Abstract
Introduction
Prior studies showed that live attenuated influenza vaccine (LAIV) is more effective than inactivated influenza vaccine (IIV) in children aged 2–8 years, supporting CDC recommendations in 2014 for preferential LAIV use in this age group. However, 2014–2015 U.S. effectiveness data indicated relatively poor effectiveness of both vaccines, leading CDC in 2015 to no longer prefer LAIV.
Methods
An age-structured model of influenza transmission and vaccination was developed, which incorporated both direct and indirect protection induced by vaccination. Based on this model, the cost effectiveness of influenza vaccination strategies in children aged 2–8 years in the U.S. was estimated. The base case assumed a mixed vaccination strategy where 33.3% and 66.7% of vaccinated children aged 2–8 years receive LAIV and IIV, respectively. Analyses were performed in 2014–2015.
Results
Using published meta-analysis vaccine effectiveness data (83% LAIV and 64% IIV), exclusive LAIV use would be a cost-effective strategy when vaccinating children aged 2–8 years, whereas IIV would not be preferred. However, when 2014–2015 U.S. effectiveness data (0% LAIV and 15% IIV) were used, IIV was likely to be preferred.
Conclusions
The cost effectiveness of influenza vaccination in children aged 2–8 years is highly dependent on vaccine effectiveness; the vaccine type with higher effectiveness is preferred. In general, exclusive IIV use is preferred over LAIV use, as long as vaccine effectiveness is higher for IIV than for LAIV.
Introduction
Influenza is a significant health and economic burden, affecting approximately 5%–20% of the U.S. population each year and resulting in 300,000 hospitalizations and 24,000 deaths each year.1 Annual U.S. direct medical costs associated with influenza are estimated to be more than $10 billion, with indirect costs exceeding $16 billion.2
Infection rates of influenza are highest among children, ranging between 23% and 49% during inter-pandemic years.3 In addition, children are more likely than adults to spread the disease. Since 2010, CDC has recommended annual influenza vaccination for individuals aged 6 months and older.2,4 Currently available influenza vaccines are either an injectable inactivated influenza vaccine (IIV) or intranasal live attenuated influenza vaccine (LAIV). LAIV is licensed in the U.S. for administration to eligible individuals aged 2–49 years, whereas IIV is licensed for eligible individuals aged 6 months or older. Studies comparing LAIV and IIV in children found LAIV more protective against culture-confirmed influenza.3 Specifically, the mean effectiveness of one and two LAIV doses among children was 77% and 87%, respectively, whereas mean IIV effectiveness in children ranged from 59% to 65%.5 As a result, the Advisory Committee on Immunization Practices recommended preferential LAIV use for healthy children aged 2–8 years in the 2014–2015 season; therefore, LAIV has been increasingly administered to children in recent years.3
However, 2014–2015 U.S. effectiveness data indicated that LAIV may not be more effective than IIV against the predominant H3N2 viruses circulating during the 2014–2015 season. The IIV effectiveness estimate against H3N2 in children aged 2–8 years was 15% (95% CI= –20, 40), compared with –23% (95% CI= –90, 21) for LAIV.6 As a result, the Advisory Committee on Immunization Practices did not renew their 2014–2015 preference for LAIV over IIV in children aged 2–8 years.
Despite increased interest in economic and epidemiologic assessment of influenza vaccination, few economic analyses have explicitly addressed LAIV use. Recent studies evaluated childhood LAIV vaccination, concluding that LAIV use is very cost effective7 or even cost saving.3 However, these studies were based on a static infection risk among unvaccinated groups, ignoring possible indirect benefits of vaccination. This paper describes the first cost-effectiveness analysis of influenza vaccination that incorporates the impact of both direct and indirect effects of influenza vaccination, based on the recently observed lower effectiveness of IIV and LAIV. Specifically, the cost effectiveness of LAIV and IIV vaccination in children aged 2–8 years is presented. In addition, the threshold vaccine effectiveness at which LAIV becomes preferred over IIV is examined.
Dynamic models implicitly capture the herd protection conferred by vaccination, and have been used in recent economic analyses of vaccination programs against infectious diseases including influenza,8,9 rotavirus,10,11 and human papillomavirus.12,13 We developed a dynamic age-structured model of influenza transmission and vaccination in order to determine the optimal cost effectiveness of vaccination strategies considering LAIV and IIV use among children aged 2–8 years.
Methods
An age-structured influenza dynamic transmission model was developed and then integrated into a cost-effectiveness analysis for vaccination. The benefit of vaccination strategies was defined as the decrease in disease burden and associated costs for children and their caregivers. The focus was on influenza among children aged 2–8 years to calculate quality-adjusted life years (QALYs) and influenza-related costs.
Mathematical Model
The mathematical model incorporated influenza transmission dynamics and vaccination programs using both LAIV and IIV (Appendix Figure 1). The influenza-related epidemiologic status of individuals was tracked in eight age-dependent classes: susceptible (Sk), vaccinated with LAIV (VLk), vaccinated with IIV (VTk), latently infected (Ek), asymptomatically or symptomatically infected (Ak or Ik), recovered (Rk), and dead due to influenza (Dk). Each epidemiologic compartment was subdivided into six age groups, denoted by the subscript k (k = 1,…, 6), corresponding to 0–6 months, 6 months to 2 years, 2–8 years, 8–19 years, 20–64 years, and ≥65 years.
