Abstract
Background
Acute decompensated heart failure (ADHF) was a frequent common outcome in the Systolic Blood Pressure (BP) Intervention Trial (SPRINT). We examined whether there was differential reduction in ADHF events from intensive BP treatment among the 6 key, pre-specified subgroups in SPRINT: age ≥75 years, prior cardiovascular (CV) disease (CVD), chronic kidney disease (CKD), women, black race, and 3 levels of baseline systolic BP (≤ 132 vs. >132 to <145 vs. ≥ 145 mm of Hg).
Methods and Results
ADHF was defined as hospitalization for ADHF, confirmed and formally adjudicated by a blinded events committee using standardized protocols. At 3.29 years follow-up, there were 103 ADHF events (2.2%) among 4683 standard arm participants and 65 ADHF events (1.4%) among 4678 intensive arm participants (Cox proportional Hazards Ratio: 0.63 [95% CI: 0.46–0.85], p value=0.003). In multivariable analyses including treatment arm, baseline covariates that were significant predictors for ADHF included CKD, CVD, age≥ 75 years, BMI, and higher systolic BP. The beneficial effect of the intervention on incident ADHF was consistent across all pre-specified subgroups. Participants who had incident ADHF had markedly increased risk of subsequent CV events, including a 27-fold increase (p<0.001) in CV death.
Conclusions
Targeting a systolic BP <120 mm Hg, compared with <140 mm Hg, significantly reduced ADHF events and the benefit was similar across all key, pre-specified subgroups. Participants who developed ADHF had markedly increased risk for subsequent CV events and death, highlighting the importance of strategies aimed at prevention of ADHF, especially intensive BP reduction.
Clinical Trial Registration
ClinicalTrials.gov number: NCT01206062.
Keywords: heart failure, hypertension, aging, clinical trial, women and minorities
Hypertension (HTN) is the most prevalent risk factor for heart failure (HF), and precedes the diagnosis of HF in 75–85% of persons who develop HF.1 In addition, HTN pathophysiology is closely linked to all key adverse outcomes in HF, including acute exacerbations, chronic symptoms, and mortality.1 Although elevated systolic blood pressure (BP) is associated with an increased risk of developing HF, and BP reduction prevents incident HF, the optimal target for BP lowering for HF prevention has been uncertain.2 In the recently reported SPRINT trial which was terminated early due to benefit, intensive BP reduction reduced the risk of the primary outcome (a composite of a myocardial infarction (MI), acute coronary syndrome (ACS) not resulting in MI, stroke, acute decompensated HF (ADHF), or death from cardiovascular (CV) causes) by 25% and reduced total mortality by 27%.3 The risk for ADHF was reduced by 38% by intensive BP reduction in SPRINT.3
SPRINT included a highly diverse group of participants, including those in 13 key, pre-specified subgroups: age (≥75 years and < 75 years); women and men; black race and non-black race; chronic kidney disease (CKD) and no CKD; prior cardiovascular disease (CVD) and no prior CVD; and the 3 tertiles of baseline systolic BP. Multiple lines of evidence suggest the potential for disparate responses to intensive BP lowering among participants in these subgroups. For example, in very elderly patients in whom the risk of HF is the highest,4 arterial stiffening is common and may contribute to the elevation of systolic BP and the depression of diastolic BP.5 Although isolated elevation of systolic BP without a concomitant elevation of diastolic BP is a known risk factor for incident HF in older adults,6, 7 low diastolic BP has been associated with adverse CV outcomes.8 This is important since myocardial perfusion depends upon adequate diastolic BP, and myocardial perfusion requirements are increased in HTN, such that intensive DBP reduction could reduce myocardial perfusion, and promote myocardial ischemia, adverse left ventricular (LV) dilation, and subsequent HF. Some studies have suggested that anti-hypertensive treatment can be associated with increased risk of coronary events at low levels of diastolic BP, particularly in those with CVD.9
In women, HTN contributes more to the population burden of HF than MI; the 5-year mortality after the onset of hypertensive HF in women is high, about 69 %.4, 10, 11 In addition, women develop concentric LV hypertrophy and maintain normal chamber size, whereas men most frequently develop eccentric LV hypertrophy with chamber dilation.12, 13 Thus, treating to lower BP goals in SPRINT may produce divergent responses by sex, due to differences in both ischemic heart disease burden and LV hypertrophic remodeling.
Compared to non-blacks, HF is more prevalent in blacks, and has higher rates of death and morbidity.14–16 Among patients with CKD, HF is the leading CV complication and its prevalence increases with declining kidney function.17 In both blacks and those with CKD, studies suggest that anti-hypertensive therapy may be of benefit in reducing CV events; however there is uncertainty regarding the optimal range of BP-lowering.15, 16, 18–22
The purpose of this analysis was to determine the relative risk for developing ADHF among the pre-specified subgroups in SPRINT, the impact of intensive BP treatment on development of ADHF among each of these subgroups, the predictors of incident ADHF, and the impact of assignment to the intensive BP treatment arm for subsequent outcomes among participants who developed ADHF in SPRINT.
Methods
Study population
The study population included all participants in SPRINT; the details of the design and primary results of SPRINT have been previously reported.3, 23 Briefly, 9361 participants ≥ 50 years of age with systolic BP ≥ 130 mm Hg, without a history of diabetes or stroke, and an increased risk of CV events were enrolled from November 2010 to March 2013. Increased CV risk was defined by one or more of the following: clinical or subclinical CVD other than stroke; CKD, excluding polycystic kidney disease; a 10-year risk of CV disease of 15% or greater on the basis of the Framingham risk score; or an age of 75 years or older. Detailed inclusion and exclusion criteria were previously reported.23 The study was approved by the institutional review board at each participating study site. All participants provided written informed consent.
The CVD subgroup included participants with clinical or subclinical CVD other than stroke. Clinical CVD was defined as previous MI, percutaneous intervention, coronary artery bypass grafting, carotid endarterectomy, or carotid stenting; peripheral arterial disease with revascularization; ACS with or without resting ECG change, ECG changes on a graded exercise test, or positive cardiac imaging study; at least a 50% diameter stenosis of a coronary, carotid, or lower extremity artery; or abdominal aortic aneurysm ≥ 5 cm with or without repair. Subclinical CVD was defined as coronary artery calcium score ≥ 400 Agatston units within the past 2 years; ankle brachial index ≤ 0.90 within the past 2 years; or LV hypertrophy by ECG (based on computer reading), echocardiogram report, or other cardiac imaging procedure report within the past 2 years. CKD was defined as estimated glomerular filtration rate (eGFR) of 20 to less than 60 ml per minute per 1.73 m2 of body-surface area, calculated with the use of the four-variable Modification of Diet in Renal Disease equation.3 Persons with symptomatic HF within the past 6 months or left ventricular ejection fraction (by any method) < 35% at the time of randomization were not included in the study.
Study measurements
Demographic data (age, race, ethnicity and gender) and physical measurements (weight and height) were collected during participant screening, and confirmed at the baseline randomization visit. Systolic and Diastolic BP were recorded as the calculated average of three seated readings. Participants answering a self-administered questionnaire as ‘Yes” to the question “Do you now smoke cigarettes?” were considered current smokers.
