Abstract
The American Diabetes Association, JDRF, the European Association for the Study of Diabetes, and the American Association of Clinical Endocrinologists convened a research symposium, “The Differentiation of Diabetes by Pathophysiology, Natural History and Prognosis” on 10–12 October 2015. International experts in genetics, immunology, metabolism, endocrinology, and systems biology discussed genetic and environmental determinants of type 1 and type 2 diabetes risk and progression, as well as complications. The participants debated how to determine appropriate therapeutic approaches based on disease pathophysiology and stage and defined remaining research gaps hindering a personalized medical approach for diabetes to drive the field to address these gaps. The authors recommend a structure for data stratification to define the phenotypes and genotypes of subtypes of diabetes that will facilitate individualized treatment.
Introduction
Though therapeutic algorithms for diabetes encourage individualization of approaches (1), they are often broadly applied in treatment and reimbursement decisions, reinforcing the “one-size-fits-all” approach (2). However, if individualized approaches are successful (if they improve morbidity/mortality and are cost-effective), health care systems are persuaded to adopt them. For example, better insights into the pathophysiology of different types of cancer have led to tailored diagnostic tools and therapies, which have dramatically improved outcomes (3). A similar approach should be realized for diabetes.
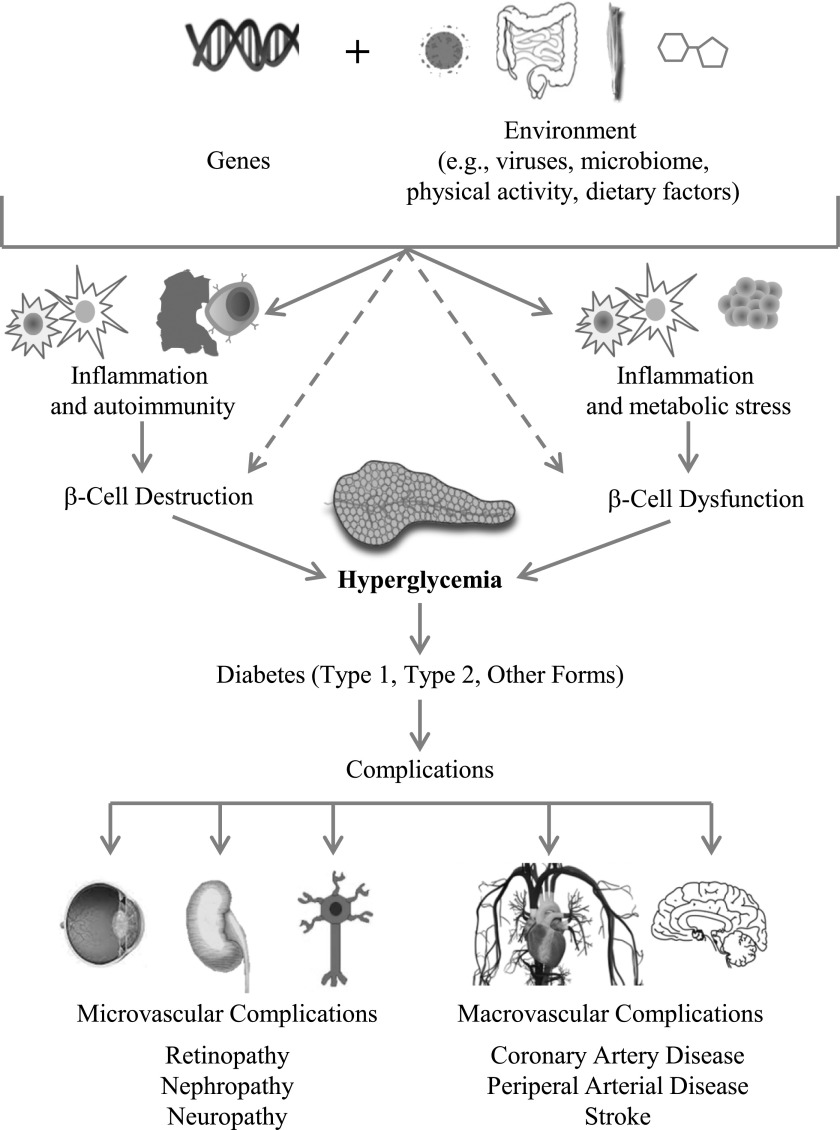
Many different paths, driven by various genetic and environmental factors, result in the progressive loss of β-cell mass (4,5) and/or function (6) that manifests clinically as hyperglycemia. Once hyperglycemia occurs, people with all forms of diabetes are at risk for developing the same complications (Fig. 1), though rates of progression may differ. The present challenge is to characterize the many paths to β-cell dysfunction or demise and identify therapeutic approaches that best target each path. By reviewing the current evidence and addressing remaining research gaps, we aim to identify subtypes of diabetes that may be associated with differential rates of progression and differential risks of complications. A personalized approach to intensive therapy to prevent or treat specific complications may help resolve the burden of diabetes complications, particularly in those at highest risk.
Figure 1.
Genetic and environmental risk factors impact inflammation, autoimmunity, and metabolic stress. These states affect β-cell mass and/or function such that insulin levels are eventually unable to respond sufficiently to insulin demands, leading to hyperglycemia levels sufficient to diagnose diabetes. In some cases, genetic and environmental risk factors and gene–environment interactions can directly impact β-cell mass and/or function. Regardless of the pathophysiology of diabetes, chronic high blood glucose levels are associated with microvascular and macrovascular complications that increase morbidity and mortality for people with diabetes. This model positions β-cell destruction and/or dysfunction as the necessary common factor to all forms of diabetes.
Pathophysiology of Diabetes
Demographics
Type 1 diabetes and type 2 diabetes differentially impact populations based on age, race, ethnicity, geography, and socioeconomic status.
Type 1 Diabetes
Between 2001 and 2009, there was a 21% increase in the number of youth with type 1 diabetes in the U.S. (7). Its prevalence is increasing at a rate of ∼3% per year globally (8). Though diagnosis of type 1 diabetes frequently occurs in childhood, 84% of people living with type 1 diabetes are adults (9). Type 1 diabetes affects males and females equally (10) and decreases life expectancy by an estimated 13 years (11). An estimated 5–15% of adults diagnosed with type 2 diabetes actually have type 1 diabetes or latent autoimmune diabetes of adults (LADA) (12).
Europoid Caucasians have the highest prevalence of type 1 diabetes among U.S. youth, representing 72% of reported cases. Hispanic Caucasians represent 16%, and non-Hispanic blacks represent 9% (7).
Incidence and prevalence rates for type 1 diabetes vary dramatically across the globe. At the extremes, China has an incidence of 0.1/100,000 per year and Finland has an incidence of 60/100,000 per year (13). With some exceptions, type 1 diabetes incidence is positively related to geographic distance north of the equator (13). Colder seasons are correlated with diagnosis and progression of type 1 diabetes. Both onset of disease and the appearance of islet autoimmunity appear to be higher in autumn and winter than in spring and summer (14).
Type 2 Diabetes
In the U.S., an estimated 95% of the nearly 30 million people living with diabetes have type 2 diabetes. An additional 86 million have prediabetes, putting them at high risk for developing type 2 diabetes (9). Among the demographic associations for type 2 diabetes are older age, race/ethnicity, male sex, and socioeconomic status (9).
Type 2 diabetes incidence is increasing in youth, especially among the racial and ethnic groups with disproportionately high risk for developing type 2 diabetes and its complications: American Indians, African Americans, Hispanics/Latinos, Asians, and Pacific Islanders (9). Older age is very closely correlated to risk for developing type 2 diabetes. More than one in four Americans over the age of 65 years have diabetes, and more than half in this age-group have prediabetes (9). The prevalence of type 2 diabetes in the U.S. is higher for males (6.9%) than for females (5.9%) (15).
There is a high degree of variability for prevalence of type 2 diabetes across the globe. East Asia, South Asia, and Australia have more adults with diabetes than any other region (153 million). North America and the Caribbean have the highest prevalence rate, with one in eight affected (8).
Independent of geography, the risk of developing type 2 diabetes is associated with low socioeconomic status. Low educational level increases risk by 41%, low occupation level by 31%, and low income level by 40% (16).
Research Gaps
The assembled experts agreed that research efforts are needed to define causative factors that account for the established correlations among different demographic subsets and the corresponding variable risks for diabetes. Factors associated with race/ethnicity and geography that differentially increase risk for type 1 diabetes and for type 2 diabetes need to be defined. For type 2 diabetes, the drivers of increased risk in individuals of low socioeconomic status also need to be established.
Genetics
Both type 1 and type 2 diabetes are polygenic diseases where many common variants, largely with small effect size, contribute to overall disease risk. Disease heritability (h2), defined as sibling-relative risk, is 3 for type 2 diabetes and 15 for type 1 diabetes (17). The lifetime risk of developing type 2 diabetes is ∼40% if one parent has type 2 diabetes and higher if the mother has the disease (18). The risk for type 1 diabetes is ∼5% if a parent has type 1 diabetes and higher if the father has the disease (19). Maturity-onset diabetes of the young (MODY) is a monogenic disease and has a high h2 of ∼50 (20). Mutations in any 1 of 13 different individual genes have been identified to cause MODY (21), and a genetic diagnosis can be critical for selecting the most appropriate therapy. For example, children with mutations in KCJN11 causing MODY should be treated with sulfonylureas rather than insulin.
Type 1 Diabetes
The higher type 1 diabetes prevalence observed in relatives implies a genetic risk, and the degree of genetic identity with the proband correlates with risk (22–26). Gene variants in one major locus, human leukocyte antigen (HLA) (27), confer 50–60% of the genetic risk by affecting HLA protein binding to antigenic peptides and antigen presentation to T cells (28). Approximately 50 additional genes individually contribute smaller effects (25,29). These contributors include gene variants that modulate immune regulation and tolerance (30–33), variants that modify viral responses (34,35), and variants that influence responses to environmental signals and endocrine function (36), as well as some that are expressed in pancreatic β-cells (37). Genetic influences on the triggering of islet autoimmunity and disease progression are being defined in relatives (38,39). Together, these gene variants explain ∼80% of type 1 diabetes heritability. Epigenetic (40), gene expression, and regulatory RNA profiles (36) may vary over time and reflect disease activity, providing a dynamic readout of risk.
Genetic variants can also identify patients at higher risk, predict rates of C-peptide decline, and predict response to various therapies (41). With a better understanding of inheritance profiles, it may become possible to realize new targets for individualized intervention.
