Abstract
Introduction
The Philadelphia chromosome negative myeloproliferative neoplasms (MPN) mainly comprise polycythemia vera (PV), essential thrombocythemia (ET) and myelofibrosis (MF, primary or post-PV/ET). Therapy in PV and ET focuses on minimizing thrombosis and bleeding risk, while in MF, prolongation of survival is an important goal. Different cytoreductive agents are employed in high risk PV and ET, while the JAK inhibtior ruxolitinib is the cornerstone of therapy in MF. Histone deacetylase inhibitors (HDACi) are pleiotropic agents with diverse epigenetic and non-epigenetic actions, selectively in transformed cells. A number of HDACi have been or are being investigated in MPN.
Areas covered
The mechanisms of action of HDACI in neoplastic cells are summarized, and the preclinical rationale and data supporting their development in MPN specifically examined, particularly their synergism with JAK inhibitors. Major findings of clinical trials of HDACi, both alone and in combination with ruxolitinib, in MPN are then discussed, with particular attention to their toxicities and disease-modifying effects.
Expert opinion
HDACi are clearly active in MPN, and there is good preclinical rationale for this. Their combination with ruxolitinib in MF is promising, but the long-term tolerability of these agents is an important concern. Further development in PV or ET appears unlikely.
Keywords: histone deacetylase inhibitors, myeloproliferative neoplasms, epigenetic, myelofibrosis, ruxolitinib, JAK inhibitors, rational combinations, polycythemia vera, essential thrombocythemia
1. Introduction
The term “myeloproliferative syndromes”, coined by William Dameshek in 1951,(1) has classically been used to refer to chronic myeloid leukemia (CML), polycythemia vera (PV), essential thrombocythemia (ET) and primary myelofibrosis (PMF), disorders characterized by the overproduction of mostly mature blood cells of one or more myeloid lineages. The modern category of “myeloproliferative neoplasms” (MPN) additionally encompasses chronic neutrophilic leukemia (CNL), chronic eosinophilic leukemia (CEL), not otherwise specified, MPN, unclassifiable (MPN-U) and mastocytosis, although the most recent iteration of the World Health Organization (WHO) classification of myeloid neoplasms recognizes mastocytosis as a separate disease entity owing to its unique clinical and pathological features.(2) The identification of clonal markers underlying most or all of these conditions, e.g., the Philadelphia chromosome (Ph)(3) and its fusion protein product BCR-ABL(4) in CML, JAK2,(5–9) MPL(10, 11) and CALR(12, 13) mutations in PV, ET and PMF, CSF3R mutations(14) in CNL, KIT mutations in mastocytosis,(15) etc. was key to the change in terminology from “syndromes” or “disorders” to “neoplasms”.
While CML represents a molecularly defined entity, the Ph-negative MPN, generally referred to as MPN, are much more molecularly heterogenous, and although the activating JAK2 V617F mutation is encountered frequently (approximately 95% of cases of PV and 50% of cases of ET and PMF) and is a phenotypic “driver” mutation, it is not considered the disease-initiating mutation.(16–18) Median survival in the three classic Ph-negative MPN ranges from being similar to that of the general population in ET(19) and 18.9 years in PV(20) to about 6.5 years in PMF.(21) Accordingly, the goal of therapy in ET and PV centers on prevention of thrombosis and bleeding, whereas in PMF and in post-ET/PV myelofibrosis, prolongation of survival takes precedence.(22) There is evidence of universal activation of JAK-STAT (Janus kinase - signal transducer and activator of transcription) signaling across the spectrum of MPN,(23, 24) and the JAK1/2 inhibitor ruxolitinib is the first agent to improve survival in myelofibrosis in randomized, controlled trials.(25–27) However, the survival benefit of ruxolitinib in myelofibrosis is modest, and has been attributed to the dramatic reduction in circulating pro-inflammatory cytokine levels with associated improvements in appetite, weight, hypocholesterolemia, performance status and cachexia.(28, 29) Thus, there still remains a major unmet need for additional disease-modifying agents in myelofibrosis. Many classes of agents have been investigated, especially in combination with ruxolitinib,(30) prominent among them being histone deacetylase inhibitors (HDACi).
2. Histone deacetylase inhibitors (HDACi)
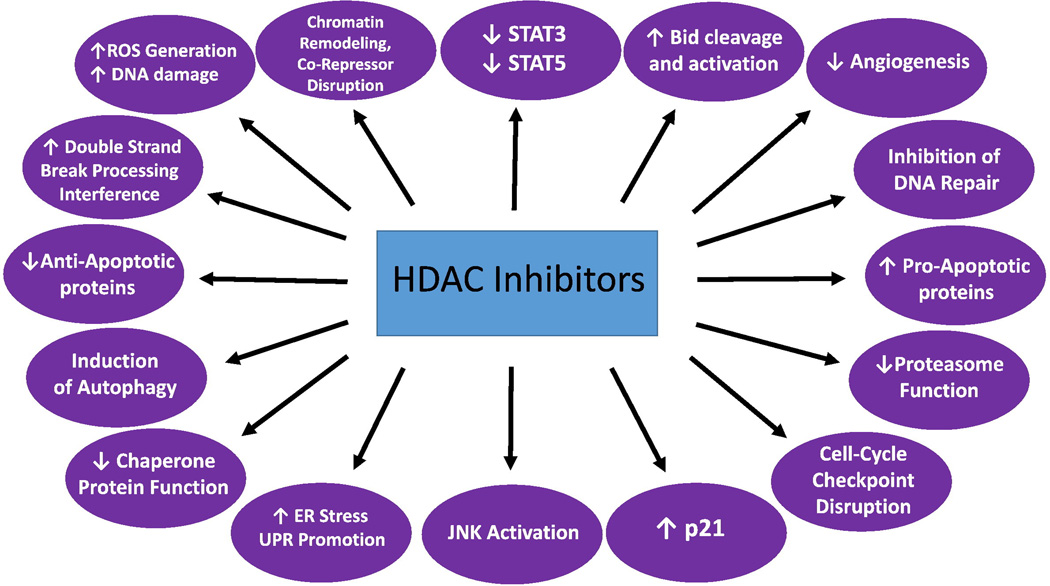
The acetylation status of histones regulates access of transcription factors to DNA and influences levels of gene expression.(31) In general, while increased histone acetylation is associated with open and active chromatin and increased transcription, deacetylated histones are associated with condensed chromatin and transcriptional repression.(32) Histone deacetylase (HDAC) activity diminishes acetylation of histones, causing compaction of the DNA/histone complex.(31) However, this is an over-simplified view, and acetylation status is often associated with the functionality of a genomic element, beyond simply determining open versus closed chromatin structure. For example, “bivalent” promoters, carrying both trimethylated H3K4 and H3K27, are not closed or condensed, but not acetylated, either.(33) The same applies to regions of DNA occupied by the zinc finger protein CTCF (reviewed in ref. (34)). Bivalent promoters are not active transcriptionally because of the need for recruitment of other factors for transcription, while CTCF-connected domains maintain the spatial organization of DNA, and are not transcriptionally active themselves.(33, 34) There are at least 18 human HDACs (Table 1), grouped by their homology to yeast proteins into four classes: classes I (HDACs 1, 2, 3 and 8), II (HDACs 4, 5, 6, 7, 9 and 10) and IV (HDAC 11) all contain a zinc ion in their active site and are inhibited by the “pan”-HDACi, while the class III HDACs (sirtuins 1–7) are nicotinamide adenine dinucleotide (NAD+)-dependent enzymes that are not inhibited by currently available HDACi.(31, 32) Besides histones, HDACs deacetylate a host of non-histone proteins of fundamental importance in cancer, such as the nuclear transcription factors p53, E2F1, GATA1, FoxO3A, c-Myc and nuclear factor kappa B (NF-κB), hypoxia-inducible factor 1α (HIF-1α), the estrogen and androgen receptor complexes, the DNA repair enzymes Ku70 and WRN, the chaperone protein heat shock protein 90 (HSP90), STAT3, β-catenin, α-tubulin and the nuclear import protein importin-α7; indeed, these enzymes may be better termed “protein deacetylases”, or simply “deacetylases”.(31, 32) Although the most accurate nomenclature is “lysine deacetylases” (KDACs, and “lysine acetyltransferases” (KATs) rather than histone acertyltransferases (HATs) for enzymes that catalyze the opposite reaction),(35) the original terminology has largely persisted in the literature, and these enzymes and their pharmacologic inhibitors continue to be referred to as HDACs and HDACi, respectively. HDACs also have a critical role in modulating the balance between pro- and anti-apoptotic proteins, and activate death receptor pathways.(31) Consequently, HDACi have wide-ranging effects, selectively in transformed cells, such as induction of cell cycle arrest (through induction of the endogenous cyclin-dependent kinase (CDK) inhibitor p21, among other mechanisms), differentiation, and apoptosis.(31, 32) HDAC inhibition may also affect tumor cell survival by blocking tumor angiogenesis, and by inhibiting intracellular stress response pathways.(31) More recent work has revealed many new insights into the mechanisms of action of HDACi (reviewed in ref. (36) and summarized in Figure 1). Importantly, HDACi disrupt cell cycle checkpoints, induce DNA damage and proteotoxic (endoplasmic reticulum) stress, and inhibit both homologous and non-homologous end-joining (NHEJ) DNA repair mechanisms.(36)
Table 1.
Human histone deacetylase: classification, localization, and inhibitors. Reproduced, with permission, from (120).
| Class | Enzymes | Localization | Expression | Drugs |
|---|---|---|---|---|
| I | HDAC 1, 2, 3, and 8 | Nucleus | Ubiquitous | Vorinostat, trichostatin A, belinostat, panobinostat, romidepsin, etinostat, and mocetinostat |
| IIa | HDAC 4, 5, 7, and 9 | Nucleus and cytoplasm |
Tissue-specific | Vorinostat, trichostatin A, belinostat, panobinostat, romidepsin, and ricolinostat (HDAC6 inhibitor) |
| IIb | HDAC 6 and 10 | Cytoplasm | ||
| III | Sirtuins 1–7 | Variable | Variable | Nicotinamide, dihydrocoumarin, and naphthopyranone |
| IV | HDAC 11 | Nucleus and cytoplasm |
Ubiquitous | Vorinostat, trichostatin A, belinostat, and panobinostat |
Abbreviations: HDAC, histone deacetylase.
Figure 1.
Mechanisms of HDACi lethality. Reproduced, with permission, from (36).
