Abstract
A bacterial etiology of rheumatoid arthritis (RA) has been suspected since the beginnings of modern germ theory. Recent studies implicate mucosal surfaces as sites of disease initiation. The common occurrence of periodontal dysbiosis in RA suggests that oral pathogens may trigger the production of disease-specific autoantibodies and arthritis in susceptible individuals. We used mass spectrometry to define the microbial composition and antigenic repertoire of gingival crevicular fluid in patients with periodontal disease and healthy controls. Periodontitis was characterized by the presence of citrullinated autoantigens that are primary immune targets in RA. The citrullinome in periodontitis mirrored patterns of hypercitrullination observed in the rheumatoid joint, implicating this mucosal site in RA pathogenesis. Proteomic signatures of several microbial species were detected in hypercitrullinated periodontitis samples. Among these, Aggregatibacter actinomycetemcomitans (Aa), but not other candidate pathogens, induced hypercitrullination in host neutrophils. We identified the pore-forming toxin leukotoxin-A (LtxA) as the molecular mechanism by which Aa triggers dysregulated activation of citrullinating enzymes in neutrophils, mimicking membranolytic pathways that sustain autoantigen citrullination in the RA joint. Moreover, LtxA induced changes in neutrophil morphology mimicking extracellular trap formation, thereby releasing the hypercitrullinated cargo. Exposure to leukotoxic Aa strains was confirmed in patients with RA and was associated with both anti-citrullinated protein antibodies (ACPAs) and rheumatoid factor (RF). The effect of HLA-DRB1 shared epitope alleles on autoantibody positivity was limited to RA patients that were exposed to Aa. These studies identify the periodontal pathogen Aa as a candidate bacterial trigger of autoimmunity in RA.
Introduction
Rheumatoid arthritis (RA) is a systemic autoimmune disease of unknown etiology characterized by synovial inflammation, joint destruction, and autoantibodies against citrullinated proteins (ACPAs) (1). Posttranslational protein modification of RA autoantigens catalyzed by peptidylarginine deiminase enzymes (PADs) is thought to drive immune events that precipitate and propagate the disease (1, 2). However, factors that underlie loss of tolerance to citrullinated proteins and disease initiation in RA remain elusive.
Recent studies have suggested mucosal surfaces, specifically the periodontium, the gut, and the lungs, as sites of disease initiation in RA (3). Periodontal disease (periodontitis), a bacterial-induced chronic inflammatory disease of the periodontium, is commonly observed in RA, implicating periodontal pathogens as potential triggers of autoimmunity (4). Although multiple bacterial species are associated with periodontitis (5), the expression of a bacterial PAD by Porphyromonas gingivalis has focused research on this oral pathogen as a putative link between periodontal infection and RA (6). The roles of other keystone pathogens for RA have not been explored.
Here, we studied the periodontal microenvironment in patients with periodontitis to define mechanisms underlying mucosal inflammation and autoimmunity in RA. Among the microbial species associated with periodontal disease, we identified Aggregatibacter actinomycetemcomitans (Aa) as the only pathogen with the ability to reproduce the repertoire of citrullinated antigens found in the RA joint. These studies provide a framework to understand autoantigen citrullination in RA as a consequence of microbial-immune cell interactions, and suggest bacterial pore-forming toxins as a unifying principle underlying abnormal activation of endogenous PADs in host target cells. As such, Aa may be a primary oral microbe that can trigger autoimmunity in RA.
