype of stroke was classified with the TOAST criteria (Trial of Org 10172).34
All patients had face-to-face follow-up at one and six-months after the event. Institutionalization was defined as living in a nursing home, residential home or community hospital. Disabling/fatal stroke or systemic emboli were defined as having a modified Rankin scale (mRS) score >2 at 6-months. Pre-morbid disability was also defined as mRS>2, with major disability defined as mRS>3 (i.e. not independently mobile).
Incidence of AF-related ischaemic stroke in OXVASC was compared with that in the Oxfordshire Community Stroke Project (OCSP), which was a high-quality population-based study of first-ever stroke in 1981-86 in an overlapping general practice population. Analysis of event rates in OCSP was restricted to the nine practices that were common to both studies. The methods of OCSP have been reported previously,30 and are summarized in supplemental methods. Case-ascertainment, assessment and follow-up were similar to OXVASC.
The definitions of incident ischaemic stroke and of AF were the same in OCSP and OXVASC and rates of brain imaging or autopsy after stroke were similar.2, 23,30 Baseline and one-month cardiovascular examinations were similar and ECG was done routinely at baseline, but ambulatory cardiac monitoring was not.
5-year costs after AF-related stroke and systemic emboli
We estimated the one-year hospital costs and 5-year residential care costs incurred by patients with incident AF-related ischaemic stroke and systemic emboli in the first 5-years of the study to ensure a minimum 5-year follow-up. Hospital records were reviewed for any accident & emergency (A&E) visit, emergency transport, outpatient care visit, day case or hospitalization for the initial event and during the first year thereafter. Details of costing study methodology in OXVASC have been published previously.35 Only hospital costs in the first year after the index event were included given that we have shown previously that subsequent hospital service use and costs revert back to pre-morbid levels.35 All resource use was priced using 2008/09 unit costs and derived from the schedule of reference costs for NHS trusts.36 We restricted the long-term costs of institutionalization into a nursing or residential care home following stroke/systemic emboli to patients who were institutionalized within the first 6 months after the event to avoid over-estimation of costs due to non-related conditions. Institutionalization was costed as the cost per week in a private nursing home (£715 in 2008), with costs incurred after the first year discounted using an annual rate of 3.5%.
Statistical analysis
Sex-specific rates (per 1000 population per year) of AF-related incident ischaemic strokes and systemic emboli were calculated in 10-year age bands, with confidence intervals (CI) estimated assuming a Poisson distribution. We used original individual patient data and the 1981-1986 age-sex study population data from OCSP to recalculate the rate of AF-related incident ischaemic strokes in OCSP for the OXVASC general practices, and standardized all rates in both studies to the 2010 census population of UK.37 We used Poisson regression models to compare the standardized incidence of AF-related ischaemic strokes in OXVASC versus OCSP. We used Chi-squared or Fisher’s Exact test to compare categorical variables and Student’s t-test for continuous variables. Costs were reported as means together with their standard deviations (SD).
We extrapolated numbers of AF-related incident ischaemic strokes from age-specific incidence rates in OCSP to the 1984 UK population structure and extrapolated rates of all AF-related embolic events in OXVASC to the 2010 UK population structure. We projected likely future numbers of AF-related embolic events using 2002-2012 incidence rates from OXVASC and the Office of National Statistics (ONS) predictions of UK population structures in 2030 and 205037 assuming no change in age-specific incidence. Numbers of events observed and projected were determined in 10-year age bands and also separately for age ≥80 years. We performed statistical analysis and graphical presentation using SPSS software version 20.0, Microsoft Excel 2010 for Windows and SAS software version 9.2.
Results
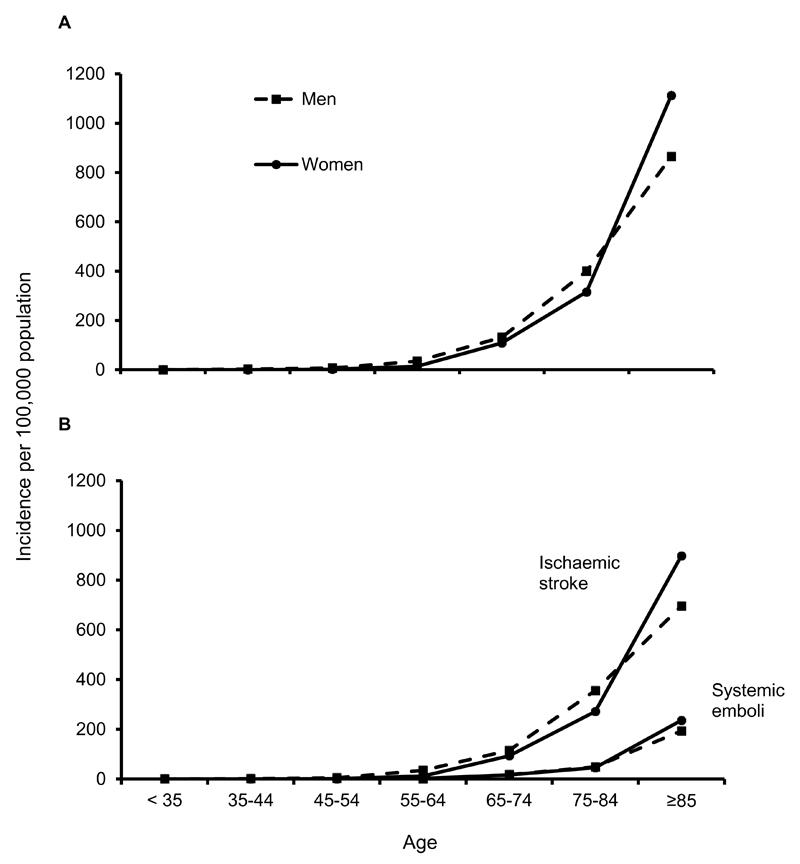
Among 3096 acute incident (2497) and recurrent (599) cerebral or peripheral vascular events in OXVASC during 2002-2012, 748 (24.2%) were AF-related, of which 454 were incident ischaemic strokes (383) or systemic emboli (71). Of these 454 patients, 274 with ischaemic stroke and 62 with systemic emboli had documented prior AF. Incidence rates of these AF-related embolic events were similar in men and women but increased steeply with age (figure 1), with 272/454 (59.9%) events occurring at ≥80 years (table 1).