Despite their pleiotropic actions and relative selectivity for malignant cells, the clinical utility of HDACi has largely been restricted to T-cell lymphomas. Thus, the pan-HDACi, vorinostat (Zolinza®, Merck)(37) and belinostat (Beleodaq®, Spectrum),(38) and the class I HDACi romidepsin (Istodax®, Celgene)(39) are all approved for the treatment of cutaneous and/or peripheral T-cell lymphoma (CTCL/PTCL). Additionally, the pan-HDACi, panobinostat (Farydak®, Novartis), is approved in combination with bortezomib and dexamethasone for previously treated patients with multiple myeloma. HDACi have also been studied extensively in myelodysplastic sydromes (MDS) and acute myeloid leukemia (AML, but responses to monotherapy have been modest (10–20%),(40) and it does not appear that the combination of HDACi with either hypomethylating agents in MDS (41, 42) or intensive chemotherapy in AML adds therapeutic benefit. HDACi continue to be investigated in MPN in both preclinical and clinical settings. In the following sections, we summarize the current status of these agents in MPN and speculate about their future role in their therapeutic arena.
3. HDACi in MPN
Therapy in ET and PV is risk-adapted with the goal of minimizing thrombosis and bleeding risk. In both diseases, hydroxyurea is the preferred agent when cytoreductive therapy is indicated, and most patients should receive aspirin.(22) Additionally, all patients should have aggressive management of cardiovascular risk factors.(22) The hematocrit goal in PV is <45%,(43) and this is usually accomplished with the use of phlebotomy in low risk patients. Anagrelide is the preferred second line cytoreductive agent in ET.(22) Recently, ruxolitinib was approved for the treatment of patients with PV who have an inadequate response to or are unable to tolerate hydroxyurea.(44) Therapy of myelofibrosis (PMF or post-ET/PV MF) centers around ruxolitinib, which has salutary effects on constitutional symptoms and splenomegaly, as well as a proven survival benefit.(25–27) Finally, allogeneic hematopoietic stem cell transplantation should be offered to higher risk MF patients with a less than 5-year life expectancy and remains the only potentially curative option.(22, 45)
Preclinical data with HDACi in MPN (Table 2)
Table 2.
Mechanisms of action of histone deacetylase inhibitors in myeloproliferative neoplasms
| Preclinical observation |
Mechanistic explanation |
Related preclinical findings, if any |
Author and reference |
|---|---|---|---|
| Increased HDAC expression in MPN |
HDACi inhibit the function of HDACs |
Wang et al.(64) Skov et al.(65) |
|
| HDACi up-regulate SOCS1 and SOCS3 |
Class I HDACs, mainly HDAC8, down-regulate SOCS1/3, negative regulators of JAK- STAT signaling |
Chen et al.(67) Gao et al.(68) |
|
| HDACi down- regulate JAK2, a client of HSP90 |
HDAC6 inhibitors disrupt chaperone function of HSP90 through acetylation |
HDAC inhibitors and HSP90 inhibitors synergize with JAK2 inhibitors in MPN models |
Guerini et al.(69) Akada et al.(73) Wang et al.(78) Fiskus et al.(76) |
| HDACi down- regulate the transcription factor, NF-E2 |
NF-E2 is overexpressed in MPN patients and its overexpression confers a transplantable MPN phenotype in mice |
Amaru Calzada et al.(70) Kaufmann et al.(71) |
Abbreviations: HDAC, histone deacetylase; HDACi, histone deacetylase inhibitor; HSP90 heat shock protein 90; MPN, myeloproliferative neoplasm; SOCS, suppressor of cytokine signaling; JAK, Janus kinase; STAT, signal transducer and activator of transcription.
Apart from the canonical actions of JAKs in transducing cytokine signals from their cognate receptors to activate intracellular pathways,(46) JAK2 has a number of nuclear actions. Both wild type JAK2 and JAK2 V617F translocate to the nucleus and phosphorylate histone H3 (HH3) at Tyr41, thereby preventing the binding of heterochromatin protein 1 alfa (HP1α) to chromatin at this residue, leading to derepression of transcription.(47) JAK2 V617F also phosphorylates the arginine methyltransferase PRMT5, greatly impairing its ability to methylate its histone substrates and thus regulates chromatin modification by facilitating transcription, promoting myeloproliferation.(48) JAK2 additionally phosphorylates p27, an endogenous CDK inhibitor involved in cell cycle regulation, thus promoting its degradation in the cytoplasm.(49) Finally, JAK2 V617F up-regulates the CDC25A phosphatase, a key regulator of the G1/S cell cycle checkpoint, through a translational mechanism involving STAT5 and the translational initiation factor eIF2α, potentially invoking another explanation for JAK2 V617F-driven expansion of hematopoietic cells.(50) HDACi induce p21(51) and p27,(32) trigger DNA damage which transformed cells are unable to repair,(52, 53) down-regulate checkpoint kinase 1 (Chk1),(54) a key regulator of the G2/M cell cycle checkpoint, interfere with the intra-S-phase(55) and mitotic spindle checkpoints(56–58) and impair multiple processes of DNA repair.(59–61) Finally, HDAC activity is required for STAT5-induced activation of transcription,(62) and panobinostat has been shown to down-regulate phospho-STAT5 (p-STAT5) and phospho-STAT3 (p-STAT3) in CTCL models in vitro and in vivo.(63)
There is preclinical evidence for increased expression of HDACs in Ph-negative MPN, particularly PMF.(64, 65) The suppressor of cytokine signaling (SOCS) proteins are negative regulators of JAK-STAT signaling, competing with STATs for docking at the cytokine receptor, promoting ubiquitinylation and proteasomal degradation of JAK2, and interfering with its catalytic function.(66) Class I HDACs, primarily HDAC8, down-regulate SOCS1 and SOCS3, and HDACi inhibit JAK-STAT signaling by increasing the expression of SOCS1 and SOCS3 in primary MPN cells.(67, 68) The HDACi givinostat has been shown to inhibit proliferation of MPN cells bearing JAK2 V617F through a specific down-modulation of the JAK2 V617F protein and inhibition of its downstream signaling (i.e., phospho-JAK2 V617F, p-STAT5, p-STAT3).(69) Besides these inhibitory effects on JAK2-STAT5-extracellular signal regulated kinase 1/2 (ERK1/2) phosphorylation, givinostat also down-regulates the hematopoietic transcription factors NF-E2 and C-MYB.(70) NF-E2 is often overexpressed in MPN patients, and mice expressing an NF-E2 transgene in hematopoietic cells exhibit many features of MPN, a phenotype transplantable to secondary recipient mice.(71) Givinostat and hydroxyurea at low doses potentiate each other’s pro-apoptotic effects in JAK2 V617F+ cell lines and are additive in freshly isolated PV cells bearing JAK2 V617F.(72) Vorinostat has also been shown to be highly effective in preclinical studies in PV, both in vitro and in vivo.(73) Vorinostat markedly inhibited proliferation and induced apoptosis in cells expressing JAK2 V617F, accompanied by inhibition of phosphorylation of JAK2, STAT5, STAT3, AKT, and ERK1/2, and significantly inhibited JAK2 V617F-expressing mouse and human PV hematopoietic progenitor cells (HPCs).(73) Furthermore, vorinostat significantly down-regulated NF-E2 and up-regulated SOCS1 and SOCS3.(73) Finally, vorinostat normalized blood counts and markedly reduced splenomegaly in JAK2 V617F knock-in mice compared with placebo, and also decreased the mutant allele burden in these mice.(73) NF-E2 transgenic mice exhibit hypoacetylation of HH3; vorinostat treatment restores physiological levels of HH3 acetylation, reduces NF-E2 expression and normalizes platelet counts in these mice.(71)
JAK2 is a client of the chaperone protein, HSP90, and treatment of PV and ET cell lines and mouse models with HSP90 inhibitors leads to degradation of JAK2, inhibition of JAK-STAT signaling, normalization of peripheral blood counts and improved survival, without degradation of JAK2 in normal tissues or substantial toxicity.(74) Synergistic induction of apoptosis is observed in cultured and primary MPN cells with combined inhibition of JAK2 and HSP90, with marked reduction in phospho-JAK2, p-STAT5, phospho-AKT (p-AKT; Akt activation is an important downstream consequence of JAK2 V617F signaling in MPN)(75) and the anti-apoptotic protein, Bcl-xL, with concomitant induction of the pro-apoptotic protein, Bim.(76) Acetylation of HSP90 with resultant down-regulation of HSP90 client proteins is an important action of HDAC6 inhibitors.(77) Thus, panobinostat blocks the autophosphorylation and downstream signaling of JAK2 V617F, and disrupts the chaperone association of JAK2 V617F with HSP90, promoting proteasomal degradation of JAK2 V617F.(78) Panobinostat induces apoptosis of JAK2 V617F+ cells and synergizes with JAK2 inhibitors in doing so, with greater cytotoxicity against primary CD34+ MPN cells than normal CD34+ HPCs.(78) The combination of ruxolitinib and panobinostat had a more profound effect on splenomegaly, as well as on bone marrow and spleen histology, in mouse models of JAK2 V617F-driven MPN, than either agent alone.(79) These findings have fueled interest in clinical trials of ruxolitinib-based combinations with HDACi. However, the bone marrow microenvironment has been shown to protect MPN cells from the combination of ruxolitinib and vorinostat through the secretion of soluble factors from the stroma; this protective effect was completely abrogated by pharmacological inhibition of the c-Jun N-terminal kinase (JNK) and phosphatidylinositol-3-kinase (PI3K) pathways.(80) Sequential treatment of CD34+ cells from patients with PMF with DNA methyltransferase inhibitors (DNMTi) followed by HDACi has been shown to reduce the number of total cells, CD34+ cells and HPCs, particularly JAK2 V617F+ HPCs.(81) This effect is, in part, due to up-regulation of the chemokine receptor, CXCR4, which is suppressed by promoter methylation in PMF.(82, 83)
Clinical trials of HDACi in MPN (Tables 3 and 4)
Table 3.