Type 2 Diabetes
While a subset of genetic variants are linked to both type 1 and type 2 diabetes (42,43), the two diseases have a largely distinct genetic basis, which could be leveraged toward classification of diabetes (44). Genome-wide association studies have identified more than 130 genetic variants associated with type 2 diabetes, glucose levels, or insulin levels; however, these variants explain less than 15% of disease heritability (45–47). There are many possibilities for explaining the majority of type 2 diabetes heritability, including disease heterogeneity, gene–gene interactions, and epigenetics. Most type 2 variants are in noncoding genomic regions. Some variants, such as those in KCNQ1, show strong parent-of-origin effects (48). It is possible that children of mothers carrying KCNQ1 are born with a reduced functional β-cell mass and thereby are less able to increase their insulin secretion when exposed to insulin resistance (49). Another area of particular interest has been the search for rare variants protecting from type 2 diabetes, such as loss-of-function mutations in SLC30A8 (50), which could offer potential new drug targets for type 2 diabetes.
To date, however, the improvement in predictive value of known genetic variants over that of classic clinical risk factors (BMI, family history, glucose) has proven minimal in type 2 diabetes.
The rapid development of molecular genetic tools and decreasing costs for next-generation sequencing should make dissection of the black box of genetics of diabetes possible in the near future, but at this point, apart from the profiles that distinguish between type 1 and type 2 diabetes and a limited number of specific variants that identify small subgroups of patients (MODY), genetics has not been successful in further differentiating subclasses of diabetes.
Research Gaps
After consideration of the known genetic associations with diabetes risk, consensus developed that the field is not yet at a place where genetics has provided actionable information to guide treatment decisions, with a few notable exceptions, namely in MODY. The experts agreed there is a need to use the increasingly accessible and affordable technologies to further refine our understanding of how genetic variations affect the rate of progression of diabetes and its complications. The expert committee also highlighted the importance of determining categorical phenotypic subtypes of diabetes in order to link specific genetic associations to these phenotypic subtypes. These types of information are necessary to develop the tools to predict response to—and side effects of—therapeutic approaches for diabetes in patient populations.
Environmental Influences
Despite the genetic underpinnings of the diseases, the prevalence of both type 1 and type 2 diabetes is increasing globally at a rate that outpaces genetic variation, suggesting that environmental factors also play a key role in both types of diabetes. Common environmental factors are associated with type 1 and type 2 diabetes, including dietary factors, endocrine disruptors and other environmental polluters, and gut microbiome composition. In addition to well-established roles in type 2 diabetes, obesity and insulin resistance may be accelerators of type 1 diabetes. Conversely, islet autoimmunity associated with possible environmental triggers (e.g., diet, infection) may have a role in a subset of people diagnosed with type 2 diabetes.
Type 1 Diabetes
Discordance rates in twins, the rise in global incidence, variance in geographic prevalence, and assimilation of local disease incidence rates when individuals migrate from low- to high-incidence countries all support an environmental influence on risk for developing type 1 diabetes. Furthermore, many lines of evidence suggest that environmental factors interact with genetic factors in both the triggering of autoimmunity and the subsequent progression to type 1 diabetes. Supporting this gene–environment interaction is the fact that most subjects with the highest-risk HLA haplotypes do not develop type 1 diabetes.
The timing of exposure to environmental triggers may also be critical. The variability of age at disease onset complicates the study of environmental exposures, though the early age of onset of islet autoantibodies associated with childhood-onset type 1 diabetes suggests that environmental exposures in the first few years of life may be contributors.
Among the environmental associations linked to type 1 diabetes are enteroviral and other infections (51,52) and altered intestinal microbiome composition (53). The timing of exposure to foods including cereal (54) and nutrients such as gluten (55) may influence β-cell autoimmunity. Low serum concentrations of vitamin D have been linked to type 1 diabetes. Perinatal risk factors and toxic doses of nitrosamine compounds have been implicated in the genesis of diabetes.
The effects of any environmental toxin on type 1 diabetes need further exploration. Studies on the environmental contributions to type 1 diabetes have been small and somewhat contradictory, highlighting the need for larger collaborative investigations such as The Environmental Determinants of Diabetes in the Young (TEDDY) (56), which aims to identify infectious agents, dietary factors, and other environmental factors that trigger islet autoimmunity and/or type 1 diabetes.
Type 2 Diabetes
Type 2 diabetes develops when β-cells fail to secrete sufficient insulin to keep up with demand, usually in the context of increased insulin resistance. A minority of people diagnosed with type 2 diabetes also have evidence of islet autoimmunity (57,58). Obesity is a major risk factor for type 2 diabetes (59,60) with complex genetic and environmental etiology.
Insulin resistance develops with ectopic fat deposition in the liver and muscle. Fat may also accumulate in the pancreas and contribute to the decline in β-cell function, islet inflammation, and eventual β-cell death (61). Type 2 diabetes occurs at different levels of BMI/body fat composition in different individuals and at lower BMI for Asians and Asian Americans (62). For susceptible people, there may be a personal “fat threshold” at which ectopic fat accumulation occurs, worsening insulin resistance and resulting in β-cell decompensation.
Weight loss improves insulin sensitivity in liver and skeletal muscle (63) and may also reduce pancreatic fat accumulation (64). Defects in insulin secretion are at least partially reversible with energy restriction and weight loss in prediabetes and recent-onset type 2 diabetes (65). Unfortunately, it is difficult to reverse long-standing diabetes, even with the large weight loss associated with bariatric surgery (66).
Both reduced sleep time and increased sleep time are associated with the development of obesity and diabetes. Obstructive sleep apnea reduces sleep time and sleep quality and is associated with type 2 diabetes and metabolic syndrome. The modern “24-hour culture” may reduce sleep time and thereby also contribute to increased risk of type 2 diabetes. And while associations with additional environmental factors exist, there have been no direct causal relationships defined to date.
Research Gaps
There is a clear correlation of environmental influences to diabetes risk. Yet, the assembled experts agreed that hypothesis-driven research is needed to define direct causal relationships between specific environmental factors and pathophysiologies leading to diabetes. Research efforts need to address environmental etiologies of type 1 diabetes and determine their relative contribution to onset of autoimmunity and progression to symptomatic disease. Whether there is a direct causal role of the intestinal microbiota in pathogenesis of type 1 and type 2 diabetes and response to therapies needs to be determined. Public health interventions that successfully reduce the levels of consumption of energy-dense foods and/or reduce sedentary time and increase time spent in physical activity need to be evaluated to determine whether they can reduce type 2 diabetes incidence at a population level.
Natural History and Prognosis
Regardless of the particular pathophysiology of an individual’s diabetes, the unifying characteristic of the vast majority of diabetes is hyperglycemia resulting from β-cell destruction or dysfunction. There is a continuum of progressive dysglycemia as insulin insufficiency increases over time. Understanding the natural history related to β-cell mass and function is key to staging the diseases and identifying where and how interventions can best be made to prevent or delay disease progression and complications.
β-Cell Mass and Function
While type 1 diabetes results from immune-mediated destruction of β-cells and type 2 diabetes is primarily associated with glucose-specific insulin secretory defects, there is growing evidence of significant overlap across the spectrum of diabetes. For example, β-cell mass is also reduced in people with type 2 diabetes (67). In both type 1 and type 2 diabetes, the stress response induced by hyperglycemia may play a role in β-cell apoptosis (68). Changes in β-cell phenotype associated with hyperglycemia may reflect a dedifferentiation of β-cells important to the natural history and staging of diabetes (69). Clearly, an insufficient number or functional decline of β-cells is central to hyperglycemia and the downstream complications of diabetes. Understanding the state of the β-cell is key to defining subtypes of diabetes.
Type 1 Diabetes
Abnormal insulin secretion can occur well before the diagnosis of type 1 diabetes (70–73), with a gradual decline beginning at least 2 years before diagnosis and accelerating proximal to diagnosis (74,75). A decline in β-cell sensitivity to glucose (76) appears to occur on a similar timeframe. As the early insulin response falters, the later insulin response becomes greater, indicating a possible compensatory mechanism. The accelerated loss of insulin response continues into the early postdiagnostic period (77).
Insulin secretion decline during the first few years after diagnosis has been described as biphasic, steeper during the first year than during the second year after diagnosis. Data also suggest that the rate of decline is slower in adults (78). The loss of insulin secretion can continue for years after diagnosis until little or no insulin secretion remains. However, low levels of C-peptide are detectable in the majority of patients after 30 years of type 1 diabetes (79).
Glucose levels are also frequently elevated years before the diagnosis of type 1 diabetes (80–82). Even within the normal range, higher glucose levels are predictive of type 1 diabetes (83). There are wide fluctuations of glucose during the progression to type 1 diabetes (84). Metabolic markers of progression, such as the occurrence of dysglycemia, could be utilized to more precisely predict the onset of diabetes in at-risk individuals (41,85). Risk scores that combine dynamic changes in glucose and C-peptide can further enhance prediction (86,87).
Type 2 Diabetes
Defective insulin secretion is central to the pathophysiology of type 2 diabetes. To maintain normal glucose levels, insulin secretion varies over a wide range in response to insulin sensitivity. The relationship between insulin secretion and insulin sensitivity is curvilinear and is expressed as the disposition index. People with type 2 diabetes cannot adequately increase insulin secretion to overcome insulin resistance and have a low disposition index (88). Consequently, while absolute insulin levels may be higher in obese subjects with type 2 diabetes who are insulin resistant than they are in lean control subjects who are insulin sensitive, they are lower than appropriate for their degree of insulin resistance. First-phase insulin secretion, especially in response to stimulation by glucose, is markedly impaired or lost (89). Maximal insulin secretion and potentiation by hyperglycemia of insulin responses to nonglucose stimuli are severely reduced (90), and the ratio of proinsulin to insulin (C-peptide) is high in type 2 diabetes (91). Over time, hyperglycemia tends to become more severe and more difficult to treat. This progressive nature of type 2 diabetes is usually due to ongoing deterioration of β-cell function.
While prediabetes and diabetes are diagnosed by absolute thresholds (92), dysglycemia is a continuum progressing from normal to overt diabetes. Early screening offers a window for treatment that may prevent or delay progression of the disease and its complications (93,94). In prediabetes, impaired glucose tolerance or impaired fasting glucose indicates glucose levels higher than normal but not in the diabetes range (92). Currently, most clinicians do not treat these patients to completely control blood glucose levels. Even after initiation of therapy in frank diabetes, intensification of therapy is often delayed (95–97), exposing people to hyperglycemia for years (93).
Several studies have shown that treatment with lifestyle change or medication can reduce the progression from prediabetes to diabetes (98,99). Furthermore, a clinical benefit of early therapy has been demonstrated (100,101), with reductions in retinopathy and cardiovascular and all-cause mortality (102). This evidence suggests that identifying prediabetes at an early stage and keeping glucose levels close to normal could change the natural history of the disease (93).
Research Gaps.