Figure 1.
Age- and sex-specific rates per 100,000 population per year in OXVASC for all incident AF-related ischaemic stroke and systemic embolism combined (A) and separately (B).
Table 1.
Age-specific rates per 1000 population per year of first ever AF-related incident ischaemic stroke and systemic embolism in OXVASC
| Ischaemic stroke | Men | Rate per 1000 per year (95% CI) | Women | Rate per 1000 per year (95% CI) | Total | Rate per 1000 per year (95% CI) |
|---|---|---|---|---|---|---|
| <60 | 11/38736 | 0.03 (0.01,0.05) | 1/35656 | 0.00 (0.00,0.02) | 12/74392 | 0.02 (0.01,0.03) |
| 60-69 | 20/4308 | 0.46 (0.28,0.72) | 20/4332 | 0.46 (0.28,0.71) | 40/8640 | 0.46 (0.33,0.63) |
| 70-79 | 57/2848 | 2.00 (1.52,2.59) | 50/3187 | 1.57 (1.16,2.07) | 107/6035 | 1.77 (1.45,2.14) |
| 80-89 | 72/1207 | 5.97 (4.67,7.51) | 91/1914 | 4.75 (3.83,5.84) | 163/3121 | 5.22 (4.45,6.09) |
| ≥90 | 11/147 | 7.50 (3.74,13.41) | 50/393 | 12.73 (9.45,16.79) | 61/540 | 11.31 (8.65,14.53) |
| Total | 171/47246 | 0.36 (0.31,0.42) | 212/45482 | 0.47 (0.41,0.53) | 383/92728 | 0.41 (0.37,0.46) |
| 0.48 (0.33,0.63)* | ||||||
| Premorbid AF | 274/92728 | 0.30 (0.26,0.33) | ||||
| 0.34 (0.21,0.47)* | ||||||
| New AF | 109/92728 | 0.12 (0.10,0.14) | ||||
| 0.14 (0.05,0.22)* | ||||||
| Systemic embolism | ||||||
| <60 | 2/38736 | 0.01 (0.00,0.02) | 0/35656 | -- | 2/74392 | 0.00 (0.00,0.01) |
| 60-69 | 1/4308 | 0.02 (0.00,0.13) | 1/4332 | 0.02 (0.00,0.13) | 2/8640 | 0.02 (0.00,0.08) |
| 70-79 | 8/2848 | 0.28 (0.12,0.55) | 11/3187 | 0.35 (0.17,0.62) | 19/6035 | 0.31 (0.19,0.49) |
| 80-89 | 11/1207 | 0.91 (0.46,1.63) | 20/1914 | 1.04 (0.64,1.61) | 31/3121 | 0.99 (0.67,1.41) |
| ≥90 | 5/147 | 3.41 (1.11,7.95) | 12/393 | 3.06 (1.58,5.34) | 17/540 | 3.15 (1.84,5.05) |
| Total | 27/47246 | 0.06 (0.04,0.08) | 44/45482 | 0.10 (0.07,0.13) | 71/92728 | 0.08 (0.06,0.10) |
| 0.09 (0.02,0.16)* | ||||||
| Premorbid AF | 62/92728 | 0.07 (0.05,0.09) | ||||
| 0.08 (0.02,0.14)* | ||||||
| New AF | 9/92728 | 0.01 (0.00,0.02) | ||||
| 0.01 (0.00,0.03)* | ||||||
Standardized to UK 2010 population
Of the 454 patients with AF-related incident events, 436 (96.0%) had non-valvular AF, 129 (28.4%) had paroxysmal AF and 325 (71.6%) had permanent AF (web-tables II-III). Of the 1248 incident ischaemic strokes in OXVASC, AF-related strokes were more likely to be major (NIHSS = 5-9 vs 0-4; age-adjusted OR=1.71, 95%CI 1.21-2.41, p=0.002) or severe (NIHSS≥10 vs 0-4; age-adjusted OR=3.18, 2.32-4.36, p<0.0001) than non-AF-related strokes. Of all 597 incident ischaemic strokes that were fatal or disabling at six months, 262 (43.9%) were AF-related and 193 (32.3%) occurred in patients with known prior AF.
Of 474 patients with incident ischaemic stroke from 1981-86 in the OCSP, 56 (11.8%) had known prior AF and 29 (6.1%) had new or missed AF (web-table IV). The proportions of incident ischaemic strokes in which AF was purely paroxysmal were similar in the two studies (111/383 in OXVASC vs 23/85 in OCSP, p=0.72), including among those at age <80 years with new paroxysmal AF (16/29 vs 3/7, p=0.68; web-table V) and at age ≥80 with paroxysmal AF and non-disabling ischaemic stroke at one month follow-up (6/37 vs 2/7, p=0.59; web-table VI). The standardized incidence of AF-related ischaemic stroke was higher in OXVASC vs OCSP (RR standardized to 2010 UK population=1.26, 1.01-1.57, p=0.041), as was the mean/SD age at AF-related stroke (80.0/9.7 vs 77.3/9.0; p=0.016). The standardized incidence increased from 0.38 (0.21-0.54) events/1000 population/year in OCSP to 0.48 (0.33-0.63) in OXVASC (web-table IV), due entirely to increased incidence at older ages (figure 2). Incidence at age ≥80 years increased significantly (6.12/1000/year vs 4.02/1000/year; age/sex adjusted RR=1.52, 1.31-1.77, p=0.001) in contrast (interaction p=0.036) to incidence at <80 years (0.18/1000/year vs 0.15/1000/year). Extrapolating these stroke incidence rates at age ≥80 to the UK populations in 1984 (OCSP) and 2010 (OXVASC) yielded an increase in expected annual number of events from 6621 to 18,176.