Completed, published clinical trials of HDACi monotherapy in myeloproliferative neoplasms.
| HDACi studied |
Phase | Population studied |
Efficacy | Toxicity | Author and reference |
|---|---|---|---|---|---|
| Givinostat (starting dose 50 mg bid × 24 weeks) |
IIA | 12 PV, 1 ET, 16 MF, all JAK2 V617F+ |
PV/ET: 1 CR, 6 PR; MF: 3 major responses; spleen ↓ in 75% of PV/ET and 38% of MF; pruritus resolved in most patients |
Well tolerated; dose ↓ in 10; interrupted in 15; 2 stopped due to AEs |
Rambaldi et al.(84) |
| Givinostat (50 or 100 mg/d plus MTD of hydroxyurea for maximum 24 weeks) |
II | PV (N = 44); all unresponsive to MTD of hydroxyurea |
ORR 55% at 50 mg/d; 50% at 100 mg/d; 64% and 67% had control of pruritus |
Grade 3 AEs in only 1 patient (4.5%) in each arm |
Finazzi et al.(88) |
| Vorinostat (400 mg/d × 24 weeks) |
II | 44 PV, 19 ET | Pruritus 19% to 0%; splenomegaly 50% to 27%; ↓ in JAK2 V617F allele burden in 65% |
High rate (44%) of discontinuation due to toxicity |
Andersen et al.(90) |
| Panobinostat (20, 30 or 25 mg tiw in 4- week cycles) |
I | MF (N = 18); PMF 56%, post-PV MF 28%; post- ET MF 17% |
Only 5 evaluable: 3 CI-spleen, 2 SD. Anemia improved in 2; responses deepened with time |
DLT was reversible thrombocytopenia |
Mascarenhas et al.(91) |
| Panobinostat (40 mg tiw in 4-week cycles) |
II | MF (N = 35); PMF 68.6%, post-PV MF 17.1%; post- ET MF 14.3% |
1 patient achieved an IWG-MRT response (3%) |
Poorly tolerated; diarrhea 80%, thrombocytopenia 71.4%; high early rate of discontinuation |
DeAngelo et al.(92) |
| Pracinostat (60 mg qod × 3 weeks per month) |
II | MF (N = 22) | IWG-MRT CI-anemia in 9%; 36% had clinical benefit; ↓ spleen (27%) |
Fatigue (91%); grade 3/4 neutropenia in 13%, thrombocytopenia in 21% |
Quintas- Cardama et al.(93) |
Abbreviations: PV, polycythemia vera; ET, essential thrombocythemia; MF, myelofibrosis; bid, twice daily; tiw; three times a week; qod, every other day; MTD, maximum tolerated dose; DLT, dose-limiting toxicity; AE, adverse event; SD, stable disease; CR, complete response; PR, partial response; CI, clinical improvement; IWG-MRT, International Working Group for Myelofibrosis Research and Treatment; JAK2, Janus kinase; HDACi, histone deacetylase inhibitor; PMF, primary myelofibrosis.
Table 4.
Ongoing clinical trials of ruxolitinib-HDACi combinations in myelofibrosis.
| HDACi studied |
Phas e |
Clinicaltrials.gov identifier |
Populatio n studied |
Efficacy | Toxicity | Author and reference, if available |
|---|---|---|---|---|---|---|
| Panobinosta t (tiw qow or every week) |
I/II |
NCT01693601 (PRIME) |
PMF or post- PV/ET MF, int-2 or high risk, in CP or AP |
N/A | N/A | N/A |
| Panobinosta t |
I/II | NCT01433445 | PMF or post- PV/ET MF, int or high risk, palpable spleen ≥ 5 cm below LCM (N = 38 in escalation phase and 23 in expansion phase) |
In expansio n phase (n = 23), 57% and 39% had ≥ 35% SVR at week 24 and 48; 29% had ≥ 20% ↓ in JAK2 V617F allele burden |
RP2D: RUX 15 mg bid and PAN 25 mg tiw qow; 21% at RP2D discontinued due to AEs; grade (32%), 3/4 AEs: anemia (32%), thrombocytopeni a (29%), diarrhea (18%), asthenia (12%), fatigue (9%) |
Harrison et al.(94) |
| Pracinostat (started after RUX alone for first 3 months; starting dose 60 mg qod × 3 days every 3 weeks) |
II | NCT02267278 | PMF or post- PV/ET MF, int or high risk, palpable spleen ≥ 5 cm below LCM |
N/A | N/A | N/A |
Abbreviations: HDACi, histone deacetylase inhibitor; RUX, ruxolitinib; PMF, primary myelofibrosis; post-PV/ET MF, post-polycythemia vera/essential thrombocythemia myelofibrosis; LCM, left costal margin; N/A, not applicable; CP, chronic phase; AP, accelerated phase; tiw, three times a week; qow, every other week; qod, every other day; int, intermediate; PAN, panobinostat; RP2D, recommended phase 2 dose; bid, twice daily; SVR, spleen volume reduction; JAK2, Janus kinase 2.
Givinostat (50 mg twice daily for 24 weeks) was evaluated in a pilot study in JAK2 V617F+ patients with PV (n = 12), ET (n = 1) or MF (n = 16).(84) Patients with a baseline platelet count < 100 × 109/L were excluded. The median treatment duration was 20 weeks and reasons for treatment discontinuation included disease progression in six patients, grade 2 thrombocytopenia in one, unrelated psychiatric symptoms in one and withdrawal of consent in two. One MF patient had a prolonged QTc at baseline and was therefore ineligible; this patient experienced a further slight QTc prolongation on study treatment, which was discontinued after day 9. Eleven PV/ET patients and seven MF patients completed 24 weeks of treatment. Dose reduction was necessary in 10 patients, and temporary withholding of the drug in 15. Among the 13 PV/ET patients, 7 (54%) had a clinical response by the 2009 European LeukemiaNet (ELN) criteria,(85) including 1 complete response (CR), and six of eight had a spleen response at the end of the study. Among the 16 patients with myelofibrosis (6 PMF + 5 post-ET MF + 5 post-PV MF), 3 (19%) achieved a major response according to EUMNET criteria,(86) but by the 2006 IWG-MRT criteria,(87) 2 patients had clinical improvement (CI) and 5 stable disease (SD). 38% of MF patients had a spleen response. The mean JAK2 V617F allele burden decreased from 55% at study entry to 47% and 41% after 12 and 24 weeks, respectively, of givinostat treatment in PV/ET patients, but there was no change among MF patients. Most toxicities were grade 1 or 2, and no grade 4 toxicity occurred. The most frequent drug-related adverse events (AEs) were diarrhea, nausea, abdominal pain, thrombocytopenia, fatigue, QTc prolongation, weight loss, anorexia and rash. Pruritus disappeared in most patients.(84)
Givinostat (50 or 100 mg daily) was also tested in combination with hydroxyurea in 44 patients with PV who were unresponsive to maximum tolerated doses (MTD) of hydroxyurea in a phase II study.(88) Patients had to have JAK2 V617F+ disease, and be unresponsive to hydroxyurea at the MTD for at least 3 months, as defined by the ELN.(89) Thrombocytopenia < 100 × 109/L was an exclusion criterion. Treatment lasted up to 24 weeks, and assessment of response occurred at 12 and 24 weeks. Responses, by ELN 2009 criteria,(85) were achieved in 55% and 50% of patients receiving 50 or 100 mg of givinostat, respectively. Pruritus was controlled in 64% and 67% of patients in the 50 and 100 mg groups, respectively. The regimen was well tolerated: eight patients (18%) discontinued, four in each treatment arm; grade 3 AEs were reported in one patient (4.5%) in each treatment arm. The most frequent drug-related grade 2 AEs were thrombocytopenia and diarrhea. The only grade 3 toxicities were nausea and anemia in the 50 mg and 100 mg groups, respectively. No grade 4 toxicity was reported.(88)
A phase II study of vorinostat (400 mg once daily) enrolled 19 patients with ET and 44 with PV.(90) There was a high discontinuation rate (52%), mainly because of non-hematologic AEs, such as fatigue, renal impairment, diarrhea, hair loss, weight loss, nausea, unspecified pain, headache and leg ulcers. 48% of patients completed 24 weeks of therapy. Most AEs reported by patients who completed the study were fatigue and gastrointestinal. The intention-to-treat response rate (RR) was 35%, and responses were independent of JAK2 mutational status among ET patients (only one PV patient was JAK2 V617F-negative). Pruritus, present in 19% of patients at study initiation, resolved in all patients, while the prevalence of splenomegaly decreased from 50% to 27% (p = 0.03). 65% of 43 evaluable patients had a decrease in the JAK2 V617F allele burden (p = 0.006), but the median decrease among molecularly responding patients was only 5.6%, and no patient had a complete molecular response.(90)
Mascarenhas et al. studied panobinostat in a phase I trial in 18 patients (10 PMF + 5 post-PV MF + 3 post-ET MF) with myelofibrosis.(91) 12 (67%) patients were positive for JAK2 V617F. Panobinostat was administered three times weekly; three successive cohorts of six patients each received 20 mg, 30 mg and 25 mg, respectively. Reversible thrombocytopenia was the dose limiting toxicity (DLT) in both the 20 mg and 30 mg dose cohorts. No patient developed clinically significant bleeding attributable to the study drug. Grade 3/4 neutropenia occurred in 17%, but there were no cases of febrile neutropenia. There were no grade 3/4 non-hematologic AEs. Diarrhea (89%), musculoskeletal AEs (33%) and fatigue (33%) were common. Only five patients received ≥6 cycles and were evaluable for response (using the 2006 IWG-MRT criteria)(87); 3 of these patients experienced CI with 100% reduction in palpable splenomegaly, and 2 SD. Two of these five patients also experienced improvement in their anemia on panobinostat. One patient had a sustained, dramatic reversal of bone marrow histopathologic abnormalities after cycles 16 and 30, and another had a significant reduction in marrow fibrosis (from grade 4 to grade 1) after 24 cycles of therapy. Most patients treated for longer than a month had symptomatic benefit, and significant reduction in splenomegaly was observed at the end of six cycles. The authors concluded that a minimum of 12 cycles of treatment with single agent panobinostat was required in order to achieve improvement in blood counts and possibly longer to achieve reversal of bone marrow histopathological abnormalities.(91)
Panobinostat was also investigated in a phase II trial in myelofibrosis (n = 35, 24 PMF + 5 post-ET MF + 6 post-PV MF) at a dose of 40 mg three times a week.(92) About two-thirds (65.7%) of patients were JAK2 V617F+. 33 patients discontinued study treatment, 19 before completing cycle 3, mostly because of AEs. The median number of days on study was only 57. Twenty patients had a dose modification, and of the remaining 15, 12 had a treatment duration of < 33 days. Thrombocytopenia occurred in 71.4% of patients, and was grade 3/4 in 60%. For neutropenia, the corresponding percentages were 20% and 14.3%. Gastrointestinal AEs were extremely frequent (diarrhea, 80%; nausea, 57.1%; anorexia, 54.3%; vomiting 40%) but mostly grades 1 and 2 in severity. Fatigue occurred in 57.1% of patients, and was grade 3/4 in 31.4%. Only one patient met the 2006 IWG-MRT criteria(87) for CI. Correlative studies demonstrated increased acetylation of intracellular targets, e.g., tubacin, HH3 and HSP90, inhibition of JAK-STAT downstream signaling (p-STAT5, p-STAT3, p-AKT, phospho-mitogen activated protein kinase 3/1 (p-MAPK3/1)), down-regulation of STAT5, STAT3 and AKT, anti-apoptotic PIM1 and Mcl-1 and the pro-inflammatory cytokines interleukin-6 (IL-6), tumor necrosis factor α (TNFα)and MIP1β, induction of Bim and a reduction in the median JAK2 V617F allele burden from 36.82% pre-treatment to 14.88% before cycle 2 to 0.86% before cycle 4.(92)