It was assumed that the vaccine provides partial protection, resulting in vaccinated individuals being less susceptible than unvaccinated ones. Individuals vaccinated with LAIV become infected at a fraction (1- σk) of the rate at which unvaccinated susceptible individuals become infected, where σk is a function of LAIV effectiveness for age group k (Appendix). Similarly, it was assumed that infection risk for IIV vaccinees is reduced by a factor, ωk. Ψk and θk are defined as the vaccination rate in age group k using LAIV and IIV, respectively. Owing to lack of data for vaccine-specific coverage across age groups, IIV was assumed to be the only vaccine used to immunize individuals in Age Groups 2, 4, 5, and 6 against influenza (Ψ2 = Ψ4 = Ψ5 = Ψ6 = 0). By contrast, age Group 3 (age 2–8 years) was eligible for either LAIV or IIV. Specifically, it was assumed that a proportion Ψ3 / (Ψ3 + θ3) of vaccinated individuals in Group 3 receive LAIV, while a proportion θ3 / (Ψ3 + θ3) receive IIV. Individuals aged <6 months receive no influenza vaccination (Ψ1 = θ1 =0).
When susceptible or vaccinated individuals are infected, they enter a latency period. The latent period (1/h) was assumed to be 1.2 days on average.14 Latently infected individuals become infectious, and the probability of symptomatic illness is denoted by p (p=0.67).15 People with asymptomatic disease were assumed to be half as infectious as those with symptomatic illness, (i.e., b=0.5).16 The average infectious period, 1/γ, was assumed to be 4 days. Influenza-induced death rates in age group k are denoted by αk, whereas recovered individuals were assumed to be protected against further influenza infection for the remainder of the outbreak.
Based on these assumptions and definitions, the model can be described as:
Here, the total population size is denoted by N(t) = Σk Sk(t) + VLk(t) + VTk(t) + Ek(t) + Ak(t) + Ik(t) + Rk(t), and the force of infection as λk = βk Σj ϕkj {bAj(t) + Ij(t)}/N(t). ϕkj is defined as the contact rate for infectious individuals of age group j (Aj or Ij) with susceptible individuals of age group k (Sk), where βk represents the transmission probability per contact in age group k. In the transmission model, contact rates (ϕkj) were parameterized based on the European age-dependent contact matrix that describes age group mixing.17 The transmission probability per contact in age group k, βk, is calibrated using U.S.-based data on influenza illness rates and vaccine coverage levels in the respective age groups.18 The model was run over a 10-month time horizon (i.e., a single influenza season) from August through May. Given this short time horizon, aging was ignored in this model.
To calibrate the model, 2012–2013 influenza season vaccination coverage was used, which ranged between 35.8% in adults (aged 20–64 years) and 69.3% in younger children (aged 6 months to 4 years) 18. For children aged 2–8 years, vaccination coverage was 58%, resulting in overall symptomatic infection rates of 7.2% for this age group.18 Thus, yearly influenza incidence for aged 2–8 years (7.2%) was used for the base case analysis where it was assumed that 33% and 67% of vaccinated children aged 2–8 years receive LAIV and IIV, respectively, with 83% LAIV effectiveness and 64% IIV effectiveness. In a sensitivity analysis, results were examined with 0% LAIV effectiveness and 15% IIV effectiveness, consistent with their recently observed variability in effectiveness. Under such assumptions, “IIV only,” “LAIV only,” and “mixed” vaccine strategies (i.e., LAIV use in 33% and IIV use in 67%) for children aged 2–8 years were considered. MATLAB 2015b was used for numerical simulations and sensitivity analyses.
The relative reduction in infection risk among the vaccinated compared with the unvaccinated when LAIV and IIV are used, σk and ωk , were parameterized as follows.
From a case-control study, vaccine effectiveness is defined as 1 – OR, where OR represents the OR of vaccination in cases and controls, or the OR of disease in vaccinated and unvaccinated population.19 Here, ΩA0 and ΩA1 are defined as the vaccine effectiveness (VE), where ΩA0 and ΩA1 represent the attack rates (cumulative incidence) in the unvaccinated and vaccinated groups, respectively:
Shim et al.20 derived the expression for ΩA0 and ΩA1 as functions of attack rates using a dynamic model of disease transmission. Those results are used to calculate ΩA0 and ΩA1, and to parameterize σk and ωk (Appendix).
Quality-Adjusted Life Years and Utilities
To calculate QALYs, utilities were weighted by time spent healthy and the number of symptom days. On a scale from 0 (death) to 1 (perfect health), the model used utility of 0.933 for healthy children unaffected by influenza (Table 1).3 The utility lost for children with influenza infections is presented in Table 1.3 In addition, utility lost due to medically significant wheezing was assumed to be 0.082, with an average duration of 12.78 days.3 Because most reactogenicity and injection site events related to vaccination are of short duration and mild severity, the only adverse event considered in QALY calculation was medically significant wheezing.3 To determine life years lost because of influenza-related deaths, it was assumed a life expectancy of 77.9 years.3 As is customary in cost-effectiveness analyses, remaining life years were discounted by 3%.