Figure 2.
Age-specific rates (per 100,000 population per year) of incident AF-related ischaemic stroke in OCSP (1981-1986) and OXVASC (2002-2012). Error bars signify the upper 95% confidence interval.
The characteristics of patients with AF-related ischaemic stroke in OCSP vs OXVASC were broadly similar (table 2, web-tables VIIA-D and VIII). However, although premorbid use of antihypertensive and antiplatelet agents was substantially higher in OXVASC (both p<0·0001), warfarin use remained low (47/383 vs 3/85, p=0.018). Among 336 patients with incident stroke or systemic embolism and known prior AF in OXVASC (figure 1), only 56 (16.7%) were anticoagulated at the time of the event (46/274 ischaemic stroke and 10/62 systemic emboli), with no increase in rates of anticoagulation more recently (24/158 in 2002-2007 vs 32/178 in 2007-2012, p=0.49) or in patients with a previous TIA (11/56; 20%). Anticoagulation rates were somewhat higher (difference - p=0.02) for persistent/permanent AF (46/233) than for paroxysmal AF (10/103). Of the 56 patients who were anticoagulated at the time of the event, the INR was subtherapeutic (usually INR<2) in 34 (60.7%). Further details on the under-treatment of patients with incident embolic event and known prior AF are in table 3, web-appendix 1, web-tables IX-XIX, and web-figures I-III. Of 128 patients with incident intracerebral bleed in OXVASC, 19 had known prior AF, of whom 12 were on anticoagulants (4 aged ≥80 years).
Table 2.
Baseline characteristics and medication use in patients with AF-related incident ischaemic stroke in OXVASC and OCSP
| OCSP (1981-86; n=85) | OXVASC (2002-2012; n=383) | p | |
|---|---|---|---|
| Baseline characteristics | |||
| Male sex (%) | 39 (45.9) | 171 (44.6) | 0.836 |
| Mean age (SD) | 77.3 (9.0) | 80.0 (9.7) | 0.016 |
| Premorbid risk factor | |||
| Congestive cardiac failure | 17 (20.0) | 99 (25.8) | 0.259 |
| Hypertension | 61 (71.8) | 282 (73.6) | 0.725 |
| Age≥75 | 52 (61.2) | 280 (73.1) | 0.028 |
| Diabetes | 14 (16.5) | 58 (15.1) | 0.759 |
| Previous TIA | 22 (25.9) | 60 (15.7) | 0.025 |
| Previous MI | 15 (17.6) | 72 (18.8) | 0.805 |
| Angina | 13 (15.3) | 102 (26.6) | 0.028 |
| Current smoking | 22 (25.9) | 30 (7.8) | <0.0001 |
| Hypercholesterolaemia# | 39 (45.9) | 118 (30.8) | 0.008 |
| Peripheral vascular disease | 8 (9.4) | 56 (14.6) | 0.206 |
| Valvular heart disease | 22 (25.9) | 82 (21.4) | 0.370 |
| Premorbid medications | |||
| Antiplatelet agent(s) | 8 (9.4) | 202 (52.7) | <0.0001 |
| Lipid lowering agent | 0 | 102 (26.6) | <0.0001 |
| Antihypertensive(s) | 19 (22.4) | 280 (73.1) | <0.0001 |
| Anticoagulant | 3 (3.5) | 47 (12.3) | 0.018 |
defined as ≥6.0 mmol/l
Table 3.
Relationship between premorbid antithrombotic therapy, pre-morbid CHADS2 score and age of onset of incident AF-related ischaemic stroke or systemic embolism
| Age group | Warfarin | No anti-thrombotics | Mono-antiplatelet | Dual-antiplatelet |
|---|---|---|---|---|
| n, (%) | n, (%) | n, (%) | n, (%) | |
| <60 | 5 (55.6) | 1 (11.1) | 3 (33.3) | 0 |
| 60-69 | 8 (28.6) | 7 (25.0) | 13 (46.4) | 0 |
| 70-79 | 24 (26.4) | 19 (20.9) | 44 (48.4) | 4 (4.4) |
| 80-89 | 19 (12.9) | 42 (28.6) | 83 (56.5) | 3 (2.0) |
| ≥90 | 0 | 17 (27.9) | 39 (63.9) | 5 (8.2) |
| Total | 56 (16.7) | 86 (25.6) | 182 (54.2) | 12 (3.6) |
| CHADS2 <2 | ||||
| <60 | 1 (20.0) | 1 (20.0) | 3 (60.0) | 0 |
| 60-69 | 6 (33.3) | 4 (22.2) | 8 (44.4) | 0 |
| 70-79 | 6 (19.4) | 6 (19.4) | 19 (61.3) | 0 |
| 80-89 | 3 (17.6) | 8 (47.1) | 5 (29.4) | 1 (5.9) |
| ≥90 | 0 | 3 (37.5) | 4 (50.0) | 1 (12.5) |
| Total | 16 (20.3) | 22 (27.8) | 39 (49.4) | 2 (2.5) |
| CHADS2 ≥2 | ||||
| <60 | 4 (100) | 0 | 0 | 0 |
| 60-69 | 2 (20.0) | 3 (30.0) | 5 (50.0) | 0 |
| 70-79 | 18 (30.0) | 13 (21.7) | 25 (41.7) | 4 (6.7) |
| 80-89 | 16 (12.3) | 34 (26.2) | 78 (60.0) | 2 (1.5) |
| ≥90 | 0 | 14 (26.4) | 35 (66.0) | 4 (7.5) |
| Total | 40 (15.6) | 64 (24.9) | 143 (55.6) | 10 (3.9) |
Of the 383 AF-related ischaemic strokes, 190 (77 patients aged <80 and 113 aged ≥80 at time of incident event) occurred during the first 5 years of OXVASC. Mean total care costs were £22,423, of which £12,417 were due to hospital care (i.e. inpatient, outpatient and emergency visits) costs and £10,007 due to long-term institutionalization (table 4). Although patients aged <80 incurred similar hospital costs to those aged ≥80 (£12,934 vs. £12,064 respectively), they incurred lower institutionalization costs (£6,670 vs. £12,280 respectively). The 5-year risk of institutionalization in long-term care homes is reported in web-figure IV. Of the 71 AF-related systemic emboli, 32 (10 patients aged <80 and 22 aged ≥80 at time of incident event) occurred during the first 5 years of OXVASC. Mean hospital care costs were £13,720. Systemic emboli patients incurred no long-term institutionalization costs over the period of interest (i.e. two patients were institutionalized in long-term care homes, but this occurred 6 months after the event). For the subset of potentially preventable AF-related events in patients who were aged ≥80 and not anticoagulated, total care costs averaged £26,454 for stroke and £14,482 for systemic emboli; £374 million per year when extrapolated across the UK (web-tables XVA-B).
