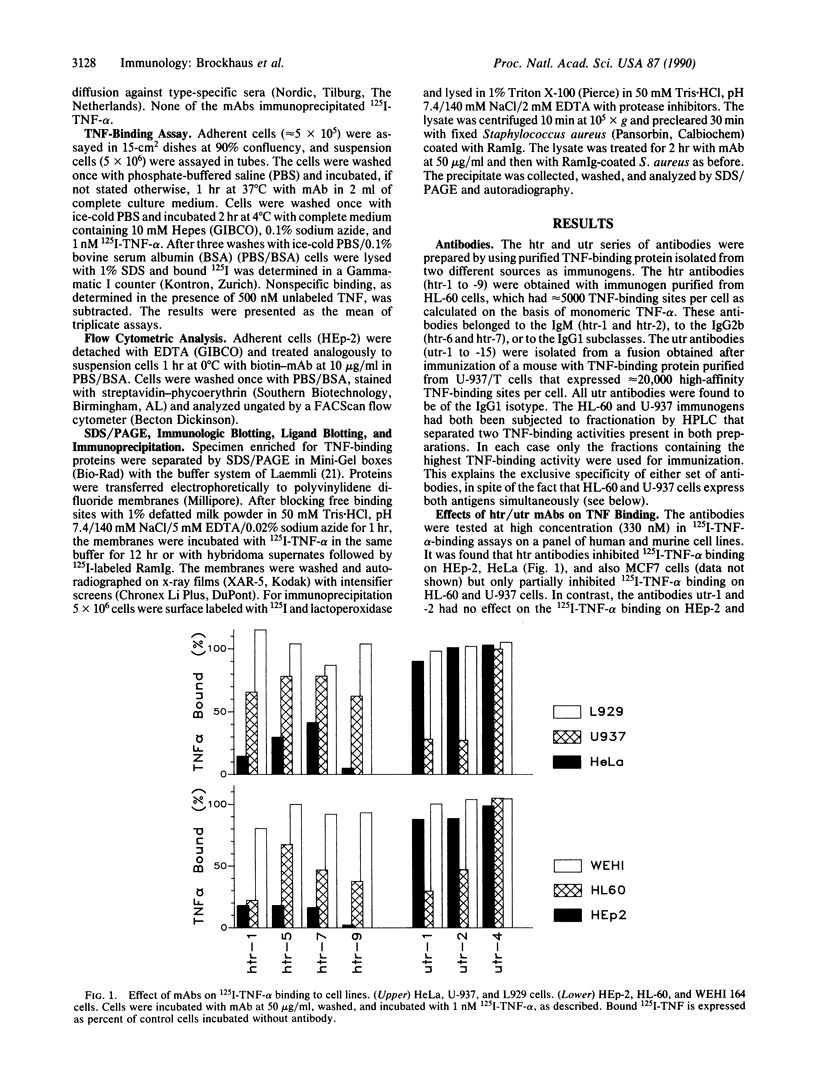
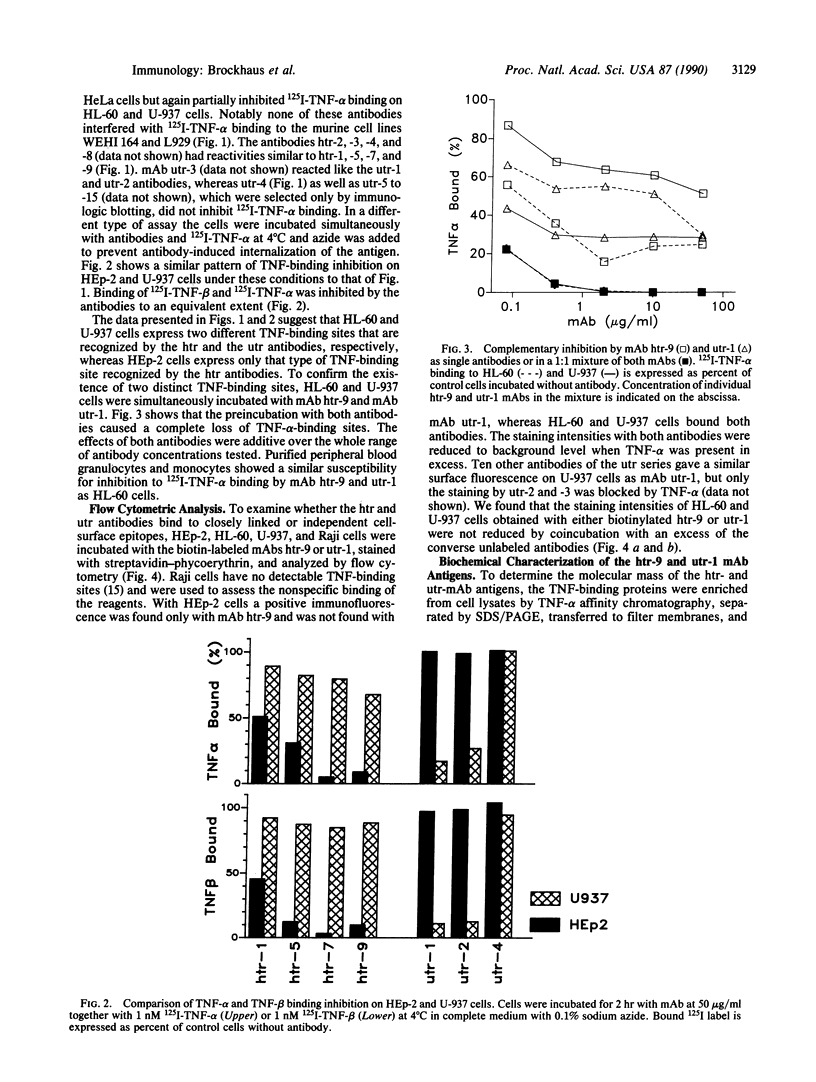
Abstract
The pleiotropic cyto/lymphokine tumor necrosis factor (TNF) exerts its functions by binding to specific cell-surface receptors. We have prepared two sets of monoclonal antibodies (mAbs) against TNF-binding proteins from the HL-60 (htr-mAb series) and U-937 (utr-mAb series) cell lines. The htr antibodies inhibit the binding of 125I-labeled TNF-alpha to HL-60 cells only partially, whereas they block the TNF-alpha binding to several adenocarcinoma cell lines (HEp-2, HeLa, and MCF7) almost completely. In contrast, the utr antibodies have no effect on TNF-alpha binding to the adenocarcinoma cell lines but partially inhibit TNF-alpha binding to HL-60 and U-937 cells. However, htr-9 and utr-1 antibodies in combination fully inhibit the TNF-alpha binding to HL-60 and U-937 cells. The binding of TNF-beta to HEp-2 and U-937 cells is also inhibited by htr and utr antibodies. Neither htr nor utr mAb has an effect on the TNF-sensitive murine cell lines L929 and WEHI 164. Flow cytometry studies show that mAbs htr-9 and utr-1 detect two distinct TNF-binding sites on human cell lines. Immunologic blot and immunoprecipitation analyses indicate that mAbs htr-9 and utr-1 recognize proteins of approximately 55 kDa and 75 kDa, respectively. These data provide evidence for the existence of two distinct TNF receptor molecules that contribute to varying extent to the TNF binding by different human cells.