Table 1.
Epidemiological and Economic Parameters, Baseline Values, Distribution, and Associated References
| Model parameter | Baseline value | Distribution | Ref |
|---|---|---|---|
| Transmission probability per contact in age group k, βk | 0.1162 for k=1, 2 | – | 18 |
| 0.0743 for k=3 | |||
| 0.0324 for k=4 | |||
| 0.0294 for k=5 | |||
| 0.0300 for k=6 | |||
|
| |||
| Rate of LAIV vaccination in age group k at time t, Ψk(t) | Fit to the current vaccine coverage data (Appendix Figure 2) | – | 23 |
|
| |||
| Rate of IIV vaccination in age group k at time t, θk(t) | Fit to the current vaccine coverage data (Appendix Figure 2) | – | 23 |
|
| |||
| Relative reduction in risk of infection among the vaccinated compared to the unvaccinated (LAIV), σk | 0.82 for VE of 83% | – | 3,24, 6 |
| 0 (2014-15 only) | |||
|
| |||
| Relative reduction in risk of infection among the vaccinated compared to the unvaccinated (IIV), ωk | 0.63 for VE of 64% | – | 3,24, 6 |
| 0.13 for VE of 15% (2014-15 only) | |||
|
| |||
| Latency period (days), 1/h | 1.2 | – | 14 |
|
| |||
| Proportion of infected people who become symptomatic, p | 0.67 | – | 15 |
|
| |||
| Relative infectiousness of asymptomatic infection compared to symptomatic ones, b | 0.5 | – | 16 |
|
| |||
| Infectious period (days), 1/γ | 4.5 | – | 14 |
|
| |||
| Influenza-related death rate, α2 | 2.3114 × 10-5 | Triangular (1.1557×10-5 – 3.4671×10-5) | 31 |
|
| |||
| Breakdown of symptomatic influenza | 30 | ||
| Uncomplicated influenza | 69% | – | |
| Complicated influenza | |||
| Influenza + AOM | 11% | – | |
| Influenza + LRI + (AOM) | 20% | – | |
|
| |||
| Probability of influenza-related events | |||
| OTC medication, conditional on symptomatic influenza | 0.433 | Triangular (0.217 – 0.650) | 31 |
| Outpatient visit, conditional on symptomatic influenza | 0.35 | Triangular (0.175 – 0.525) | 7 |
| Hospitalization, conditional on symptomatic influenza | 0.0053 | Triangular (0.0027 – 0.0080) | 7,9 |
| Death (LR and HR (10%) weighted average), conditional on symptomatic influenza | 2.3114 × 10-5 | Triangular (1.1557×10-5 – 3.4671×10-5) | 31 |
|
| |||
| LAIV-associated adverse events (%) | 30 | ||
| MSW | 2.10 | Beta (α=47, β=2140) | |
| Reactogenicity event | |||
| Injection site event | 35.4 | – | |
| 16.7 | – | ||
|
| |||
| IIV-associated adverse events (%) | 30 | ||
| MSW | 2.33 | Beta (α=56, β=2347) | |
| Reactogenicity event | |||
| Injection site event | 24.8 | – | |
| 24.1 | – | ||
|
| |||
| Unit costs for children 2-8 years of age | |||
|
| |||
| Vaccine-related costs (per dose) | 3,28 | ||
| LAIV | $23.42 | – | |
| IIV | $14.61 | Triangular (13.05 – 18.27) | |
| Administration of LAIV | $12.92 | – | |
| Administration of IIV | $12.92 | – | |
|
| |||
| Adverse event-related costs (per episode) | 3 | ||
| MSW | $82.10 | Triangular (81.50 – 82.71) | |
| Reactogenicity event | |||
| Injection site event | $2.36 | – | |
| $2.36 | – | ||
|
| |||
| Influenza-related direct costs | |||
| Hospitalization for influenza | $5850 | Triangular (2372 – 10,760) | 31, 31 |
| Outpatient visit (including prescription medication) | $145 | Triangular (133 – 170) | 31 |
| OTC medication | $6.23 | – | |
|
| |||
| Direct non-medical costs | |||
| Transportation costs | $7.50 | – | 3 |
|
| |||
| Indirect costs | |||
| Cost of caregiver work loss per influenza illness | $178 | – | 31 |
|
| |||
| Health state utilities and durations | |||
|
| |||
| Health state utility | 3 | ||
| Non influenza; no wheezing | 0.933 | – | |
| MSW | 0.851 | – | |
| Influenza (uncomplicated and complicated) | 0.558 | – | |
|
| |||
| Average number of febrile days | 3 | ||
| Uncomplicated influenza | 3.00 | – | |
| Influenza + AOM | 3.52 | – | |
| Influenza + LRI | 3.33 | – | |
|
| |||
| Average number of symptom days | 3 | ||
| MSW | 12.78 | – | |
LAIV, live attenuated influenza vaccine; IIV, inactivated influenza vaccine; AOM, acute otitis media; LRI, lower respiratory tract infection; LR, low risk; HR, high risk; OTC, over-the-counter; MSW, medically significant wheezing
Costs
In children, 43% of symptomatic influenza infections require over-the-counter medication, 35% require outpatient visits, and 0.53% require hospitalization (Table 1). Vaccination costs included vaccine prices, cost of administration, and cost of adverse events. Influenza-related direct costs included hospitalization, outpatient visits, over-the-counter medications, and transportation costs. Influenza-related indirect costs consisted of caregiver time lost from work attributable to influenza. Costs were adjusted to 2015 U.S. dollars.