Table 4.
Mean hospital and long-term institutionalization care costs after AF-related incident embolic vascular events, and incident embolic vascular events in patients with known prior AF
| All patients | <80 years | ≥80 years | ≥80 years & not treated with warfarin | |
|---|---|---|---|---|
| AF-related incident embolic vascular events | ||||
| Ischaemic stroke, n | 190 | 77 | 113 | 105 |
| Hospital costs | £12,417 (14,759) | £12,934 (15,016) | £12,065 (14,638) | £12,425 (15,048) |
| Long-term costs* | £10,007 (35,034) | £6,670 (28,732) | £12,280 (38,706) | £13,216 (40,011) |
| Total care costs | £22,423 (41,802) | £19,603 (35,676) | £24,345 (45,561) | £25,641 (47,000) |
| Systemic emboli, n | 32 | 10 | 22 | 19 |
| Hospital/total costs | £13,720 (21,593) | £13,969 (19,905) | £13,606 (22,769) | £14,842 (24,283) |
| Incident embolic vascular events in patients with known AF | ||||
| Ischaemic stroke, n | 126 | 54 | 72 | 65 |
| Hospital costs | £12,042 (14,920) | £12,801 (16,000) | £11,474 (14,144) | £11,849 (14,725) |
| Long-term costs* | £11,303 (37,778) | £8,792 (33,782) | £13,185 (40,650) | £14,605 (42,568) |
| Total care costs | £23,345 (44,456) | £21,593 (40,822) | £24,659 (47,237) | £26,454 (49,377) |
| Systemic emboli, n | 31 | 9 | 22 | 19 |
| Hospital/total costs | £13,634 (21,944) | £13,702 (21,094) | £13,606 (22,769) | £14,842 (24,283) |
Unless stated, costs are reported as means and standard deviation (S.D.)
Excludes institutionalization costs for 5 patients (all aged ≥ 80 years) who were already institutionalized before the index event.
Extrapolating current age and sex-specific rates of AF-related incident embolic events in OXVASC to the 2010 UK population yields an estimate of 34,258 events per year, 22,152 (64.7%) at age ≥80 years (figure 3, web-tables XVIA-D). Assuming age-specific incidence rates do not change, population projections suggest that there will be 87,353 incident AF-related events (71,178 ischaemic strokes and 16,175 systemic emboli) in 2050 (figure 3, web-tables XVIE-G), 72,974 (83.5%) at age ≥80 years and 35,603 (40.8%) at age ≥90 years. By 2050, the estimated care costs, at 2008 prices, of AF-related incident ischaemic stroke would be £1.7 billion, and £221 million for systemic emboli. In those aged ≥80 years, the costs due to stroke alone would be £1.4 billion, with over 50% of costs (£720 million) due to institutionalization in long term nursing or residential care (web-tables XVA-B).
Figure 3.
Projected number of AF-related incident ischaemic strokes (dark gray bar) and incident systemic emboli (light gray bar) extrapolated from OXVASC to the UK population in 2010, 2030 and 2050 stratified by age and based on the current incidence rate in OXVASC.
Discussion
Although the high prevalence of AF in the elderly and the widespread under-use of anticoagulation are well described, the impact on event rates at the population level has not previously been determined. By studying the combined burden of AF-related stroke and peripheral embolic events we have made several observations that have important implications for the future burden of disease and for improving prevention. As expected in view of the aging population, the number of AF-related ischaemic strokes (and presumably also other embolic events) at older ages has increased over the last 25 years. However, this increase in absolute numbers is greater than expected on the basis of demographic change alone because of a concomitant increase in incidence of AF-related ischaemic stroke at age ≥80 years, despite the advent of convincing trial evidence of the effectiveness of anticoagulation. Crucially from an overall burden of disease perspective, this increase in events at older ages has not been counterbalanced by a reduction in incidence of events at younger ages i.e. there has been no “right-shift” in age-specific incidence. Even assuming as we have that age-specific incidence of AF-related stroke at older ages does not continue to increase, without improved prevention the absolute number of AF-related ischaemic strokes and systemic emboli will treble again by 2050, based on demographic change alone.
