Abstract
Background
Plaque neovascularization accompanies local inflammation and critically contributes to plaque instability. Correct identification of intraplaque neovascularization by contrast–enhanced ultrasound (CEUS) may provide an additional risk marker in carotid stenosis. This pilot study investigates the correlation between histological evaluation of carotid plaque specimens and pre-surgery CEUS to identify neovascularization.
Methods
17 patients with high-grade internal carotid artery (ICA) stenosis were studied. CEUS was performed in all patients shortly before carotid endarterectomy. Neovascularization, infiltration of T cells and macrophages along with intraplaque hemorrhage were studied in excised plaques by immunohistochemistry. Ultrasound-based four-level and two-level classification systems for neovascularization were used. CEUS findings were compared with histological findings.
Results
Scores on the CEUS-based four-level and two-level classifications were robustly correlated with the density of intraplaque vessels (r = 0.635, p = 0.006 and r = 0.578, p = 0.015, respectively). Histological evaluation of regions with strong and prolonged intraplaque enhancement typically showed strong intraplaque neovascularization in conjunction with acute intraplaque hemorrhage. Moreover, higher grades of intraplaque neovascularization as determined by ultrasound were associated with a higher percentage of macrophage-rich areas.
Conclusion
CEUS is a technique well suited to gauge the degree of neovascularization of carotid plaques. Future research will have to define the reliability and validity of CEUS in everyday clinical practice. Further, our study suggests that CEUS may also be useful to pick up features of vulnerable plaques such as acute intraplaque hemorrhages.
Introduction
Carotid stenosis may precipitate life-threatening complications such as ischemic stroke [1]. The rupture or erosion of a carotid plaque may lead to thromboemboli that occlude vessels in a distal branch. Stroke risk in patients with carotid plaques may vary widely [2]. Well-established risk predictors are the degree of internal carotid artery (ICA) stenosis as well as prior ischemic events in the territory of the internal carotid artery [3,4]. In symptomatic ICA stenosis, the risk of a further ischemic event within the subsequent 12 weeks may be as high as 32%. By contrast, the risk of suffering an ischemic stroke as a result of asymptomatic ICA stenosis ranges between 1 and 2% annually [5,6]. Typical characteristics of high-risk stenosis include a thin fibrous cap alongside a large necrotic core, intraplaque hemorrhage as well as high numbers of infiltrated inflammatory cells [7–11]. Inflammation plays a key role in plaque progression and vulnerability to rupture [7–9]. High intraplaque neovascularization seems to be an important mechanism that sustains local inflammation and may thus serve as an indirect marker of plaque inflammation. In this context, it is not surprising that a number of studies have shown that high plaque neovascularization is associated with a higher risk of symptomatic stenosis [9–11].
Nowadays, ultrasound is able to capture and discern many properties of carotid plaques that might have previously gone undetected [12–17]. Importantly, there appears to be a good correlation between ultrasound imaging and subsequent histopathological findings [2,11,18,19]. In particular, ultrasound investigations into intraplaque neovascularization have garnered increasing attention since intraplaque neovascularization holds promise as an indirect marker of the amount of infiltrated inflammatory cells [2,11,19].
Regarding the use of the CEUS technique, several advantages are worth emphasizing: CEUS is a mobile and a relatively low-cost technique that is suitable for almost every patient. Other techniques such as MRI have more contraindications and are more difficult to conduct, e.g. in patients with claustrophobia, a pacemaker, patients who require intensive care etc. Finally, CEUS is a technique which can be learnt relatively easily.
This study aims to test the hypothesis that contrast-enhanced ultrasound (CEUS) of carotid stenosis is able to quantify intraplaque neovascularization. CEUS was performed in patients before carotid endarterectomy (CEA). Plaque specimens obtained during CEA were then examined using immunohistochemistry.
Methods
Patients
Nineteen patients with high-grade carotid stenosis who were about to undergo CEA were enrolled into the study. One patient withdrew his consent prior to scheduled CEA. The carotid specimen of another patient was so calcified that, despite multiple decalcification steps, it could not be processed further. In total, 17 patients completed the study. Patients were classified according to the North American Symptomatic Endarterectomy Trial criteria (NASCET) as having asymptomatic or symptomatic ICA stenosis [5]. Symptomatic ICA stenosis is defined by the occurrence of neurologic symptoms referable to the ipsilateral carotid artery within the preceding 120 days. Other potential causes of stroke such as cardiac embolism had to be ruled out. None of the patients suffering from asymptomatic ICA stenosis had suffered a previous ischemic event attributable to carotid stenosis [5,6].