Our group has evaluated the pan-HDACi, pracinostat, at a dose of 60 mg three times weekly for three out of every four weeks in a phase II study in 22 patients with myelofibrosis.(93) 82% of patients were positive for JAK2 V617F, and the majority of patients were symptomatic at study entry and had significant palpable splenomegaly. 27% of patients experienced a reduction in splenomegaly, but none of these met the 2006 IWG-MRT criteria(87) for CI. Two patients had CI in terms of anemia. 17% of JAK2 V617F+ patients had a reduction in allele burden (median 11.4%), although this was not significant when the entire cohort of JAK2 V617F+ patients was analyzed as a whole. The major toxicity of pracinostat in this trial was fatigue, occurring in 91% of patients. 13% and 21% of patients, respectively, had grade 3/4 neutropenia and thrombocytopenia. At the time of publication, all but one patient had discontinued pracinostat, mostly due to lack of response or disease progression.(93)
In light of these observations, and the established role of ruxolitinib in myelofibrosis, efforts are ongoing to combine HDACi such as pracinostat (NCT02267278) or panobinostat (NCT01693601, NCT01433445) with ruxolitinib. Preliminary results from a phase Ib trial of panobinostat and ruxolitinib in patients with MF have been published in abstract form.(94) Patients (n = 61, 38 escalation phase + 23 expansion phase) were required to have palpable splenomegaly ≥5 cm below the left costal margin and intermediate-1, -2 or high risk disease by the International Prognostic Scoring System,(95) and received ruxolitinib 5–15 mg twice daily and panobinostat 10–25 mg three times a week, every other week. Grade 4 thrombocytopenia and grade 3 nausea were identified as DLTs, and the higher doses of both drugs were confirmed to be the recommended phase 2 doses (RP2D). Among 34 patients treated at the RP2D, 21% discontinued due to AEs. The most common grade 3/4 hematologic AEs in these patients, regardless of attribution, were anemia (32%) and thrombocytopenia (29%), while grade 3/4 non-hematologic AEs included diarrhea (18%), asthenia (12%) and fatigue (9%). 57% and 39% of patients achieved a ≥35% reduction in spleen volume at weeks 24 and 48, respectively. 4 of 12 evaluable patients had improved bone marrow fibrosis at week 48, and 5 of 17 (29%) JAK2 V617F+ patients in the expansion phase of the trial had a ≥20% decrease in allele burden by week 48.(94)
4. Conclusion
HDACi are active in MPN, both preclinically and in the clinic. However, these agents have chronic, predominantly low grade, toxicities that make their long-term tolerability a concern, especially since these drugs need to be administered for long periods of time in order for disease-modifying effects to emerge, at least in MF, where the need to alter the underlying disease biology is greatest. Current development strategies are focusing on combinations with ruxolitinib, employing low doses of HDACi.
5. Expert Opinion
As discussed in this review, there is strong preclinical rationale and modest evidence of clinical efficacy to support the continued development of HDACi in MPN. However, considering that the MPN are chronic disorders with relatively long survival, the long-term tolerability of these drugs becomes an important concern. This is particularly relevant because the disease-modifying effects of these drugs in MF appear to take time to emerge.(91)
In PV, a disease with a long natural history, while HDACi are clearly active, their continued development is uncertain and appears unlikely. The recent approval of ruxolitinib for the treatment of hydroxyurea-resistant or -intolerant patients with PV represents an important advance in the management of this disease.(44) While HDACi appear to control troublesome symptoms of PV such as pruritus,(84, 88, 90) so does ruxolitinib.(44) Longer term follow-up of the RESPONSE trial has shown a steady decrease in the JAK2 V617F allele burden among patients originally randomized to ruxolitinib (22% mean reduction at weak 80, 40% by week 208).(96, 97) While the significance of JAK2 V617F allele burden reduction in MPN remains unknown, these findings underscore the fact that this action is not unique to HDACI. Finally, given the success of pegylated interferon alfa-2a in PV,(98, 99) a longer acting interferon, ropeginterferon alfa-2b, which allows administration every 2 weeks, has been developed.(100) In a phase 1/2 study, it produced a cumulative overall response rate of 90% (47% CR plus 43% partial response, PR). A complete molecular response was achieved in 21% and a partial molecular response in 47%. There were no DLTs.(100) Given the above, it is difficult to envision a role for long-term therapy with HDACI with their attendant toxicities (fatigue, gastrointestinal, thrombocytopenia, neutropenia) in PV.
Thrombocytopenia is an on-target effect of HDACI due to hyperacetylation of tubulin, an important constituent of the platelet microtubule cytoskeleton, leading to decreased megakaryocyte maturation and proplatelet formation, ultimately impeding the release of platelets from megakaryocytes.(101, 102) This has led some to propose development of HDACI as a treatment for ET, which is characterized by thrombocytosis.(103) However, the there is no evidence that the platelet count per se affects the risk of thrombosis or hemorrhage in ET (Falchi L, Bose P, Newberry KJ, Verstovsek S, submitted), which raises the question whether simply lowering the platelet count is a clinically relevant therapeutic goal in ET, the most indolent among the classic MPN. Risk factors for inferior survival in ET include age ≥60, leukocytosis ≥11 × 109/L and prior thrombosis.(104) Risk factors for thrombosis in ET include age over 60, history of thrombosis, presence of cardiovascular risk factors and the JAK2 V617F mutation.(105, 106) Patients with ET can have significant symptoms,(107) and ruxolitinib is being developed for this indication.
The need for disease-modifying agents is greatest in MF, where ruxolitinib currently is the cornerstone of treatment. A number of JAK inhibitors have been investigated in the clinic, but many of them have been discontinued due to toxicity.(108) Of the ones still in development, pacritinib,(109) which appears to be nonmyelosuppressive, and momelotinib,(110) which may actually improve the anemia of MF, are in the most advanced stages of clinical testing; however, the “full clinical hold” recently placed on the development of pacritinib by the US Food and Drug Administration is of concern. This underscores the importance of developing rational ruxolitinib-based combinations(30). Many classes of agents are being tested, either alone or in combination with ruxolitinib: these include HDACi, DNMTi, HSP90 inhibitors, PI3K/Akt/mTOR pathway inhibitors, hedgehog inhibitors, anti-fibrotic agents and telomerase inhibitors (reviewed in ref. (111)). Currently available JAK inhibitors are “type I” inhibitors that bind to and stabilize the active conformation of JAK2.(112) An interesting mechanism of reactivation of JAK-STAT signaling despite the presence of a type I inhibitor is “JAK2 inhibitor persistence”, characterized by heterodimerization between activated JAK2 and JAK1 or TYK2.(113) This may be circumvented by “type II” JAK2 inhibitors, which stabilize the inactive state of the kinase.(112, 114) The anti-fibrotic agent PRM-151 (recombinant human pentraxin-2) has shown encouraging results in MF as a single agent and trials are ongoing.(115) The telomerase inhibitor imetelstat produced responses in 21% of patients in a pilot study in patients with higher risk MF, including reversal of bone marrow fibrosis in those patients who obtained a CR, albeit at the expense of significant myelosuppression, and responses did not correlate with baseline telomere length.(116) Ruxolitinib-based combinations with PI3K inhibitors(117) and hedgehog inhibitors(118) have so far been disappointing. The combination of ruxolitinib with DNMTis may be most efficacious in patients with accelerated or blast phase MPN and those with myelodysplastic syndrome/MPN “overlap” syndromes.(119) As discussed above, the combination of ruxolitinib and panobinostat in MF is promising thus far,(94) and appears to lead to faster spleen responses and JAK2 V617F allele burden reductions than ruxolitinib alone,(25, 26) although the clinical relevance of the latter phenomenon remains unproven. Evaluation of pracinostat in combination with ruxolitinib is ongoing (NCT02267278). The key to the future role, if any, of HDACi in MF will be their long-term tolerability and disease-modifying benefits over those of ruxolitinib alone,(25, 26) which may be best assessed by regression/reversal of bone marrow fibrosis and, of course, improved overall survival.
Highlights box.
HDACi are active, both preclinically and clinically, in MPN.
HDAC6 inhibitors down-regulate JAK2 and JAK2 V617F by disrupting the chaperone function of HSP90 and synergize with JAK2 inhibitors.
HDACi have significant chronic toxicities, e.g., diarrhea, fatigue, thrombocytopenia.
Long-term therapy is required for disease-modifying effects of HDACi in MF to emerge.
Ongoing trials are investigating combinations of HDACi with ruxolitinib in myelofibrosis.
Acknowledgments
Funding
This paper has been supported in part by the MD Anderson Cancer Center Support Grant from the National Cancer Institute (P30CA016672).
P Bose has participated on the advisory board for Incyte Corporation. S Verstovsek has received research support from the National Cancer Institute.
Footnotes
Disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Bibliography
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Dameshek W. Some speculations on the myeloproliferative syndromes. Blood. 1951 Apr;6(4):372–375. [PubMed] [Google Scholar]
- 2. Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, et al. The 2016 revision to the world health organization classification of myeloid neoplasms and acute leukemia. Blood. 2016 May 19;127(20):2391–2405. doi: 10.1182/blood-2016-03-643544. •Key paper summarizing changes to the WHO classification of myeloid malignancies in the 2016 revision.
- 3. Rowley JD. Letter: A new consistent chromosomal abnormality in chronic myelogenous leukaemia identified by quinacrine fluorescence and giemsa staining. Nature. 1973 Jun 1;243(5405):290–293. doi: 10.1038/243290a0. ••Discovery of the Philadelphia chromosome, hallmark of CML.
- 4. Ben-Neriah Y, Daley GQ, Mes-Masson AM, Witte ON, Baltimore D. The chronic myelogenous leukemia-specific P210 protein is the product of the bcr/abl hybrid gene. Science. 1986 Jul 11;233(4760):212–214. doi: 10.1126/science.3460176. ••Demonstration that the protein product of the Philadelphia chromosome, BCR-ABL, causes CML.