We also documented in detail the consequences and costs of potentially preventable AF-related ischaemic events at the population level. Only 9% of patients aged ≥80 years with ischaemic stroke or systemic emboli related to known prior AF were on premorbid anticoagulants, despite the vast majority having a high CHADS2 score and low bleeding risk, and despite the low rate of documented contraindications (webappendix 1, web-tables IX-XIV, web-figures I-III). Consequently, there was a 50-fold imbalance in this age group between numbers of AF-related embolic events (n=208) and numbers of anticoagulant-associated intracranial haemorrhages (n=4). Importantly, over half of patients aged ≥80 and not anticoagulated were previously independent (mRS≤2), and nearly 90% were independently mobile (mRS≤2), but nearly three quarters were dead or more disabled six months after the event (web-tables XIVA-B). Consequently, half of all disabling or fatal incident ischaemic strokes at age ≥80 in the OXVASC population were AF-related. This under-use of anticoagulation was in stark contrast to the high rates of use of antihypertensive drugs and statins in these patients (table 2). Given our projection that by 2050, over 80% of AF-related embolic events will occur at age ≥80 and over 40% at age ≥90, this under-use of anticoagulants in relatively fit older individuals is a major problem for public health.
Rates of anticoagulation for AF at age ≥80 years in the UK range from 21% to 46% (web-tables IA-C), despite the BAFTA trial22 having shown safety and effectiveness in the elderly. We found relatively high rates of pre-morbid antiplatelet drug use in older patients with known prior AF, consistent with evidence that physicians overestimate the bleeding risks of warfarin and underestimate its benefits and overestimate the benefits of antiplatelet drugs for stroke prevention in AF.16 Although the benefit of anticoagulants over antiplatelet drugs in high-risk patients with AF is maintained at older ages,22,38 there is a particular reticence to prescribe warfarin in healthy elderly patients with AF.39 The availability of new oral agents, which post-dated our study period, might change this, but there is little evidence so far of any impact on rates of anticoagulation in older age groups.40,41
We found no evidence of any reduction in AF-related ischaemic events during 2002-2012, despite the introduction in 2006 of AF registers in primary care as part of a remuneration scheme in UK and despite publication of the BAFTA trial in 2007.22 AF registers are clearly potentially useful tools, but only 118 (26%) of 454 patients with incident AF-related ischaemic strokes or systemic emboli in OXVASC did not have documented prior AF and were not aware of the diagnosis and in some of these apparently undocumented cases, the AF would have been either very recent or potentially induced by the ischaemic event itself. Overall, therefore, our findings show that most of the clinical burden of potentially preventable embolic events is due to under-treatment and not under-detection. It is noteworthy that until April 2012 the same scheme rewarded primary care physicians equally for use of anticoagulants or antiplatelet agents in patients with AF (web-table XVII).
More incentives for physicians to prescribe anticoagulants might help but it is paramount that international clinical guidelines and reimbursement systems should focus on older age groups. More research into barriers to prescribing anticoagulants and patients’ reluctance to take them might be useful, but the long-standing and apparently intractable nature of the problem suggests that other approaches will also be required. The considerable current burden and cost of potentially preventable AF-related events, the projected future cost (£1.4 billion/year by 2050), and a population prevalence of AF of over 10% at age 80 would justify consideration of direct public education about the importance of AF. The lack of data on the safety and effectiveness of new anticoagulants at older ages12, 21 must also be addressed, and further trials of non-anticoagulant approaches to prevention42 should be a priority, including pragmatic comparisons with antiplatelet treatment alone rather than anticoagulants.
Projections of future event rates will always be imprecise. We think that our estimates are reasonably valid since demographic changes in our study population that underlie the results of under-anticoagulation and projected numbers of future events are applicable to most developed countries. Although studies of the likely future burden of AF itself have been confined to the USA,7, 8 numbers of individuals with AF are projected to increase 2.5-fold by 2050 (5.6 to 12.1 million) if age-specific prevalence remains stable,8 or by 3-fold (15.9 million) if prevalence continues to increase.7 Our findings relating to the incidence of AF-related embolic events, rather than the prevalence of AF, suggest that unless current rates of anticoagulation increase in the elderly, the number of events will simply parallel the future increase in prevalence. A very substantial fall in age-specific prevalence would be required to offset the increasing number of embolic events due simply to the increasing numbers of older people. Crucially, in contrast to more purely atherosclerotic vascular events, such as acute coronary events, and other high-risk conditions, such as abdominal aortic aneurysms, which are moving to older age groups, a fall in age-specific prevalence of AF seems unlikely since other major risk factors for AF, such as diabetes and obesity, are increasing in prevalence.43 Indeed, it is possible that prevalence of AF at older ages will increase more rapidly in future as premature death due to acute coronary events continues to fall, thereby increasing the numbers of individuals with risk factors for AF who survive to older ages, and as long-term survival in patients with established cardiac disease continues to improve. Moreover, although the association between most vascular risk factors and risk of stroke diminishes with age, the importance of AF as a cause of stroke increases steeply with age.44 It should also be noted that the trebling in numbers of events at age ≥80 years over the last 25 years occurred despite awareness amongst clinicians of the importance of stroke prevention in AF, despite screening in primary care, despite the availability of warfarin and despite high rates of use of antihypertensive drugs and statins.