All patients were on antiplatelet drugs and received a statin. No patient received immunosuppressive treatment (e.g., corticosteroids, azathioprine, monoclonal antibodies etc.). Approval for this study was obtained from the institutional ethics committee of Charité Hospital Berlin (Ethikkommission 1). Informed consent was obtained from all participants prior to enrollment.
Contrast-enhanced ultrasound imaging of carotid stenosis
CEUS was performed using the AplioTM 500 ultrasound system and a 5-MHz wideband transducer for contrast harmonic imaging (Toshiba, Otawara, Japan) after a bolus injection of 2.4 ml SonoVue echocontrast agent (Bracco, Milan, Italy). Directly after the injection of the contrast agent, the i.v. access was flushed with 5 ml 0.9% sodium chloride. The interval between injection of SonoVue and the beginning of CEUS imaging of the plaque ranged between 10 and 60 seconds depending on arrival time in the carotid artery. The digital recording of the dynamic sequence at the level of the carotid bifurcation was started when the contrast agent reached the carotid bifurcation.
Micro Flow Imaging (Toshiba), which employs summation of microbubble signals, was used to study neovascularization. A video clip in longitudinal and transverse orientation was saved for subsequent analysis. Ten frames per second were acquired over a span of 50 seconds. For the separate imaging of the plaque area in the longitudinal and transverse planes, the procedure was repeated after an interval of five minutes. In order to evaluate intraplaque neovascularization, the radiologist only took into account the flow dynamics of contrast agent within the plaque. Vascularization associated with adventitia was disregarded because these structures are not removed by CEA and thus cannot be examined histologically [20]. T. F. performed all CEUS investigations.
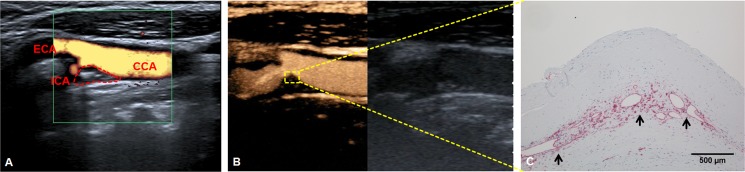
A typical false-color Doppler ultrasound scan of the carotid bifurcation is given in Fig 1. Important anatomical landmarks including the carotid plaque are highlighted. A freeze-frame shot from a CEUS video clip (S1 Video) of the same patient is provided in Fig 1. The flow dynamics of contrast agent within the vessel and plaque are illustrated. Fig 1 then allows the direct comparison of CEUS findings with the histological evaluation (CD31 vessel staining) of the identical area within the carotid plaque of the same patient.
Fig 1. Contrast–enhanced ultrasound (CEUS) for assessing neo-vascularization of carotid plaque.
(A) Color Doppler ultrasound of carotid bifurcation. The dotted area represents the stenotic carotid plaque. CCA: Common carotid artery. ICA: Internal carotid artery. ECA: External carotid artery. (B) CEUS of the same area as shown in A. Note yellow-orange color of the contrast agent filling the lumen of the carotid artery. Furthermore, CEUS contrast effects are visible within the carotid plaque (yellow square), indicating plaque neovascularization. (C) Immunohistological evaluation of the plaque area shown in B with anti-CD31 antibody staining. The arrows mark CD31-positive neovessels.
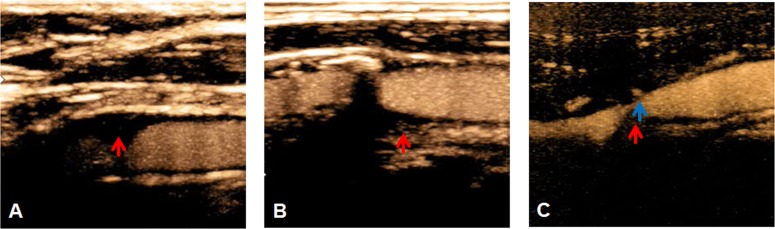
Assessment of intraplaque neovascularization followed the classification of Huang and co-workers [21]: Grade I: non-enhancement; grade II: arterial wall vasa vasorum enhancement; grade III: arterial wall vasa vasorum as well as plaque shoulder enhancement; grade IV: extensive and internal plaque enhancement (Fig 2). For clinical simplicity, we dichotomized CEUS findings into carotid stenosis with low (‘A’; grades I and II) and high (‘B’; grades III and IV) intraplaque contrast agent enhancement.