- 5.Baxter EJ, Scott LM, Campbell PJ, East C, Fourouclas N, Swanton S, et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005 Mar 19–25;365(9464):1054–1061. doi: 10.1016/S0140-6736(05)71142-9. [DOI] [PubMed] [Google Scholar]
- 6.James C, Ugo V, Le Couedic JP, Staerk J, Delhommeau F, Lacout C, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2005 Apr 28;434(7037):1144–1148. doi: 10.1038/nature03546. [DOI] [PubMed] [Google Scholar]
- 7.Kralovics R, Passamonti F, Buser AS, Teo SS, Tiedt R, Passweg JR, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med. 2005 Apr 28;352(17):1779–1790. doi: 10.1056/NEJMoa051113. [DOI] [PubMed] [Google Scholar]
- 8. Levine RL, Wadleigh M, Cools J, Ebert BL, Wernig G, Huntly BJ, et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell. 2005 Apr;7(4):387–397. doi: 10.1016/j.ccr.2005.03.023. ••References 5–8, from different groups, represent the discovery of JAK2 V617F as a common activating mutation in MPN.
- 9. Scott LM, Tong W, Levine RL, Scott MA, Beer PA, Stratton MR, et al. JAK2 exon 12 mutations in polycythemia vera and idiopathic erythrocytosis. N Engl J Med. 2007 Feb 1;356(5):459–468. doi: 10.1056/NEJMoa065202. •Discovery of JAK2 exon 12 mutations in PV.
- 10. Pikman Y, Lee BH, Mercher T, McDowell E, Ebert BL, Gozo M, et al. MPLW515L is a novel somatic activating mutation in myelofibrosis with myeloid metaplasia. PLoS Med. 2006 Jul;3(7):e270. doi: 10.1371/journal.pmed.0030270. •Discovery of MPL mutations in MF.
- 11.Pardanani AD, Levine RL, Lasho T, Pikman Y, Mesa RA, Wadleigh M, et al. MPL515 mutations in myeloproliferative and other myeloid disorders: A study of 1182 patients. Blood. 2006 Nov 15;108(10):3472–3476. doi: 10.1182/blood-2006-04-018879. [DOI] [PubMed] [Google Scholar]
- 12. Klampfl T, Gisslinger H, Harutyunyan AS, Nivarthi H, Rumi E, Milosevic JD, et al. Somatic mutations of calreticulin in myeloproliferative neoplasms. N Engl J Med. 2013 Dec 19;369(25):2379–2390. doi: 10.1056/NEJMoa1311347. ••Discovery of CALR mutations in the majority of JAK2- and MPL-wild type patients with ET or MF.
- 13. Nangalia J, Massie CE, Baxter EJ, Nice FL, Gundem G, Wedge DC, et al. Somatic CALR mutations in myeloproliferative neoplasms with nonmutated JAK2. N Engl J Med. 2013 Dec 19;369(25):2391–2405. doi: 10.1056/NEJMoa1312542. ••Discovery of CALR mutations in the majority of JAK2- and MPL-wild type patients with ET or MF.
- 14. Maxson JE, Gotlib J, Pollyea DA, Fleischman AG, Agarwal A, Eide CA, et al. Oncogenic CSF3R mutations in chronic neutrophilic leukemia and atypical CML. N Engl J Med. 2013 May 9;368(19):1781–1790. doi: 10.1056/NEJMoa1214514. •Discovery of CSF3R mutations in the majority of patients with atypical CML and CNL.
- 15. Furitsu T, Tsujimura T, Tono T, Ikeda H, Kitayama H, Koshimizu U, et al. Identification of mutations in the coding sequence of the proto-oncogene c-kit in a human mast cell leukemia cell line causing ligand-independent activation of c-kit product. J Clin Invest. 1993 Oct;92(4):1736–1744. doi: 10.1172/JCI116761. •Discovery of KIT mutations in SM.
- 16. Kralovics R, Teo SS, Li S, Theocharides A, Buser AS, Tichelli A, et al. Acquisition of the V617F mutation of JAK2 is a late genetic event in a subset of patients with myeloproliferative disorders. Blood. 2006 Aug 15;108(4):1377–1380. doi: 10.1182/blood-2005-11-009605. •Demonstration that JAK2 V617F is not necessarily the disease-initiating mutation in MPN.
- 17. Li J, Kent DG, Godfrey AL, Manning H, Nangalia J, Aziz A, et al. JAK2V617F homozygosity drives a phenotypic switch in myeloproliferative neoplasms, but is insufficient to sustain disease. Blood. 2014 May 15;123(20):3139–3151. doi: 10.1182/blood-2013-06-510222. •Demonstration that JAK2 V617F is a phenotypic driver mutation in MPN, but not the causal genetic event.
- 18.Vainchenker W, Delhommeau F, Constantinescu SN, Bernard OA. New mutations and pathogenesis of myeloproliferative neoplasms. Blood. 2011 Aug 18;118(7):1723–1735. doi: 10.1182/blood-2011-02-292102. [DOI] [PubMed] [Google Scholar]
- 19.Passamonti F, Rumi E, Pungolino E, Malabarba L, Bertazzoni P, Valentini M, et al. Life expectancy and prognostic factors for survival in patients with polycythemia vera and essential thrombocythemia. Am J Med. 2004 Nov 15;117(10):755–761. doi: 10.1016/j.amjmed.2004.06.032. [DOI] [PubMed] [Google Scholar]
- 20.Tefferi A, Rumi E, Finazzi G, Gisslinger H, Vannucchi AM, Rodeghiero F, et al. Survival and prognosis among 1545 patients with contemporary polycythemia vera: An international study. Leukemia. 2013 Sep;27(9):1874–1881. doi: 10.1038/leu.2013.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cervantes F, Dupriez B, Passamonti F, Vannucchi AM, Morra E, Reilly JT, et al. Improving survival trends in primary myelofibrosis: An international study. J Clin Oncol. 2012 Aug 20;30(24):2981–2987. doi: 10.1200/JCO.2012.42.0240. [DOI] [PubMed] [Google Scholar]
- 22. Barbui T, Barosi G, Birgegard G, Cervantes F, Finazzi G, Griesshammer M, et al. Philadelphia-negative classical myeloproliferative neoplasms: Critical concepts and management recommendations from european LeukemiaNet. J Clin Oncol. 2011 Feb 20;29(6):761–770. doi: 10.1200/JCO.2010.31.8436. •ELN guidelines on management of MPN.
- 23. Anand S, Stedham F, Gudgin E, Campbell P, Beer P, Green AR, et al. Increased basal intracellular signaling patterns do not correlate with JAK2 genotype in human myeloproliferative neoplasms. Blood. 2011 Aug 11;118(6):1610–1621. doi: 10.1182/blood-2011-02-335042. •Demonstration that JAK-STAT activation in MPN does not correlate with mutational status of JAK2.
- 24. Rampal R, Al-Shahrour F, Abdel-Wahab O, Patel JP, Brunel JP, Mermel CH, et al. Integrated genomic analysis illustrates the central role of JAK-STAT pathway activation in myeloproliferative neoplasm pathogenesis. Blood. 2014 May 29;123(22):e123–e133. doi: 10.1182/blood-2014-02-554634. •Demonstration that JAK-STAT signaling is universal across MPNs and key to MPN pathogenesis.
- 25. Verstovsek S, Mesa RA, Gotlib JR, Gupta V, DiPersio JF, Catalano JV, et al. LONG-TERM OUTCOMES OF RUXOLITINIB (RUX) THERAPY IN PATIENTS (PTS) WITH MYELOFIBROSIS (MF): 5-YEAR FINAL EFFICACY AND SAFETY ANALYSIS FROM COMFORT-I. European haematology association 21st congress; June 9–12; Copenhagen, Denmark. 2016. p. S452. •Final results of the COMFORT-I trial, one of the pivotal studies leading to the regulatory approval of ruxolitinib.
- 26. Harrison CN, Vannucchi AM, Kiladjian J, Al-Ali HK, Gisslinger H, Knoops L, et al. Long-term efficacy and safety in COMFORT-II, a phase 3 study comparing ruxolitinib with best available therapy for the treatment of myelofibrosis: 5-year final study results. Blood. 2015;126(23):59. •Final results of the COMFORT-II trial, one of the pivotal studies leading to the regulatory approval of ruxolitinib.
- 27.Vannucchi AM, Kantarjian HM, Kiladjian JJ, Gotlib J, Cervantes F, Mesa RA, et al. A pooled analysis of overall survival in COMFORT-I and COMFORT-II, 2 randomized phase 3 trials of ruxolitinib for the treatment of myelofibrosis. Haematologica. 2015 Jun 11; doi: 10.3324/haematol.2014.119545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mascarenhas J, Hoffman R. A comprehensive review and analysis of the effect of ruxolitinib therapy on the survival of patients with myelofibrosis. Blood. 2013 Jun 13;121(24):4832–4837. doi: 10.1182/blood-2013-02-482232. [DOI] [PubMed] [Google Scholar]
- 29.Savona MR. Are we altering the natural history of primary myelofibrosis? Leuk Res. 2014 Sep;38(9):1004–1012. doi: 10.1016/j.leukres.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 30.Stein BL, Swords R, Hochhaus A, Giles F. Novel myelofibrosis treatment strategies: Potential partners for combination therapies. Leukemia. 2014 Nov;28(11):2139–2147. doi: 10.1038/leu.2014.176. [DOI] [PubMed] [Google Scholar]
- 31.Lane AA, Chabner BA. Histone deacetylase inhibitors in cancer therapy. Journal of Clinical Oncology. 2009 Nov 10;27(32):5459–5468. doi: 10.1200/JCO.2009.22.1291. [DOI] [PubMed] [Google Scholar]
- 32.Bhalla KN. Epigenetic and chromatin modifiers as targeted therapy of hematologic malignancies. [June 10, 2005];Journal of Clinical Oncology. 2005 Jun 10;23(17):3971–3993. doi: 10.1200/JCO.2005.16.600. [DOI] [PubMed] [Google Scholar]
- 33.Mikkelsen TS, Ku M, Jaffe DB, Issac B, Lieberman E, Giannoukos G, et al. Genome-wide maps of chromatin state in pluripotent and lineage-committed cells. Nature. 2007 Aug 2;448(7153):553–560. doi: 10.1038/nature06008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ghirlando R, Felsenfeld G. CTCF: Making the right connections. Genes Dev. 2016 Apr 15;30(8):881–891. doi: 10.1101/gad.277863.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang XJ, Seto E. The Rpd3/Hda1 family of lysine deacetylases: From bacteria and yeast to mice and men. Nat Rev Mol Cell Biol. 2008 Mar;9(3):206–218. doi: 10.1038/nrm2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bose P, Dai Y, Grant S. Histone deacetylase inhibitor (HDACI) mechanisms of action: Emerging insights. Pharmacol Ther. 2014 Apr 24; doi: 10.1016/j.pharmthera.2014.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Olsen EA, Kim YH, Kuzel TM, Pacheco TR, Foss FM, Parker S, et al. Phase IIb multicenter trial of vorinostat in patients with persistent, progressive, or treatment refractory cutaneous T-cell lymphoma. J Clin Oncol. 2007 Jul 20;25(21):3109–3115. doi: 10.1200/JCO.2006.10.2434. •Pivotal trial of vorinostat in CTCL.