Results
Periodontitis mirrors the antigenic microenvironment of the RA joint
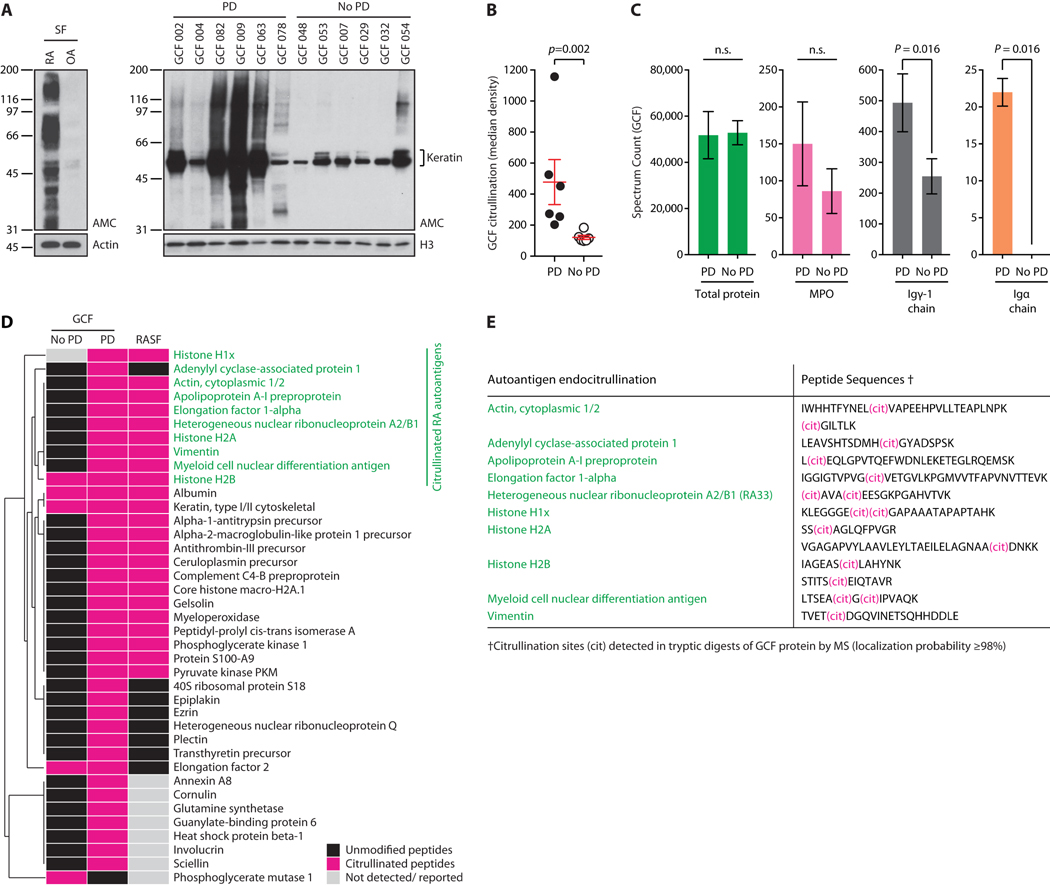
To study periodontitis-associated pathogens as possible environmental triggers of autoimmunity in RA, we initially analyzed the antigenic composition of the periodontal microenvironment in patients with periodontitis and healthy controls. Gingival crevicular fluid (GCF) collected from the gingival sulcus, the space between the gingival mucosa and tooth, has been widely used to study the microbial and inflammatory components of the periodontal pocket (7). In periodontitis, analysis of GCF revealed extensive protein citrullination (Fig. 1A-B), mirroring patterns of cellular hypercitrullination previously observed in the RA joint (Fig. 1A, left panel) (2). Hypercitrullination was minimal in healthy subjects without periodontitis, where protein citrullination was limited to physiologic substrates such as keratins (Fig. 1A). Mass spectrometry (MS) analysis of GCF from patients with periodontitis and controls without periodontal disease showed comparable total peptide counts, but significant enrichment for inflammatory markers such as IgG and IgA in periodontal disease (p=0.016 and p=0.016, respectively) (Fig. 1C). In addition, posttranslational protein modification analysis confirmed that citrullinated proteins were highly enriched in periodontitis (Fig. 1D). The citrullinome of the periodontal pocket in periodontitis mirrored the spectrum of protein citrullination found in the RA joint, including major citrullinated autoantigens targeted by disease-specific autoantibodies in RA (citrullinated actin, α-enolase, hnRNP A2/B1 (RA33) and vimentin, among others) (Fig. 1D) (2, 8-10).