Fig 2. Grades of intraplaque neovascularization as assessed by contrast–enhanced ultrasound (CEUS).
The arrows indicate specific plaque characteristics. (A) Grade II: very low diffuse enhancement, no focal intraplaque enhancement. (B) Grade III: clear focal plaque shoulder enhancement in proximal part of carotid plaque. (C) Grade IV: clear focal plaque shoulder enhancement (blue arrow) with strong diffuse intraplaque enhancement in an additional region (red arrow).
Tissue preparation, quantification and imaging
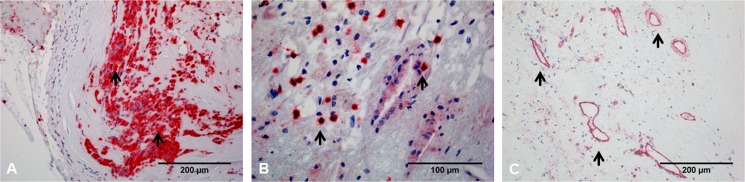
CEA specimens were processed as described in detail previously [7]. Briefly, immunohistochemistry of 4 μm paraffin sections followed the alkaline phosphatase method [7]. For immunohistochemical detection of macrophages (CD68), T-cells (CD3), and neovascularization (CD31), specimens were stained automatically using the Ventana Discovery XT staining system (Ventana, Tucson, USA). CD31 is a transmenbrane glycoprotein that is mainly expressed by endothelial cells. Thus, CD31 labels neovascularization within carotid plaques [22]. Additional stains with van Gieson’s and hematoxylin/eosin were performed for verification and spatial classification of immunohistochemical results. For quantitative evaluation, the StereoInvestigator system (Micro Brightfield Europe, Magdeburg, Germany) was used in combination with a spectral confocal microscope and a digital camera (Leica Microsystems, Heidelberg, Germany). As macrophages tend to appear as confluent infiltrates (Fig 3), the total area occupied by macrophages was measured. The density of CD31-positive vessels and the density of T cells (Fig 3) were determined by counting the cells in relation to the total section area (n/mm2). The extent of macrophage infiltration was quantified by calculating the percentage of the area occupied by macrophages relative to the total section area.
Fig 3. Immunohistochemical evaluation of carotid plaque.
Immunohistochemical stainings of macrophages (CD68), T-cells (CD3), and neovascularization (CD31). Arrows highlight distinctive features of the immunohistological staining patterns. (A) Macrophages often appear as confluent infiltrates. (B) T cells typically present as single cells. (C) CD31-positive vessels within atherosclerotic plaque.
Statistical analysis and blinding
Great care was taken to ensure adequate blinding of all investigators. In particular, the histological investigation of CEA specimens (C.S.) and the radiological examination (T.F.) of carotid stenosis occurred entirely independently. Statistical analysis was performed using IBM SPSS Statistics 22 (IBM Corporation, Armonk, U.S.A). Fisher’s exact test was applied to evaluate group differences in gender composition between patients with and patients without symptomatic ICA stenosis. The Mann-Whitney-U test was used to examine differences between groups. Correlations between CEUS grading and histological findings were performed using Spearman’s rho.
Results
The main clinical characteristics of our sample are summarized in Table 1. The sample consisted of mainly male patients. Median age was 66 years. Seven patients had symptomatic ICA stenosis. The median time interval between CEA and the most recent symptoms related to symptomatic ICA stenosis was 9 days. The median time interval between CEUS and CEA was 1 day. Patients with symptomatic ICA stenosis did not differ from those with asymptomatic stenosis in terms of gender (p = 0.35), age (p = 0.23), the degree of stenosis (p>0.99), or the time interval between the CEUS examination and CEA (p = 0.60).
Table 1. Baseline characteristics of the patient sample.
| Variables | Patients (n = 17) |
|---|---|
| Age, years | 66 (58–76) |
| Males,% | 10 (58.8) |
| Grade of ICA stenosis, % | 85 (60–95) |
| Symptomatic ICA stenosis, % | 7 (41.2) |
| Days CEUS to CEA, days | 1 (0–9) |
| Days last symptoms to CEA, days | 9 (2–35) |
Categorical variables are presented as number (%), continuous variables as median and range (minimum—maximum).
The four-level CEUS classification of intraplaque neovascularization was strongly and positively correlated with increased density of intraplaque vessels on histology (r = 0.635, p = 0.006). Relatively similar results were obtained when the two-level CEUS classification was used (r = 0.578, p = 0.015). Table 2 provides correlations between ultrasound classification of neovascularization and key histological measures.