- 38. O'Connor OA, Horwitz S, Masszi T, Van Hoof A, Brown P, Doorduijn J, et al. Belinostat in patients with relapsed or refractory peripheral T-cell lymphoma: Results of the pivotal phase II BELIEF (CLN-19) study. J Clin Oncol. 2015 Aug 10;33(23):2492–2499. doi: 10.1200/JCO.2014.59.2782. •Pivotal trial of belinostat in PTCL.
- 39. Coiffier B, Pro B, Prince HM, Foss F, Sokol L, Greenwood M, et al. Results from a pivotal, open-label, phase II study of romidepsin in relapsed or refractory peripheral T-cell lymphoma after prior systemic therapy. J Clin Oncol. 2012 Feb 20;30(6):631–636. doi: 10.1200/JCO.2011.37.4223. •Pivotal trial of romidepsin in PTCL.
- 40.Quintas-Cardama A, Santos FP, Garcia-Manero G. Histone deacetylase inhibitors for the treatment of myelodysplastic syndrome and acute myeloid leukemia. Leukemia. 2011 Feb;25(2):226–235. doi: 10.1038/leu.2010.276. [DOI] [PubMed] [Google Scholar]
- 41.Prebet T, Sun Z, Figueroa ME, Ketterling R, Melnick A, Greenberg PL, et al. Prolonged administration of azacitidine with or without entinostat for myelodysplastic syndrome and acute myeloid leukemia with myelodysplasia-related changes: Results of the US leukemia intergroup trial E1905. J Clin Oncol. 2014 Apr 20;32(12):1242–1248. doi: 10.1200/JCO.2013.50.3102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sekeres MA, Othus M, List AF, Odenike O, Stone RM, Gore SD, et al. Additional analyses of a randomized phase II study of azacitidine combined with lenalidomide or with vorinostat vs. azacitidine monotherapy in higher-risk myelodysplastic syndromes (MDS) and chronic myelomonocytic leukemia (CMML): North american intergroup study SWOG S1117. Blood. 2015;126(23):908-. doi: 10.1200/JCO.2015.66.2510. •Large cooperative group study showing lack of benefit of the addition of lenalidomide or vorinostat to azacitidine in higher risk MDS and CMML.
- 43. Marchioli R, Finazzi G, Specchia G, Cacciola R, Cavazzina R, Cilloni D, et al. Cardiovascular events and intensity of treatment in polycythemia vera. N Engl J Med. 2013 Jan 3;368(1):22–33. doi: 10.1056/NEJMoa1208500. ••Study that established 45% as the Hct goal in PV.
- 44. Vannucchi AM, Kiladjian JJ, Griesshammer M, Masszi T, Durrant S, Passamonti F, et al. Ruxolitinib versus standard therapy for the treatment of polycythemia vera. N Engl J Med. 2015 Jan 29;372(5):426–435. doi: 10.1056/NEJMoa1409002. ••Pivotal trial that led to approval of ruxolitinib for hydroxyurea-resistant/intolerant PV.
- 45.Kroger NM, Deeg JH, Olavarria E, Niederwieser D, Bacigalupo A, Barbui T, et al. Indication and management of allogeneic stem cell transplantation in primary myelofibrosis: A consensus process by an EBMT/ELN international working group. Leukemia. 2015 Aug 21; doi: 10.1038/leu.2015.233. [DOI] [PubMed] [Google Scholar]
- 46. Parganas E, Wang D, Stravopodis D, Topham DJ, Marine JC, Teglund S, et al. Jak2 is essential for signaling through a variety of cytokine receptors. Cell. 1998 May 1;93(3):385–395. doi: 10.1016/s0092-8674(00)81167-8. ••Seminal paper showing fundamental role of JAK2 in cytokine signaling.
- 47. Dawson MA, Bannister AJ, Gottgens B, Foster SD, Bartke T, Green AR, et al. JAK2 phosphorylates histone H3Y41 and excludes HP1alpha from chromatin. Nature. 2009 Oct 8;461(7265):819–822. doi: 10.1038/nature08448. ••Seminal paper showing nuclear, non-canonical action of JAK2 in regulating gene expression.
- 48. Liu F, Zhao X, Perna F, Wang L, Koppikar P, Abdel-Wahab O, et al. JAK2V617F-mediated phosphorylation of PRMT5 downregulates its methyltransferase activity and promotes myeloproliferation. Cancer Cell. 2011 Feb 15;19(2):283–294. doi: 10.1016/j.ccr.2010.12.020. ••Seminal paper showing nuclear, non-canonical action of JAK2 in regulating gene expression.
- 49.Meyer SC, Levine RL. Molecular pathways: Molecular basis for sensitivity and resistance to JAK kinase inhibitors. Clin Cancer Res. 2014 Apr 15;20(8):2051–2059. doi: 10.1158/1078-0432.CCR-13-0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gautier EF, Picard M, Laurent C, Marty C, Villeval JL, Demur C, et al. The cell cycle regulator CDC25A is a target for JAK2V617F oncogene. Blood. 2012 Feb 2;119(5):1190–1199. doi: 10.1182/blood-2011-01-327742. [DOI] [PubMed] [Google Scholar]
- 51.Richon VM, Sandhoff TW, Rifkind RA, Marks PA. Histone deacetylase inhibitor selectively induces p21WAF1 expression and gene-associated histone acetylation. Proc Natl Acad Sci U S A. 2000 Aug 29;97(18):10014–10019. doi: 10.1073/pnas.180316197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ungerstedt JS, Sowa Y, Xu WS, Shao Y, Dokmanovic M, Perez G, et al. Role of thioredoxin in the response of normal and transformed cells to histone deacetylase inhibitors. Proc Natl Acad Sci U S A. 2005 Jan 18;102(3):673–678. doi: 10.1073/pnas.0408732102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee JH, Choy ML, Ngo L, Foster SS, Marks PA. Histone deacetylase inhibitor induces DNA damage, which normal but not transformed cells can repair. Proc Natl Acad Sci U S A. 2010 Aug 17;107(33):14639–14644. doi: 10.1073/pnas.1008522107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brazelle W, Kreahling JM, Gemmer J, Ma Y, Cress WD, Haura E, et al. Histone deacetylase inhibitors downregulate checkpoint kinase 1 expression to induce cell death in non-small cell lung cancer cells. PLoS One. 2010 Dec 14;5(12):e14335. doi: 10.1371/journal.pone.0014335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bhaskara S, Chyla BJ, Amann JM, Knutson SK, Cortez D, Sun ZW, et al. Deletion of histone deacetylase 3 reveals critical roles in S phase progression and DNA damage control. Mol Cell. 2008 Apr 11;30(1):61–72. doi: 10.1016/j.molcel.2008.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ishii S, Kurasawa Y, Wong J, Yu-Lee LY. Histone deacetylase 3 localizes to the mitotic spindle and is required for kinetochore-microtubule attachment. Proc Natl Acad Sci U S A. 2008 Mar 18;105(11):4179–4184. doi: 10.1073/pnas.0710140105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Magnaghi-Jaulin L, Eot-Houllier G, Fulcrand G, Jaulin C. Histone deacetylase inhibitors induce premature sister chromatid separation and override the mitotic spindle assembly checkpoint. Cancer Res. 2007 Jul 1;67(13):6360–6367. doi: 10.1158/0008-5472.CAN-06-3012. [DOI] [PubMed] [Google Scholar]
- 58.Stevens FE, Beamish H, Warrener R, Gabrielli B. Histone deacetylase inhibitors induce mitotic slippage. Oncogene. 2008 Feb 28;27(10):1345–1354. doi: 10.1038/sj.onc.1210779. [DOI] [PubMed] [Google Scholar]
- 59.Adimoolam S, Sirisawad M, Chen J, Thiemann P, Ford JM, Buggy JJ. HDAC inhibitor PCI-24781 decreases RAD51 expression and inhibits homologous recombination. Proc Natl Acad Sci U S A. 2007 Dec 4;104(49):19482–19487. doi: 10.1073/pnas.0707828104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kachhap SK, Rosmus N, Collis SJ, Kortenhorst MS, Wissing MD, Hedayati M, et al. Downregulation of homologous recombination DNA repair genes by HDAC inhibition in prostate cancer is mediated through the E2F1 transcription factor. PLoS One. 2010 Jun 18;5(6):e11208. doi: 10.1371/journal.pone.0011208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Miller KM, Tjeertes JV, Coates J, Legube G, Polo SE, Britton S, et al. Human HDAC1 and HDAC2 function in the DNA-damage response to promote DNA nonhomologous end-joining. Nat Struct Mol Biol. 2010 Sep;17(9):1144–1151. doi: 10.1038/nsmb.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rascle A, Johnston JA, Amati B. Deacetylase activity is required for recruitment of the basal transcription machinery and transactivation by STAT5. Mol Cell Biol. 2003 Jun;23(12):4162–4173. doi: 10.1128/MCB.23.12.4162-4173.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shao W, Growney JD, Feng Y, O'Connor G, Pu M, Zhu W, et al. Activity of deacetylase inhibitor panobinostat (LBH589) in cutaneous T-cell lymphoma models: Defining molecular mechanisms of resistance. Int J Cancer. 2010 Nov 1;127(9):2199–2208. doi: 10.1002/ijc.25218. [DOI] [PubMed] [Google Scholar]
- 64.Wang JC, Chen C, Dumlao T, Naik S, Chang T, Xiao YY, et al. Enhanced histone deacetylase enzyme activity in primary myelofibrosis. Leuk Lymphoma. 2008 Dec;49(12):2321–2327. doi: 10.1080/10428190802527699. [DOI] [PubMed] [Google Scholar]
- 65.Skov V, Larsen TS, Thomassen M, Riley CH, Jensen MK, Bjerrum OW, et al. Increased gene expression of histone deacetylases in patients with philadelphia-negative chronic myeloproliferative neoplasms. Leuk Lymphoma. 2012 Jan;53(1):123–129. doi: 10.3109/10428194.2011.597905. [DOI] [PubMed] [Google Scholar]
- 66.Haan S, Wuller S, Kaczor J, Rolvering C, Nocker T, Behrmann I, et al. SOCS-mediated downregulation of mutant Jak2 (V617F, T875N and K539L) counteracts cytokine-independent signaling. Oncogene. 2009 Aug 27;28(34):3069–3080. doi: 10.1038/onc.2009.155. [DOI] [PubMed] [Google Scholar]
- 67.Chen CQ, Yu K, Yan QX, Xing CY, Chen Y, Yan Z, et al. Pure curcumin increases the expression of SOCS1 and SOCS3 in myeloproliferative neoplasms through suppressing class I histone deacetylases. Carcinogenesis. 2013 Jul;34(7):1442–1449. doi: 10.1093/carcin/bgt070. [DOI] [PubMed] [Google Scholar]
- 68.Gao SM, Chen CQ, Wang LY, Hong LL, Wu JB, Dong PH, et al. Histone deacetylases inhibitor sodium butyrate inhibits JAK2/STAT signaling through upregulation of SOCS1 and SOCS3 mediated by HDAC8 inhibition in myeloproliferative neoplasms. Exp Hematol. 2013 Mar;41(3):261, 70.e4. doi: 10.1016/j.exphem.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 69. Guerini V, Barbui V, Spinelli O, Salvi A, Dellacasa C, Carobbio A, et al. The histone deacetylase inhibitor ITF2357 selectively targets cells bearing mutated JAK2(V617F) Leukemia. 2008 Apr;22(4):740–747. doi: 10.1038/sj.leu.2405049. •Important preclinical paper demonstrating efficacy of givinostat against JAK2 V617F+ MPN.