Influenza costs depend on infection rates and disease severity. The expected influenza cost can be calculated as , where each k corresponds to a different clinical outcome: hospitalization, outpatient visit, and the use of over-the-counter medication. C1 and C3(k) are transportation costs and clinical outcome costs, respectively, where P(k) is the probability of each outcome. C2 is the cost of caregiver work loss per influenza illness when the cost is calculated from societal perspective, whereas it is assumed C2 = 0 when a healthcare perspective is taken. Finally, vaccination program cost was calculated as
where each h corresponds to different adverse events: medically significant wheezing, reactogenicity, and infection site event. CA(k) is the cost of each adverse event, where TLAIV(h) and TIIV(h) refer to the probability of each event associated with LAIV and IIV use, respectively. CLAIV and CIIV are LAIV and IIV vaccination costs, respectively, including administration cost. This cost expectation, UVAC, is a summation over a time horizon, from time 0 to T.
Cost-Effectiveness Analysis
A cost-effectiveness analysis of influenza vaccination for children aged 2–8 years was performed to estimate their influenza vaccination cost and incremental health effects. The economic analyses compared LAIV (or IIV) against a mixed vaccination strategy using current vaccine uptake of LAIV and IIV among children aged 2–8 years. For a mixed strategy, the combined use of both LAIV and IIV was considered, based on National Immunization Survey-Flu parental reported data for the 2013–2014 influenza seasons. Specifically, in the 2013–2014 season, 33.3% of vaccinated children aged 2–8 years received LAIV whereas 66.7% of vaccinated children received IIV, which was used to parameterize this strategy.21
Cost-effectiveness ratios were evaluated as the incremental cost-effectiveness ratio (ICER) in terms of dollars per QALY gained, , Where ΔUINF is the cost saved because of outcomes averted by an alternate vaccination program. ΔQINF is the total discounted QALYs gained as a result of a new vaccination intervention. Cost-effectiveness analyses were conducted from two perspectives: healthcare system (which included only direct costs) and societal (which included direct and indirect costs).
A probabilistic sensitivity analysis was conducted via Monte Carlo sampling that produced a distribution of realistic influenza outcomes and their costs (Table 1). This probabilistic sensitivity analysis generated a range of feasible predictions of influenza outcomes among children aged 2–8 years under different scenarios of vaccination over which cost effectiveness was calculated.
Analyses were performed in 2014–2015, and the World Health Report 2002 standard of cost effectiveness was used.22 This standard suggests that “very cost-effective interventions” are those that gain each additional QALY at a cost less than gross domestic product per capita, whereas “cost-effective interventions” are those that gain each additional QALY at a cost less than three times the gross domestic product per capita.22
Results
The base case assumed published meta-analysis vaccine effectiveness data (i.e., IIV 64% and LAIV 83%) and a mixed vaccination strategy as previously described.23 Under base case assumptions, the model predicted symptomatic influenza in children aged 2–8 years of 7.2%, assuming 58% vaccination, which resulted in 41 deaths, 9,342 hospitalizations, and 616,940 hospital outpatient visits in this age group. The base case scenario also resulted in costs of $664 million to the healthcare system and $978 million to society, in this age group.
The probabilistic sensitivity analysis, varying parameters over their distributions when published meta-analysis vaccine effectiveness data were used, showed that the LAIV strategy versus a base case scenario was cost saving in 32% of the model iterations from societal perspective, being very cost effective 97% of the time (Figure 1A). Similarly, from a healthcare perspective, LAIV strategy was cost effective 99% of the time with an incremental cost-effectiveness ratio of $53,960/QALY gained (95% CI=18,400, 113,430). On the other hand, the IIV strategy was not preferred owing to higher net cost and lower effectiveness (Figure 1C).
Figure 1.