Table 2. Correlations of CEUS-based classification of intraplaque neovascularization and histological parameters.
| Variables | US neovasculization grading I—IV | US neovasculization grading A and B |
|---|---|---|
| intraplaque vessels, vessels/mm2 | 0.635 (0.006) | 0.578 (0.015) |
| T cells, cells/mm2 | 0.263 (0.308) | 0.452 (0.068) |
| macrophage rich area, % | 0.499 (0.042) | 0.503 (0.040) |
Correlations are presented using Spearman’s rho (p).
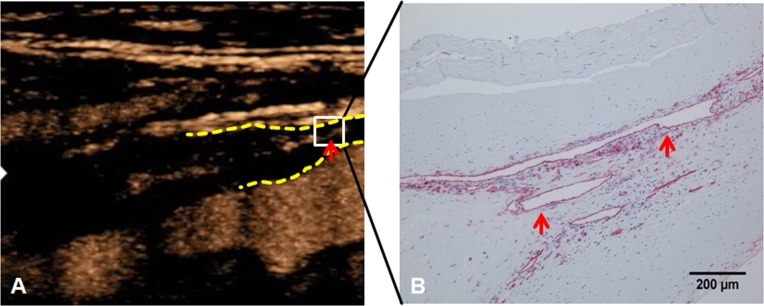
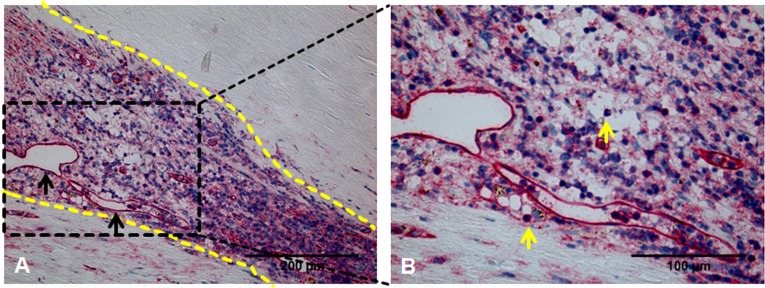
So far, only few studies by us and others have directly compared CEUS and histopathology [2,18,23]. In this investigation, focal enhancements of contrast corresponded well to intraplaque neovascularization (Figs 1 and 4).
Fig 4. Neovascularization on contrast-enhanced ultrasound (CEUS) and immunohistochemistry.
(A) Contrast-enhanced ultrasound (CEUS) of carotid stenosis. The dotted yellow lines mark the proximal beginning of the carotid plaque. The red arrow marks an area of focal neovascularization. (B) The area corresponding to the white square in A was further analyzed with anti-cd31 immunohistochemistry. Note pronounced neovascularization (red arrows) in the surgical specimen.
Moreover, histological evaluation of regions with intense and prolonged intraplaque enhancement regularly yielded neovascularization and acute intraplaque hemorrhage (Fig 5). CEUS is not able to distinguish between contrast agent located within neovessels and contrast agent moving freely within the plaque, such as may be the case in acute neovessel rupture and subsequent intraplaque hemorrhage.
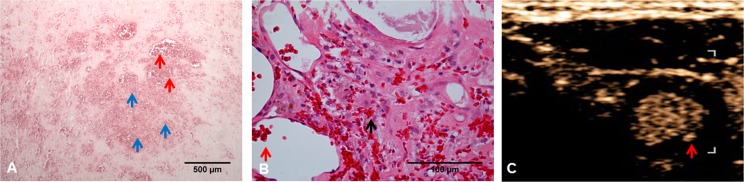
Fig 5. Plaque neovascularization and intra-plaque hemorrhages.
(A) Hematoxylin/eosin staining of carotid plaque reveals intraplaque vessels densely filled with erythrocytes (red arrows) alongside acute intraplaque hemorrhages (blue arrows). (B) Higher magnification demonstrates extravascular erythrocytes indicative of intraplaque hemorrhages (black arrow). The intraplaque hemorrhages shown here must be relatively fresh because individual erythrocytes are still clearly demarcated. (C) Axial imaging of carotid artery by contrast-enhanced ultrasound (CEUS). The red arrow points to the area that corresponds to the histological image (A). CEUS is not able to distinguish between contrast agent located within neovessels and contrast agent moving freely within the plaque, such as may be the case in neovessel rupture followed by intraplaque hemorrhage.
An example of massive infiltration of inflammatory cells in the area of neovascularization is given in Fig 6.