Fig. 1. The periodontal microenvironment in patients with periodontitis recreates the antigenic repertoire of the RA joint.
(A) GCF from patients with periodontitis (PD) and without PD (No PD) was analyzed by anti-modified citrulline (AMC) immunoblotting to detect citrullination (right panel) (60). Histone H3 (H3) is shown to demonstrate loading. AMC of RA and osteoarthritis (OA) synovial fluid cells (SF) is shown for comparison (top left panel). Actin was used to demonstrate loading. (B) Hypercitrullination in PD and No PD samples from A was quantified by chemiluminescence. Red lines represent mean values±SEM (Mann-Whitney test). (C) GCF was analyzed by MS. Exclusive spectrum counts in GCF samples from patients with PD and No PD are shown for total protein, myeloperoxidase (MPO), immunoglobulin (Ig) gamma-1 chain, and Ig alpha chain. Data are expressed as mean±SD. (Mann-Whitney test). (D) GCF samples were analyzed by MS to detect citrullinated peptides. Hierarchical clustering of citrullinated proteins (pink) in GCF from patients with PD and No PD as compared to RA SF (2, 8-10). Native peptides are shown in black, and proteins for which no peptides were identified or reported in gray. Citrullinated RA autoantigens are highlighted in green. (E) Peptide sequences and citrullination sites of RA autoantigens identified in GCF.
Whereas the calcium-dependent mammalian PADs citrullinate specific arginine residues within polypeptide chains (endocitrullination), bacterial PAD from P gingivalis (PPAD) modifies only C-terminal arginines exposed after substrate cleavage by P gingivalis arginine gingipains (C-terminal citrullination) (11). Peptide spectra of citrullinated RA autoantigens detected in periodontitis GCF invariably showed peptidylarginine endocitrullination (Fig. 1E), which is consistent with the activity of human (host), but not bacterial PADs.
The periodontal pathogen Aa induces cellular hypercitrullination in neutrophils
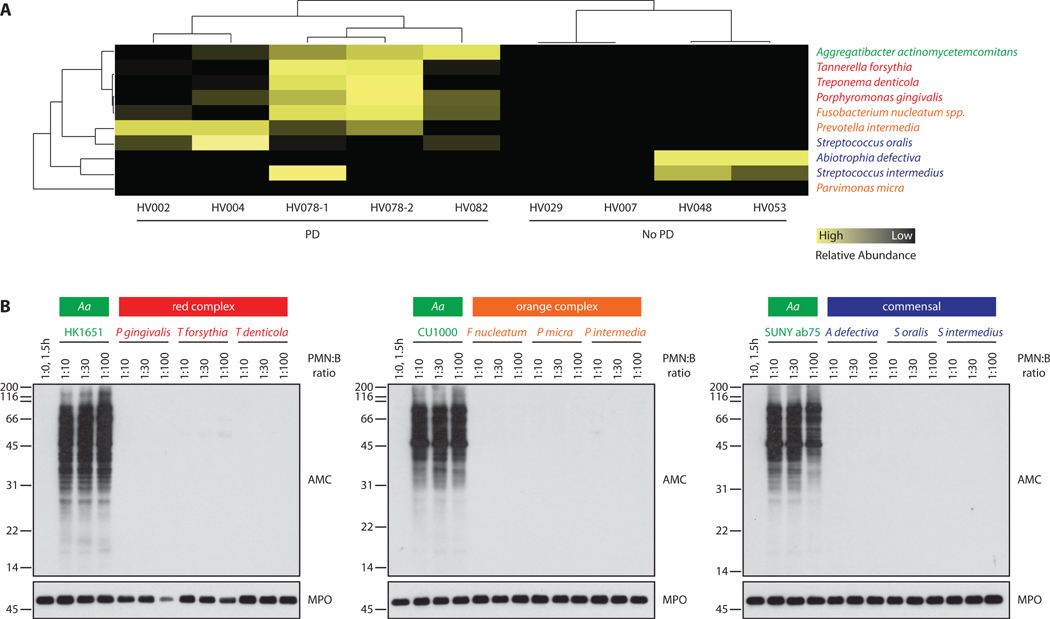
When analyzing GCF samples for proteomic signatures of the subgingival microbiome (table S1A-I), several bacterial species strongly associated with periodontal disease were found enriched in hypercitrullinated periodontitis samples (5); these included the “red complex” bacteria (P gingivalis, Tannerella forsythia, Treponema denticola), “orange complex” bacteria (Fusobacterium nucleatum ssp., Prevotella intermedia), and Aa (Fig. 2A). In contrast, peptide spectra of oral commensal bacteria (Abiotrophia defectiva, Streptococcus intermedius) did not cluster with periodontal disease and were more common in the