The strong consensus of this group was that the primary defect resulting in hyperglycemia is insufficient β-cell number and/or β-cell function (of various etiologies). From this β-cell–centric view, it is imperative to determine what etiological factors are the basis for abnormal insulin secretion patterns in type 1 diabetes and type 2 diabetes. Biomarkers and imaging tools are needed to assess β-cell mass and loss of functional mass and to monitor progression and response to therapeutic interventions. The point at which β-cell dysfunction becomes irreversible needs to be determined. The molecular basis for the glucose-specific insulin secretory defect and the role of β-cell dedifferentiation in type 1 diabetes and in type 2 diabetes need to be determined. The extent to which insulin resistance contributes to glycemia and the complications of type 1 diabetes remains unknown. Research is needed to determine whether increased β-cell activity, stimulated by insulin resistance, enhances or accelerates the β-cell lesion in type 1 diabetes and in type 2 diabetes and to identify mechanisms by which β-cells can overcome an insulin-resistant environment.
Autoimmunity
Circulating autoantibodies against insulin, glutamic acid decarboxylase (GAD), the protein tyrosine phosphatase IA-2, and/or zinc transporter 8 can be detected prior to clinical diagnosis of type 1 diabetes (103). While individuals with single autoantibody positivity frequently revert to negative, reversion is rare in people with multiple autoantibodies (104). Currently, we lack sufficient biomarkers and imaging techniques to monitor autoantibody flare-ups, reversions, and progression to type 1 diabetes. The presence of two or more islet autoantibodies in children with HLA risk genotypes or with relatives who have type 1 diabetes is associated with a 75% risk of developing clinical diabetes within 10 years (105). Risk is incremental with detection of increasing numbers of autoantibodies (105–107). A positive test for at least two autoantibodies is now considered a diagnostic stage of type 1 diabetes (Table 1) (41). The presence of islet autoantibodies reflects an underlying immune B- and T-cell response to β-cell antigens. Autoimmune responses to β-cells lead to loss of β-cell mass and function and onset of glucose intolerance, representing the next distinct stage prior to onset of clinical symptoms of diabetes.
Table 1.
Staging of type 1 diabetes
| Stage 1 | Stage 2 | Stage 3 | |
|---|---|---|---|
| Phenotypic characteristics | • Autoimmunity• Normoglycemia• Presymptomatic | • Autoimmunity• Dysglycemia• Presymptomatic | • New onset• Hyperglycemia• Symptomatic |
| Diagnostic criteria | • Multiple autoantibodies• No impaired glucose tolerance or impaired fasting glucose | • Multiple autoantibodies• Dysglycemia: impaired fasting glucose and/or impaired glucose tolerance• Fasting plasma glucose 100–125 mg/dL• 2-h glasma glucose 140–199 mg/dL• HbA1c 5.7–6.4% or ≥10% increase in HbA1c | • Clinical symptoms• Diabetes by standard criteria |
Despite the strong prognostic value of autoimmunity in type 1 diabetes, there is no successful strategy to prevent or treat it. HLA confers strong susceptibility for the development of two or more islet autoantibodies (108). For primary prevention of β-cell autoimmunity in children, data suggest there may be a critical period in the first 2 years of life (109–111).
Interestingly, autoantibodies against GAD are present in ∼5% of individuals diagnosed with type 2 diabetes (112). As compared with GAD antibody–negative patients with type 2 diabetes, these patients have lower BMI and residual β-cell function. Further, they carry a genetic profile more similar to that of patients with type 1 diabetes and an earlier requirement for insulin therapy (112), suggesting that autoimmune diabetes in adults may actually be a form of type 1 diabetes that exhibits slow progression associated with later age of onset.
Research Gaps
The assembled group agreed that while it is clear that inflammation and autoimmunity lead to β-cell destruction characteristic of type 1 diabetes, much more information is needed to understand the pathophysiology and progression of autoimmunity related to diabetes in order to develop rational approaches to prevent or reverse it. We do not have a clear understanding of whether different antigenic targets, single-antibody positivity, or other contributing factors have variable prognostic, genetic and environmental correlates that can be used to better develop and apply stage-appropriate personalized therapies. The molecular mechanisms by which β-cells die or fail in the presence of β-cell autoimmunity need determination. Biomarkers and imaging tools are needed to define reversion or stable autoimmunity versus active or flaring autoimmunity. Furthermore, inexpensive specific and sensitive assays to identify β-cell autoimmunity are needed, to be deployed on a population-wide level and beyond the confines of specialized laboratories.
Therapeutics
Aside from insulin and insulin analogs, therapies for diabetes include those that enhance insulin secretion, those that stimulate insulin action, those that reduce hepatic and endogenous glucose production, and those that impact glycemia through other mechanisms. By better understanding the pathophysiology and natural history of various subtypes of diabetes and applying what we know about the modes of action and pharmacogenomics of existing therapies, we can better apply a personalized approach to diabetes management. There is a growing body of evidence regarding which phenotypic and genotypic subsets of patients with diabetes respond best, or are resistant to, specific therapies (113), including sulfonylureas (114,115), metformin (116,117), thiazolidinediones (118,119), incretin therapies (120), and inhibitors of sodium–glucose cotransporter 2 (SGLT2) (121,122).
Type 1 Diabetes
Individuals with type 1 diabetes require intensive therapy, characterized by exogenous insulin administration through multiple daily injections with both fast-acting insulin with meals and basal insulin, or with continuous subcutaneous insulin infusion through pumps. There are no significant generalizable differences in efficacy or safety between the two approaches (123).
The goal of intensive insulin therapy is to maintain as close to normal glucose concentration as possible while avoiding hypoglycemia. Achieving this goal requires individualization of treatment and targets, which may also change over time within individuals. The American Diabetes Association’s glycemic target for adults is HbA1c <7%. However, consideration of individual circumstances is critical. Pediatric patients are recommended to target <7.5%, whereas adults who are able to do so safely should target <6.5% (92).
Both long-acting and short-acting insulin analog preparations with more predictable time-action profiles have been developed, allowing patients to achieve more physiological insulin delivery and, therefore, tighter glucose control with fewer side effects. Technologies for self-monitoring blood glucose and continuous glucose monitoring have advanced in recent years and are becoming more widespread. Continuous glucose monitoring allows patients to visualize changes in glucose levels and tailor their treatment in real time (124). The amylin analog pramlintide is approved for use as an adjunct to insulin in patients with type 1 diabetes who have not achieved glycemic goals despite optimized insulin therapy. Pramlintide lowers postprandial glucose (125), thereby improving overall glycemic control, and it has a modest but significant weight loss effect. However, pramlintide added to insulin may increase the risk of hypoglycemia (126,127).
A number of agents currently approved for the treatment of type 2 diabetes have also been investigated for use in type 1 diabetes, including α-glucosidase inhibitors (128,129), thiazolidinediones (130–132), metformin (133), glucagon-like peptide 1 (GLP-1) receptor agonists (134,135), dipeptidyl peptidase 4 (DPP-4) inhibitors (136), and SGLT2 inhibitors (137,138). The benefits of these agents in type 1 diabetes are not well established, and their eventual use in this population will depend on further demonstration of efficacy and safety.
Type 2 Diabetes
There are many agents now available to treat hyperglycemia in type 2 diabetes, with varying mechanisms of action and targeting different pathophysiological components of the disease. Many agents are not always able to achieve adequate control unless they are started earlier in disease progression or are used in combinations (metformin, SGLT2 inhibitors, DPP-4 inhibitors, GLP-1 receptor agonists, peroxisome proliferator–activated receptor γ agonists). This limitation in efficacy may be due in part to the fact that these agents are often initiated after β-cell function or mass has deteriorated beyond a critical level or to their limited effects on insulin secretion. Many people with type 2 diabetes ultimately require insulin therapy, which reflects long-standing type 2 diabetes and greatly diminished β-cell function but also likely includes individuals who have slowly progressing autoimmune diabetes with adult onset (LADA) or other ambiguous forms of diabetes.
Age
Data from randomized controlled trials in people with type 2 diabetes under the age of 18 years or over the age of 65 years are scarce. Beneficial effects of tight glucose control on complications take years to be realized (139,140). Targets of glucose control should be adapted to life expectancy, frailty, biological age, and social situation rather than just calendar age. HbA1c targets in this population need to be adjusted when using agents that cause side effects such as hypoglycemia. However, overt hyperglycemia needs to be addressed to avoid acute complications of diabetes and a catabolic state (141).
Comorbidities: Kidney Impairment.
Kidney impairment is a prevalent complication of diabetes. It is also an independent comorbidity, very often caused by vascular complications in people with type 2 diabetes. Therapeutic choices become more limited because of contraindications (e.g., metformin) or the need for good kidney function for efficacy (e.g., SGLT2 inhibitors), leaving many patients with only insulin therapy (142). Targets for glucose control in the population with kidney impairment may need to be adapted, as kidney impairment also predisposes to hypoglycemia (143). The use of HbA1c is also problematic in people with kidney impairment because of reduced red blood cell survival, use of erythropoietin, modifications of hemoglobin (e.g., carbamylation), and mechanical destruction of red blood cells on dialysis (144).
Comorbidities: Cardiovascular Complications.
Cardiovascular complications require a multifactorial approach, including blood pressure and lipid control. Hypoglycemia is linked to arrhythmias and mortality in people with a history of cardiovascular events (145). However, when agents that do not cause hypoglycemia can be used, tight glucose control should be sought. Agents such as DPP-4 inhibitors (146–148) and GLP-1 receptor agonists (149) have been shown to be safe in this population. Some agents, such as pioglitazone (150) and metformin (151), may even be cardioprotective. Empagliflozin (152) and liraglutide (153) reduce cardiovascular and all-cause mortality over 2.5–5 years of therapy in patients at high risk of cardiovascular disease. Nephropathy is a recognized risk factor for cardiovascular complications, especially in type 1 diabetes (143).
Weight
To avoid comorbidities and complications associated with obesity, weight management should be a priority in all patients, independent of BMI. Weight loss can be achieved by lifestyle intervention, choosing glucose-lowering drugs that promote weight loss, and incorporating obesity pharmacotherapy or bariatric surgery in appropriate patients (154).
Research Gaps
While research and development efforts over the past few decades have led to the availability of several new classes of medications and new insulin formulations and delivery methods, we still lack a clear understanding of the ideal approaches to selecting appropriate treatment regimens for particular individuals. With a more in-depth characterization of the pathophysiology and natural history of subtypes of diabetes coupled with the pharmacogenomics of new and existing therapies, we can begin to develop a more personalized approach to diabetes management.
Several areas can be immediately addressed. This includes performing clinical trials in vulnerable and understudied populations, including the elderly and children, that are critical to validate more precise evidence-based treatments in these populations. Studies examining the appropriate application of immune therapies in combination (sequentially or simultaneously) to target β-cell specific immune response, islet inflammation, and more global defective immunoregulation are critical. For type 2 diabetes, the early use of combinations of glucose-lowering agents needs to be studied. For people with diabetes who are overweight or obese, studies are needed to determine whether weight loss medication and bariatric surgery could be used to support diabetes treatment goals.