Intervention
All participants provided written informed consent. Participants were randomly assigned to have systolic BP targeted to < 140 mm Hg (n = 4683) or < 120 mm Hg (n = 4678). Extensive details regarding the randomization and intervention have been previously reported.3, 23, 24
Study Outcomes
Pre-specified subgroups of interest for all outcomes were defined according to status with respect to CVD at baseline (yes vs. no), status with respect to CKD at baseline (yes vs. no), sex, race (black vs. nonblack), age (<75 vs. ≥75 years), and baseline systolic BP tertiles (≤132 mm Hg, >132 to <145 mm Hg, and ≥145 mm Hg).
Clinical and laboratory data were obtained at baseline and every 3 months for the first year, then every 6 months. Data regarding potential outcomes were assessed every 3 months using a structured interview to minimize ascertainment bias,23 and a standard protocol, with centralized monitoring by the coordinating center, was used to obtain relevant information, including medical records, lab reports, and electrocardiograms. Deaths were investigated whenever clinic staff became aware of them.
All clinical events, including ADHF, were formally adjudicated by a Morbidity and Mortality committee using a standardized electronic form. Adjudicators were blinded to treatment assignment. ADHF was defined as hospitalization or emergency department (ED) visit requiring treatment with infusion therapy (diuretic or inotropic agents) for a clinical syndrome that presented with multiple signs and symptoms consistent with cardiac decompensation and inadequate cardiac pump function. A detailed manual of operations for adjudication was developed based initially on that used and validated in the Atherosclerosis Risk in Communities study.25 All ADHF events were new (incident) HF events. Positive symptoms supporting ADHF included: new onset or increasing shortness of breath; peripheral edema; orthopnea; or paroxysmal nocturnal dyspnea. Positive signs supporting ADHF included: hypoxia; pulmonary rales on clinical examination; pulmonary vascular congestion on chest X–ray; elevation of biomarker B–type natriuretic peptide (BNP) or pro-N-terminal BNP above diagnostic threshold; reduced left ventricular ejection fraction or diastolic dysfunction; new or increased treatment specifically for ADHF, such as intravenous loop diuretic or inotrope; documented response to therapy; and evidence in the treating physician’s notes that the primary reason for the hospitalization or ED visit was ADHF.
Confirmation of ADHF events relied on multiple pieces of key clinical data as well as adjudicators’ clinical judgment, guided by specific, pre-specified definitions and operational rules. Identification of ADHF did not rely on any single piece of data such as the presence of dyspnea, edema, a low LV ejection fraction, or an increased BNP value. For participants seen in the ED alone, positive adjudication as an ADHF event required unequivocal documentation in the medical record of administration of intravenous loop diuretic or inotrope and an appropriate response to therapy, regardless of the strength of the history, physical exam, and other evaluations supporting ADHF.
Chronic stable HF during a hospitalization, with no evidence of decompensation or augmentation of therapy for worsening HF, was not considered an endpoint in SPRINT. Reduced LV ejection fraction in the absence of symptoms, right sided HF, and volume overload due to inadequate dialysis in patients with end-stage renal disease were not considered SPRINT HF endpoints.
The SPRINT BP intervention was stopped early at the recommendation of the data and safety monitoring board (and accepted by NIH) on August 20, 2015, due to benefit in the intensive arm on the primary outcome. Data, in this paper were frozen on March 10, 2016 but only used adjudicated events that occurred on or prior to August 20, 2015, that included additional six events from the original report,3 three in each treatment arm.
Statistical analyses
All statistical analyses were conducted at the coordinating center with the use of SAS software, version 9.4 (SAS Institute). Continuous variables are presented as mean and standard deviation (SD) or median and interquartile range, and categorical variables are presented as number with percent. Baseline characteristics were compared among participants who did and did not experience ADHF during the trial, with the use of the chi-square test, Wilcoxon rank-sum test, and two-sample t-tests where appropriate.
Time until first occurrence of ADHF was compared between the two treatment arms with the use of the intention-to-treat approach for all randomized participants. For this analysis, we used Cox proportional-hazards regression with two-sided tests at the 5% level of significance, with stratification by clinical site. Follow-up time was censored on the date of the last event ascertainment. Interactions between pre-specified subgroups and the treatment arm were assessed with a likelihood ratio test for interaction. As a sensitivity analysis, baseline variables likely related to the development of ADHF were assessed in univariate models, and added as a group to the primary analysis model.
Among the subset of participants experiencing HF during the trial, a Cox proportional hazards model, including baseline covariates and treatment arm, was used to examine which baseline covariates were predictive of recurrent ADHF. Only the first recurrence of ADHF was used in this analysis. Time to event was calculated as the number of days from the first ADHF to the first recurrence, and censoring time was calculated as the time from first ADHF to the last participant visit. The effect of ADHF on other clinical outcomes (all-cause mortality, CVD mortality, non-MI ACS, and stroke) was evaluated using a time-dependent Cox proportional hazards model, with a time-varying covariate for first ADHF event to model the effect of participants moving from the non-ADHF state to the ADHF state on these other clinical outcomes.26
No adjustments were made for multiple testing. Nominal P-values are reported throughout as simple guides to possible associations.
RESULTS
A total of 9361 participants were enrolled between November 2010 and March 2013. The BP intervention was stopped early at the recommendation of the data and safety monitoring board (and accepted by NIH) on August 20, 2015, due to benefit in the intensive arm on the primary outcome.3 The median follow-up at that time was 3.29 years of the planned average of 5 years. However, processing and adjudication of events that occurred prior to August 20, 2016 continued such that as of the date of this analysis (March 10, 2016), there were a total of 103 ADHF events among 4683 (2.2%) participants in the standard arm and 65 ADHF events among 4678 (1.4%) intensive arm participants (Cox proportional hazards ratio stratified by clinical site: 0.63 [95% CI: 0.46–0.85], p value= 0.003) for a risk reduction of 37%. Separation between groups in ADHF events was apparent at 6 months (Figure 1). The number needed to treat27 to prevent an ADHF event during the median 3.29 years of the trial was 130.
Figure 1.
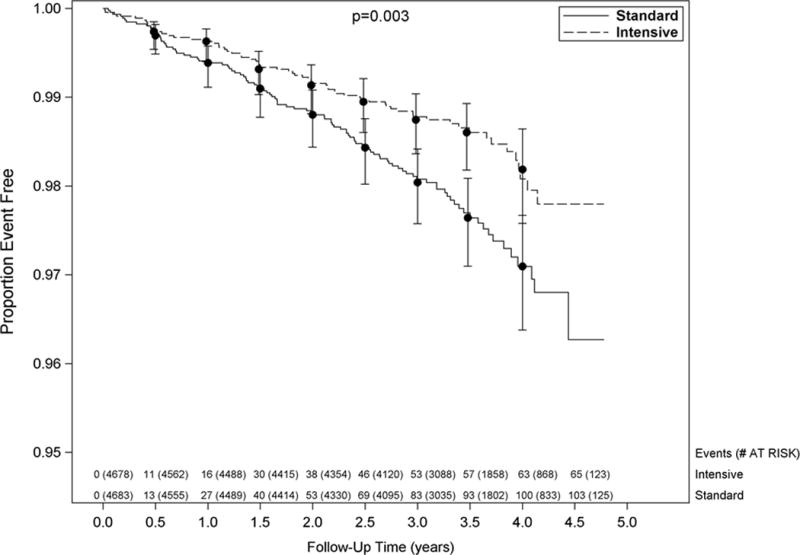
Kaplan-Meier curves for the SPRINT acute decompensated heart failure outcome by treatment group. Vertical bars indicate 95% confidence intervals. P-value is from a Cox proportional hazards model stratified by clinical site. Number at risk and number of events is shown every six months.