- 70.Amaru Calzada A, Todoerti K, Donadoni L, Pellicioli A, Tuana G, Gatta R, et al. The HDAC inhibitor givinostat modulates the hematopoietic transcription factors NFE2 and C-MYB in JAK2(V617F) myeloproliferative neoplasm cells. Exp Hematol. 2012 Aug;40(8):634, 45.e10. doi: 10.1016/j.exphem.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 71.Kaufmann KB, Grunder A, Hadlich T, Wehrle J, Gothwal M, Bogeska R, et al. A novel murine model of myeloproliferative disorders generated by overexpression of the transcription factor NF-E2. J Exp Med. 2012 Jan 16;209(1):35–50. doi: 10.1084/jem.20110540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Amaru Calzada A, Pedrini O, Finazzi G, Leoni F, Mascagni P, Introna M, et al. Givinostat and hydroxyurea synergize in vitro to induce apoptosis of cells from JAK2(V617F) myeloproliferative neoplasm patients. Exp Hematol. 2013 Mar;41(3):253, 60.e2. doi: 10.1016/j.exphem.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 73. Akada H, Akada S, Gajra A, Bair A, Graziano S, Hutchison RE, et al. Efficacy of vorinostat in a murine model of polycythemia vera. Blood. 2012 Apr 19;119(16):3779–3789. doi: 10.1182/blood-2011-02-336743. •Important preclinical paper demonstrating efficacy of vorinostat in an animal model of PV.
- 74. Marubayashi S, Koppikar P, Taldone T, Abdel-Wahab O, West N, Bhagwat N, et al. HSP90 is a therapeutic target in JAK2-dependent myeloproliferative neoplasms in mice and humans. J Clin Invest. 2010 Oct;120(10):3578–3593. doi: 10.1172/JCI42442. •Established potential of therapeutically targeting the chaperone protein, HSP90, in MPN.
- 75.Kamishimoto J, Tago K, Kasahara T, Funakoshi-Tago M. Akt activation through the phosphorylation of erythropoietin receptor at tyrosine 479 is required for myeloproliferative disorder-associated JAK2 V617F mutant-induced cellular transformation. Cell Signal. 2011 May;23(5):849–856. doi: 10.1016/j.cellsig.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 76. Fiskus W, Verstovsek S, Manshouri T, Rao R, Balusu R, Venkannagari S, et al. Heat shock protein 90 inhibitor is synergistic with JAK2 inhibitor and overcomes resistance to JAK2-TKI in human myeloproliferative neoplasm cells. Clin Cancer Res. 2011 Dec 1;17(23):7347–7358. doi: 10.1158/1078-0432.CCR-11-1541. •Demonstration of synergism between HSP90 inhibitor and JAK2 inhibitor in MPN cells.
- 77. Bali P, Pranpat M, Bradner J, Balasis M, Fiskus W, Guo F, et al. Inhibition of histone deacetylase 6 acetylates and disrupts the chaperone function of heat shock protein 90: A novel basis for antileukemia activity of histone deacetylase inhibitors. J Biol Chem. 2005 Jul 22;280(29):26729–26734. doi: 10.1074/jbc.C500186200. •Showed that HDAC6 inhibitors acetylate and disrupt the function of HSP90, down-regulating its client proteins.
- 78. Wang Y, Fiskus W, Chong DG, Buckley KM, Natarajan K, Rao R, et al. Cotreatment with panobinostat and JAK2 inhibitor TG101209 attenuates JAK2V617F levels and signaling and exerts synergistic cytotoxic effects against human myeloproliferative neoplastic cells. Blood. 2009 Dec 3;114(24):5024–5033. doi: 10.1182/blood-2009-05-222133. •Demonstration of synergism between HDAC inhibitor and JAK2 inhibitor in MPN cells.
- 79. Evrot E, Ebel N, Romanet V, Roelli C, Andraos R, Qian Z, et al. JAK1/2 and pan-deacetylase inhibitor combination therapy yields improved efficacy in preclinical mouse models of JAK2V617F-driven disease. Clin Cancer Res. 2013 Nov 15;19(22):6230–6241. doi: 10.1158/1078-0432.CCR-13-0905. •Demonstration of synergism between HDAC inhibitor and JAK2 inhibitor in animal models of MPN.
- 80.Cardoso BA, Belo H, Barata JT, Almeida AM. The bone marrow-mediated protection of myeloproliferative neoplastic cells to vorinostat and ruxolitinib relies on the activation of JNK and PI3K signalling pathways. PLoS One. 2015 Dec 1;10(12):e0143897. doi: 10.1371/journal.pone.0143897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shi J, Zhao Y, Ishii T, Hu W, Sozer S, Zhang W, et al. Effects of chromatin-modifying agents on CD34+ cells from patients with idiopathic myelofibrosis. Cancer Res. 2007 Jul 1;67(13):6417–6424. doi: 10.1158/0008-5472.CAN-07-0572. [DOI] [PubMed] [Google Scholar]
- 82.Rosti V, Massa M, Vannucchi AM, Bergamaschi G, Campanelli R, Pecci A, et al. The expression of CXCR4 is down-regulated on the CD34+ cells of patients with myelofibrosis with myeloid metaplasia. Blood Cells Mol Dis. 2007 May-Jun;38(3):280–286. doi: 10.1016/j.bcmd.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 83.Bogani C, Ponziani V, Guglielmelli P, Desterke C, Rosti V, Bosi A, et al. Hypermethylation of CXCR4 promoter in CD34+ cells from patients with primary myelofibrosis. Stem Cells. 2008 Aug;26(8):1920–1930. doi: 10.1634/stemcells.2008-0377. [DOI] [PubMed] [Google Scholar]
- 84. Rambaldi A, Dellacasa CM, Finazzi G, Carobbio A, Ferrari ML, Guglielmelli P, et al. A pilot study of the histone-deacetylase inhibitor givinostat in patients with JAK2V617F positive chronic myeloproliferative neoplasms. Br J Haematol. 2010 Aug;150(4):446–455. doi: 10.1111/j.1365-2141.2010.08266.x. •The first clinical study to show activity of HDACi in MPN.
- 85. Barosi G, Birgegard G, Finazzi G, Griesshammer M, Harrison C, Hasselbalch HC, et al. Response criteria for essential thrombocythemia and polycythemia vera: Result of a european LeukemiaNet consensus conference. Blood. 2009 May 14;113(20):4829–4833. doi: 10.1182/blood-2008-09-176818. •ELN response criteria for PV and ET.
- 86.Barosi G, Bordessoule D, Briere J, Cervantes F, Demory JL, Dupriez B, et al. Response criteria for myelofibrosis with myeloid metaplasia: Results of an initiative of the european myelofibrosis network (EUMNET) Blood. 2005 Oct 15;106(8):2849–2853. doi: 10.1182/blood-2005-04-1520. [DOI] [PubMed] [Google Scholar]
- 87.Tefferi A, Barosi G, Mesa RA, Cervantes F, Deeg HJ, Reilly JT, et al. International working group (IWG) consensus criteria for treatment response in myelofibrosis with myeloid metaplasia, for the IWG for myelofibrosis research and treatment (IWG-MRT) Blood. 2006 Sep 1;108(5):1497–1503. doi: 10.1182/blood-2006-03-009746. [DOI] [PubMed] [Google Scholar]
- 88.Finazzi G, Vannucchi AM, Martinelli V, Ruggeri M, Nobile F, Specchia G, et al. A phase II study of givinostat in combination with hydroxycarbamide in patients with polycythaemia vera unresponsive to hydroxycarbamide monotherapy. Br J Haematol. 2013 Jun;161(5):688–694. doi: 10.1111/bjh.12332. [DOI] [PubMed] [Google Scholar]
- 89. Barosi G, Birgegard G, Finazzi G, Griesshammer M, Harrison C, Hasselbalch H, et al. A unified definition of clinical resistance and intolerance to hydroxycarbamide in polycythaemia vera and primary myelofibrosis: Results of a european LeukemiaNet (ELN) consensus process. Br J Haematol. 2010 Mar;148(6):961–963. doi: 10.1111/j.1365-2141.2009.08019.x. •Definitions of hydroxyurea resistance and intolerance in PV and MF.
- 90.Andersen CL, McMullin MF, Ejerblad E, Zweegman S, Harrison C, Fernandes S, et al. A phase II study of vorinostat (MK-0683) in patients with polycythaemia vera and essential thrombocythaemia. Br J Haematol. 2013 Aug;162(4):498–508. doi: 10.1111/bjh.12416. [DOI] [PubMed] [Google Scholar]
- 91. Mascarenhas J, Lu M, Li T, Petersen B, Hochman T, Najfeld V, et al. A phase I study of panobinostat (LBH589) in patients with primary myelofibrosis (PMF) and post-polycythaemia vera/essential thrombocythaemia myelofibrosis (post-PV/ET MF) Br J Haematol. 2013 Apr;161(1):68–75. doi: 10.1111/bjh.12220. •Study suggesting that HDACi need to be given at low doses for prolonged periods for disease modification in MF.