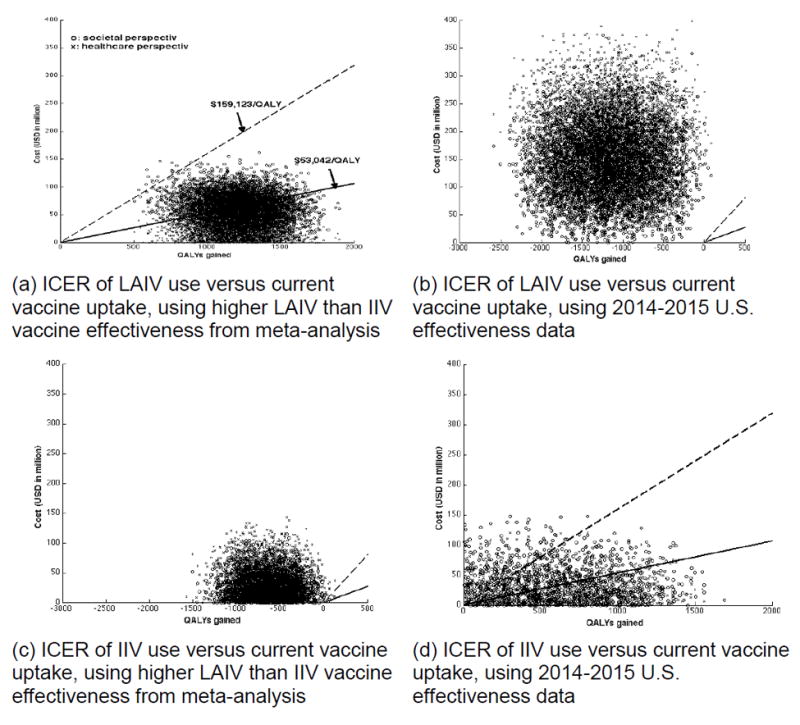
Association between discounted QALYs gained and costs of vaccination.
Note: The crosses are when societal perspective is taken, and circles when healthcare perspective is taken. Regions below the dashed lines indicate cost-effective (<$159,123/QALY), whereas regions below the solid lines indicate very cost-effective (<$53,042/QALY). The different panels constitute different vaccination scenarios and vaccine effectiveness.
ICER, incremental cost-effectiveness ratio; LAIV, live attenuated influenza vaccine; IIV, inactivated influenza vaccine; QALY, quality-adjusted life years
In light of recently observed variations in vaccine effectiveness, the 2014–2015 U.S. effectiveness data (0% LAIV and 15% IIV) were applied and probabilistic sensitivity analysis (Figures 1B and 1D) was carried out. In this analysis, IIV was cost saving in 73% and 85% of the model iterations, from healthcare and societal perspectives, respectively. Furthermore, IIV was considered very cost effective 86% of the time from the societal perspective. On the other hand, the LAIV strategy was not preferred in this analysis, resulting in lower effectiveness and higher net cost than the base case scenario (Figure 1B).
For direct comparison of LAIV and IIV strategies, analyses incorporating a range of LAIV and IIV effectiveness were carried out. If LAIV effectiveness was <43%, LAIV was never favored when a cost-savings threshold was used (Figure 2A). In addition, if IIV effectiveness was greater than 45%, IIV use was cost saving over the entire range of LAIV effectiveness from the healthcare perspective (Figure 2A). As shown in Figure 2, the favorability threshold for LAIV effectiveness increases as IIV effectiveness increases. Thus, if IIV effectiveness in children aged 2–8 years remains at its 2014-15 level, 15%, then LAIV would not be favored unless its effectiveness was >63% from the healthcare perspective, or >31% from the societal perspective. If IIV effectiveness returns to 64%, LAIV effectiveness would have to be >85% for LAIV to be favored from the societal perspective, whereas LAIV would not be favored over the entire range of IIV effectiveness from the healthcare perspective (Figure 2).
Figure 2.
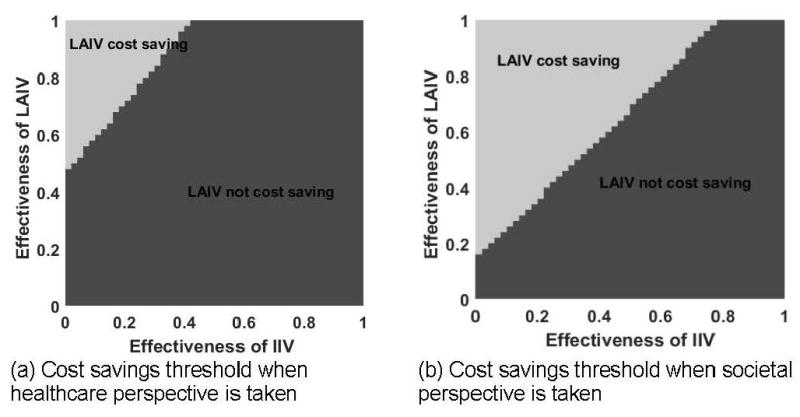
Two-way sensitivity analysis – LAIV vs. IIV effectiveness, cost savings threshold in 2-8 year-olds.
Note: The area where LAIV is cost saving compared to IIV is depicted for hypothetical improved LAIV effectiveness values (y-axis) over IIV (x-axis). In panel (a), healthcare perspective is taken; panel (b) societal perspective is taken.
LAIV, live attenuated influenza vaccine; IIV, inactivated influenza vaccine
These findings were confirmed when the incremental cost-effectiveness ratios of LAIV versus IIV among children aged 2–8 years were computed at various levels of vaccine effectiveness (Table 2). In this two-way sensitivity analysis, if LAIV effectiveness in children aged 2–8 years remains at its 2014–2015 level, 0%, then IIV would be favored unless as long as its effectiveness was >0% from both healthcare and societal perspectives. If LAIV effectiveness becomes as high as 75%, IIV effectiveness would have to be >90% for IIV to be favored, whereas switching to IIV would be considered very cost effective from the healthcare perspective if the effectiveness of IIV is between 30% and 45% (Table 2). From the societal perspective, if LAIV effectiveness is 75%, LAIV would be a favored over IIV unless IIV effectiveness is >45%.