setting of oral health (Fig. 2A). We hypothesized that one or several of the periodontitis-associated bacterial species may hold potential to activate endogenous host PADs, explaining endocitrullination patterns observed in periodontal disease. To screen for bacterial species that activate hypercitrullination in host cells, we initially studied the effects of periodontal pathogens and commensals on primary human neutrophils, the major source of citrullinated autoantigens in RA and the predominant immune cell in the periodontal pocket (2, 7). Incubation of neutrophils with different Aa serotypes (serotypes a, b, and f), but not with other candidate pathogens of the red and orange complex or with oral commensals, reproduced patterns of cellular hypercitrullination observed in GCF of patients with periodontitis and synovial fluid of patients with RA (Fig. 2B) (2). These findings were further confirmed by dot blotting, which also captures low molecular weight cleavage products that may be acted on by P gingivalis PAD (6) (fig. S1). Citrullination was not detected in bacteria alone or control neutrophils (fig. S1), demonstrating that hypercitrullination is dependent on both bacterial factors and components of the host immune cell. These data highlight Aa as an oral pathogen with the potential to dysregulate protein citrullination in human cells.
Fig. 2. Aa, but not other periodontal pathogens or oral commensal bacteria, induces cellular hypercitrullination in neutrophils.
(A) Hierarchical clustering of bacterial peptides from periodontal pathogens and commensals as identified by MS in individual GCF samples. Relative abundance of total bacterial spectra across samples is shown (yellow=high abundance; black=low abundance). Specific peptides identified for individual bacterial species are listed in table S1. (B) Aa HK1651 (serotype b), Aa CU1000 (serotype f), Aa SUNY Ab75 (serotype a), red complex bacteria, orange complex bacteria, and oral commensals were grown in liquid culture and adjusted by optical density (OD600=0.350). Increasing amounts of bacteria were incubated with human neutrophils at neutrophil:bacteria (PMN:B) ratio of 1:10 to 1:100. Citrullination was visualized by AMC immunoblotting (top panels). Detection of myeloperoxidase (MPO) is shown to demonstrate loading (bottom panels). MPO degradation was seen with the highest amount of P gingivalis bacteria (PMN:P gingivalis ratio=1:100); this was likely a consequence of proteolytic cleavage after lysis of neutrophils in SDS buffer. The experiments were performed on three separate occasions with similar results.