Table 1 shows the baseline clinical characteristics for the cohort based on ADHF occurrence during the trial and by treatment arm. Patients who developed ADHF were older, had higher prevalence of baseline CKD, clinical CVD, increased albuminuria and lower eGFR and diastolic BP at baseline irrespective of treatment arm. Race and gender were similar in the ADHF and no ADHF groups in both treatment arms. Univariate predictors for development of ADHF were randomization to the standard treatment arm, age ≥ 75 years, subgroups with CKD and CVD (Table 2). In multivariable analyses that included treatment arm, the baseline covariates that were statistically significant predictors for development of ADHF included age ≥75 years, CKD, CVD, higher baseline SBP, body mass index, smoking status, and lower baseline diastolic BP (Table 3). The effects of the intervention on the rate of ADHF events were consistent across the pre-specified subgroups (Figure 2). There were no statistically significant interactions (p-values ranged from 0.21–0.99) between treatment arm and subgroup with respect to the risk of ADHF events, and all subgroups had reduced risk of ADHF when assigned to the intensive BP intervention (Figure 2).
Table 1.
Baseline Clinical Characteristics By On–trial ADHF Occurrence
| Characteristics | Intensive Treatment | Standard Treatment | ||
|---|---|---|---|---|
| No ADHF (N=4613) |
ADHF (N=65) |
No ADHF (N=4580) |
ADHF (N=103) |
|
| Age≥ 75 years-no (%) | 1282 (28%) | 35 (54%) | 1261 (28%) | 58 (56%) |
| Chronic Kidney disease- no (%) | 1289 (28%) | 41 (63%) | 1264 (28%) | 52 (51%) |
| Cardiovascular disease- no (%) | 914 (20%) | 26 (40%) | 897 (20%) | 40 (39%) |
| Subclinical | 241 (5%) | 6 (9%) | 235 (5%) | 11 (11%) |
| Clinical | 754 (16%) | 25 (36%) | 749 (16%) | 34 (33%) |
| Framingham 10-year CVD risk score | 25 ± 13 | 33 ± 18 | 25 ± 12 | 33 ± 16 |
| Framingham Risk ≥15%- no (%) | 3502 (76%) | 54 (83%) | 3458 (76%) | 89 (86%) |
| Female sex-no. (%) | 1661 (36%) | 23 (35%) | 1618 (35%) | 30 (29%) |
| Age in years | 68 ± 9 | 75 ± 11 | 68 ± 9 | 74 ± 10 |
| Race or Ethnic Group-no. (%) | ||||
| Black Race | 1357 (29%) | 22 (34%) | 1395 (31%) | 28 (27%) |
| Hispanic | 499 (11%) | 4 (6%) | 476 (10%) | 5 (4.9%) |
| Other | 98 (2.1%) | 0 (0%) | 77 (1.7%) | 1 (1%) |
| White | 2659 (56%) | 39 (60%) | 2632 (56%) | 69 (67%) |
| Smoking Status- no. (%) | ||||
| Never | 2022 (44%) | 28 (43%) | 2035 (45%) | 37 (36%) |
| Former | 1947 (42%) | 30 (46%) | 1946 (43%) | 50 (49%) |
| Current | 632 (14%) | 7 (11%) | 585 (13%) | 16 (16%) |
| BMI (kg/m2) | 30 ± 6 | 30 ± 6 | 30 ± 6 | 30 ± 6 |
| Systolic BP mm of Hg | 140 ± 16 | 142 ± 16 | 140 ± 15 | 141 ± 19 |
| Diastolic BP mm of Hg | 78 ± 12 | 73 ± 14 | 78 ± 12 | 74 ± 13 |
| Heart Rate/min | 66 ± 11 | 66 ± 16 | 66 ± 12 | 67 ± 12 |
| SBP Tertile- no (%) | ||||
| ≤ 132 mm Hg | 1567 (34%) | 16 (25%) | 1523 (33%) | 30 (29%) |
| > 132 mm Hg to < 145 mm Hg | 1468 (32%) | 21 (32%) | 1517 (33%) | 32 (31%) |
| ≥ 145 mm Hg | 1578 (34%) | 28 (43%) | 1540 (34%) | 41 (40%) |
| Creatinine- mg/dl | 1.1 ± 0.3 | 1.4 ± 0.5 | 1.1 ± 0.3 | 1.2 ± 0.4 |
| eGFR MDRD [mL/min/1.73m2] | 72 ± 21 | 56 ± 24 | 72 ± 215 | 63 ± 22 |
| Ratio of urinary albumin to creatinine | 41 ± 166 | 230 ± 552 | 39 ± 134 | 133 ± 494 |
| Total Cholesterol- mg/dl | 190 ± 42 | 187 ± 37 | 190 ± 41 | 181 ± 42 |
| LDL – mg/dl | 113 ± 36 | 111 ± 32 | 112 ± 35 | 104 ± 34 |
| HDL –mg/dl | 53 ± 14 | 53 ± 15 | 53 ± 15 | 50 ± 13 |
| Triglycerides, median and Q1, Q3 –mg/dl | 106 [76,148] | 112 [80,143] | 106 [77,152] | 116.0 [90,157] |
| Fasting Glucose –mg/dl | 99 ± 14 | 99± 13 | 99 ± 13 | 100 ± 11 |
| ACE Inhibitors –no (%) | 1736 (38%) | 27 (42%) | 1652 (36%) | 41 (40%) |
| Angiotensin II antagonists–no (%) | 979 (21%) | 14 (22%) | 969 (21%) | 23 (22%) |
| Beta blockers –no (%) | 1455 (32%) | 27 (42%) | 1359 (30%) | 40 (39%) |
| CCBs-Dihydropyridines- –no (%) | 1344 (29%) | 33 (51%) | 1368 (30%) | 37 (36%) |
| Thiazide diuretics –no (%) | 1774 (39%) | 12 (19%) | 1838 (40%) | 35 (34%) |
| Statin Use- no (%) | 1946 (43%) | 32 (51%) | 2025 (45%) | 51 (50%) |
| Aspirin Use- no (%) | 2362 (51%) | 44 (68%) | 2286 (50%) | 64 (62%) |
ADHF=acute decompensated heart failure, CVD=cardiovascular disease, BMI=body mass index, BP=blood pressure, eGFR =estimated glomerular filtration rate, LDL=low –density lipoprotein, HDL= high-density lipoprotein, Q1= 25th and Q3=75th percentile. ACE =angiotensin converting enzyme, CCB=calcium channel blocker. Subclinical cardiovascular disease includes: ≥ 50% stenosis of a coronary, carotid, or lower extremity artery; abdominal aortic aneurysm ≥ 5 cm with or without repair; coronary artery calcium score ≥400 Agatston units; low ankle brachial index (≤ 0.90); left ventricular hypertrophy by computer ECG reading, echocardiogram report, or other cardiac imaging procedure. Clinical cardiovascular disease includes: myocardial infarction; acute coronary syndrome with or without resting ECG changes, ECG changes on graded exercise test, or positive cardiac imaging study; coronary revascularization; carotid endarterectomy or carotid stenting; peripheral arterial disease with revascularization.
Table 2.