Our study has several limitations. First, our findings cannot necessarily be generalized to other populations or healthcare systems. For example, rates of prior anticoagulation in patients with incident ischaemic stroke in OXVASC are somewhat lower than those in other recent stroke incidence studies (web-figure V). However, under-use of anticoagulants is widely documented in all countries in which it has been studied, population aging is widespread, and other studies show that a high proportion of AF-related strokes occur at age over 80 years.45,46 In addition, the OXVASC population has a lower mean deprivation level than the overall UK population. Second, it is possible that better detection of paroxysmal AF might have biased our comparisons of OCSP and OXVASC. However, the proportion of stroke cases with paroxysmal AF was similar in both time periods, including among those at age <80 years with new paroxysmal AF. Third, it is also possible that ascertainment of more minor strokes was more effective in OXVASC than in OCSP, possibly accounting for the apparent increase in age-specific incidence. However, study methods were designed to be very similar, previous comparisons showed no evidence of a difference in effectiveness of ascertainment,2 and AF-related strokes at age ≥80 years tend to be severe (web-table XIVA-B) such that ascertainment will be high. Indeed, the proportion of non-disabling AF-related strokes at age ≥80 years was similarly low in both studies (web-table VI) and overall severity and case-fatality of AF-related strokes in the two studies were virtually identical (web-table VIII). Fourth, not all apparently AF-associated systemic emboli or stroke will definitely have been due to AF and not all would have been prevented by prior anticoagulation. However, warfarin reduces the rate of ischaemic stroke and systemic emboli in primary prevention in AF by about 70% compared with placebo,10, 11 and so most of the events in untreated patients would have been preventable. In keeping with this conclusion, we found an alternative aetiology for stroke in only a small proportion of cases (web-table III). Fifth, we did not have individual patient data on anticoagulation in our underlying study population. However, the overall anticoagulation rate in Oxfordshire for individuals with a CHADS2 score ≥2 is somewhat greater than elsewhere in the UK (web-table IC), suggesting that our measure of the burden of potentially preventable AF-related events is unlikely to be an over-estimate, particularly since we exclude AF-associated TIAs and also all recurrent AF-related embolic events. Sixth, ambulatory cardiac monitoring was not routinely performed in either study, which might have led to under-ascertainment of paroxysmal AF. Seventh, as we only included institutional costs (hospital and care homes), our costs associated with AF-related embolic events are likely to be an underestimate since we did not include the indirect costs borne by relatives/friends when providing unpaid care to AF-related embolic event patients. Eighth, we could not meaningfully determine whether premorbid anticoagulation was associated with a lower overall cost due to the small number of patients receiving anticoagulation prior to their index event (n=17) in the first 5 years of our study (web-table XVIII). However, there was a small trend for more favourable functional outcome at 6 months for patients with ischaemic stroke that received premorbid anticoagulation (web-table XIX). Finally, we do not have individual patient risk factor data for the underlying population in OCSP to adjust the comparative incidence rates for differences in the characteristics of the underlying population over and above age and sex.
In conclusion, without improved prevention, the UK faces a substantial increase in potentially preventable AF-related embolic events due to the multiplicative effects of increased life-expectancy and increased prevalence rates at older ages, without any evidence of a counterbalancing reduction in age-specific incidence at younger ages. AF projection studies in the USA7,8 suggest that our findings will be generalizable to North America. Improved prevention of embolic events in people with AF should be a major public health priority throughout the developed world and will become increasingly important in the developing world as populations age.
Supplementary Material
Acknowledgements
We are grateful to all the staff in the general practices that collaborated in OXVASC: Abingdon Surgery, Stert St, Abingdon; Malthouse Surgery, Abingdon; Marcham Road Family Health Centre, Abingdon; The Health Centre, Berinsfield; Exeter Surgery, Kidlington; Kidlington and Yarnton Medical Group, Kidlington; 19 Beaumont St, Oxford; Bartlemas Surgery, East Oxford; Church Street Practice, Wantage. We also acknowledge the use of the facilities of the Acute Vascular Imaging Centre, Oxford. We are grateful to Charles Warlow for access to additional unpublished data from the Oxfordshire Community Stroke Project.
Sources of Funding
The Oxford Vascular Study is funded by the Wellcome Trust, Wolfson Foundation, Medical Research Council, Dunhill Medical Trust, UK Stroke Association, Medical Research Council, National Institute for Health Research (NIHR), and the NIHR Biomedical Research Centre, Oxford. PMR has a Wellcome Trust Senior Investigator Award and an NIHR Senior Investigator Award.
Footnotes
Conflicts of interest: none.
Ethics: The Oxford Vascular Study has been approved by our local research ethics committee.
References
- 1.Feigin VL, Lawes CM, Bennett DA, Barker-Collo SL, Parag V. Worldwide stroke incidence and early case fatality reported in 56 population-based studies: A systematic review. Lancet Neurol. 2009;8:355–369. doi: 10.1016/S1474-4422(09)70025-0. [DOI] [PubMed] [Google Scholar]