Complications
Intensive glycemic control can reduce diabetes complications (140,155). In fact, in the decades since these studies were first published, rates of microvascular and macrovascular complications of diabetes and deaths from hyperglycemic crisis have substantially decreased (156). However, complications of diabetes remain the greatest health threat to people living with diabetes. Research efforts to identify clinical variables and biomarkers that indicate the presence or progression of complications may lead to a better understanding of risk and help identify individuals who may benefit from particular therapies to reduce the impact of diabetes.
Type 1 Diabetes
The underlying pathophysiology driving an increased risk of cardiovascular complications in type 1 diabetes remains unclear. It is in part related to nephropathy and appears to be distinct from the pathophysiology of cardiovascular complications of type 2 diabetes (157). Intensive treatment of type 1 diabetes with insulin often leads to weight gain. Concurrent with the population-wide rise in incidence of obesity, many people with type 1 diabetes have begun to exhibit features of obesity and metabolic syndrome, likely increasing the development of cardiovascular disease. Current treatment recommendations for management of cardiovascular risk factors predominantly derive from studies on type 2 diabetes or populations that did not discriminate between diabetes type. Risk factors should be monitored and treated in type 1 diabetes to recommended targets, but research is needed to determine distinctions in cardiovascular risk pathophysiology in type 1 diabetes and to identify appropriate therapies to reduce risk.
Kidney disease predicts cardiovascular disease in people with type 1 diabetes (143) and is associated with development of additional microvascular and macrovascular complications over time. People with type 1 diabetes show signs of premature arterial stiffening that is further exaggerated in those with diabetic nephropathy.
There is a genetic propensity for diabetic nephropathy that peaks at 10–14 years duration of type 1 diabetes (158). The risk plateaus after 15 years duration, and the incidence of microalbuminuria matches this pattern (FinnDiane Study Group, unpublished observations). The peak incidence of macroalbuminuria and end-stage kidney disease lags 10 to 15 years behind the appearance of microalbuminuria. Progression to end-stage kidney disease is linked to age of onset and duration of diabetes (159). Female sex seems to be protective if age of onset occurs during or after puberty. Similar factors influence risk for and progression of diabetic retinopathy. Intensive glucose control significantly reduces the risk of diabetic peripheral neuropathy and cardiovascular autonomic neuropathy in type 1 diabetes (160).
Average HbA1c and HbA1c variability are higher in people who progress to diabetic kidney disease (161). Those with more components of metabolic syndrome have more kidney disease and higher HbA1c. A person with type 1 diabetes is much more likely to develop diabetic kidney disease if a sibling with type 1 diabetes has it. The risk of diabetic nephropathy in type 1 diabetes is fourfold higher in children whose mothers have type 1 diabetes than in those without a parent with diabetes (162), indicating a role for epigenetics in the development of kidney disease. Urine metabolites have been identified that highlight potential involvement of mitochondrial dysfunction in diabetic kidney disease (163).
Type 2 Diabetes
A large proportion of people with type 2 diabetes also have nonhyperglycemic components of the metabolic syndrome (164), including hypertension, hyperlipidemia, and increased risk for cardiovascular disease. These metabolic features are interrelated and must be considered collectively. Multiple risk factor reduction is critical. Lipoprotein metabolism is often abnormal in diabetic nephropathy, but treatment strategies to avoid cardiovascular disease in this population are unclear. Statins appear to be ineffective at preventing cardiovascular disease in people with end-stage kidney diease (165,166). Once on statins, fibrates may not be beneficial for preventing cardiovascular disease in this population but might have microvascular benefits through anti-inflammatory actions (167). There are reasonably good data indicating that cholesterol absorption is higher in diabetes, suggesting that ezetimibe might have unique effects in diabetes (168,169).
Cardiovascular disease risk increases substantially when estimated glomerular filtration rate falls below 45 mL/min/1.73 m2. Microalbuminuria is not always due to diabetic nephropathy (170), but it is a marker of inflammation that indicates vascular leakage and increased cardiovascular risk. Albuminuria has been used as a marker of diabetic nephropathy for three decades. Yet, its power is limited. It varies by 25–30% daily in individuals (171–174). It is transient and patients can revert to normal albuminuria without treatment.
Interestingly, the urinary metabolomics signature of diabetic kidney disease is similar in people with type 1 and type 2 diabetes (163). Newly identified biomarkers such as urinary adiponectin and serum tumor necrosis factor-α receptor 1 may be better predictors of nephropathy than albumin excretion rate; however, they require greater evaluation in prospective studies.
Tight glycemic control is the only strategy known to prevent or delay the development of peripheral neuropathy, and cardiac autonomic neuropathy is perhaps even more important in relation to cardiovascular mortality (175). However, randomized clinical trials to determine appropriate targets are lacking. Outcomes for cardiovascular disease and mortality have been mixed in different studies.
Research Gaps
The assembled experts agreed that the means to determine which individuals with diabetes will develop particular complications remain unclear. Research efforts are needed to delineate the mechanisms underpinning the development of complications in type 1 diabetes and type 2 diabetes and identifying the differences between them. For example, the contributions of genetics to development of complications in specific populations need to be determined. The benefits of screening and early treatment to control glucose levels in people with presymptomatic diabetes on the development of complications also needs to be assessed.
In some cases, the data supporting current treatment recommendations are drawn from populations that are too heterogeneous to be sufficiently representative of subtypes of diabetes. For example, current treatment recommendations for management of cardiovascular complications derive predominantly from data in type 2 diabetes or in populations that did not discriminate between diabetes type. Thus, data to support evidence-based targets to avoid cardiovascular complications in type 1 diabetes are needed.
There are also some targeted issues that need to be addressed around specific complications to better inform treatment. For example, because of inconclusive associations, trials are needed to determine whether fibrates are able to modify the natural history of retinopathy and, if so, by what mechanisms. Given the limitations of current predictors of kidney disease progression, better biomarkers are needed. Finally, a better understanding of how complications of diabetes affect one another and how they impact treatment approaches is needed. This underlines a need for studies comparing the effectiveness of different strategies for glucose control in subpopulations with comorbidities.
Conclusions
Diabetes is currently broadly classified as type 1, type 2, gestational, and a group of “other specific syndromes.” However, increasing evidence suggests that there are populations of individuals within these broad categories that have subtypes of disease with a well-defined etiology that may be clinically characterized (e.g., LADA, MODY). These developments suggest that perhaps, with more focused research in critical areas, we are approaching a point where it would be possible to categorize diabetes in a more precise manner that can inform individual treatment decisions.
Characterization of disease progression is much more developed for type 1 diabetes than for type 2 diabetes. Studies of first-degree relatives of people with type 1 diabetes suggest that persistent presence of two or more autoantibodies is an almost certain predictor of clinical hyperglycemia and diabetes. The rate of progression depends on the age of antibody onset, the number of antibodies, antibody specificity, and titer. Rising glucose and HbA1c levels substantially precede the clinical onset of diabetes, making diagnosis feasible well before the onset of diabetic ketoacidosis. Three distinct stages of type 1 diabetes can be identified (Table 1) and serve as a framework for future research and regulatory decision-making (41).
The paths to β-cell demise and dysfunction are less well defined, but deficient β-cell insulin secretion in the face of hyperglycemia appears to be the common denominator. Future classification schemes for diabetes will likely focus on the pathophysiology of the underlying β-cell dysfunction and the stage of disease as indicated by glucose status (normal, impaired, or diabetes).
Recently, the All New Diabetics in Scania (ANDIS) study reported five distinct subtypes of diabetes on the basis of clustering of clinical, blood-based, and genetic information in newly diagnosed patients in Sweden (176). Importantly, these subtypes of diabetes appear to be differentially linked to risk for particular complications. The researchers confirmed similar groupings and relationships among patients in Finland. This model represents a notable example of an approach that, with additional information, could be refined in more diverse populations to begin developing meaningful classifications based on clinical characteristics, demographics, and novel biomarkers for disease risk, progression, and complications in discreet populations.
Remaining critical research gaps are currently preventing the realization of true precision medicine for people with diabetes. The authors have outlined some of these key gaps (Supplementary Table 1) and call for the diabetes research community to address these open questions to better understand genetic and molecular mechanisms of diabetes and its complications, define phenotypes and genotypes of subtypes of diabetes, and use this understanding in the development and application of therapies to prevent and treat diabetes and complications.
Understanding the pathways to loss of β-cell mass and function is key to addressing all forms of diabetes and avoiding complications of diabetes; therefore, the gaps in these topic areas are highlighted as particular priorities among the many critical areas that remain to be investigated. By addressing the noted research gaps, we will be able to further refine models and make meaningful distinctions to stage diabetes.
Supplementary Material
Article Information
Acknowledgments. The authors gratefully acknowledge the Differentiation of Diabetes by Pathophysiology, Natural History and Prognosis research symposium steering committee members and speakers for the excellent presentations, discussions, and contributions to the conference. J.S.S., G.L.B., E.B., R.H.E., L.G., P.-H.G., R.A.I., C.M., J.P.P., A.P., D.A.S., J.M.S., J.P.H.W., and R.E.R. were presenters. Other faculty included Michael Bergman, New York University School of Medicine; Barbara E. Corkey, Boston University School of Medicine; James R. Gavin III, Emory University School of Medicine; Stanley Schwartz, University of Pennsylvania; and Kumar Sharma, University of California at San Diego. The conference was supported in part by an unrestricted educational grant from Novo Nordisk Inc. The sponsor had no influence on the selection of speakers, selection of writing group members, topics or content covered at the conference, or the content of this report. The authors thank Shirley Ash of the American Diabetes Association for assistance with the conference.
Duality of Interest. J.S.S. reports personal fees from Adocia, AstraZeneca, Boehringer Ingelheim, Dance Biopharm, Debiopharm, DexCom Inc., Genentech, Gilead, Intarcia Therapeutics, Merck, Regeneron, Sanofi, vTv Therapeutics, and Valeritas outside the submitted work. G.L.B. reports personal fees from AstraZeneca, Bayer, Boehringer Ingelheim, GlaxoSmithKline, Janssen, Merck, NxStage, and Sanofi outside the submitted work. G.L.B. is a special government employee of the U.S. Food and Drug Administration and the Centers for Medicare & Medicaid Services. T.D., A.T.M., and R.E.R. are employees of the American Diabetes Association, which received an unrestricted educational grant from Novo Nordisk Inc. to support the research symposium. P.-H.G. reports personal fees from AbbVie, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Genzyme, Janssen, Medscape, MSD, Novartis, Novo Nordisk, and Sanofi outside the submitted work. Y.H. reports grants and personal fees from Amgen, AstraZeneca, Bristol-Myers Squibb, Boehringer Ingelheim, GlaxoSmithKline, Merck, Novo Nordisk, and Sanofi; grants from Esparion, Grifolis, Hamni, Intarcia, and Lexicon; and personal fees from Amarin, Eli Lilly, Eisai, Janssen, Regeneron, and Vivus (all outside the submitted work). R.A.I. reports personal fees from Janssen outside the submitted work. J.P.H.W. reports grants from the Novo Nordisk Research Foundation during the conduct of the study. J.P.H.W. reports grants from AztraZeneca and Novo Nordisk; personal fees from AstraZeneca, Janssen Pharmaceuticals, Orexigen, and Novo Nordisk; and other institutional support from AstraZeneca, Boehringer Ingelheim, Janssen Pharmaceuticals, Orexigen, and Novo Nordisk (all outside the submitted work). No other potential conflicts of interest relevant to this article were reported.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db16-0806/-/DC1.