Aa triggers hypercitrullination through its pore-forming toxin leukotoxin A
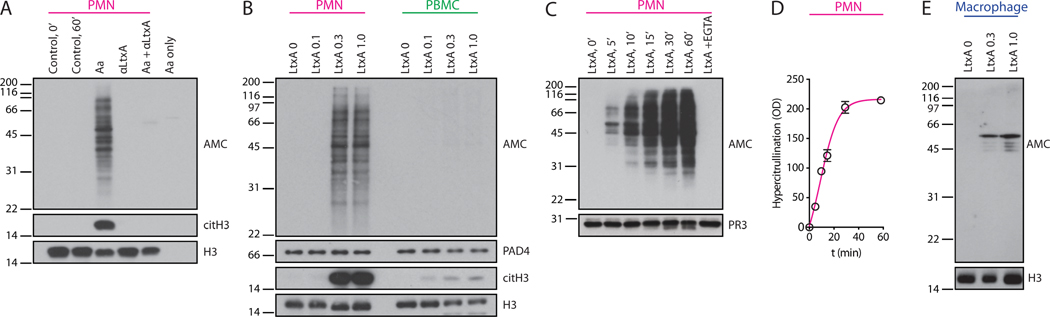
Leukotoxin A (LtxA) is the major virulence factor of Aa (12). A member of the repeats-in-toxin (RTX) family of pore-forming proteins, LtxA induces plasma membrane permeabilization and unregulated calcium influx into toxin-susceptible cells (13). LtxA expression varies among Aa strains and correlates with periodontal disease severity in colonized individuals (14). We hypothesized that similar to membranolytic pathways that mediate hypercitrullination in the RA joint (2), transient disruption of neutrophil membrane integrity by LtxA may drive the dysregulated activation of PAD enzymes through influx of extracellular calcium. Exposure of neutrophils to live Aa cells in the presence of a blocking antibody against LtxA abrogated hypercitrullination, suggesting that Aa-induced citrullination is indeed dependent on LtxA activity (Fig. 3A). Conversely, neutrophil hypercitrullination was reproduced with purified LtxA protein from culture supernatants of Aa (Fig. 3B). Cellular hypercitrullination induced by LtxA was rapid (detectable as early as 5 minutes into incubation) and plateaued within the first hour (Fig. 3C-D). Although the calcium concentration of saliva is similar to serum (1.25-1.5 mM) (15), we confirmed that LtxA-mediated neutrophil hypercitrullination can occur in human saliva and likely also in other extracellular compartments at calcium concentrations below 0.2 mM (fig. S2A and B, respectively).
Fig. 3. The pore-forming toxin of Aa induces cellular hypercitrullination in neutrophils.
(A) Human neutrophils (PMNs) and Aa HK1651 cells were incubated alone or co-incubated in the presence or absence of a blocking antibody against LtxA (αLtxA) for 1 h at 37°C. Total citrullinated protein (AMC), citrullinated H3 (citH3), and histone H3 (H3) (loading control) were detected by immunoblotting. (B) PMNs and PBMCs were incubated with increasing concentrations of purified LtxA (0-1.0 μg/mL) for 1 h at 37°C. AMC and citH3 by immunoblotting are shown. H3 and PAD4 were visualized to demonstrate loading. (C) PMNs were incubated with purified LtxA (0.3 μg/mL) at 37°C for 0-60 min. PMNs incubated with LtxA + 5 mM EGTA for 60 min were used as controls. AMC and proteinase 3 (PR3) (loading control) were detected by immunoblotting. (D) Total citrullinated protein at each time point in C was quantified by densitometry (mean optical density ±SEM). Data are from two independent experiments. (E) Human monocyte-derived macrophages were incubated with increasing amounts of purified LtxA (0-1.0 μg/mL) for 3 h at 37°C. AMC and H3 (loading control) by immunoblotting are shown. The experiments in A, B, and E were performed on at least 3 separated occasions, with similar results.
LtxA-mediated hypercitrullination was not observed in human peripheral blood mononuclear cells (PBMCs) or macrophages treated with purified LtxA (Fig. 3B and E), suggesting a major role for neutrophils as a primary source of citrullinated proteins induced by LtxA. Activation of LtxA-stimulated PBMCs was evident by trace citrullination of histone H3 (Fig. 3B). Degrees of histone H3 citrullination differed dramatically between LtxA-treated neutrophils and PBMCs despite similar expression of PAD4 (Fig. 3B). These qualitative differences in histone H3 citrullination have previously been observed in neutrophils exposed to hypercitrullinating (complement and perforin) vs non-hypercitrullinating stimuli (LPS and PMA, among others) (2). In macrophages, LtxA induced citrullination of a ~45-55 kDa protein complex that likely corresponds to citrullinated vimentin (Fig. 3E).