Univariate Baseline Predictors of Acute Decompensated HF
| Baseline Variables | Hazard Ratio | 95% Confidence Interval | P value | |
|---|---|---|---|---|
| Randomized to Intensive Blood Pressure Arm | 0.63 | 0.46 | 0.85 | 0.003 |
| Chronic kidney disease | 2.71 | 1.97 | 3.73 | <0.001 |
| Age ≥ 75 years | 2.93 | 2.12 | 4.10 | <0.001 |
| History of Cardiovascular disease | 2.37 | 1.70 | 3.29 | <0.001 |
| Female | 0.83 | 0.58 | 1.19 | 0.31 |
| Black Race | 1.01 | 0.68 | 1.50 | 0.94 |
| Seated Systolic Blood Pressure (10 unit increase) | 1.09 | 0.99 | 1.20 | 0.082 |
| Seated Diastolic Blood Pressure (10 unit increase) | 0.73 | 0.64 | 0.85 | <0.001 |
| Body mass index (kg/m2) | 1.01 | 0.98 | 1.04 | 0.48 |
| Current Smoker | 1.20 | 0.73 | 1.81 | 0.49 |
HF=heart failure
Table 3.
Multivariable Model: Baseline Predictors of Acute Decompensated HF
| Baseline Variables | Hazard Ratio | 95% Confidence Interval | P value | |
|---|---|---|---|---|
| Randomized to Intensive Blood Pressure Arm | 0.60 | 0.44 | 0.82 | 0.001 |
| Chronic kidney disease | 2.14 | 1.54 | 2.99 | <.0001 |
| Age≥ 75 years | 2.35 | 1.59 | 3.48 | <.0001 |
| History of Cardiovascular disease | 2.02 | 1.43 | 2.81 | <.0001 |
| Female | 0.72 | 0.49 | 1.03 | 0.077 |
| Black Race | 1.31 | 0.85 | 1.98 | 0.22 |
| Seated Systolic Blood Pressure (10 unit increase) | 1.16 | 1.04 | 1.30 | 0.008 |
| Seated Diastolic Blood Pressure (10 unit increase) | 0.80 | 0.68 | 0.95 | 0.012 |
| Body mass index (kg/m2) | 1.04 | 1.02 | 1.07 | 0.002 |
| Current Smoker | 1.66 | 1.01 | 2.65 | 0.038 |
HF=heart failure
Figure 2.
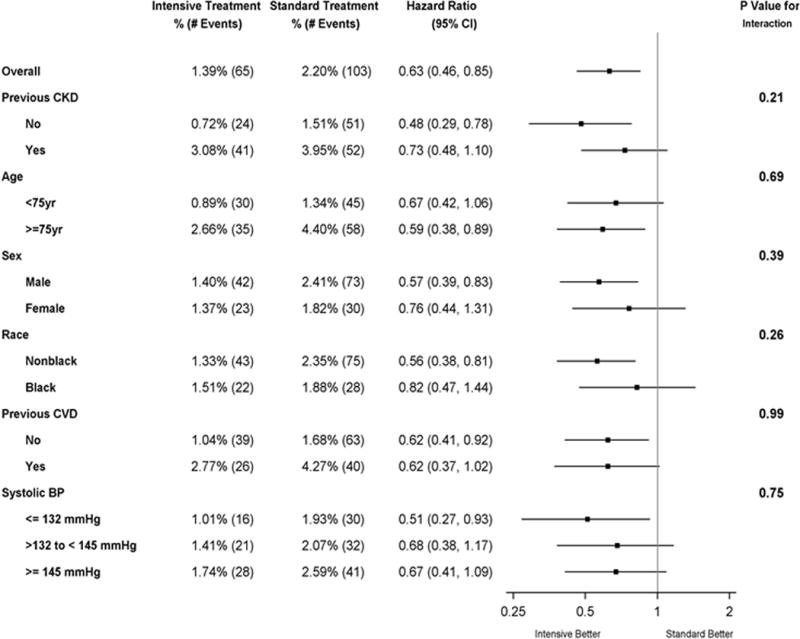
Forest plot of acute decompensated heart failure according to subgroups. The dashed vertical line represents the hazard ratio for the overall study population. The box sizes are proportional to the precision of the estimates (with larger boxes indicating a greater degree of precision). The subgroup of no previous chronic kidney disease (CKD) includes some participants with unknown CKD status at baseline. Black race includes Hispanic black and black as part of a multiracial identification.
Clinical outcomes following ADHF events
Participants who were adjudicated as having an incident ADHF event had markedly increased risk of subsequent CV outcomes and death (regardless of treatment arm. Even after adjusting for treatment arm, gender, baseline age, baseline CKD, and baseline CVD, the hazard ratio for subsequent CV events and death among participants who experienced an initial ADHF event remained very high (Table 4). Among the 168 participants who had an initial ADHF event, 48 (29%) had at least one recurrent ADHF event. There was no significant difference in this regard between treatment group: 29 of 103 (28%) standard arm and 19 of 65 (29%) intensive arm; HR 0.93 (95% CI 0.50–1.67, p-value 0.81). Black race and CKD sub group were the most significant baseline predictors of recurrent ADHF events (Table 5).
Table 4.
Subsequent Clinical Outcomes Based on Initial ADHF Occurrence
| Outcome | No ADHF (n=9193) |
ADHF (n=168) |
Hazard Ratio | Lower 95% CI | Higher 95% CI | P value |
|---|---|---|---|---|---|---|
| No. (%) | No (%) | |||||
| Death from any cause - no (%) | 323 (3.5) | 44 (26.2) | 9.5 | 6.7 | 13.1 | <0.001 |
| Death from cardiovascular causes- no (%) | 79 (0.9) | 26 (15.5) | 26.8 | 16.2 | 43.0 | <0.001 |
| Myocardial infarction (MI) -no (%) | 180 (2.0) | 38 (22.6) | 15.7 | 9.9 | 24.0 | <0.001 |
| Non MI acute coronary syndrome - no (%) | 75 (0.8) | 7 (4.2) | 9.9 | 3.7 | 21.6 | <0.001 |
| Stroke - no (%) | 122 (1.3) | 16 (9.5) | 4.0 | 1.4 | 8.8 | 0.003 |
ADHF=acute decompensated heart failure, CI=confidence interval. Data are adjusted for treatment arm, gender, baseline age (in years), baseline chronic kidney disease, and baseline cardiovascular disease.
ADHF was a time-dependent covariate in these models
Table 5.
Multivariable Model Showing Predictors of Recurrence of ADHF Among 168 Participants Experiencing a First ADHF
| Baseline Variables | Hazard Ratio | 95% Confidence Interval | P value | |
|---|---|---|---|---|
| Intensive Blood Pressure Treatment Arm | 0.93 | 0.50 | 1.67 | 0.81 |
| Subgroup with Age ≥ 75 years | 0.98 | 0.51 | 1.90 | 0.94 |
| Female sex | 0.79 | 0.40 | 1.52 | 0.49 |
| Black Race | 2.14 | 1.07 | 4.22 | 0.029 |
| Subgroup with CVD History | 0.78 | 0.42 | 1.42 | 0.43 |
| Subgroup CKD | 1.99 | 1.04 | 3.93 | 0.042 |
| Systolic Blood Pressure (units of 10) | 1.11 | 0.92 | 1.33 | 0.26 |
| Diastolic Blood Pressure (units of 10) | 1.12 | 0.85 | 1.44 | 0.41 |
| Body Mass Index (kg/m2) | 1.03 | 0.98 | 1.08 | 0.31 |
| Current Smoker | 0.34 | 0.08 | 0.98 | 0.081 |
ADHF=acute decompensated heart failure, CVD=cardiovascular disease, CKD=chronic kidney disease
DISCUSSION
ADHF was one of the most frequent outcomes in the SPRINT trial, which was terminated early due to a 25% reduction in the primary outcome, a composite of CV events, including ADHF.3 The present report, which includes six additional ADHF events adjudicated since the main trial outcome report, showed that among participants with systolic hypertension who were age ≥ 50 years and at high risk for CV events, treatment that targets a systolic BP of less than 120 mm Hg, as compared with less than 140 mm Hg, resulted in a 36% lower rate of ADHF. The beneficial effect of the intervention on the ADHF event rate became apparent early, at 6-month follow-up, and increased with duration of follow-up. The beneficial effect was consistent across all the key pre-specified subgroups, including age ≥75 years or < 75 years, with or without prior CVD, with or without CKD, women or men, black race or non-black race, and the tertiles of baseline systolic BP. In multivariable analyses that included treatment arm, most subgroup characteristics except black race and female sex were significant independent predictors of development of ADHF. Finally, participants who had an initial ADHF event had markedly increased risk of subsequent events, including recurrent ADHF. In comparison to participants without an initial ADHF event, these participants had 27-fold higher risk of CV death, 16-fold higher risk of MI and 10-fold higher risk of all-cause death, even after adjustment for relevant covariates. These data highlight the importance of preventing ADHF in high risk hypertensive patients by optimal BP reduction.