References
- 1.Inzucchi SE, Bergenstal RM, Buse JB, et al. . Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2015;38:140–149 [DOI] [PubMed] [Google Scholar]
- 2.National Institute for Health and Care Excellence. NICE guidance [Internet]. Available from https://www.nice.org.uk/guidance/conditions-and-diseases/diabetes-and-other-endocrinal--nutritional-and-metabolic-conditions/diabetes?unlid=957964380201659104345. Accessed 27 January 2016
- 3.Dietel M, Jöhrens K, Laffert MV, et al. . A 2015 update on predictive molecular pathology and its role in targeted cancer therapy: a review focussing on clinical relevance. Cancer Gene Ther 2015;22:417–430 [DOI] [PubMed] [Google Scholar]
- 4.Matveyenko AV, Butler PC. Relationship between beta-cell mass and diabetes onset. Diabetes Obes Metab 2008;10(Suppl. 4):23–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campbell-Thompson M, Fu A, Kaddis JS, et al. Insulitis and β-cell mass in the natural history of type 1 diabetes. Diabetes 2016;65:719–731 [DOI] [PMC free article] [PubMed]
- 6.Ferrannini E, Gastaldelli A, Miyazaki Y, Matsuda M, Mari A, DeFronzo RA. β-Cell function in subjects spanning the range from normal glucose tolerance to overt diabetes: a new analysis. J Clin Endocrinol Metab 2005;90:493–500 [DOI] [PubMed] [Google Scholar]
- 7.Dabelea D, Mayer-Davis EJ, Saydah S, et al.; SEARCH for Diabetes in Youth Study . Prevalence of type 1 and type 2 diabetes among children and adolescents from 2001 to 2009. JAMA 2014;311:1778–1786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.International Diabetes Federation. IDF Diabetes Atlas, 7th edition [Internet], 2015. Available from http://diabetesatlas.org. Accessed 7 January 2016 [Google Scholar]
- 9.Centers for Disease Control and Prevention National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States, 2014. U.S. Department of Health and Human Services, 2014 [Google Scholar]
- 10.Maahs DM, West NA, Lawrence JM, Mayer-Davis EJ. Epidemiology of type 1 diabetes. Endocrinol Metab Clin North Am 2010;39:481–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Livingstone SJ, Levin D, Looker HC, et al.; Scottish Diabetes Research Network epidemiology group; Scottish Renal Registry . Estimated life expectancy in a Scottish cohort with type 1 diabetes, 2008-2010. JAMA 2015;313:37–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stenström G, Gottsäter A, Bakhtadze E, Berger B, Sundkvist G. Latent autoimmune diabetes in adults: definition, prevalence, β-cell function, and treatment. Diabetes 2005;54(Suppl. 2):S68–S72 [DOI] [PubMed] [Google Scholar]
- 13.Karvonen M, Viik-Kajander M, Moltchanova E, Libman I, LaPorte R, Tuomilehto J. Incidence of childhood type 1 diabetes worldwide. Diabetes Mondiale (DiaMond) Project Group. Diabetes Care 2000;23:1516–1526 [DOI] [PubMed] [Google Scholar]
- 14.Knip M, Veijola R, Virtanen SM, Hyöty H, Vaarala O, Akerblom HK. Environmental triggers and determinants of type 1 diabetes. Diabetes 2005;54(Suppl. 2):S125–S136 [DOI] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention. Age-adjusted rates of diagnosed diabetes per 100 civilian, non-institutionalized population, by sex, United States, 1980–2014 [Internet]. Available from: http://www.cdc.gov/diabetes/statistics/prev/national/figbysex.htm. Accessed 19 November 2015
- 16.Agardh E, Allebeck P, Hallqvist J, Moradi T, Sidorchuk A. Type 2 diabetes incidence and socio-economic position: a systematic review and meta-analysis. Int J Epidemiol 2011;40:804–818 [DOI] [PubMed] [Google Scholar]
- 17.Prasad RB, Groop L. Genetics of type 2 diabetes-pitfalls and possibilities. Genes (Basel) 2015;6:87–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Groop L, Forsblom C, Lehtovirta M, et al. . Metabolic consequences of a family history of NIDDM (the Botnia study): evidence for sex-specific parental effects. Diabetes 1996;45:1585–1593 [DOI] [PubMed] [Google Scholar]
- 19.Hämäläinen A-M, Knip M. Autoimmunity and familial risk of type 1 diabetes. Curr Diab Rep 2002;2:347–353 [DOI] [PubMed] [Google Scholar]
- 20.Hemminki K, Li X, Sundquist K, Sundquist J. Familial risks for type 2 diabetes in Sweden. Diabetes Care 2010;33:293–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murphy R, Ellard S, Hattersley AT. Clinical implications of a molecular genetic classification of monogenic beta-cell diabetes. Nat Clin Pract Endocrinol Metab 2008;4:200–213 [DOI] [PubMed] [Google Scholar]
- 22.Barnett AH, Eff C, Leslie RD, Pyke DA. Diabetes in identical twins. A study of 200 pairs. Diabetologia 1981;20:87–93 [DOI] [PubMed] [Google Scholar]
- 23.Srikanta S, Ganda OP, Eisenbarth GS, Soeldner JS. Islet-cell antibodies and beta-cell function in monozygotic triplets and twins initially discordant for type I diabetes mellitus. N Engl J Med 1983;308:322–325 [DOI] [PubMed] [Google Scholar]
- 24.Redondo MJ, Jeffrey J, Fain PR, Eisenbarth GS, Orban T. Concordance for islet autoimmunity among monozygotic twins. N Engl J Med 2008;359:2849–2850 [DOI] [PubMed] [Google Scholar]
- 25.Rich SS. Mapping genes in diabetes. Genetic epidemiological perspective. Diabetes 1990;39:1315–1319 [DOI] [PubMed] [Google Scholar]
- 26.Aly TA, Ide A, Jahromi MM, et al. . Extreme genetic risk for type 1A diabetes. Proc Natl Acad Sci USA 2006;103:14074–14079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Noble JA, Valdes AM, Varney MD, et al.; Type 1 Diabetes Genetics Consortium . HLA class I and genetic susceptibility to type 1 diabetes: results from the Type 1 Diabetes Genetics Consortium. Diabetes 2010;59:2972–2979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu X, Deutsch AJ, Lenz TL, et al. . Additive and interaction effects at three amino acid positions in HLA-DQ and HLA-DR molecules drive type 1 diabetes risk. Nat Genet 2015;47:898–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cooper JD, Howson JMM, Smyth D, et al.; Type 1 Diabetes Genetics Consortium . Confirmation of novel type 1 diabetes risk loci in families. Diabetologia 2012;55:996–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Long SA, Cerosaletti K, Bollyky PL, et al. . Defects in IL-2R signaling contribute to diminished maintenance of FOXP3 expression in CD4+CD25+ regulatory T-cells of type 1 diabetic subjects. Diabetes 2010;59:407–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Long SA, Cerosaletti K, Wan JY, et al. . An autoimmune-associated variant in PTPN2 reveals an impairment of IL-2R signaling in CD4(+) T cells. Genes Immun 2011;12:116–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pugliese A, Zeller M, Fernandez A Jr, et al. . The insulin gene is transcribed in the human thymus and transcription levels correlated with allelic variation at the INS VNTR-IDDM2 susceptibility locus for type 1 diabetes. Nat Genet 1997;15:293–297 [DOI] [PubMed] [Google Scholar]
- 33.Sosinowski T, Eisenbarth GS. Type 1 diabetes: primary antigen/peptide/register/trimolecular complex. Immunol Res 2013;55:270–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Colli ML, Moore F, Gurzov EN, Ortis F, Eizirik DL. MDA5 and PTPN2, two candidate genes for type 1 diabetes, modify pancreatic beta-cell responses to the viral by-product double-stranded RNA. Hum Mol Genet 2010;19:135–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marroqui L, Dos Santos RS, Fløyel T, et al. . TYK2, a candidate gene for type 1 diabetes, modulates apoptosis and the innate immune response in human pancreatic β-cells. Diabetes 2015;64:3808–3817 [DOI] [PubMed] [Google Scholar]
- 36.Fløyel T, Kaur S, Pociot F. Genes affecting β-cell function in type 1 diabetes. Curr Diab Rep 2015;15:97. [DOI] [PubMed] [Google Scholar]
- 37.Santin I, Eizirik DL. Candidate genes for type 1 diabetes modulate pancreatic islet inflammation and β-cell apoptosis. Diabetes Obes Metab 2013;15(Suppl. 3):71–81 [DOI] [PubMed] [Google Scholar]
- 38.Törn C, Hadley D, Lee H-S, et al.; TEDDY Study Group . Role of type 1 diabetes-associated SNPs on risk of autoantibody positivity in the TEDDY study. Diabetes 2015;64:1818–1829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Steck AK, Wong R, Wagner B, et al. . Effects of non-HLA gene polymorphisms on development of islet autoimmunity and type 1 diabetes in a population with high-risk HLA-DR,DQ genotypes. Diabetes 2012;61:753–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stankov K, Benc D, Draskovic D. Genetic and epigenetic factors in etiology of diabetes mellitus type 1. Pediatrics 2013;132:1112–1122 [DOI] [PubMed] [Google Scholar]
- 41.Insel RA, Dunne JL, Atkinson MA, et al. . Staging presymptomatic type 1 diabetes: a scientific statement of JDRF, the Endocrine Society, and the American Diabetes Association. Diabetes Care 2015;38:1964–1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Redondo MJ, Muniz J, Rodriguez LM, et al. . Association of TCF7L2 variation with single islet autoantibody expression in children with type 1 diabetes. BMJ Open Diabetes Res Care 2014;2:e000008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Basile KJ, Guy VC, Schwartz S, Grant SFA. Overlap of genetic susceptibility to type 1 diabetes, type 2 diabetes, and latent autoimmune diabetes in adults. Curr Diab Rep 2014;14:550. [DOI] [PubMed] [Google Scholar]