LtxA generates citrullinated autoantigens targeted in RA
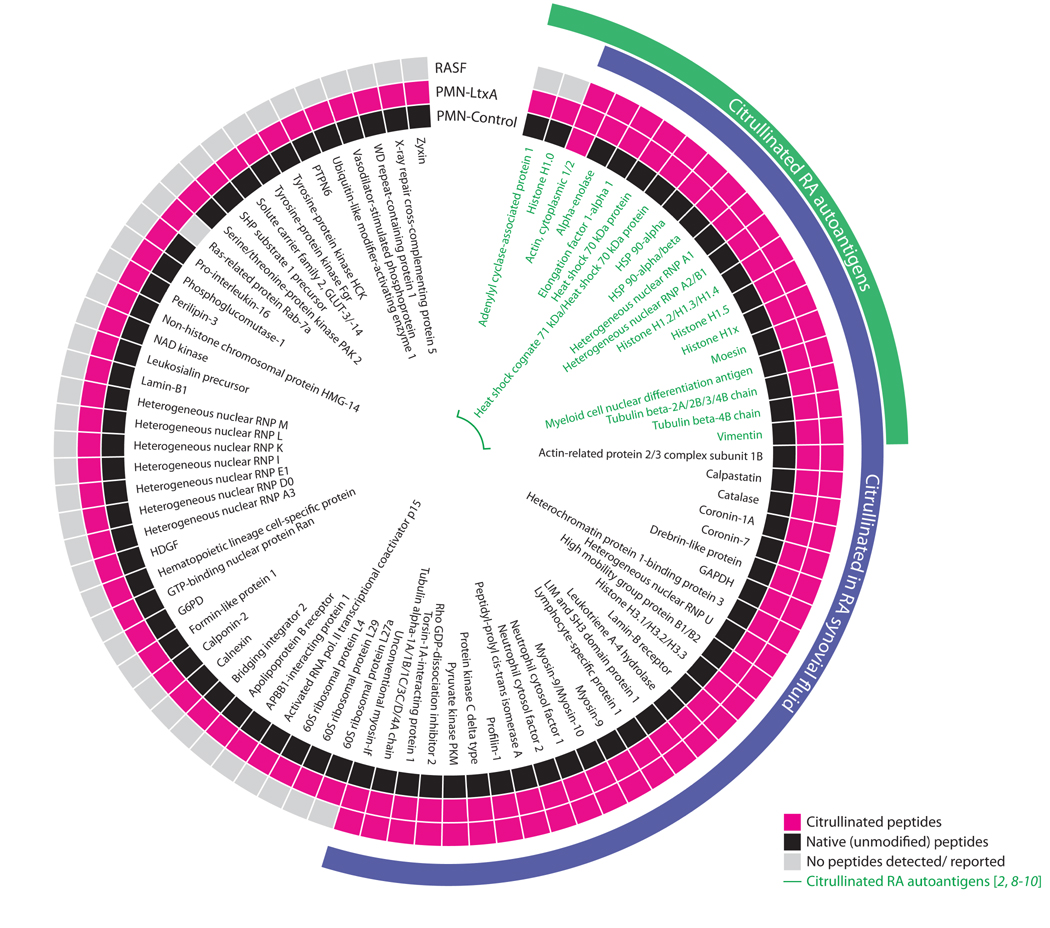
To define the spectrum of citrullinated proteins (the citrullinome) induced during LtxA-mediated membranolysis, we analyzed neutrophils treated with either buffer alone of purified LtxA by MS (table S2 and S3, respectively). The LtxA-induced citrullinome showed marked overlap with the synovial fluid citrullinomes previously identified in patients with RA (44/86 proteins) (2, 8-10) (Fig. 4). With the exception of actin (2 vs. 311 citrullinated spectra in control vs. LtxA-treated neutrophils), citrullinated peptides were exclusively detected in neutrophils treated with LtxA (table S2, S3 and Fig. 4). LtxA distinctly induced generation of citrullinated RA autoantigens (Fig. 4), suggesting that Aa-mediated neutrophil damage in the context of periodontal infection may be sufficient to generate the antigenic determinants recognized by disease-specific autoantibodies in RA.