The robustness of the current results from SPRINT is supported by the large magnitude of the risk reduction, the early separation of groups during follow-up, and the consistency of the effect across all key pre-specified subgroups. The results are further supported by including only ADHF events, use of a formal, robust, blinded adjudication process, and the finding of markedly worsened prognosis after a positively adjudicated ADHF event.
These results from the large SPRINT trial significantly extend our knowledge of the impact of BP lowering on HF prevention in older persons with systolic HTN. Several prior trials in older patients with systolic HTN showed large reductions in new HF events (64% in the HYVET study, 50% in the SHEP trial, and 36% in the Syst-Eur) resulting from systolic BP reductions to 140–45 mmHg.28–30 HYVET included 3845 participants aged 80 years or older (mean age, 83.6 years; mean BP, 173/91 mm Hg) and of whom 11.8% had a history of CVD. SHEP included 4736 persons aged ≥60 years with mean baseline BP of 170/77 mm Hg. Syst-Eur included 4695 persons aged ≥60 years with mean baseline BP of 174/86 mmHg. The particularly large reduction in HF events in HYVET likely reflects the older age of the participants compared to the other two trials. A larger benefit was also observed in participants aged >80 years in the SHEP trial.
Although the benefits of lowering SBP to the 140–145 mmHg range for preventing HF events was well established by previous trials,27–29 there has been a paucity of information regarding the potential benefit and risk of lowering BP further. Data regarding the effect of BP reduction to <130 mmHg on HF events have come primarily from observational studies and have been variable. Analysis from ONTARGET suggested that BP control to <130/80 mmHg conferred additional protection against stroke and renal disease but not MI or HF.31 ACCORD was the only previous large randomized trial that specifically addressed the potential benefit of lowering systolic BP to <130 mmHg (the target was 120 mmHg). The effects on HF outcomes in SPRINT and the ACCORD trial are directionally consistent,3, 32 although HF event reduction in the ACCORD trial was smaller in magnitude and not statistically significant (HR 0.94; CI 0.70–1.26). Several important differences between these trials should be noted. ACCORD recruited patients with diabetes mellitus only, while SPRINT specifically excluded these patients. In addition, the event rate in the standard-therapy group in ACCORD was almost 50% lower than expected. This lower event rate was likely due to several factors. First, inclusion criteria directed participants with dyslipidemia into the ACCORD lipid trial, leaving participants who were at lower risk for cardiovascular events to be enrolled into the BP trial. Second, the sample size of ACCORD was only half that of SPRINT (4733 versus 9361), and excluded persons with CKD and those aged >79 years, and so may not have been adequately powered to examine HF. SPRINT enrolled an older cohort (mean age 68 years versus 62 years in the ACCORD trial), with 28% of participants ≥75 years and mandated inclusion of significant proportions of patients with pre-existing CVD and CKD. ACCORD also used a factorial design that included comparisons of standard and intensive glycemic and lipid treatment targets in the same trial. Thus, differences in results between SPRINT and ACCORD could be due to differences in study design, study population, and treatment interactions. As a result of these issues, the lower power in ACCORD was such that the confidence limits for HF events could not exclude a benefit. Consistent with this finding, the recently published SPRINT SENIOR study, which included 2636 elderly participants who were age ≥75 years (mean age, 79.9 years), showed a significantly lower rate of the primary composite outcome, ADHF events and all-cause mortality in the intensive treatment group compared to the standard treatment group (the mean SBP in the intensive treatment group was 123.4 mm Hg vs. 134.8 mm Hg in the standard treatment group). The overall serious adverse event rate was comparable by treatment group, including among the frailest participants. There were no differences between treatment groups in injurious falls, orthostatic hypotension or syncope.24
The present results are consistent with the findings of two recent meta-analyses. The first included 613,825 subjects and showed that every 10 mmHg reduction in systolic BP reduced the risk of major HF events by 28%.33 The proportional reductions per 10 mmHg decrease in systolic BP were greater for stroke and HF than for coronary heart disease, and there was a trend towards decreased HF events even with baseline systolic BP <130 mmHg.33 The second meta-analysis included 35 HTN treatment trials with HF events and showed a strong, significant correlation between the extent of SBP and DBP reduction and the reduction in HF events.34 The relation between BP reduction and reduction in events was stronger for HF than for mortality and coronary events.
In the present study, both higher baseline systolic BP and lower baseline diastolic BP were associated with higher ADHF risk. Because coronary perfusion occurs predominantly during diastole, excessively diastolic BP could potentially pose a risk in patients with coronary artery disease.35 Similarly, a recent study demonstrated that among adults with a SBP ≥ 120 mm Hg, low DBP, particularly < 60 mm of Hg was associated with subclinical myocardial damage and coronary artery disease events.36 However, in both the main SPRINT and the SPRINT SENIORS trials, ADHF events were lower in the intensive arm compared to the standard arm despite lower diastolic BPs (SPRINT: 68.7 versus 76.3 mmHg; SPRINT SENIORS: 62.0 versus 67.2 mm Hg).3, 24 In the SHEP trial, although the incidence of CVD events was higher among participants in the treatment arm whose diastolic BPs were lowered to <70 mmHg compared to those ≥ 70mmHg, the incidence of CVD events was still lower than in the participants in the placebo arm.9 Finally, in a recent meta-analysis, the reduction in HF events was related to the degree of DBP reduction.34
Participants who developed ADHF in SPRINT had markedly increased risk of subsequent all-cause mortality, CV mortality, CV events, and readmission for recurrent ADHF. These results are similar to findings from multiple other studies,37–39 and support the validity of adjudicated ADHF events in SPRINT. Among baseline characteristics, black race was a significant predictor of ADHF recurrence in SPRINT. Assignment to the intensive BP treatment arm appeared to have a neutral effect on recurrence of ADHF events (and other subsequent CV outcomes). However, because the trial was stopped early due to benefit, the duration of follow-up was much shorter than planned, and the number of recurrent HF events was relatively small. In addition, after an ADHF event, there were usually marked changes in medications in order to treat the ADHF. These factors likely confounded any ability to discern an effect of initial SPRINT treatment assignment on recurrent ADHF events. However, the grave prognosis of participants who developed ADHF strongly emphasize the importance of measures to prevent incident ADHF, including intensive BP reduction.