Table 2.
ICER of LAIV Strategy Versus IIV Strategy (in 103 USD per QALY)
| ICER from healthcare perspective
| ||||||||
|---|---|---|---|---|---|---|---|---|
| Effectiveness of IIV | ||||||||
| 0% | 15% | 30% | 45% | 60% | 75% | 90% | ||
|
| ||||||||
| Effectiveness of LAIV | 0% | 1670.2 | IIV dominant | IIV dominant | IIV dominant | IIV dominant | IIV dominant | IIV dominant |
|
|
||||||||
| 15% | 45.4 | 1670.2 | IIV dominant | IIV dominant | IIV dominant | IIV dominant | IIV dominant | |
|
|
||||||||
| 30% | 12.2 | 47.9 | 1670.2 | IIV dominant | IIV dominant | IIV dominant | IIV dominant | |
|
|
||||||||
| 45% | 1.0 | 13.9 | 51.7 | 1670.2 | IIV dominant | IIV dominant | IIV dominant | |
|
|
||||||||
| 60% | LAIV dominant | 2.3 | 16.1 | 56.3 | 1670.2 | IIV dominant | IIV dominant | |
|
|
||||||||
| 75% | LAIV dominant | LAIV dominant | 3.9 | 18.8 | 62.3 | 1670.2 | IIV dominant | |
|
|
||||||||
| 90% | LAIV dominant | LAIV dominant | LAIV dominant | 6.1 | 22.4 | 70.0 | 1670.2 | |
|
| ||||||||
| ICER from societal perspective
| ||||||||
| Effectiveness of IIV | ||||||||
| 0% | 15% | 30% | 45% | 60% | 75% | 90% | ||
|
| ||||||||
| Effectiveness of LAIV | 0% | 1670.2 | IIV dominant | IIV dominant | IIV dominant | IIV dominant | IIV dominant | IIV dominant |
|
|
||||||||
| 15% | 0.3 | 1670.2 | IIV dominant | IIV dominant | IIV dominant | IIV dominant | IIV dominant | |
|
|
||||||||
| 30% | LAIV dominant | 2.8 | 1670.2 | IIV dominant | IIV dominant | IIV dominant | IIV dominant | |
|
|
||||||||
| 45% | LAIV dominant | LAIV dominant | 6.7 | 1670.2 | IIV dominant | IIV dominant | IIV dominant | |
|
|
||||||||
| 60% | LAIV dominant | LAIV dominant | LAIV dominant | 11.4 | 1670.2 | IIV dominant | IIV dominant | |
|
|
||||||||
| 75% | LAIV dominant | LAIV dominant | LAIV dominant | LAIV dominant | 17.7 | 1670.2 | IIV dominant | |
|
|
||||||||
| 90% | LAIV dominant | LAIV dominant | LAIV dominant | LAIV dominant | LAIV dominant | 25.6 | 1670.2 | |
ICER, incremental cost-effectiveness ratio; LAIV, live attenuated influenza vaccine; IIV, inactivated influenza vaccine; USD, United States dollars; QALY, quality-adjusted life years
Discussion
For many reasons, LAIV is being increasingly administered to children, despite its higher cost compared with IIV.24-28 In 2014, CDC recommended preferential LAIV use in children aged 2–8 years.24-27 However, recent data did not confirm LAIV superiority, and thus this recommendation was subsequently changed by CDC in 2015, stating no preference for either vaccine. In fact, relatively poor effectiveness for both vaccines has been demonstrated in certain situations.29
To incorporate observed variation in vaccine effectiveness, wide effectiveness ranges for both vaccines were considered. In general, when the IIV effectiveness was higher than LAIV effectiveness, exclusive IIV use was preferred over LAIV vaccination (Table 2). Similarly, IIV is expected to be cost saving when IIV effectiveness is superior to LAIV (Figure 2). Furthermore, the IIV-only strategy is highly like to be cost effective compared with the mixed vaccine strategy, consistent with the current uptake levels (i.e., LAIV given to 36% of vaccine recipients aged 2–8 years) when 2014–2015 U.S. effectiveness data (0% LAIV and 15% IIV) are applied. These results provide insight into vaccination program effectiveness for influenza seasons when LAIV effectiveness was relatively low against the H1N1 influenza strain, as in 2013–2014, and the H3N2 influenza strain, as in 2014–2015.
For probabilistic sensitivity analyses, key epidemiologic parameters were simultaneously varied, showing that LAIV would be almost never favored when 2014–2015 U.S. effectiveness data (0% LAIV and 15% IIV) were used. If LAIV effectiveness returns to 83%, LAIV vaccination would be again a cost-effective strategy compared with both IIV and mixed strategies. Prior analyses of LAIV versus IIV administration in children also concluded that LAIV was cost effective,3,7,30 with some discrepancies between results in this paper and those of previous analyses.3,7 They may be due to differences in methodology, specifically the inclusion of a mixed strategy where both LAIV and IIV are simultaneously used, and the incorporation of vaccine-induced indirect protection.