Fig. 4. The citrullinome induced by LtxA in neutrophils overlaps with the spectrum of citrullinated proteins in the RA joint.
Citrullinated proteins in neutrophils stimulated with purified LtxA (PMN-LtxA) or buffer alone (PMN-Control) were identified by MS analysis and compared to published data of the citrullinome in RA SF (2, 8-10). Citrullinated proteins are shown in pink, and known citrullinated RA autoantigens are highlighted in green. Native peptides identified are shown in black; proteins for which no peptides were identified or previously reported are shown in gray. Actin was the only protein that was found to be citrullinated at baseline (2 vs. 311 citrullinated spectra in control vs. LtxA-treated neutrophils).
LtxA induces neutrophil lysis and the extracellular release of hypercitrullinated proteins
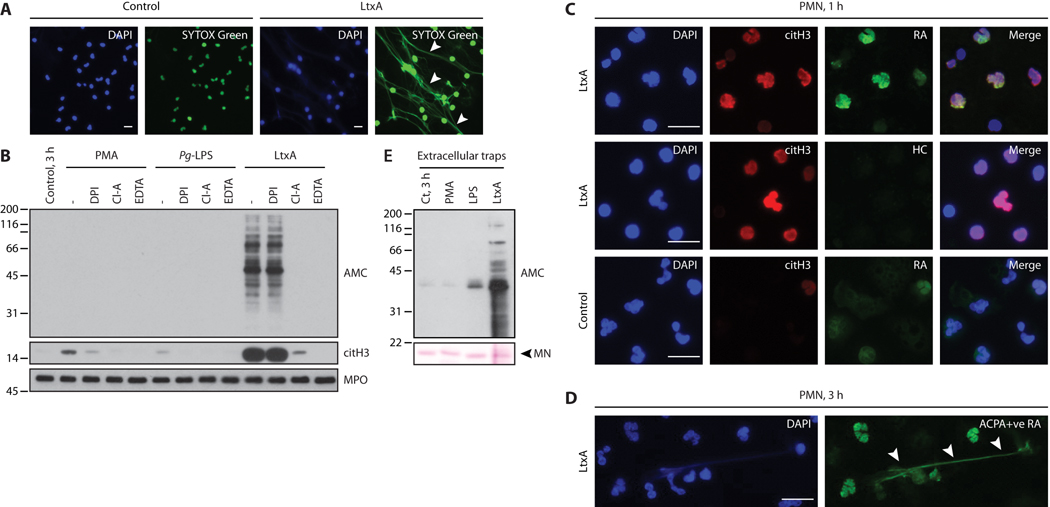
Cell death is a core process in the pathogenesis of systemic autoimmune diseases during which autoantigens become accessible to immune effector pathways. Whereas LtxA has been reported to induce target cell apoptosis and necrosis (12), immunofluorescence of dying neutrophils treated with purified LtxA showed DNA extrusion similar to extracellular traps (Fig. 5A), an antimicrobial form of neutrophil death also known as NETosis (16). Although neutrophil-specific cytolysis induced by LtxA can explain the formation of structures similar to neutrophil extracellular traps (NETs) (17), we addressed whether LtxA-induced cellular hypercitrullination may be linked to the process of NETosis. Whereas the inhibitor of NET formation diphenyleneiodonium (DPI) decreased histone H3 citrullination during both phorbol 12-myristate 13-acetate (PMA)- and lipopolysaccharide (LPS)-induced NETosis, LtxA-induced hypercitrullination was unaffected by DPI (Fig. 5B). In contrast, the PAD inhibitor Cl-amidine and EDTA completely abrogated hypercitrullination induced by LtxA, demonstrating that this process is dependent on extracellular calcium and PAD activity (Fig. 5B). Unlike LtxA, conventional NET-inducing stimuli were not associated with hypercitrullination as previously described (2, 17, 18). Citrullination induced with these stimuli was restricted to protein substrates of the chromatin complex (for example, histone H3) (Fig. 5B).