- 44.Oram RA, Patel K, Hill A, et al. . A type 1 diabetes genetic risk score can aid discrimination between type 1 and type 2 diabetes in young adults. Diabetes Care 2016;39:337–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morris AP, Voight BF, Teslovich TM, et al.; Wellcome Trust Case Control Consortium; Meta-Analyses of Glucose and Insulin-related traits Consortium (MAGIC) Investigators; Genetic Investigation of ANthropometric Traits (GIANT) Consortium; Asian Genetic Epidemiology Network–Type 2 Diabetes (AGEN-T2D) Consortium; South Asian Type 2 Diabetes (SAT2D) Consortium; DIAbetes Genetics Replication And Meta-analysis (DIAGRAM) Consortium . Large-scale association analysis provides insights into the genetic architecture and pathophysiology of type 2 diabetes. Nat Genet 2012;44:981–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Scott RA, Lagou V, Welch RP, et al.; DIAbetes Genetics Replication and Meta-analysis (DIAGRAM) Consortium . Large-scale association analyses identify new loci influencing glycemic traits and provide insight into the underlying biological pathways. Nat Genet 2012;44:991–1005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gaulton KJ, Ferreira T, Lee Y, et al.; DIAbetes Genetics Replication And Meta-analysis (DIAGRAM) Consortium . Genetic fine mapping and genomic annotation defines causal mechanisms at type 2 diabetes susceptibility loci. Nat Genet 2015;47:1415–1425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Travers ME, Mackay DJG, Dekker Nitert M, et al. . Insights into the molecular mechanism for type 2 diabetes susceptibility at the KCNQ1 locus from temporal changes in imprinting status in human islets. Diabetes 2013;62:987–992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Portha B, Chavey A, Movassat J. Early-life origins of type 2 diabetes: fetal programming of the beta-cell mass. Exp Diabetes Res 2011;2011:105076 [DOI] [PMC free article] [PubMed]
- 50.Flannick J, Thorleifsson G, Beer NL, et al.; Go-T2D Consortium; T2D-GENES Consortium . Loss-of-function mutations in SLC30A8 protect against type 2 diabetes. Nat Genet 2014;46:357–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schneider DA, von Herrath MG. Potential viral pathogenic mechanism in human type 1 diabetes. Diabetologia 2014;57:2009–2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Roivainen M, Klingel K. Virus infections and type 1 diabetes risk. Curr Diab Rep 2010;10:350–356 [DOI] [PubMed] [Google Scholar]
- 53.Giongo A, Gano KA, Crabb DB, et al. . Toward defining the autoimmune microbiome for type 1 diabetes. ISME J 2011;5:82–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Norris JM, Barriga K, Klingensmith G, et al. . Timing of initial cereal exposure in infancy and risk of islet autoimmunity. JAMA 2003;290:1713–1720 [DOI] [PubMed] [Google Scholar]
- 55.Ziegler A-G, Schmid S, Huber D, Hummel M, Bonifacio E. Early infant feeding and risk of developing type 1 diabetes-associated autoantibodies. JAMA 2003;290:1721–1728 [DOI] [PubMed] [Google Scholar]
- 56.Hagopian WA, Lernmark A, Rewers MJ, et al. . TEDDY--The Environmental Determinants of Diabetes in the Young: an observational clinical trial. Ann N Y Acad Sci 2006;1079:320–326 [DOI] [PubMed] [Google Scholar]
- 57.Tiberti C, Giordano C, Locatelli M, et al. . Identification of tyrosine phosphatase 2(256-760) construct as a new, sensitive marker for the detection of islet autoimmunity in type 2 diabetic patients: the non-insulin requiring autoimmune diabetes (NIRAD) study 2. Diabetes 2008;57:1276–1283 [DOI] [PubMed] [Google Scholar]
- 58.Brooks-Worrell BM, Boyko EJ, Palmer JP. Impact of islet autoimmunity on the progressive β-cell functional decline in type 2 diabetes. Diabetes Care 2014;37:3286–3293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chan JM, Rimm EB, Colditz GA, Stampfer MJ, Willett WC. Obesity, fat distribution, and weight gain as risk factors for clinical diabetes in men. Diabetes Care 1994;17:961–969 [DOI] [PubMed] [Google Scholar]
- 60.Colditz GA, Willett WC, Rotnitzky A, Manson JE. Weight gain as a risk factor for clinical diabetes mellitus in women. Ann Intern Med 1995;122:481–486 [DOI] [PubMed] [Google Scholar]
- 61.van der Zijl NJ, Goossens GH, Moors CCM, et al. . Ectopic fat storage in the pancreas, liver, and abdominal fat depots: impact on β-cell function in individuals with impaired glucose metabolism. J Clin Endocrinol Metab 2011;96:459–467 [DOI] [PubMed] [Google Scholar]
- 62.Araneta MRG, Kanaya AM, Hsu WC, et al. . Optimum BMI cut points to screen Asian Americans for type 2 diabetes. Diabetes Care 2015;38:814–820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Henry RR, Wallace P, Olefsky JM. Effects of weight loss on mechanisms of hyperglycemia in obese non-insulin-dependent diabetes mellitus. Diabetes 1986;35:990–998 [DOI] [PubMed] [Google Scholar]
- 64.Lim EL, Hollingsworth KG, Aribisala BS, Chen MJ, Mathers JC, Taylor R. Reversal of type 2 diabetes: normalisation of beta cell function in association with decreased pancreas and liver triacylglycerol. Diabetologia 2011;54:2506–2514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McCaffery JM, Jablonski KA, Franks PW, et al.; Diabetes Prevention Program Research Group . TCF7L2 polymorphism, weight loss and proinsulin:insulin ratio in the diabetes prevention program. PLoS One 2011;6:e21518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Panunzi S, Carlsson L, De Gaetano A, et al. . Determinants of diabetes remission and glycemic control after bariatric surgery. Diabetes Care 2016;39:166–174 [DOI] [PubMed] [Google Scholar]
- 67.Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. β-Cell deficit and increased β-cell apoptosis in humans with type 2 diabetes. Diabetes 2003;52:102–110 [DOI] [PubMed] [Google Scholar]
- 68.Laybutt DR, Kaneto H, Hasenkamp W, et al. . Increased expression of antioxidant and antiapoptotic genes in islets that may contribute to β-cell survival during chronic hyperglycemia. Diabetes 2002;51:413–423 [DOI] [PubMed] [Google Scholar]
- 69.Weir GC, Bonner-Weir S. Five stages of evolving β-cell dysfunction during progression to diabetes. Diabetes 2004;53(Suppl. 3):S16–S21 [DOI] [PubMed] [Google Scholar]
- 70.Vardi P, Crisa L, Jackson RA. Predictive value of intravenous glucose tolerance test insulin secretion less than or greater than the first percentile in islet cell antibody positive relatives of type 1 (insulin-dependent) diabetic patients. Diabetologia 1991;34:93–102 [DOI] [PubMed] [Google Scholar]
- 71.Chase HP, Voss MA, Butler-Simon N, Hoops S, O’Brien D, Dobersen MJ. Diagnosis of pre-type I diabetes. J Pediatr 1987;111:807–812 [DOI] [PubMed] [Google Scholar]
- 72.Srikanta S, Ganda OP, Rabizadeh A, Soeldner JS, Eisenbarth GS. First-degree relatives of patients with type I diabetes mellitus. Islet-cell antibodies and abnormal insulin secretion. N Engl J Med 1985;313:461–464 [DOI] [PubMed] [Google Scholar]
- 73.Ginsberg-Fellner F, Witt ME, Franklin BH, et al. . Triad of markers for identifying children at high risk of developing insulin-dependent diabetes mellitus. JAMA 1985;254:1469–1472 [PubMed] [Google Scholar]
- 74.Sosenko JM, Skyler JS, Beam CA, et al.; Type 1 Diabetes TrialNet and Diabetes Prevention Trial–Type 1 Study Groups . Acceleration of the loss of the first-phase insulin response during the progression to type 1 diabetes in diabetes prevention trial-type 1 participants. Diabetes 2013;62:4179–4183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sosenko JM, Palmer JP, Rafkin LE, et al.; Diabetes Prevention Trial-Type 1 Study Group . Trends of earlier and later responses of C-peptide to oral glucose challenges with progression to type 1 diabetes in diabetes prevention trial-type 1 participants. Diabetes Care 2010;33:620–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ferrannini E, Mari A, Nofrate V, Sosenko JM, Skyler JS; DPT-1 Study Group . Progression to diabetes in relatives of type 1 diabetic patients: mechanisms and mode of onset. Diabetes 2010;59:679–685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sosenko JM, Palmer JP, Rafkin-Mervis L, et al. . Glucose and C-peptide changes in the perionset period of type 1 diabetes in the Diabetes Prevention Trial-Type 1. Diabetes Care 2008;31:2188–2192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Greenbaum CJ, Beam CA, Boulware D, et al.; Type 1 Diabetes TrialNet Study Group . Fall in C-peptide during first 2 years from diagnosis: evidence of at least two distinct phases from composite Type 1 Diabetes TrialNet data. Diabetes 2012;61:2066–2073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Oram RA, Jones AG, Besser REJ, et al. . The majority of patients with long-duration type 1 diabetes are insulin microsecretors and have functioning beta cells. Diabetologia 2014;57:187–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rosenbloom AL, Hunt SS, Rosenbloom EK, Maclaren NK. Ten-year prognosis of impaired glucose tolerance in siblings of patients with insulin-dependent diabetes. Diabetes 1982;31:385–387 [DOI] [PubMed] [Google Scholar]
- 81.Tarn AC, Smith CP, Spencer KM, Bottazzo GF, Gale EA. Type I (insulin dependent) diabetes: a disease of slow clinical onset? Br Med J (Clin Res Ed) 1987;294:342–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Beer SF, Heaton DA, Alberti KG, Pyke DA, Leslie RD. Impaired glucose tolerance precedes but does not predict insulin-dependent diabetes mellitus: a study of identical twins. Diabetologia 1990;33:497–502 [DOI] [PubMed] [Google Scholar]
- 83.Sosenko JM, Palmer JP, Greenbaum CJ, et al.; Diabetes Prevention Trial-Type 1 Study Group . Increasing the accuracy of oral glucose tolerance testing and extending its application to individuals with normal glucose tolerance for the prediction of type 1 diabetes: the Diabetes Prevention Trial-Type 1. Diabetes Care 2007;30:38–42 [DOI] [PubMed] [Google Scholar]
- 84.Sosenko JM, Skyler JS, Krischer JP, et al.; Diabetes Prevention Trial-Type 1 Study Group . Glucose excursions between states of glycemia with progression to type 1 diabetes in the diabetes prevention trial-type 1 (DPT-1). Diabetes 2010;59:2386–2389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sosenko JM, Palmer JP, Rafkin-Mervis L, et al.; Diabetes Prevention Trial-Type 1 Study Group . Incident dysglycemia and progression to type 1 diabetes among participants in the Diabetes Prevention Trial-Type 1. Diabetes Care 2009;32:1603–1607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sosenko JM, Krischer JP, Palmer JP, et al.; Diabetes Prevention Trial-Type 1 Study Group . A risk score for type 1 diabetes derived from autoantibody-positive participants in the diabetes prevention trial-type 1. Diabetes Care 2008;31:528–533 [DOI] [PubMed] [Google Scholar]