The study has several strengths, including the large sample size, a diverse population at high risk for CV events, a large proportion of participants age ≥ 75 years who have previously been under-represented in hypertension trials, and designation of key subgroups prior to trial launch. Another strength of the study is that, since ADHF was a component of the composite primary trial outcome, robust procedures were undertaken to ensure validity of the ADHF events in SPRINT, including: protocols for capture of events and obtaining medical records from field sites; regularly scheduled participant queries to ascertain events; formal review by an events adjudication committee; formal event definitions that required fulfillment of multiple, specific criteria; inclusion of acute, decompensated HF that resulted in hospitalization or emergency department visit with intravenous diuretic or inotrope infusion and appropriate response to therapy; and exclusion of chronic, stable HF, which is a more challenging and variable event for trial adjudication.
Potential limitations include that this is a secondary analysis, and that there is uncertainty regarding generalizability of the findings to populations not included in the trial, including those with diabetes mellitus, prior stroke, low risk for CV events, age < 50 years, and persons residing in nursing homes or assisted-living facilities. Lowering BP in individuals with diabetes is an area of significant current controversy, with particular debate surrounding who should be offered therapy and the BP targets to be achieved. A recent meta-analysis of treatment trials in people with type 2 diabetes mellitus who had systolic BP ≤135 mmHg in the intensive BP group and ≤140 mmHg in the standard BP group found a 10% (95% CI 2–17%) reduction in total mortality.40 In addition, a secondary analysis of the ACCORD results showed that, as compared with the combined standard glycemia and BP arm, intensive BP treatment alone reduced major CV outcomes by 26% without an additional benefit from combining the two intensive treatments.41 However, although the beneficial effect of intensive BP reduction on CV outcomes appears to be potentially similar between the populations with and without diabetes, the optimal BP target in DM is still uncertain. Our data do not include a pre-diabetes subgroup, which is another group of significant interest.
Although the subgroups examined in this manuscript were pre-specified in the SPRINT protocol, the study was not powered to detect an interaction between treatment arm and any subgroup. Thus, we have presented unadjusted p-values, along with hazard ratios and confidence intervals, as simple guides to possible associations. Our finding that black race was not a significant predictor of incident ADHF may have been due to the somewhat lower (4 years) average age compared to non-blacks.
Since new outpatient HF was not included as an event, we may have underestimated the effect of the intervention on overall incident HF.
CONCLUSIONS
Acute decompensated HF was one of the most frequent CV outcomes in SPRINT. In patients at high risk for CV events, targeting a systolic BP of less than 120 mm Hg, as compared with less than 140 mm Hg, significantly reduced the risk of developing ADHF by 36%. This benefit was similar across all key, pre-determined subgroups. Participants who developed ADHF had a markedly increased risk for subsequent death and CV events, even during the truncated duration of follow-up due to early trial termination. These findings highlight the importance of strategies aimed at the prevention of ADHF, especially intensive BP reduction.
Clinical Perspective.
This manuscript from the large Systolic Blood Pressure Reduction Intervention Trial (SPRINT) trial reports the incidence, predictors, and outcomes of participants who developed acute decompensated heart failure (ADHF), one of the main trial outcomes, during the 3.5 years of follow-up before the trial was prematurely terminated due to benefit. This the first report from a prospective, randomized trial of the effect of intensive blood pressure reduction (to 120mmHg or less) in older persons with systolic hypertension. The robustness of the current results from SPRINT is supported by the large magnitude of the risk reduction, the early separation of groups during follow-up, and the consistency of the effect across all key pre-specified subgroups. The trial addresses several points of controversy, including the optimal level of blood pressure reduction and whether benefit is similar across multiple, pre-defined subsets of patients, including elderly, women, minorities, patients with pre-existing cardiovascular and / or kidney disease, and by 3 different levels of baseline blood pressure. The results are potentially paradigm shifting for the prevention of heart failure through intensive blood pressure management.
Acknowledgments
The SPRINT investigators acknowledge the contribution of study medications (azilsartan and azilsartan combined with chlorthalidone) from Takeda Pharmaceuticals International, Inc. All components of the SPRINT study protocol were designed and implemented by the investigators. The investigative team collected, analyzed, and interpreted the data. All aspects of manuscript writing and revision were carried out by the coauthors. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH, the U.S. Department of Veterans Affairs, or the United States Government. For a full list of contributors to SPRINT, please see the supplementary acknowledgement list: https://www.sprinttrial.org/public/dspScience.cfm. Role of the Funder/Sponsor: The SPRINT steering committee was responsible for the design and conduct of the study, including the collection and management of the data. Scientists at the National Institutes of Health as a group and the principal investigator of the Veterans Affairs clinical network had 1 vote on the steering committee of the trial. There were 7 voting members of the steering committee. The National Institutes of Health, the US Department of Veterans Affairs, and the US government had no role in analysis and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Sources of Funding: The Systolic Blood Pressure Intervention Trial is funded with Federal funds from the National Institutes of Health (NIH), including the National Heart, Lung, and Blood Institute (NHLBI), the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), the National Institute on Aging (NIA), and the National Institute of Neurological Disorders and Stroke (NINDS), under Contract Numbers HHSN268200900040C, HHSN268200900046C, HHSN268200900047C, HHSN268200900048C, HHSN268200900049C, and Inter-Agency Agreement Number A-HL-13-002-001. It was also supported in part with resources and use of facilities through the Department of Veterans Affairs. We also acknowledge the support from the following Clinical and Transitional Science Awards (CTSAs) funded by National Center For Advancing Translational Sciences (NCATS): CWRU: UL1TR000439, OSU: UL1RR025755, U Penn: UL1RR024134& UL1TR000003, Boston: UL1RR025771, Stanford: UL1TR000093, Tufts: UL1RR025752, UL1TR000073 & UL1TR001064, University of Illinois: UL1TR000050, University of Pittsburgh: UL1TR000005, UT Southwestern: 9U54TR000017-06, University of Utah: UL1TR000105-05, Vanderbilt University: UL1 TR000445, George Washington University: UL1TR000075, University of CA, Davis: UL1 TR000002, University of Florida: UL1 TR000064, University of Michigan: UL1TR000433, Tulane University: P30GM103337 COBRE Award NIGMS. This work was also supported in part by NIH grants R01AG18915, 1R01HL107257, P30AG021332, and R01AG020583 (Dr. Kitzman).
Footnotes
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, the US Department of Veterans Affairs, or the US government.
Previous Presentation: Presented in part at the American Society of Hypertension Annual Meeting; May 14, 2016; New York, NY.
Conflict of Interest Disclosures: The authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Kitzman reported receiving personal fees from Merck, Forest Labs, and Abbvie; personal fees and other from Gilead and Relypsa; and grants from Novartis outside the submitted work. Dr Oparil reported receiving personal fees from Forest Laboratories Inc; grants, personal fees, and nonfinancial support from Medtronic; personal fees from Amgen (Onyx is subsidiary); grants and personal fees from AstraZeneca and Bayer Healthcare Pharmaceuticals Inc; personal fees from Boehringer-Ingelheim and GlaxoSmithKline; grants from Merck and Co; and serving as co-chair for the Eighth Joint National Committee. Dr. Lewis reported research funding from Novo Nordisk. Dr. Cushman reported receiving institutional grants from Eli Lilly and Boehringer Ingelheim, and has provided uncompensated consultation to Takeda Pharmaceuticals. No other disclosures were reported.