Full text
PDF
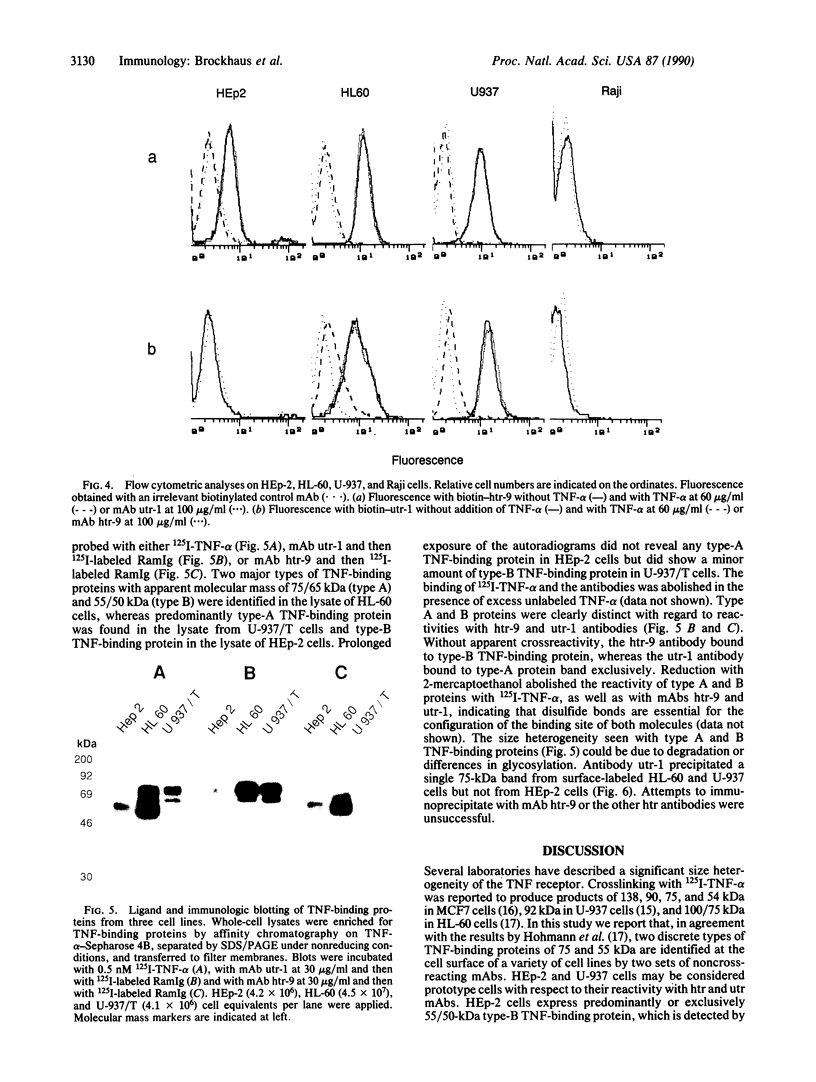

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beutler B., Cerami A. Cachectin: more than a tumor necrosis factor. N Engl J Med. 1987 Feb 12;316(7):379–385. doi: 10.1056/NEJM198702123160705. [DOI] [PubMed] [Google Scholar]
- Carswell E. A., Old L. J., Kassel R. L., Green S., Fiore N., Williamson B. An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3666–3670. doi: 10.1073/pnas.72.9.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creasey A. A., Yamamoto R., Vitt C. R. A high molecular weight component of the human tumor necrosis factor receptor is associated with cytotoxicity. Proc Natl Acad Sci U S A. 1987 May;84(10):3293–3297. doi: 10.1073/pnas.84.10.3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dukovich M., Wano Y., Le thi Bich Thuy, Katz P., Cullen B. R., Kehrl J. H., Greene W. C. A second human interleukin-2 binding protein that may be a component of high-affinity interleukin-2 receptors. Nature. 1987 Jun 11;327(6122):518–522. doi: 10.1038/327518a0. [DOI] [PubMed] [Google Scholar]
- Espevik T., Brockhaus M., Loetscher H., Nonstad U., Shalaby R. Characterization of binding and biological effects of monoclonal antibodies against a human tumor necrosis factor receptor. J Exp Med. 1990 Feb 1;171(2):415–426. doi: 10.1084/jem.171.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Grau G. E., Fajardo L. F., Piguet P. F., Allet B., Lambert P. H., Vassalli P. Tumor necrosis factor (cachectin) as an essential mediator in murine cerebral malaria. Science. 1987 Sep 4;237(4819):1210–1212. doi: 10.1126/science.3306918. [DOI] [PubMed] [Google Scholar]
- Helson L., Green S., Carswell E., Old L. J. Effect of tumour necrosis factor on cultured human melanoma cells. Nature. 1975 Dec 25;258(5537):731–732. doi: 10.1038/258731a0. [DOI] [PubMed] [Google Scholar]
- Hohmann H. P., Remy R., Brockhaus M., van Loon A. P. Two different cell types have different major receptors for human tumor necrosis factor (TNF alpha). J Biol Chem. 1989 Sep 5;264(25):14927–14934. [PubMed] [Google Scholar]
- Ishikura H., Hori K., Bloch A. Differential biologic effects resulting from bimodal binding of recombinant human tumor necrosis factor to myeloid leukemia cells. Blood. 1989 Feb;73(2):419–424. [PubMed] [Google Scholar]
- Jacob C. O., McDevitt H. O. Tumour necrosis factor-alpha in murine autoimmune 'lupus' nephritis. Nature. 1988 Jan 28;331(6154):356–358. doi: 10.1038/331356a0. [DOI] [PubMed] [Google Scholar]
- Jones E. Y., Stuart D. I., Walker N. P. Structure of tumour necrosis factor. Nature. 1989 Mar 16;338(6212):225–228. doi: 10.1038/338225a0. [DOI] [PubMed] [Google Scholar]
- Kindler V., Sappino A. P., Grau G. E., Piguet P. F., Vassalli P. The inducing role of tumor necrosis factor in the development of bactericidal granulomas during BCG infection. Cell. 1989 Mar 10;56(5):731–740. doi: 10.1016/0092-8674(89)90676-4. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Munker R., DiPersio J., Koeffler H. P. Tumor necrosis factor: receptors on hematopoietic cells. Blood. 1987 Dec;70(6):1730–1734. [PubMed] [Google Scholar]