Conclusions
With variations in LAIV and IIV effectiveness, evaluation of various vaccination strategies might provide insight for successful influenza immunization programs. Incorporation of indirect effects of vaccination and age-dependent contact patterns enhanced the cost-effectiveness analysis of influenza vaccination. New findings should strengthen understanding of the economic benefits of influenza vaccination, and elucidate the epidemiologic and economic burdens associated with influenza in the U.S.
Supplementary Material
Acknowledgments
Research was supported by the National Institute of General Medical Sciences of NIH under Award Number R01GM111121. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Richard Zimmerman has active research grants from Sanofi Pasteur, Pfizer Inc., and Merck & Co., Inc. Mary Patricia Nowalk has received or currently receives grant funding from Pfizer, Inc. and Merck & Co., Inc. Jonathan Raviotta currently receives grant funding from Pfizer, Inc. No other financial disclosures were reported by the authors of this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.CDC. Estimates of deaths associated with seasonal influenza --- United States, 1976-2007. MMWR Morb Mortal Wkly Rep. 2010;59(33):1057–1062. [PubMed] [Google Scholar]
- 2.Clements KM, Meier G, McGarry LJ, Pruttivarasin N, Misurski DA. Cost-effectiveness analysis of universal influenza vaccination with quadrivalent inactivated vaccine in the United States. Hum Vaccin Immunother. 2014;10(5):1171–1180. doi: 10.4161/hv.28221. http://dx.doi.org/10.4161/hv.28221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luce BR, Nichol KL, Belshe RB, et al. Cost-effectiveness of live attenuated influenza vaccine versus inactivated influenza vaccine among children aged 24-59 months in the United States. Vaccine. 2008;26(23):2841–2848. doi: 10.1016/j.vaccine.2008.03.046. http://dx.doi.org/10.1016/j.vaccine.2008.03.046. [DOI] [PubMed] [Google Scholar]
- 4.Fiore AE, Uyeki TM, Broder K, et al. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Recomm Rep. 2010;59(RR-8):1–62. [PubMed] [Google Scholar]
- 5.Ambrose CS, Levin MJ, Belshe RB. The relative efficacy of trivalent live attenuated and inactivated influenza vaccines in children and adults. Influenza Other Respir Viruses. 2011;5(2):67–75. doi: 10.1111/j.1750-2659.2010.00183.x. http://dx.doi.org/10.1111/j.1750-2659.2010.00183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.CDC. Advisory Committee on Immunization Practices (ACIP) reaffirms recommendation for annual influenza vaccination. [April 14, 2015];2015 www.cdc.gov/media/releases/2015/s0226-acip.html.
- 7.Prosser LA, Bridges CB, Uyeki TM, et al. Health benefits, risks, and cost-effectiveness of influenza vaccination of children. Emerg Infect Dis. 2006;12(10):1548–1558. doi: 10.3201/eid1210.051015. http://dx.doi.org/10.3201/eid1210.051015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thommes EW, Ismaila A, Chit A, Meier G, Bauch CT. Cost-effectiveness evaluation of quadrivalent influenza vaccines for seasonal influenza prevention: a dynamic modeling study of Canada and the United Kingdom. BMC Infect Dis. 2015;15:465. doi: 10.1186/s12879-015-1193-4. http://dx.doi.org/10.1186/s12879-015-1193-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Damm O, Eichner M, Rose MA, et al. Public health impact and cost-effectiveness of intranasal live attenuated influenza vaccination of children in Germany. Eur J Health Econ. 2015;16(5):471–488. doi: 10.1007/s10198-014-0586-4. http://dx.doi.org/10.1007/s10198-014-0586-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shim E, Galvani AP. Impact of transmission dynamics on the cost-effectiveness of rotavirus vaccination. Vaccine. 2009;27(30):4025–4030. doi: 10.1016/j.vaccine.2009.04.030. http://dx.doi.org/10.1016/j.vaccine.2009.04.030. [DOI] [PubMed] [Google Scholar]
- 11.Atkins KE, Shim E, Carroll S, Quilici S, Galvani AP. The cost-effectiveness of pentavalent rotavirus vaccination in England and Wales. Vaccine. 2012;30(48):6766–6776. doi: 10.1016/j.vaccine.2012.09.025. http://dx.doi.org/10.1016/j.vaccine.2012.09.025. [DOI] [PubMed] [Google Scholar]
- 12.Jit M, Brisson M, Laprise JF, Choi YH. Comparison of two dose and three dose human papillomavirus vaccine schedules: cost effectiveness analysis based on transmission model. BMJ. 2015;350:g7584. doi: 10.1136/bmj.g7584. http://dx.doi.org/10.1136/bmj.g7584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laprise JF, Drolet M, Boily MC, et al. Comparing the cost-effectiveness of two- and three-dose schedules of human papillomavirus vaccination: a transmission-dynamic modelling study. Vaccine. 2014;32(44):5845–5853. doi: 10.1016/j.vaccine.2014.07.099. http://dx.doi.org/10.1016/j.vaccine.2014.07.099. [DOI] [PubMed] [Google Scholar]