Reference List
- 1.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–259. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 2.Levy D, Larson MG, Vasan RS, Kannel WB, Ho KK. The progression from hypertension to congestive heart failure. JAMA. 1996;275:1557–1562. [PubMed] [Google Scholar]
- 3.Wright JT, Jr, Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, Reboussin DM, Rahman M, Oparil S, Lewis CE, Kimmel PL, Johnson KC, Goff DC, Jr, Fine LJ, Cutler JA, Cushman WC, Cheung AK, Ambrosius WT. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N Engl J Med. 2015;373:2103–2116. doi: 10.1056/NEJMoa1511939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lloyd-Jones DM, Larson MG, Leip EP, Beiser A, D’Agostino RB, Kannel WB, Murabito JM, Vasan RS, Benjamin EJ, Levy D. Lifetime risk for developing congestive heart failure: the Framingham Heart Study. Circulation. 2002;106:3068–3072. doi: 10.1161/01.cir.0000039105.49749.6f. [DOI] [PubMed] [Google Scholar]
- 5.Franklin SS, Gustin W, Wong ND, Larson MG, Weber MA, Kannel WB, Levy D. Hemodynamic patterns of age-related changes in blood pressure. The Framingham Heart Study. Circulation. 1997;96:308–315. doi: 10.1161/01.cir.96.1.308. [DOI] [PubMed] [Google Scholar]
- 6.Psaty BM, Furberg CD, Kuller LH, Borhani NO, Rautaharju PM, O’Leary DH, Bild DE, Robbins J, Fried LP, Reid C. Isolated systolic hypertension and subclinical cardiovascular disease in the elderly. Initial findings from the Cardiovascular Health Study. JAMA. 1992;268:1287–1291. [PubMed] [Google Scholar]
- 7.Ekundayo OJ, Allman RM, Sanders PW, Aban I, Love TE, Arnett D, Ahmed A. Isolated systolic hypertension and incident heart failure in older adults: a propensity-matched study. Hypertension. 2009;53:458–465. doi: 10.1161/HYPERTENSIONAHA.108.119792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benetos A, Zureik M, Morcet J, Thomas F, Bean K, Safar M, Ducimetiere P, Guize L. A decrease in diastolic blood pressure combined with an increase in systolic blood pressure is associated with a higher cardiovascular mortality in men. J Am Coll Cardiol. 2000;35:673–680. doi: 10.1016/s0735-1097(99)00586-0. [DOI] [PubMed] [Google Scholar]
- 9.Somes GW, Pahor M, Shorr RJ, Cushman WC, Applegate W. The role of diastolic blood pressure when treating isolated systolic hypertension. Arch Intern Med. 1999;159:2004–2009. doi: 10.1001/archinte.159.17.2004. [DOI] [PubMed] [Google Scholar]
- 10.Mosterd A, Hoes AW, de Bruyne MC, Deckers JW, Linker DT, Hofman A, Grobbee DE. Prevalence of heart failure and left ventricular dysfunction in the general population: The Rotterdam Study. Eur Heart J. 1999;20:447–455. [PubMed] [Google Scholar]
- 11.Bui AL, Horwich TB, Fonarow GC. Epidemiology and risk profile of heart failure. Nat Rev Cardiol. 2011;8:30–41. doi: 10.1038/nrcardio.2010.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lam CSP, Carson P, Anand IS, Rector TS, Kuskowski M, Komajda M, McKelvie RS, McMurray JJ, Zile M, Massie BM, Kitzman DW. Sex differences in clinical characteristics and outcomes in elderly patients with heart failure and preserved ejection fraction: The I-PRESERVED Trial. Circ Heart Fail. 2012;5:571–578. doi: 10.1161/CIRCHEARTFAILURE.112.970061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Douglas PS, Katz SE, Weinberg EO, Chen MH, Bishop SP, Lorell BH. Hypertrophic remodeling: gender differences in the early response to left ventricular pressure overload. J Am Coll Cardiol. 1998;32:1118–1125. doi: 10.1016/s0735-1097(98)00347-7. [DOI] [PubMed] [Google Scholar]
- 14.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Mackey RH, Magid DJ, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, III, Moy CS, Mussolino ME, Neumar RW, Nichol G, Pandey DK, Paynter NP, Reeves MJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Heart disease and stroke statistics–2014 update: a report from the American Heart Association. Circulation. 2014;129:e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bahrami H, Kronmal R, Bluemke DA, Olson J, Shea S, Liu K, Burke GL, Lima JA. Differences in the incidence of congestive heart failure by ethnicity: the multi-ethnic study of atherosclerosis. Arch Intern Med. 2008;168:2138–2145. doi: 10.1001/archinte.168.19.2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kalogeropoulos A, Georgiopoulou V, Kritchevsky SB, Psaty BM, Smith NL, Newman AB, Rodondi N, Satterfield S, Bauer DC, Bibbins-Domingo K, Smith AL, Wilson PW, Vasan RS, Harris TB, Butler J. Epidemiology of incident heart failure in a contemporary elderly cohort: the health, aging, and body composition study. Arch Intern Med. 2009;169:708–715. doi: 10.1001/archinternmed.2009.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bagshaw SM, Cruz DN, Aspromonte N, Daliento L, Ronco F, Sheinfeld G, Anker SD, Anand I, Bellomo R, Berl T, Bobek I, Davenport A, Haapio M, Hillege H, House A, Katz N, Maisel A, Mankad S, McCullough P, Mebazaa A, Palazzuoli A, Ponikowski P, Shaw A, Soni S, Vescovo G, Zamperetti N, Zanco P, Ronco C. Epidemiology of cardio-renal syndromes: workgroup statements from the 7th ADQI Consensus Conference. Nephrol Dial Transplant. 2010;25:1406–1416. doi: 10.1093/ndt/gfq066. [DOI] [PubMed] [Google Scholar]
- 18.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Mackey RH, Magid DJ, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, III, Moy CS, Mussolino ME, Neumar RW, Nichol G, Pandey DK, Paynter NP, Reeves MJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Executive summary: heart disease and stroke statistics–2014 update: a report from the American Heart Association. Circulation. 2014;129:399–410. doi: 10.1161/01.cir.0000442015.53336.12. [DOI] [PubMed] [Google Scholar]
- 19.Loehr LR, Rosamond WD, Chang PP, Folsom AR, Chambless LE. Heart Failure Incidence and Survival (from the Atherosclerosis Risk in Communities Study) Am J Cardiol. 2008;101:1016–22. doi: 10.1016/j.amjcard.2007.11.061. [DOI] [PubMed] [Google Scholar]
- 20.Dries DL, Exner DV, Gersh BJ, Cooper HA, Carson PE, Domanski MJ. Racial differences in the outcome of left ventricular dysfunction [see comments] [published erratum appears in N Engl J Med 1999 Jul 22;341(4):298] N Engl J Med. 1999;340:609–616. doi: 10.1056/NEJM199902253400804. [DOI] [PubMed] [Google Scholar]