- Pennica D., Nedwin G. E., Hayflick J. S., Seeburg P. H., Derynck R., Palladino M. A., Kohr W. J., Aggarwal B. B., Goeddel D. V. Human tumour necrosis factor: precursor structure, expression and homology to lymphotoxin. Nature. 1984 Dec 20;312(5996):724–729. doi: 10.1038/312724a0. [DOI] [PubMed] [Google Scholar]
- Rubin B. Y., Anderson S. L., Sullivan S. A., Williamson B. D., Carswell E. A., Old L. J. High affinity binding of 125I-labeled human tumor necrosis factor (LuKII) to specific cell surface receptors. J Exp Med. 1985 Sep 1;162(3):1099–1104. doi: 10.1084/jem.162.3.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheurich P., Ucer U., Krönke M., Pfizenmaier K. Quantification and characterization of high-affinity membrane receptors for tumor necrosis factor on human leukemic cell lines. Int J Cancer. 1986 Jul 15;38(1):127–133. doi: 10.1002/ijc.2910380120. [DOI] [PubMed] [Google Scholar]
- Stauber G. B., Aggarwal B. B. Characterization and affinity cross-linking of receptors for human recombinant lymphotoxin (tumor necrosis factor-beta) on a human histiocytic lymphoma cell line, U-937. J Biol Chem. 1989 Feb 25;264(6):3573–3576. [PubMed] [Google Scholar]
- Talmadge J. E., Phillips H., Schneider M., Rowe T., Pennington R., Bowersox O., Lenz B. Immunomodulatory properties of recombinant murine and human tumor necrosis factor. Cancer Res. 1988 Feb 1;48(3):544–550. [PubMed] [Google Scholar]
- Tracey K. J., Beutler B., Lowry S. F., Merryweather J., Wolpe S., Milsark I. W., Hariri R. J., Fahey T. J., 3rd, Zentella A., Albert J. D. Shock and tissue injury induced by recombinant human cachectin. Science. 1986 Oct 24;234(4775):470–474. doi: 10.1126/science.3764421. [DOI] [PubMed] [Google Scholar]
- Tracey K. J., Vlassara H., Cerami A. Cachectin/tumour necrosis factor. Lancet. 1989 May 20;1(8647):1122–1126. doi: 10.1016/s0140-6736(89)92394-5. [DOI] [PubMed] [Google Scholar]
- Trauth B. C., Klas C., Peters A. M., Matzku S., Möller P., Falk W., Debatin K. M., Krammer P. H. Monoclonal antibody-mediated tumor regression by induction of apoptosis. Science. 1989 Jul 21;245(4915):301–305. doi: 10.1126/science.2787530. [DOI] [PubMed] [Google Scholar]
- Updyke T. V., Nicolson G. L. Immunoaffinity isolation of membrane antigens with biotinylated monoclonal antibodies and immobilized streptavidin matrices. J Immunol Methods. 1984 Oct 12;73(1):83–95. doi: 10.1016/0022-1759(84)90034-6. [DOI] [PubMed] [Google Scholar]
- Yonehara S., Ishii A., Yonehara M. A cell-killing monoclonal antibody (anti-Fas) to a cell surface antigen co-downregulated with the receptor of tumor necrosis factor. J Exp Med. 1989 May 1;169(5):1747–1756. doi: 10.1084/jem.169.5.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]