Fig 6. Massive infiltration of inflammatory cells in area of intraplaque neovascularization.
(A) CD31 antibody staining reveals intraplaque neovascularization. Black arrows mark individual neovessels. The dotted lines delineate an area within the plaque characterized by a high density of blue-stained nuclei suggesting strong infiltration of inflammatory cells in close proximity to the area of neovascularization. (B) Higher magnification of boxed area shown in A. Yellow arrows mark individual inflammatory cells. Cell shape and nuclear geometry suggest the presence of inflammatory cells (predominantly macrophages and T cells).
Histological results were also compared between patients with symptomatic ICA stenosis and asymptomatic patients (Table 3). The percentage of area infiltrated by macrophages was significantly higher in plaques from patients with symptomatic ICA stenosis. An extended data set containing information on clinical characteristics, immunohistological und CEUS parameters of individual patients is available in S1 Table.
Table 3. Comparison of histological and CEUS parameters between asymptomatic patients and patients suffering from symptomatic ICA stenosis.
| Variables | Symptomatic (n = 7) | Asymptomatic (n = 10) | p |
|---|---|---|---|
| intraplaque vessels/mm2 | 8.14 (4.33) | 6.39 (4.70) | 0.109 |
| T cells/mm2 | 41.91 (23.69) | 21.91 (16.51) | 0.364 |
| macrophage rich area in % | 7.25 (4.31) | 2.98 (1.00) | 0.007 |
| CEUS neovasculization grade I, n(%) | 0(0) | 0(0) | 0.270 |
| CEUS neovasculization grade II, n(%) | 1(14.3) | 5(50.0) | |
| CEUS neovasculization grade III, n(%) | 5(71.4) | 4(40.0) | |
| CEUS neovasculization grade IV, n(%) | 1(14.3) | 1(10.0) | |
| CEUS neovasculization grade A, n(%) | 1(14.3) | 5(50.0) | 0.230 |
| CEUS neovasculization grade B, n(%) | 6(85.7) | 5(50.0) |
Continuous variables are given as mean (SD). Categorical variables are presented as number (%).
Discussion
Our pilot study demonstrates that CEUS of carotid stenosis is able to faithfully detect different grades of intraplaque neovascularization confirmed by immunohistochemistry. The percentage of area infiltrated by macrophages was significantly higher in plaques from patients with symptomatic ICA stenosis and in carotid stenosis with higher grades of intraplaque neovascularization as assessed by CEUS. Furthermore, our study suggests that intense and prolonged intraplaque enhancement of ultrasound contrast agent seems to be associated with intraplaque hemorrhage. Intraplaque hemorrhage contributes to plaque instability and plays an essential part in atherosclerotic plaque growth [7,24,25]. Importantly, intraplaque hemorrhage frequently results from the rupture of neovessels which may be caused by, among other factors, high levels of local inflammation. Intraplaque hemorrhage further destabilizes the carotid plaque [26–28]. Increased plaque vascularization seems to be a prerequisite for maintaining the inflammatory process within the plaque. Vasa vasorum are present in the adventitia and, under physiological conditions, nurture the outer components of the vessel wall. The intima, however, is fed by oxygen diffusion from the lumen [9]. If the intima thickens as a result of atherosclerotic progression, oxygen diffusion becomes impaired and the vasa vasorum turn into a major source of nutrients. Hypoxia and inflammation thus stimulate the extent and distribution of the vascular network [29]. Intraplaque neovascularization is based on microvessels originating from the adventitia [26]. These microvessels are immature and fragile and, for this reason, prone to rupture [9]. Furthermore, neovessels are critical for the trafficking of inflammatory cells like T-cells and macrophages into the plaque [9,26]. Intraplaque neovascularization may also be conceptualized as a healing process. In all probability, intraplaque neovascularization is a prerequisite for the removal of amorphous cell material near the lipid core by e.g. macrophages [7,24]. The precise role of plaque neovascularization in the complex pathophysiology of plaque instability and plaque healing remains to be further defined.
CEUS seems to have the potential not only to evaluate intraplaque neovascularization and hemorrhage, but also to examine other features of carotid stenosis. There are studies indicating that microbubbles of ultrasound contrast agent can be conjugated to specific vascular cell adhesion molecules and integrins expressed in endothelial cells [30,31]. Recently, CEUS as a form of molecular imaging has been suggested as a promising technique to evaluate inflammation [2,14]. Microbubbles that are phagocytized by macrophages and can adhere directly to the surface of damaged endothelium may aid in risk stratification of atherosclerotic plaques [32]. Importantly, the grade of neovascularization in CEUS was positively correlated with the size of the macrophage-infiltrated area in our study. Hence, if this result is replicated, CEUS detection of intraplaque neovascularization may also provide the clinician with a measure of macrophage infiltration. Most likely, our observation indicates that high levels of neovascularization promote macrophage infiltration and vice versa.