- 87.Sosenko JM, Skyler JS, Mahon J, et al.; Type 1 Diabetes TrialNet and Diabetes Prevention Trial-Type 1 Study Groups . Validation of the Diabetes Prevention Trial-Type 1 Risk Score in the TrialNet Natural History Study. Diabetes Care 2011;34:1785–1787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Basu A, Dalla Man C, Basu R, Toffolo G, Cobelli C, Rizza RA. Effects of type 2 diabetes on insulin secretion, insulin action, glucose effectiveness, and postprandial glucose metabolism. Diabetes Care 2009;32:866–872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.van Haeften TW, Pimenta W, Mitrakou A, et al. . Relative conributions of beta-cell function and tissue insulin sensitivity to fasting and postglucose-load glycemia. Metabolism 2000;49:1318–1325 [DOI] [PubMed] [Google Scholar]
- 90.Ward WK, Bolgiano DC, McKnight B, Halter JB, Porte D Jr. Diminished B cell secretory capacity in patients with noninsulin-dependent diabetes mellitus. J Clin Invest 1984;74:1318–1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yoshioka N, Kuzuya T, Matsuda A, Taniguchi M, Iwamoto Y. Serum proinsulin levels at fasting and after oral glucose load in patients with type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia 1988;31:355–360 [DOI] [PubMed] [Google Scholar]
- 92.American Diabetes Association Approaches to glycemic treatment. Sec. 7. In Standards of Medical Care in Diabetes—2016. Diabetes Care 2016;39(Suppl. 1):S52–S5926696682 [Google Scholar]
- 93.Phillips LS, Ratner RE, Buse JB, Kahn SE. We can change the natural history of type 2 diabetes. Diabetes Care 2014;37:2668–2676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bergman M, Dankner R, Roth J, Narayan KMV. Are current diagnostic guidelines delaying early detection of dysglycemic states? Time for new approaches. Endocrine 2013;44:66–69 [DOI] [PubMed] [Google Scholar]
- 95.Phillips LS, Twombly JG. It’s time to overcome clinical inertia. Ann Intern Med 2008;148:783–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nichols GA, Koo YH, Shah SN. Delay of insulin addition to oral combination therapy despite inadequate glycemic control: delay of insulin therapy. J Gen Intern Med 2007;22:453–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Khunti K, Wolden ML, Thorsted BL, Andersen M, Davies MJ. Clinical inertia in people with type 2 diabetes: a retrospective cohort study of more than 80,000 people. Diabetes Care 2013;36:3411–3417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tuomilehto J, Lindström J, Eriksson JG, et al.; Finnish Diabetes Prevention Study Group . Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 2001;344:1343–1350 [DOI] [PubMed] [Google Scholar]
- 99.Knowler WC, Barrett-Connor E, Fowler SE, et al.; Diabetes Prevention Program Research Group . Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gong Q, Gregg EW, Wang J, et al. . Long-term effects of a randomised trial of a 6-year lifestyle intervention in impaired glucose tolerance on diabetes-related microvascular complications: the China Da Qing Diabetes Prevention Outcome Study. Diabetologia 2011;54:300–307 [DOI] [PubMed] [Google Scholar]
- 101.Perreault L, Pan Q, Mather KJ, Watson KE, Hamman RF, Kahn SE; Diabetes Prevention Program Research Group . Effect of regression from prediabetes to normal glucose regulation on long-term reduction in diabetes risk: results from the Diabetes Prevention Program Outcomes Study. Lancet 2012;379:2243–2251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Li G, Zhang P, Wang J, et al. . Cardiovascular mortality, all-cause mortality, and diabetes incidence after lifestyle intervention for people with impaired glucose tolerance in the Da Qing Diabetes Prevention Study: a 23-year follow-up study. Lancet Diabetes Endocrinol 2014;2:474–480 [DOI] [PubMed] [Google Scholar]
- 103.Bonifacio E. Predicting type 1 diabetes using biomarkers. Diabetes Care 2015;38:989–996 [DOI] [PubMed] [Google Scholar]
- 104.Vehik K, Lynch KF, Schatz DA, et al.; TEDDY Study Group . Reversion of β-cell autoimmunity changes risk of type 1 diabetes: TEDDY study. Diabetes Care 2016;39:1535–1542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ziegler AG, Rewers M, Simell O, et al. . Seroconversion to multiple islet autoantibodies and risk of progression to diabetes in children. JAMA 2013;309:2473–2479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Steck AK, Vehik K, Bonifacio E, et al.; TEDDY Study Group . Predictors of progression from the appearance of islet autoantibodies to early childhood diabetes: The Environmental Determinants of Diabetes in the Young (TEDDY). Diabetes Care 2015;38:808–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Orban T, Sosenko JM, Cuthbertson D, et al.; Diabetes Prevention Trial-Type 1 Study Group . Pancreatic islet autoantibodies as predictors of type 1 diabetes in the Diabetes Prevention Trial-Type 1. Diabetes Care 2009;32:2269–2274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Colman PG, Steele C, Couper JJ, et al. . Islet autoimmunity in infants with a type I diabetic relative is common but is frequently restricted to one autoantibody. Diabetologia 2000;43:203–209 [DOI] [PubMed] [Google Scholar]
- 109.Ziegler A-G, Bonifacio E; BABYDIAB-BABYDIET Study Group . Age-related islet autoantibody incidence in offspring of patients with type 1 diabetes. Diabetologia 2012;55:1937–1943 [DOI] [PubMed] [Google Scholar]
- 110.Parikka V, Näntö-Salonen K, Saarinen M, et al. . Early seroconversion and rapidly increasing autoantibody concentrations predict prepubertal manifestation of type 1 diabetes in children at genetic risk. Diabetologia 2012;55:1926–1936 [DOI] [PubMed] [Google Scholar]
- 111.Krischer JP, Lynch KF, Schatz DA, et al.; TEDDY Study Group . The 6 year incidence of diabetes-associated autoantibodies in genetically at-risk children: the TEDDY study. Diabetologia 2015;58:980–987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Leslie RD, Palmer J, Schloot NC, Lernmark A. Diabetes at the crossroads: relevance of disease classification to pathophysiology and treatment. Diabetologia 2016;59:13–20 [DOI] [PubMed] [Google Scholar]
- 113.Pearson ER. Personalized medicine in diabetes: the role of ‘omics’ and biomarkers. Diabet Med 2016;33:712–717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Shepherd M, Pearson ER, Houghton J, Salt G, Ellard S, Hattersley AT. No deterioration in glycemic control in HNF-1α maturity-onset diabetes of the young following transfer from long-term insulin to sulphonylureas. Diabetes Care 2003;26:3191–3192 [DOI] [PubMed] [Google Scholar]
- 115.Holstein A, Hahn M, Patzer O, Seeringer A, Kovacs P, Stingl J. Impact of clinical factors and CYP2C9 variants for the risk of severe sulfonylurea-induced hypoglycemia. Eur J Clin Pharmacol 2011;67:471–476 [DOI] [PubMed] [Google Scholar]
- 116.Dujic T, Zhou K, Donnelly LA, Tavendale R, Palmer CNA, Pearson ER. Association of organic cation transporter 1 with intolerance to metformin in type 2 diabetes: a GoDARTS study. Diabetes 2015;64:1786–1793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Connelly PJ, Smith N, Chadwick R, Exley AR, Shneerson JM, Pearson ER. Recessive mutations in the cancer gene Ataxia Telangiectasia Mutated (ATM), at a locus previously associated with metformin response, cause dysglycaemia and insulin resistance. Diabet Med 2016;33:371–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Jones TA, Sautter M, Van Gaal LF, Jones NP. Addition of rosiglitazone to metformin is most effective in obese, insulin-resistant patients with type 2 diabetes. Diabetes Obes Metab 2003;5:163–170 [DOI] [PubMed] [Google Scholar]
- 119.Brooks-Worrell BM, Palmer JP. Attenuation of islet-specific T cell responses is associated with C-peptide improvement in autoimmune type 2 diabetes patients. Clin Exp Immunol 2013;171:164–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jones AG, McDonald TJ, Shields BM, et al.; PRIBA Study Group . Markers of β-cell failure predict poor glycemic response to GLP-1 receptor agonist therapy in type 2 diabetes. Diabetes Care 2016;39:250–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ohki T, Isogawa A, Toda N, Tagawa K. Effectiveness of ipragliflozin, a sodium-glucose co-transporter 2 inhibitor, as a second-line treatment for non-alcoholic fatty liver disease patients with type 2 diabetes mellitus who do not respond to incretin-based therapies including glucagon-like peptide-1 analogs and dipeptidyl peptidase-4 inhibitors. Clin Drug Investig 2016;36:313–319 [DOI] [PubMed] [Google Scholar]
- 122.Wilding JPH, Woo V, Soler NG, et al.; Dapagliflozin 006 Study Group . Long-term efficacy of dapagliflozin in patients with type 2 diabetes mellitus receiving high doses of insulin: a randomized trial. Ann Intern Med 2012;156:405–415 [DOI] [PubMed] [Google Scholar]
- 123.Yeh H-C, Brown TT, Maruthur N, et al. . Comparative effectiveness and safety of methods of insulin delivery and glucose monitoring for diabetes mellitus: a systematic review and meta-analysis. Ann Intern Med 2012;157:336–347 [DOI] [PubMed] [Google Scholar]
- 124.Tamborlane WV, Beck RW, Bode BW, et al.; Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group . Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med 2008;359:1464–1476 [DOI] [PubMed] [Google Scholar]
- 125.Weyer C, Gottlieb A, Kim DD, et al. . Pramlintide reduces postprandial glucose excursions when added to regular insulin or insulin lispro in subjects with type 1 diabetes: a dose-timing study. Diabetes Care 2003;26:3074–3079 [DOI] [PubMed] [Google Scholar]
- 126.Ratner RE, Dickey R, Fineman M, et al. . Amylin replacement with pramlintide as an adjunct to insulin therapy improves long-term glycaemic and weight control in type 1 diabetes mellitus: a 1-year, randomized controlled trial. Diabet Med 2004;21:1204–1212 [DOI] [PubMed] [Google Scholar]
- 127.Edelman S, Garg S, Frias J, et al. . A double-blind, placebo-controlled trial assessing pramlintide treatment in the setting of intensive insulin therapy in type 1 diabetes. Diabetes Care 2006;29:2189–2195 [DOI] [PubMed] [Google Scholar]