- 92. DeAngelo DJ, Mesa RA, Fiskus W, Tefferi A, Paley C, Wadleigh M, et al. Phase II trial of panobinostat, an oral pan-deacetylase inhibitor in patients with primary myelofibrosis, post-essential thrombocythaemia, and post-polycythaemia vera myelofibrosis. Br J Haematol. 2013 Aug;162(3):326–335. doi: 10.1111/bjh.12384. •Clinical trial of panobinostat in MF demonstrating down-regulation of JAK-STAT signaling, lowering of cytokine levels and JAK2 V617F allele burden reduction.
- 93.Quintas-Cardama A, Kantarjian H, Estrov Z, Borthakur G, Cortes J, Verstovsek S. Therapy with the histone deacetylase inhibitor pracinostat for patients with myelofibrosis. Leuk Res. 2012 Sep;36(9):1124–1127. doi: 10.1016/j.leukres.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Harrison CN, Kiladjian JJ, Heidel FH, Vannucchi AM, Passamonti F, Hayat A, et al. Efficacy, safety, and confirmation of the recommended phase 2 starting dose of the combination of ruxolitinib (RUX) and panobinostat (PAN) in patients (pts) with myelofibrosis (MF) Blood. 2015;126(23):4060-. •The most mature clinical data available so far from a ruxolitinib-HDACi combination trial in MF.
- 95. Cervantes F, Dupriez B, Pereira A, Passamonti F, Reilly JT, Morra E, et al. New prognostic scoring system for primary myelofibrosis based on a study of the international working group for myelofibrosis research and treatment. Blood. 2009 Mar 26;113(13):2895–2901. doi: 10.1182/blood-2008-07-170449. •The IPSS for PMF - the most widely used prognostic scoring system for newly diagnosed MF.
- 96.Verstovsek S, Vannucchi AM, Griesshammer M, Masszi T, Durrant S, Passamonti F, et al. Ruxolitinib versus best available therapy in patients with polycythemia vera: 80-week follow-up from the RESPONSE trial. Haematologica. 2016 Jul;101(7):821–829. doi: 10.3324/haematol.2016.143644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Vannucchi AM, Verstovsek S, Guglielmelli P, Griesshammer M, Burn TC, Naim A, et al. RUXOLITINIB (RUX) REDUCES JAK2V617F ALLELE BURDEN (AB) IN PATIENTS (PTS) WITH POLYCYTHEMIA VERA (PV) ENROLLED IN THE RESPONSE STUDY. European haematology association 21st congress; Copenhagen, Denmark. 2016. [June 9–12, 2016]. p. S454. [Google Scholar]
- 98.Kiladjian JJ, Cassinat B, Chevret S, Turlure P, Cambier N, Roussel M, et al. Pegylated interferon-alfa-2a induces complete hematologic and molecular responses with low toxicity in polycythemia vera. Blood. 2008 Oct 15;112(8):3065–3072. doi: 10.1182/blood-2008-03-143537. [DOI] [PubMed] [Google Scholar]
- 99.Quintas-Cardama A, Kantarjian H, Manshouri T, Luthra R, Estrov Z, Pierce S, et al. Pegylated interferon alfa-2a yields high rates of hematologic and molecular response in patients with advanced essential thrombocythemia and polycythemia vera. J Clin Oncol. 2009 Nov 10;27(32):5418–5424. doi: 10.1200/JCO.2009.23.6075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gisslinger H, Zagrijtschuk O, Buxhofer-Ausch V, Thaler J, Schloegl E, Gastl GA, et al. Ropeginterferon alfa-2b, a novel IFNalpha-2b, induces high response rates with low toxicity in patients with polycythemia vera. Blood. 2015 Oct 8;126(15):1762–1769. doi: 10.1182/blood-2015-04-637280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bishton MJ, Harrison SJ, Martin BP, McLaughlin N, James C, Josefsson EC, et al. Deciphering the molecular and biologic processes that mediate histone deacetylase inhibitor-induced thrombocytopenia. Blood. 2011 Mar 31;117(13):3658–3668. doi: 10.1182/blood-2010-11-318055. [DOI] [PubMed] [Google Scholar]
- 102.Iancu-Rubin C, Gajzer D, Mosoyan G, Feller F, Mascarenhas J, Hoffman R. Panobinostat (LBH589)-induced acetylation of tubulin impairs megakaryocyte maturation and platelet formation. Exp Hematol. 2012 Jul;40(7):564–574. doi: 10.1016/j.exphem.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mascarenhas J, Roper N, Chaurasia P, Hoffman R. Epigenetic abnormalities in myeloproliferative neoplasms: A target for novel therapeutic strategies. Clin Epigenetics. 2011 Aug;2(2):197–212. doi: 10.1007/s13148-011-0050-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Passamonti F, Thiele J, Girodon F, Rumi E, Carobbio A, Gisslinger H, et al. A prognostic model to predict survival in 867 world health organization-defined essential thrombocythemia at diagnosis: A study by the international working group on myelofibrosis research and treatment. Blood. 2012 Aug 9;120(6):1197–1201. doi: 10.1182/blood-2012-01-403279. •Prognostic scoring system in ET.
- 105. Barbui T, Finazzi G, Carobbio A, Thiele J, Passamonti F, Rumi E, et al. Development and validation of an international prognostic score of thrombosis in world health organization-essential thrombocythemia (IPSET-thrombosis) Blood. 2012 Dec 20;120(26):5128, 33. doi: 10.1182/blood-2012-07-444067. quiz 5252. •The most established scoring system for risk stratification for thrombosis in patients with ET.
- 106.Barbui T, Vannucchi AM, Buxhofer-Ausch V, De Stefano V, Betti S, Rambaldi A, et al. Practice-relevant revision of IPSET-thrombosis based on 1019 patients with WHO-defined essential thrombocythemia. Blood Cancer J. 2015 Nov 27;5:e369. doi: 10.1038/bcj.2015.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Geyer HL, Scherber RM, Dueck AC, Kiladjian JJ, Xiao Z, Slot S, et al. Distinct clustering of symptomatic burden among myeloproliferative neoplasm patients: Retrospective assessment in 1470 patients. Blood. 2014 Jun 12;123(24):3803–3810. doi: 10.1182/blood-2013-09-527903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Geyer HL, Mesa RA. Therapy for myeloproliferative neoplasms: When, which agent, and how? Blood. 2014 Dec 4;124(24):3529–3537. doi: 10.1182/blood-2014-05-577635. [DOI] [PubMed] [Google Scholar]
- 109.Komrokji RS, Seymour JF, Roberts AW, Wadleigh M, To LB, Scherber R, et al. Results of a phase 2 study of pacritinib (SB1518), a JAK2/JAK2(V617F) inhibitor, in patients with myelofibrosis. Blood. 2015 Apr 23;125(17):2649–2655. doi: 10.1182/blood-2013-02-484832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pardanani A, Laborde RR, Lasho TL, Finke C, Begna K, Al-Kali A, et al. Safety and efficacy of CYT387, a JAK1 and JAK2 inhibitor, in myelofibrosis. Leukemia. 2013 Jun;27(6):1322–1327. doi: 10.1038/leu.2013.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Geyer HL, Mesa RA. Emerging drugs for the treatment of myelofibrosis. Expert Opin Emerg Drugs. 2015;20(4):663–678. doi: 10.1517/14728214.2015.1061502. [DOI] [PubMed] [Google Scholar]
- 112.Andraos R, Qian Z, Bonenfant D, Rubert J, Vangrevelinghe E, Scheufler C, et al. Modulation of activation-loop phosphorylation by JAK inhibitors is binding mode dependent. Cancer Discov. 2012 Jun;2(6):512–523. doi: 10.1158/2159-8290.CD-11-0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Koppikar P, Bhagwat N, Kilpivaara O, Manshouri T, Adli M, Hricik T, et al. Heterodimeric JAK-STAT activation as a mechanism of persistence to JAK2 inhibitor therapy. Nature. 2012 Sep 6;489(7414):155–159. doi: 10.1038/nature11303. ••Preclinical demonstration of JAK2 inhibitor "persistence" as a mechanism of acquired resistance.
- 114. Meyer SC, Keller MD, Chiu S, Koppikar P, Guryanova OA, Rapaport F, et al. CHZ868, a type II JAK2 inhibitor, reverses type I JAK inhibitor persistence and demonstrates efficacy in myeloproliferative neoplasms. Cancer Cell. 2015 Jul 13;28(1):15–28. doi: 10.1016/j.ccell.2015.06.006. ••Demonstration of the ability of a new "type II" JAK2 inhibitor to overcome type I JAK2 inhibitor persistence and resistance.
- 115.Verstovsek S, Mesa RA, Foltz LM, Gupta V, Mascarenhas JO, Ritchie EK, et al. PRM-151 in myelofibrosis: Durable efficacy and safety at 72 weeks. Blood. 2015;126(23):56. [Google Scholar]
- 116.Tefferi A, Lasho TL, Begna KH, Patnaik MM, Zblewski DL, Finke CM, et al. A pilot study of the telomerase inhibitor imetelstat for myelofibrosis. N Engl J Med. 2015 Sep 3;373(10):908–919. doi: 10.1056/NEJMoa1310523. [DOI] [PubMed] [Google Scholar]
- 117.Durrant S, Nagler A, Vannucchi AM, Lavie D, Chuah C, Passamonti F, et al. An open-label, multicenter, 2-arm, dose-finding, phase 1b study of the combination of ruxolitinib and buparlisib (BKM120) in patients with myelofibrosis: Results from HARMONY study. Blood. 2015;126(23):827-. [Google Scholar]
- 118.Gupta V, Harrison CN, Hasselbalch HC, Pieri L, Koschmieder S, Cervantes F, et al. Phase 1b/2 study of the efficacy and safety of sonidegib (LDE225) in combination with ruxolitinib (INC424) in patients with myelofibrosis. Blood. 2015;126(23):825. [Google Scholar]
- 119.Daver N, Verstovsek S. Ruxolitinib and DNA methyltransferase-inhibitors: A foray into combination regimens in myelofibrosis. Leuk Lymphoma. 2015 Feb;56(2):279–280. doi: 10.3109/10428194.2014.931955. [DOI] [PubMed] [Google Scholar]
- 120.Yazbeck VY, Grant S. Romidepsin for the treatment of non-hodgkin's lymphoma. Expert Opin Investig Drugs. 2015;24(7):965–979. doi: 10.1517/13543784.2015.1041586. [DOI] [PubMed] [Google Scholar]