Algorithms for the automated analysis of the behavior of the ultrasound contrast agent within the plaque tissue may hold great promise. For example, quantification of time-signal intensity curves in CEUS of carotid stenosis may allow the examiner to differentiate between symptomatic and asymptomatic ICA stenosis [24,33]. Still, one has to point out that, in the walls of latex flow phantoms, enhancement patterns typical for vasa vasorum may actually occur in the absence of vasa vasorum [34]. Therefore, algorithms for sorting out artifacts will also have to be developed especially when using automated signal quantification of the contrast agent.
To summarize, our study provides promising initial evidence that contrast-enhanced ultrasound (CEUS) of carotid stenosis may be a useful tool to assess intraplaque neovascularization. However, the technique may require further refinement and testing before it can be broadly implemented in the clinic. Since our pilot study included only a relatively small number of patients, further investigations with a higher number of patients will also be necessary to validate our findings.
Supporting information
In S1 Video you will find a video clip of the same region like in Fig 1 showing a contrast-enhanced ultrasound (CEUS) investigation of this area. The flow dynamics of contrast agent within the vessel and in the plaque are shown to detect neovascularization.
(MP4)
(DOCX)
Data Availability
Please also note that an extended data set containing information on clinical characteristics as well as immunohistological und CEUS parameters of individual patients has been appended as S1 Table.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Ibrahimi P, Jashari F, Nicoll R, Bajraktari G, Wester P, Henein MY. Coronary and carotid atherosclerosis: How useful is the imaging? Atherosclerosis. 2013;231: 323–333. doi: 10.1016/j.atherosclerosis.2013.09.035 [DOI] [PubMed] [Google Scholar]
- 2.Kunte H, Rückert R- I, Schmidt C, Harms L, Kasper A- S, Hellweg R, et al. Detection of Unstable Carotid Plaque by Tissue Doppler Imaging and Contrast-Enhanced Ultrasound in a Patient with Recurrent Amaurosis Fugax. Case Rep Vasc Med. 2013;2013: 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barnett HJ, Taylor DW, Eliasziw M, Fox AJ, Ferguson GG, Haynes RB, et al. Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med. 1998;339: 1415–1425. doi: 10.1056/NEJM199811123392002 [DOI] [PubMed] [Google Scholar]
- 4.Rothwell PM, Gutnikov SA, Warlow CP, European Carotid Surgery Trialist’s Collaboration. Reanalysis of the final results of the European Carotid Surgery Trial. Stroke J Cereb Circ. 2003;34: 514–523. [DOI] [PubMed] [Google Scholar]
- 5.North American Symptomatic Carotid Endarterectomy Trial Collaborators. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med. 1991;325: 445–453. doi: 10.1056/NEJM199108153250701 [DOI] [PubMed] [Google Scholar]
- 6.Hennerici M, Hülsbömer HB, Hefter H, Lammerts D, Rautenberg W. Natural history of asymptomatic extracranial arterial disease. Results of a long-term prospective study. Brain J Neurol. 1987;110 (Pt 3): 777–791. [DOI] [PubMed] [Google Scholar]
- 7.Kunte H, Amberger N, Busch MA, Rückert R-I, Meiners S, Harms L. Markers of instability in high-risk carotid plaques are reduced by statins. J Vasc Surg. 2008;47: 513–522. doi: 10.1016/j.jvs.2007.11.045 [DOI] [PubMed] [Google Scholar]
- 8.Ammirati E, Moroni F, Norata GD, Magnoni M, Camici PG. Markers of inflammation associated with plaque progression and instability in patients with carotid atherosclerosis. Mediators Inflamm. 2015;2015: 718329 doi: 10.1155/2015/718329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moreno PR, Purushothaman KR, Zias E, Sanz J, Fuster V. Neovascularization in human atherosclerosis. Curr Mol Med. 2006;6: 457–477. [DOI] [PubMed] [Google Scholar]