- 2.Rothwell PM, Coull AJ, Giles MF, Howard SC, Silver LE, Bull LM, Gutnikov SA, Edwards P, Mant D, Sackley CM, Farmer A, et al. Change in stroke incidence, mortality, case-fatality, severity, and risk factors in Oxfordshire, UK from 1981 to 2004 (Oxford Vascular Study) Lancet. 2004;363:1925–1933. doi: 10.1016/S0140-6736(04)16405-2. [DOI] [PubMed] [Google Scholar]
- 3.www.escardio.org/communities/EHRA/publications/paper-interest/documents/ehra-stroke-report-recommend-document.pdf
- 4.Luengo-Fernandez R, Yiin GS, Gray AM, Rothwell PM. Population-based study of acute- and long-term care costs after stroke in patients with AF. International journal of stroke. 2013;8:308–314. doi: 10.1111/j.1747-4949.2012.00812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frost L, Engholm G, Johnsen S, Moller H, Henneberg EW, Husted S. Incident thromboembolism in the aorta and the renal, mesenteric, pelvic, and extremity arteries after discharge from the hospital with a diagnosis of atrial fibrillation. Archives of internal medicine. 2001;161:272–276. doi: 10.1001/archinte.161.2.272. [DOI] [PubMed] [Google Scholar]
- 6.Menke J, Luthje L, Kastrup A, Larsen J. Thromboembolism in atrial fibrillation. The American journal of cardiology. 2010;105:502–510. doi: 10.1016/j.amjcard.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 7.Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Bailey KR, Abhayaratna WP, Seward JB, Tsang TS. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114:119–125. doi: 10.1161/CIRCULATIONAHA.105.595140. [DOI] [PubMed] [Google Scholar]
- 8.Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, Singer DE. Prevalence of diagnosed atrial fibrillation in adults: National implications for rhythm management and stroke prevention: The AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370–2375. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 9.Heeringa J, van der Kuip DA, Hofman A, Kors JA, van Herpen G, Stricker BH, Stijnen T, Lip GY, Witteman JC. Prevalence, incidence and lifetime risk of atrial fibrillation: The Rotterdam study. European heart journal. 2006;27:949–953. doi: 10.1093/eurheartj/ehi825. [DOI] [PubMed] [Google Scholar]
- 10.Hart RG, Pearce LA, Aguilar MI. Meta-analysis: Antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146:857–867. doi: 10.7326/0003-4819-146-12-200706190-00007. [DOI] [PubMed] [Google Scholar]
- 11.Aguilar MI, Hart R, Pearce LA. Oral anticoagulants versus antiplatelet therapy for preventing stroke in patients with non-valvular atrial fibrillation and no history of stroke or transient ischemic attacks. Cochrane Database Syst Rev. 2007:CD006186. doi: 10.1002/14651858.CD006186.pub2. [DOI] [PubMed] [Google Scholar]
- 12.Ruff CT, Giugliano RP, Braunwald E, Hoffmann EB, Deenadayalu N, Ezekowitz MD, Camm AJ, Weitz JI, Lewis BS, Parkhomenko A, Yamashita T, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383:955–962. doi: 10.1016/S0140-6736(13)62343-0. [DOI] [PubMed] [Google Scholar]
- 13.Harper P, Young L, Merriman E. Bleeding risk with dabigatran in the frail elderly. The New England journal of medicine. 2012;366:864–866. doi: 10.1056/NEJMc1112874. [DOI] [PubMed] [Google Scholar]
- 14.Radecki RP. Dabigatran: Uncharted waters and potential harms. Ann Intern Med. 2012;157:66–68. doi: 10.7326/0003-4819-157-1-201207030-00467. [DOI] [PubMed] [Google Scholar]
- 15.Ogilvie IM, Newton N, Welner SA, Cowell W, Lip GY. Underuse of oral anticoagulants in atrial fibrillation: A systematic review. Am J Med. 2010;123:638–645 e634. doi: 10.1016/j.amjmed.2009.11.025. [DOI] [PubMed] [Google Scholar]
- 16.Gladstone DJ, Bui E, Fang J, Laupacis A, Lindsay MP, Tu JV, Silver FL, Kapral MK. Potentially preventable strokes in high-risk patients with atrial fibrillation who are not adequately anticoagulated. Stroke. 2009;40:235–240. doi: 10.1161/STROKEAHA.108.516344. [DOI] [PubMed] [Google Scholar]
- 17.Lakshminarayan K, Solid CA, Collins AJ, Anderson DC, Herzog CA. Atrial fibrillation and stroke in the general Medicare population: A 10-year perspective (1992 to 2002) Stroke. 2006;37:1969–1974. doi: 10.1161/01.STR.0000230607.07928.17. [DOI] [PubMed] [Google Scholar]
- 18.Perera V, Bajorek BV, Matthews S, Hilmer SN. The impact of frailty on the utilisation of antithrombotic therapy in older patients with atrial fibrillation. Age Ageing. 2009;38:156–162. doi: 10.1093/ageing/afn293. [DOI] [PubMed] [Google Scholar]
- 19.Zimetbaum PJ, Thosani A, Yu HT, Xiong Y, Lin J, Kothawala P, Emons M. Are atrial fibrillation patients receiving warfarin in accordance with stroke risk? Am J Med. 2010;123:446–453. doi: 10.1016/j.amjmed.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 20.Scowcroft AC, Lee S, Mant J. Thromboprophylaxis of elderly patients with AF in the UK: An analysis using the General Practice Research Database (GPRD) 2000-2009. Heart. 2013;99:127–132. doi: 10.1136/heartjnl-2012-302843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eikelboom JW, Wallentin L, Connolly SJ, Ezekowitz M, Healey JS, Oldgren J, Yang S, Alings M, Kaatz S, Hohnloser SH, Diener HC, et al. Risk of bleeding with 2 doses of dabigatran compared with warfarin in older and younger patients with atrial fibrillation: An analysis of the randomized evaluation of long-term anticoagulant therapy (RE-LY) trial. Circulation. 2011;123:2363–2372. doi: 10.1161/CIRCULATIONAHA.110.004747. [DOI] [PubMed] [Google Scholar]
- 22.Mant J, Hobbs FD, Fletcher K, Roalfe A, Fitzmaurice D, Lip GY, Murray E. Warfarin versus aspirin for stroke prevention in an elderly community population with atrial fibrillation (the Birmingham Atrial Fibrillation Treatment of the Aged Study, BAFTA): A randomised controlled trial. Lancet. 2007;370:493–503. doi: 10.1016/S0140-6736(07)61233-1. [DOI] [PubMed] [Google Scholar]