- 21.Agarwal R, Sinha AD. Cardiovascular protection with antihypertensive drugs in dialysis patients: systematic review and meta-analysis. Hypertension. 2009;53:860–866. doi: 10.1161/HYPERTENSIONAHA.108.128116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heerspink HJ, Ninomiya T, Zoungas S, de Zeeuw D, Grobbee DE, Jardine MJ, Gallagher M, Roberts MA, Cass A, Neal B, Perkovic V. Effect of lowering blood pressure on cardiovascular events and mortality in patients on dialysis: a systematic review and meta-analysis of randomised controlled trials. Lancet. 2009;373:1009–1015. doi: 10.1016/S0140-6736(09)60212-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ambrosius WT, Sink KM, Foy CG, Berlowitz DR, Cheung AK, Cushman WC, Fine LJ, Goff DC, Jr, Johnson KC, Killeen AA, Lewis CE, Oparil S, Reboussin DM, Rocco MV, Snyder JK, Williamson JD, Wright JT, Jr, Whelton PK. The design and rationale of a multicenter clinical trial comparing two strategies for control of systolic blood pressure: the Systolic Blood Pressure Intervention Trial (SPRINT) Clin Trials. 2014;11:532–546. doi: 10.1177/1740774514537404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Williamson JD, Supiano MA, Applegate WB, Berlowitz DR, Campbell RC, Chertow GM, Fine LJ, Haley WE, Hawfield AT, Ix JH, Kitzman DW, Kostis JB, Krousel-Wood MA, Launer LJ, Oparil S, Rodriguez CJ, Roumie CL, Shorr RI, Sink KM, Wadley VG, Whelton PK, Whittle J, Woolard NF, Wright JT, Jr, Pajewski NM. Intensive vs Standard Blood Pressure Control and Cardiovascular Disease Outcomes in Adults Aged >/=75 Years: A Randomized Clinical Trial. JAMA. 2016;315:2673–2682. doi: 10.1001/jama.2016.7050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosamond WD, Chang PP, Baggett C, Johnson A, Bertoni AG, Shahar E, Deswal A, Heiss G, Chambless LE. Classification of heart failure in the atherosclerosis risk in communities (ARIC) study: a comparison of diagnostic criteria. Circ Heart Fail. 2012;5:152–159. doi: 10.1161/CIRCHEARTFAILURE.111.963199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crowley J, Hu M. Covariance Analysis of Heart Transplant Survival Data. J Am Stat Assoc. 1977;72:27–36. [Google Scholar]
- 27.Altman DG, Andersen PK. Calculating the number needed to treat for trials where the outcome is time to an event. BMJ. 1999;319:1492–1495. doi: 10.1136/bmj.319.7223.1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beckett S, Peters R, Fletcher AE, Staessen JA, Liu L, Dumitrascu D, Stoyanovsky V, Antikainen RL, Nikitin Y, Anderson C, Belhani A, Forette F, Rajkumar C, Thijs L, Banya W, Bulpitt CJ, the HYVET Study Group Treatment of hypertension in patients 80 years of age or older. N Engl J Med. 2008;358:1887–98. doi: 10.1056/NEJMoa0801369. [DOI] [PubMed] [Google Scholar]
- 29.Kostis J, Davis BR, Cutler JA, Grimm RH, Jr, Berge KG, Cohen JD, Lacy CR, Perry HM, Jr, Blaufox MD, Wassertheil-Smoller S, Black HR, Schron E, Berkson DM, Curb JD, Smith WM, McDonald R, Applegate WB, SHEP Cooperative Research Group Prevention of heart failure by antihypertensive drug treatment in older persons with isolated systolic hypertension. JAMA. 1997;278:212–216. [PubMed] [Google Scholar]
- 30.Stassen JA, Fagard R, Thijs L, Celis H, Arabidze GG, Birkenhäger WH, Bulpitt CJ, de Leeuw PW, Dollery CT, Fletcher AE, Forette F, Leonetti G, Nachev C, O’Brien ET, Rosenfeld J, Rodicio JL, Tuomilehto J, Zanchetti A, The Systolic Hypertension in Europe (Syst-Eur) Trial Investigators Randomised double-blind comparison of placebo and active treatment for older patients with isolated systolic hypertension. Lancet. 1997;350:757–764. doi: 10.1016/s0140-6736(97)05381-6. [DOI] [PubMed] [Google Scholar]
- 31.Mancia G, Schumacher H, Redon J, Verdecchia P, Schmieder R, Jennings G, Yusoff K, Ryden L, Liu GL, Teo K, Sleight P, Yusuf S. Blood pressure targets recommended by guidelines and incidence of cardiovascular and renal events in the Ongoing Telmisartan Alone and in Combination With Ramipril Global Endpoint Trial (ONTARGET) Circulation. 2011;124:1727–1736. doi: 10.1161/CIRCULATIONAHA.110.008870. [DOI] [PubMed] [Google Scholar]
- 32.The ACCORD Study Group. Effects of Intensive Blood-Pressure Control in Type 2 Diabetes Mellitus. N Engl J Med. 2010;362:1575–1585. doi: 10.1056/NEJMoa1001286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ettehad D, Emdin CA, Kiran A, Anderson SG, Callender T, Emberson J, Chalmers J, Rodgers A, Rahimi K. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet. 2016;387:957–967. doi: 10.1016/S0140-6736(15)01225-8. [DOI] [PubMed] [Google Scholar]
- 34.Thomopoulos C, Parati G, Zanchetti A. Effects of blood pressure-lowering treatment. 6. Prevention of heart failure and new-onset heart failure - meta-analyses of randomized trials. J Hypertens. 2016;34:373–384. doi: 10.1097/HJH.0000000000000848. [DOI] [PubMed] [Google Scholar]
- 35.Messerli FH, Panjrath GS. The J-curve between blood pressure and coronary artery disease or essential hypertension: exactly how essential? J Am Coll Cardiol. 2009;54:1827–1834. doi: 10.1016/j.jacc.2009.05.073. [DOI] [PubMed] [Google Scholar]
- 36.McEvoy JW, Chen Y, Rawlings A, Hoogeveen RC, Ballantyne CM, Blumenthal RS, Coresh J, Selvin E. Diastolic Blood Pressure, Subclinical Myocardial Damage, and Cardiac Events: Implications for Blood Pressure Control. J Am Coll Cardiol. 2016;68:1713–1722. doi: 10.1016/j.jacc.2016.07.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de FS, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire DK, Mohler ER, III, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB. Heart disease and stroke statistics–2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 38.Yancy C, Lopatin M, Stevenson L, De Marco T, Fonarow G, ADHERE Scientific Advisory Committee and Investigators Clinical presentation, management, and in-hospital outcomes of patients admitted with acute decompensated heart failure with preserved systolic function: a report from the Acute Decompensated Heart Failure National Registry (ADHERE) Database. J Am Coll Cardiol. 2006;47:76–84. doi: 10.1016/j.jacc.2005.09.022. [DOI] [PubMed] [Google Scholar]
- 39.Krumholz HM, Parent EM, Tu N, Vaccarino V, Wang Y, Radford MJ, Hennen J. Readmission after hospitalization for congestive heart failure among Medicare beneficiaries. Arch Intern Med. 1997;157:99–104. [PubMed] [Google Scholar]
- 40.Bangalore S, Kumar S, Lobach I, Messerli FH. Blood pressure targets in subjects with type 2 diabetes mellitus/impaired fasting glucose: observations from traditional and bayesian random-effects meta-analyses of randomized trials. Circulation. 2011;123:2799–2810. doi: 10.1161/CIRCULATIONAHA.110.016337. [DOI] [PubMed] [Google Scholar]
- 41.Margolis KL, O’Connor PJ, Morgan TM, Buse JB, Cohen RM, Cushman WC, Cutler JA, Evans GW, Gerstein HC, Grimm RH, Jr, Lipkin EW, Narayan KM, Riddle MC, Jr, Sood A, Goff DC., Jr Outcomes of combined cardiovascular risk factor management strategies in type 2 diabetes: the ACCORD randomized trial. Diabetes Care. 2014;37:1721–1728. doi: 10.2337/dc13-2334. [DOI] [PMC free article] [PubMed] [Google Scholar]