- 10.Staub D, Patel MB, Tibrewala A, Ludden D, Johnson M, Espinosa P, et al. Vasa vasorum and plaque neovascularization on contrast-enhanced carotid ultrasound imaging correlates with cardiovascular disease and past cardiovascular events. Stroke J Cereb Circ. 2010;41: 41–47. [DOI] [PubMed] [Google Scholar]
- 11.Coli S, Magnoni M, Sangiorgi G, Marrocco-Trischitta MM, Melisurgo G, Mauriello A, et al. Contrast-enhanced ultrasound imaging of intraplaque neovascularization in carotid arteries: correlation with histology and plaque echogenicity. J Am Coll Cardiol. 2008;52: 223–230. doi: 10.1016/j.jacc.2008.02.082 [DOI] [PubMed] [Google Scholar]
- 12.Partovi S, Loebe M, Aschwanden M, Baldi T, Jäger KA, Feinstein SB, et al. Contrast-enhanced ultrasound for assessing carotid atherosclerotic plaque lesions. AJR Am J Roentgenol. 2012;198: W13–19. doi: 10.2214/AJR.11.7312 [DOI] [PubMed] [Google Scholar]
- 13.Ten Kate GL, van den Oord SCH, Sijbrands EJG, van der Lugt A, de Jong N, Bosch JG, et al. Current status and future developments of contrast-enhanced ultrasound of carotid atherosclerosis. J Vasc Surg. 2013;57: 539–546. doi: 10.1016/j.jvs.2012.07.028 [DOI] [PubMed] [Google Scholar]
- 14.Magnoni M, Ammirati E, Camici PG. Non-invasive molecular imaging of vulnerable atherosclerotic plaques. J Cardiol. 2015;65: 261–269. doi: 10.1016/j.jjcc.2015.01.004 [DOI] [PubMed] [Google Scholar]
- 15.Kunte H, Rückert R- I, Schmidt C, Harms L, Grigoryev M, Fischer T. Inverse fly-through technique in ultrasound imaging of carotid stenosis. Neurology. 2013;80: 122 doi: 10.1212/WNL.0b013e31827b1b06 [DOI] [PubMed] [Google Scholar]
- 16.Cattaneo M, Staub D, Porretta AP, Gallino JM, Santini P, Limoni C, et al. Contrast-enhanced ultrasound imaging of intraplaque neovascularization and its correlation to plaque echogenicity in human carotid arteries atherosclerosis. Int J Cardiol. 2016;223: 917–922. doi: 10.1016/j.ijcard.2016.08.261 [DOI] [PubMed] [Google Scholar]
- 17.Hjelmgren O, Gellerman K, Kjelldahl J, Lindahl P, Bergström GML. Increased Vascularization in the Vulnerable Upstream Regions of Both Early and Advanced Human Carotid Atherosclerosis. PloS One. 2016;11: e0166918 doi: 10.1371/journal.pone.0166918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kunte H, Schmidt C, Harms L, Rückert R-I, Grigoryev M, Fischer T. Contrast-enhanced ultrasound and detection of carotid plaque neovascularization. Neurology. 2012;79: 2081 doi: 10.1212/WNL.0b013e3182749f4c [DOI] [PubMed] [Google Scholar]
- 19.Li C, He W, Guo D, Chen L, Jin X, Wang W, et al. Quantification of carotid plaque neovascularization using contrast-enhanced ultrasound with histopathologic validation. Ultrasound Med Biol. 2014;40: 1827–1833. doi: 10.1016/j.ultrasmedbio.2014.02.010 [DOI] [PubMed] [Google Scholar]
- 20.Yuan C, Beach KW, Smith LH, Hatsukami TS. Measurement of Atherosclerotic Carotid Plaque Size In Vivo Using High Resolution Magnetic Resonance Imaging. Circulation. 1998;98: 2666–2671. [DOI] [PubMed] [Google Scholar]
- 21.Huang P-T, Chen C-C, Aronow WS, Wang X-T, Nair CK, Xue N-Y, et al. Assessment of neovascularization within carotid plaques in patients with ischemic stroke. World J Cardiol. 2010;2: 89–97. doi: 10.4330/wjc.v2.i4.89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pusztaszeri MP, Seelentag W, Bosman FT. Immunohistochemical expression of endothelial markers CD31, CD34, von Willebrand factor, and Fli-1 in normal human tissues. J Histochem Cytochem Off J Histochem Soc. 2006;54: 385–395. [DOI] [PubMed] [Google Scholar]
- 23.Saito K, Nagatsuka K, Ishibashi-Ueda H, Watanabe A, Kannki H, Iihara K. Contrast-enhanced ultrasound for the evaluation of neovascularization in atherosclerotic carotid artery plaques. Stroke. 2014;45: 3073–3075. doi: 10.1161/STROKEAHA.114.006483 [DOI] [PubMed] [Google Scholar]