- 14.Galvani AP, Reluga TC, Chapman GB. Long-standing influenza vaccination policy is in accord with individual self-interest but not with the utilitarian optimum. PNAS. 2007;104(13):5692–5697. doi: 10.1073/pnas.0606774104. http://dx.doi.org/10.1073/pnas.0606774104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Longini IM, Jr, Halloran ME, Nizam A, Yang Y. Containing pandemic influenza with antiviral agents. Am J Epidemiol. 2004;159(7):623–633. doi: 10.1093/aje/kwh092. http://dx.doi.org/10.1093/aje/kwh092. [DOI] [PubMed] [Google Scholar]
- 16.Hsu SB, Hsieh YH. On the role of asymptomatic infection in transmission dynamics of infectious diseases. Bull Math Biol. 2008;70(1):134–155. doi: 10.1007/s11538-007-9245-6. http://dx.doi.org/10.1007/s11538-007-9245-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mossong J, Hens N, Jit M, et al. Social contacts and mixing patterns relevant to the spread of infectious diseases. PLoS medicine. 2008;5(3):e74. doi: 10.1371/journal.pmed.0050074. http://dx.doi.org/10.1371/journal.pmed.0050074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.CDC. Estimated influenza illnesses and hospitalizations averted by influenza vaccination - United States, 2012-13 influenza season. MMWR Morb Mortal Wkly Rep. 2013;62(49):997–1000. [PMC free article] [PubMed] [Google Scholar]
- 19.Gail MH, Benichou J. Encyclopedia of Epidemiologic Methods. Wiley; 2000. [Google Scholar]
- 20.Shim E, Galvani AP. Distinguishing vaccine efficacy and effectiveness. Vaccine. 2012;30(47):6700–6705. doi: 10.1016/j.vaccine.2012.08.045. http://dx.doi.org/10.1016/j.vaccine.2012.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kahn KE, Santibanez TA, Zhai Y, Singleton JA. Influenza vaccination type, live, attenuated influenza vaccine (LAIV) versus inactivated influenza vaccine (IIV), received by children, United States, 2011-12 through 2013-14 influenza seasons. Vaccine. 2015;33(39):5196–5203. doi: 10.1016/j.vaccine.2015.07.064. http://dx.doi.org/10.1016/j.vaccine.2015.07.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.WHO. Report of the Commission on Macroeconomics and Health. Geneva: 2002. [Google Scholar]
- 23.CDC. FluVaxView. State, regional, and national vaccination coverage [Google Scholar]
- 24.Osterholm MT, Kelley NS, Sommer A, Belongia EA. Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12(1):36–44. doi: 10.1016/S1473-3099(11)70295-X. http://dx.doi.org/10.1016/S1473-3099(11)70295-X. [DOI] [PubMed] [Google Scholar]
- 25.Grohskopf LA, Olsen SJ, Sokolow LZ, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP) -- United States, 2014-15 influenza season. MMWR Morb Mortal Wkly Rep. 2014;63(32):691–697. [PMC free article] [PubMed] [Google Scholar]
- 26.Andersohn F, Bornemann R, Damm O, Martin F, Mittendorf T, Theidel U. Vaccination of children with a live-attenuated, intranasal influenza vaccine – analysis and evaluation through a Health Technology Assessment. GMS Health Technol Assess. 2014 doi: 10.3205/hta000119. http://dx.doi.org/10.3205/hta000119. [DOI] [PMC free article] [PubMed]
- 27.Jefferson T, Rivetti A, Di Pietrantonj C, Demicheli V, Ferroni E. Vaccines for preventing influenza in healthy children. Cochrane Database Syst Rev. 2012;8 doi: 10.1002/14651858.CD004879.pub4. CD004879. http://dx.doi.org/10.1002/14651858.cd004879.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.CDC. CDC Vaccine Price List. [May 2015];2015 www.cdc.gov/vaccines/programs/vfc/awardees/vaccine-management/price-list/
- 29.Flannery B, Clippard JR. Updated interim influenza vaccine effectiveness estimates by age group and vaccine type for the 2014-15 season: Updates from the U.S. Influenza Vaccine Effectiveness (Flu VE) Network. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2015-02/flu-01-Flannery.pdf.
- 30.Tarride JE, Burke N, Von Keyserlingk C, O’Reilly D, Xie F, Goeree R. Cost-effectiveness analysis of intranasal live attenuated vaccine (LAIV) versus injectable inactivated influenza vaccine (TIV) for Canadian children and adolescents. Clinicoecon Outcomes Res. 2012;4:287–298. doi: 10.2147/CEOR.S33444. http://dx.doi.org/10.2147/CEOR.S33444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weycker D, Edelsberg J, Halloran ME, et al. Population-wide benefits of routine vaccination of children against influenza. Vaccine. 2005;23(10):1284–1293. doi: 10.1016/j.vaccine.2004.08.044. http://dx.doi.org/10.1016/j.vaccine.2004.08.044. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