- 23.Rothwell PM, Coull AJ, Silver LE, Fairhead JF, Giles MF, Lovelock CE, Redgrave JN, Bull LM, Welch SJ, Cuthbertson FC, Binney LE, et al. Population-based study of event-rate, incidence, case fatality, and mortality for all acute vascular events in all arterial territories (Oxford Vascular Study) Lancet. 2005;366:1773–1783. doi: 10.1016/S0140-6736(05)67702-1. [DOI] [PubMed] [Google Scholar]
- 24.2001 census area statistics. HMSO; 2001. [Google Scholar]
- 25.Department of the Environment, Transport and the Regions. Indices of deprivation. London: Stationery Office; 2000. Aug, [Google Scholar]
- 26.Coull AJ, Silver LE, Bull LM, Giles MF, Rothwell PM. Direct assessment of completeness of ascertainment in a stroke incidence study. Stroke. 2004;35:2041–2045. doi: 10.1161/01.STR.0000137605.48864.2f. [DOI] [PubMed] [Google Scholar]
- 27.Hatano S. Experience from a multicentre stroke register: A preliminary report. Bull World Health Organ. 1976;54:541–553. [PMC free article] [PubMed] [Google Scholar]
- 28.Camm AJ, Kirchhof P, Lip GY, Schotten U, Savelieva I, Ernst S, Van Gelder IC, Al-Attar N, Hindricks G, Prendergast B, Heidbuchel H, et al. Guidelines for the management of atrial fibrillation: The task force for the management of atrial fibrillation of the European Society of Cardiology (ESC) Europace. 2010;12:1360–1420. doi: 10.1093/europace/euq350. [DOI] [PubMed] [Google Scholar]
- 29.Fuster V, Ryden LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, Halperin JL, Kay GN, Le Huezey JY, Lowe JE, Olsson SB, et al. 2011 ACCF/AHA/HRS focused updates incorporated into the ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation: A report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines developed in partnership with the European Society of Cardiology and in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Journal of the American College of Cardiology. 2011;57:e101–198. doi: 10.1016/j.jacc.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 30.Sandercock P, Bamford J, Dennis M, Burn J, Slattery J, Jones L, Boonyakarnkul S, Warlow C. Atrial fibrillation and stroke: Prevalence in different types of stroke and influence on early and long term prognosis (Oxfordshire Community Stroke Project) BMJ. 1992;305:1460–1465. doi: 10.1136/bmj.305.6867.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: Results from the National Registry of Atrial Fibrillation. JAMA. 2001;285:2864–2870. doi: 10.1001/jama.285.22.2864. [DOI] [PubMed] [Google Scholar]
- 32.Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: The euro heart survey on atrial fibrillation. Chest. 2010;137:263–272. doi: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- 33.Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: The Euro Heart Survey. Chest. 2010;138:1093–1100. doi: 10.1378/chest.10-0134. [DOI] [PubMed] [Google Scholar]
- 34.Adams HP, Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EE., 3rd Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 35.Luengo-Fernandez R, Gray AM, Rothwell PM. A population-based study of hospital care costs during 5 years after transient ischemic attack and stroke. Stroke. 2012;43:3343–3351. doi: 10.1161/STROKEAHA.112.667204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.National Health Service. NHS reference costs 2008-2009. http://www.dh.gov.uk/en/publicationsandstatistics/publications/publicationspolicyandguidance/dh_111591.
- 37.Http://www.statistics.gov.uk/hub/population/index.html
- 38.van Walraven C, Hart RG, Connolly S, Austin PC, Mant J, Hobbs FD, Koudstaal PJ, Petersen P, Perez-Gomez F, Knottnerus JA, Boode B, et al. Effect of age on stroke prevention therapy in patients with atrial fibrillation: The Atrial Fibrillation Investigators. Stroke. 2009;40:1410–1416. doi: 10.1161/STROKEAHA.108.526988. [DOI] [PubMed] [Google Scholar]
- 39.Pugh D, Pugh J, Mead GE. Attitudes of physicians regarding anticoagulation for atrial fibrillation: A systematic review. Age Ageing. 2011;40:675–683. doi: 10.1093/ageing/afr097. [DOI] [PubMed] [Google Scholar]
- 40.Kirley K, Qato DM, Kornfield R, Stafford RS, Alexander GC. National trends in oral anticoagulant use in the United States, 2007 to 2011. Circ Cardiovasc Qual Outcomes. 2012;5:615–621. doi: 10.1161/CIRCOUTCOMES.112.967299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Holt TA, Hunter TD, Gunnarsson C, Khan N, Cload P, Lip GY. Risk of stroke and oral anticoagulant use in atrial fibrillation: A cross-sectional survey. The British journal of general practice. 2012;62:e710–717. doi: 10.3399/bjgp12X656856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Holmes DR, Reddy VY, Turi ZG, Doshi SK, Sievert H, Buchbinder M, Mullin CM, Sick P. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: A randomised non-inferiority trial. Lancet. 2009;374:534–542. doi: 10.1016/S0140-6736(09)61343-X. [DOI] [PubMed] [Google Scholar]
- 43.Yoon KH, Lee JH, Kim JW, Cho JH, Choi YH, Ko SH, Zimmet P, Son HY. Epidemic obesity and type 2 diabetes in asia. Lancet. 2006;368:1681–1688. doi: 10.1016/S0140-6736(06)69703-1. [DOI] [PubMed] [Google Scholar]
- 44.Kannel WB, Wolf PA, Benjamin EJ, Levy D. Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: Population-based estimates. The American journal of cardiology. 1998;82:2N–9N. doi: 10.1016/s0002-9149(98)00583-9. [DOI] [PubMed] [Google Scholar]
- 45.Bejot Y, Ben Salem D, Osseby GV, Couvreur G, Durier J, Marie C, Cottin Y, Moreau T, Giroud M. Epidemiology of ischemic stroke from atrial fibrillation in Dijon, France, from 1985 to 2006. Neurology. 2009;72:346–353. doi: 10.1212/01.wnl.0000341280.31919.bd. [DOI] [PubMed] [Google Scholar]
- 46.Hannon N, Sheehan O, Kelly L, Marnane M, Merwick A, Moore A, Kyne L, Duggan J, Moroney J, McCormack PM, Daly L, et al. Stroke associated with atrial fibrillation--incidence and early outcomes in the north Dublin population stroke study. Cerebrovasc Dis. 2010;29:43–49. doi: 10.1159/000255973. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.