- 24.Kopriva D, Kisheev A, Meena D, Pelle S, Karnitsky M, Lavoie A, et al. The Nature of Iron Deposits Differs between Symptomatic and Asymptomatic Carotid Atherosclerotic Plaques. Hagemeyer CE, editor. PLOS ONE. 2015;10: e0143138 doi: 10.1371/journal.pone.0143138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mangla R, Kolar B, Almast J, Ekholm SE. Border Zone Infarcts: Pathophysiologic and Imaging Characteristics. RadioGraphics. 2011;31: 1201–1214. doi: 10.1148/rg.315105014 [DOI] [PubMed] [Google Scholar]
- 26.Virmani R, Kolodgie FD, Burke AP, Finn AV, Gold HK, Tulenko TN, et al. Atherosclerotic plaque progression and vulnerability to rupture: angiogenesis as a source of intraplaque hemorrhage. Arterioscler Thromb Vasc Biol. 2005;25: 2054–2061. doi: 10.1161/01.ATV.0000178991.71605.18 [DOI] [PubMed] [Google Scholar]
- 27.van Dijk AC, Truijman MTB, Hussain B, Zadi T, Saiedie G, de Rotte A a. J, et al. Intraplaque Hemorrhage and the Plaque Surface in Carotid Atherosclerosis: The Plaque At RISK Study (PARISK). AJNR Am J Neuroradiol. 2015;36: 2127–2133. doi: 10.3174/ajnr.A4414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Teng Z, Sadat U, Brown AJ, Gillard JH. Plaque hemorrhage in carotid artery disease: pathogenesis, clinical and biomechanical considerations. J Biomech. 2014;47: 847–858. doi: 10.1016/j.jbiomech.2014.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sluimer JC, Gasc J-M, van Wanroij JL, Kisters N, Groeneweg M, Sollewijn Gelpke MD, et al. Hypoxia, hypoxia-inducible transcription factor, and macrophages in human atherosclerotic plaques are correlated with intraplaque angiogenesis. J Am Coll Cardiol. 2008;51: 1258–1265. doi: 10.1016/j.jacc.2007.12.025 [DOI] [PubMed] [Google Scholar]
- 30.Kaufmann BA, Sanders JM, Davis C, Xie A, Aldred P, Sarembock IJ, et al. Molecular imaging of inflammation in atherosclerosis with targeted ultrasound detection of vascular cell adhesion molecule-1. Circulation. 2007;116: 276–284. doi: 10.1161/CIRCULATIONAHA.106.684738 [DOI] [PubMed] [Google Scholar]
- 31.Leong-Poi H, Christiansen J, Heppner P, Lewis CW, Klibanov AL, Kaul S, et al. Assessment of endogenous and therapeutic arteriogenesis by contrast ultrasound molecular imaging of integrin expression. Circulation. 2005;111: 3248–3254. doi: 10.1161/CIRCULATIONAHA.104.481515 [DOI] [PubMed] [Google Scholar]
- 32.Owen DR, Shalhoub J, Miller S, Gauthier T, Doryforou O, Davies AH, et al. Inflammation within carotid atherosclerotic plaque: assessment with late-phase contrast-enhanced US. Radiology. 2010;255: 638–644. doi: 10.1148/radiol.10091365 [DOI] [PubMed] [Google Scholar]
- 33.Xiong L, Deng Y-B, Zhu Y, Liu Y-N, Bi X-J. Correlation of carotid plaque neovascularization detected by using contrast-enhanced US with clinical symptoms. Radiology. 2009;251: 583–589. doi: 10.1148/radiol.2512081829 [DOI] [PubMed] [Google Scholar]
- 34.Thapar A, Shalhoub J, Averkiou M, Mannaris C, Davies AH, Leen ELS. Dose-dependent artifact in the far wall of the carotid artery at dynamic contrast-enhanced US. Radiology. 2012;262: 672–679. doi: 10.1148/radiol.11110968 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
In S1 Video you will find a video clip of the same region like in Fig 1 showing a contrast-enhanced ultrasound (CEUS) investigation of this area. The flow dynamics of contrast agent within the vessel and in the plaque are shown to detect neovascularization.
(MP4)
(DOCX)
Data Availability Statement
Please also note that an extended data set containing information on clinical characteristics as well as immunohistological und CEUS parameters of individual patients has been appended as S1 Table.