- 128.Raju B, Arbelaez AM, Breckenridge SM, Cryer PE. Nocturnal hypoglycemia in type 1 diabetes: an assessment of preventive bedtime treatments. J Clin Endocrinol Metab 2006;91:2087–2092 [DOI] [PubMed] [Google Scholar]
- 129.Riccardi G, Giacco R, Parillo M, et al. . Efficacy and safety of acarbose in the treatment of Type 1 diabetes mellitus: a placebo-controlled, double-blind, multicentre study. Diabet Med 1999;16:228–232 [DOI] [PubMed] [Google Scholar]
- 130.Strowig SM, Raskin P. The effect of rosiglitazone on overweight subjects with type 1 diabetes. Diabetes Care 2005;28:1562–1567 [DOI] [PubMed] [Google Scholar]
- 131.Shimada A, Shigihara T, Okubo Y, Katsuki T, Yamada Y, Oikawa Y. Pioglitazone may accelerate disease course of slowly progressive type 1 diabetes. Diabetes Metab Res Rev 2011;27:951–953 [DOI] [PubMed] [Google Scholar]
- 132.Yang Z, Zhou Z, Li X, Huang G, Lin J. Rosiglitazone preserves islet beta-cell function of adult-onset latent autoimmune diabetes in 3 years follow-up study. Diabetes Res Clin Pract 2009;83:54–60 [DOI] [PubMed] [Google Scholar]
- 133.Vella S, Buetow L, Royle P, Livingstone S, Colhoun HM, Petrie JR. The use of metformin in type 1 diabetes: a systematic review of efficacy. Diabetologia 2010;53:809–820 [DOI] [PubMed] [Google Scholar]
- 134.Kielgast U, Krarup T, Holst JJ, Madsbad S. Four weeks of treatment with liraglutide reduces insulin dose without loss of glycemic control in type 1 diabetic patients with and without residual β-cell function. Diabetes Care 2011;34:1463–1468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Crisci I, Aragona M, Politi KS, Daniele G, Del Prato S. GLP-1 receptor agonists in type 1 diabetes: a proof-of-concept approach. Acta Diabetol 2015;52:1129–1133 [DOI] [PubMed] [Google Scholar]
- 136.Garg SK, Moser EG, Bode BW, et al. . Effect of sitagliptin on post-prandial glucagon and GLP-1 levels in patients with type 1 diabetes: investigator-initiated, double-blind, randomized, placebo-controlled trial. Endocr Pract 2013;19:19–28 [DOI] [PubMed] [Google Scholar]
- 137.Mudaliar S, Armstrong DA, Mavian AA, et al. . Remogliflozin etabonate, a selective inhibitor of the sodium-glucose transporter 2, improves serum glucose profiles in type 1 diabetes. Diabetes Care 2012;35:2198–2200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Peters AL, Henry RR, Thakkar P, Tong C, Alba M. Diabetic ketoacidosis with canagliflozin, a sodium–glucose cotransporter 2 inhibitor, in patients with type 1 diabetes. Diabetes Care 2016;39:532–538 [DOI] [PubMed] [Google Scholar]
- 139.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HAW. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008;359:1577–1589 [DOI] [PubMed] [Google Scholar]
- 140.UK Prospective Diabetes Study (UKPDS) Group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352:837–853 [PubMed] [Google Scholar]
- 141.Twito O, Frankel M, Nabriski D. Impact of glucose level on morbidity and mortality in elderly with diabetes and pre-diabetes. World J Diabetes 2015;6:345–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Flynn C, Bakris GL. Noninsulin glucose-lowering agents for the treatment of patients on dialysis. Nat Rev Nephrol 2013;9:147–153 [DOI] [PubMed] [Google Scholar]
- 143.Tuttle KR, Bakris GL, Bilous RW, et al. . Diabetic kidney disease: a report from an ADA Consensus Conference. Diabetes Care 2014;37:2864–2883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Chachou A, Randoux C, Millart H, Chanard J, Gillery P. Influence of in vivo hemoglobin carbamylation on HbA1c measurements by various methods. Clin Chem Lab Med 2000;38:321–326 [DOI] [PubMed] [Google Scholar]
- 145.Chow E, Bernjak A, Williams S, et al. . Risk of cardiac arrhythmias during hypoglycemia in patients with type 2 diabetes and cardiovascular risk. Diabetes 2014;63:1738–1747 [DOI] [PubMed] [Google Scholar]
- 146.Scirica BM, Bhatt DL, Braunwald E, et al.; SAVOR-TIMI 53 Steering Committee and Investigators . Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 2013;369:1317–1326 [DOI] [PubMed] [Google Scholar]
- 147.White WB, Cannon CP, Heller SR, et al.; EXAMINE Investigators . Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med 2013;369:1327–1335 [DOI] [PubMed] [Google Scholar]
- 148.Green JB, Bethel MA, Armstrong PW, et al.; TECOS Study Group . Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med 2015;373:232–242 [DOI] [PubMed] [Google Scholar]
- 149.Pfeffer MA, Claggett B, Diaz R, et al.; ELIXA Investigators . Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med 2015;373:2247–2257 [DOI] [PubMed] [Google Scholar]
- 150.Dormandy JA, Charbonnel B, Eckland DJA, et al.; PROactive Investigators . Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet 2005;366:1279–1289 [DOI] [PubMed] [Google Scholar]
- 151.UK Prospective Diabetes Study (UKPDS) Group Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998;352:854–865 [PubMed] [Google Scholar]
- 152.Zinman B, Wanner C, Lachin JM, et al.; EMPA-REG OUTCOME Investigators . Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015;373:2117–2128 [DOI] [PubMed] [Google Scholar]
- 153.Marso SP, Daniels GH, Brown-Frandsen K, et al.; LEADER Steering Committee; LEADER Trial Investigators . Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016;375:311–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wilding JPH. The importance of weight management in type 2 diabetes mellitus. Int J Clin Pract 2014;68:682–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.The Diabetes Control and Complications Trial Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–986 [DOI] [PubMed] [Google Scholar]
- 156.Gregg EW, Li Y, Wang J, et al. . Changes in diabetes-related complications in the United States, 1990-2010. N Engl J Med 2014;370:1514–1523 [DOI] [PubMed] [Google Scholar]
- 157.de Ferranti SD, de Boer IH, Fonseca V, et al. . Type 1 diabetes mellitus and cardiovascular disease: a scientific statement from the American Heart Association and American Diabetes Association. Diabetes Care 2014;37:2843–2863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Krolewski AS, Warram JH, Christlieb AR, Busick EJ, Kahn CR. The changing natural history of nephropathy in type I diabetes. Am J Med 1985;78:785–794 [DOI] [PubMed] [Google Scholar]
- 159.Finne P, Reunanen A, Stenman S, Groop P-H, Grönhagen-Riska C. Incidence of end-stage renal disease in patients with type 1 diabetes. JAMA 2005;294:1782–1787 [DOI] [PubMed] [Google Scholar]
- 160.Martin CL, Albers JW, Pop-Busui R; DCCT/EDIC Research Group . Neuropathy and related findings in the diabetes control and complications trial/epidemiology of diabetes interventions and complications study. Diabetes Care 2014;37:31–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wadén J, Forsblom C, Thorn LM, Gordin D, Saraheimo M, Groop PH; Finnish Diabetic Nephropathy Study Group . A1C variability predicts incident cardiovascular events, microalbuminuria, and overt diabetic nephropathy in patients with type 1 diabetes. Diabetes 2009;58:2649–2655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Thorn LM, Forsblom C, Fagerudd J, Pettersson-Fernholm K, Kilpikari R, Groop PH; FinnDiane Study Group . Clustering of risk factors in parents of patients with type 1 diabetes and nephropathy. Diabetes Care 2007;30:1162–1167 [DOI] [PubMed] [Google Scholar]
- 163.Sharma K, Karl B, Mathew AV, et al. . Metabolomics reveals signature of mitochondrial dysfunction in diabetic kidney disease. J Am Soc Nephrol 2013;24:1901–1912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet 2005;365:1415–1428 [DOI] [PubMed] [Google Scholar]
- 165.Fellström B, Holdaas H, Jardine AG, et al.; AURORA Study Group . Cardiovascular disease in patients with renal disease: the role of statins. Curr Med Res Opin 2009;25:271–285 [DOI] [PubMed] [Google Scholar]
- 166.Wanner C, Krane V, März W, et al.; German Diabetes and Dialysis Study Investigators . Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med 2005;353:238–248 [DOI] [PubMed] [Google Scholar]
- 167.Simó R, Simó-Servat O, Hernández C. Is fenofibrate a reasonable treatment for diabetic microvascular disease? Curr Diab Rep 2015;15:24. [DOI] [PubMed] [Google Scholar]
- 168.Baigent C, Landray MJ, Reith C, et al.; SHARP Investigators . The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet 2011;377:2181–2192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Cannon CP, Blazing MA, Giugliano RP, et al.; IMPROVE-IT Investigators . Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med 2015;372:2387–2397 [DOI] [PubMed] [Google Scholar]
- 170.Steinke JM, Sinaiko AR, Kramer MS, Suissa S, Chavers BM, Mauer M; International Diabetic Nephopathy Study Group . The early natural history of nephropathy in Type 1 Diabetes: III. Predictors of 5-year urinary albumin excretion rate patterns in initially normoalbuminuric patients. Diabetes 2005;54:2164–2171 [DOI] [PubMed] [Google Scholar]
- 171.Kalaitzidis RG, Bakris GL. Should proteinuria reduction be the criterion for antihypertensive drug selection for patients with kidney disease? Curr Opin Nephrol Hypertens 2009;18:386–391 [DOI] [PubMed] [Google Scholar]
- 172.Weir MR, Bakris GL. Editorial perspective. Should microalbuminuria ever be considered as a renal endpoint in any clinical trial? Am J Nephrol 2010;31:469–470 [DOI] [PubMed] [Google Scholar]
- 173.Glassock RJ. Debate: CON position. Should microalbuminuria ever be considered as a renal endpoint in any clinical trial? Am J Nephrol. 2010;31:466–467 [DOI] [PubMed]
- 174.Bakris GL, Molitch M. Microalbuminuria as a risk predictor in diabetes: the continuing saga. Diabetes Care 2014;37:867–875 [DOI] [PubMed] [Google Scholar]
- 175.Serhiyenko VA, Serhiyenko AA. Diabetic cardiac autonomic neuropathy: Do we have any treatment perspectives? World J Diabetes 2015;6:245–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Storm, P, Rosengren A, Groop L. A novel fine-tuned classification of diabetes with prognostic value: steps towards precision medicine (Abstract). Diabetes 2016;65(Suppl. 1):A94
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.