Fig. 5. RA patient sera recognize LtxA-induced autoantigens in hypercitrullinated neutrophils and extracellular traps.
(A) Neutrophils were incubated alone (control) or with 0.3 μg/mL LtxA. After 3 h, cells were fixed, permeabilized, and stained for DNA with DAPI and SYTOX Green. LtxA induced the extracellular release of neutrophil DNA (arrowheads). (B) Neutrophils were incubated with/without PMA, P gingivalis (Pg)-LPS, or LtxA in the absence or presence of DPI, Cl-A, or EDTA. After 3 h, samples were analyzed by immunoblotting to detect hypercitrullination (AMC), citrullinated histone H3 (citH3), and MPO (loading control). (C) Neutrophils were stimulated for 1 h with LtxA (upper and middle panels) or buffer alone (bottom panels). After fixation/permeabilization, cells were stained with DAPI, anti-citH3 to visualize hypercitrullinated neutrophils (red), and ACPA-positive RA serum (green) to detect RA autoantigens (top and bottom panels). Healthy donor serum (HC) was used as a control (middle panels). (D) LtxA-stimulated neutrophils were incubated for 3 h to allow lysis. Fixed/permeabilized cells were stained to visualize DNA (DAPI) and autoantigens (ACPA-positive RA serum; green). (E) AMC immunoblot of proteins recovered by micrococcal nuclease (MN) digestion from neutrophils stimulated with PMA, Pg-LPS, LtxA, or buffer alone (3 h). The experiments were performed on at least 3 separate occasions, with similar results. Scale bars 10 μm.
To establish whether LtxA-mediated autoantigen production is contingent on the process of cytolysis and extracellular release of PADs, we analyzed neutrophils for the presence of citrullinated autoantigens before extrusion of their DNA (Fig. 5C). By immunofluorescence, ACPA-positive RA patient serum, but not control serum, detected citrullinated autoantigens in LtxA-exposed neutrophils that had not yet released chromatin, demonstrating that citrullination occurs intracellularly and not as consequence of extracellular PAD activity (Fig. 5C, top panels). Trace staining with RA patient serum was seen in unstimulated control neutrophils incubated for an equal amount of time (Fig. 5C, lower panels). This may represent recognition of neutrophil antigens targeted as native (unmodified) proteins in RA (9). Given the time to complete neutrophil lysis, hypercitrullinated cellular proteins were released together with neutrophil chromatin, as detected by immunofluorescence staining and in micrococcal nuclease digests (Fig. 5D and 5E, respectively). Hypercitrullination was not detectable in NETs isolated from PMA- and LPS-stimulated neutrophils (Fig. 5E).
RA is associated with exposure to leukotoxic strains of Aa
Serological quantification of antibodies against Aa and LtxA has been used as a valuable method to identify individuals with previous or current periodontal infection by Aa (19-22). Anti-Aa antibodies in patients with periodontitis show only minimal variation in titer over 15 years of follow up (19). Even with periodontal treatment, antibodies against Aa were shown to remain elevated over the duration of a 30-month period (20). As such, serology is a powerful tool to indicate exposure to Aa. Indeed, anti-Aa antibodies reliably identified a periodontal disease subset that was positive for Aa DNA in subgingival plaque samples by PCR (fig. S3A).
To screen for Aa exposure in patients with RA, we initially assessed the prevalence of anti-Aa antibodies in a large cohort of patients with established RA (ESCAPE RA) (23). Aa strains are categorized into seven antigenic groups (serotypes a-g) (14, 24). Among these, Aa serotype b strains are associated with high levels of leukotoxic activity (a direct marker of LtxA production) (14). We therefore quantified anti-Aa antibodies in patients with RA and controls using an Aa serotype b whole-cell ELISA. Serum anti-Aa serotype b antibodies were detected in 21% (41/196) of patients with RA, indicating a systemic immune response to this subset of Aa (fig. S3B). Anti-Aa antibodies were strikingly associated with RA when compared with controls without periodontitis (21% vs 3%; p<0.001) (fig. S3B).
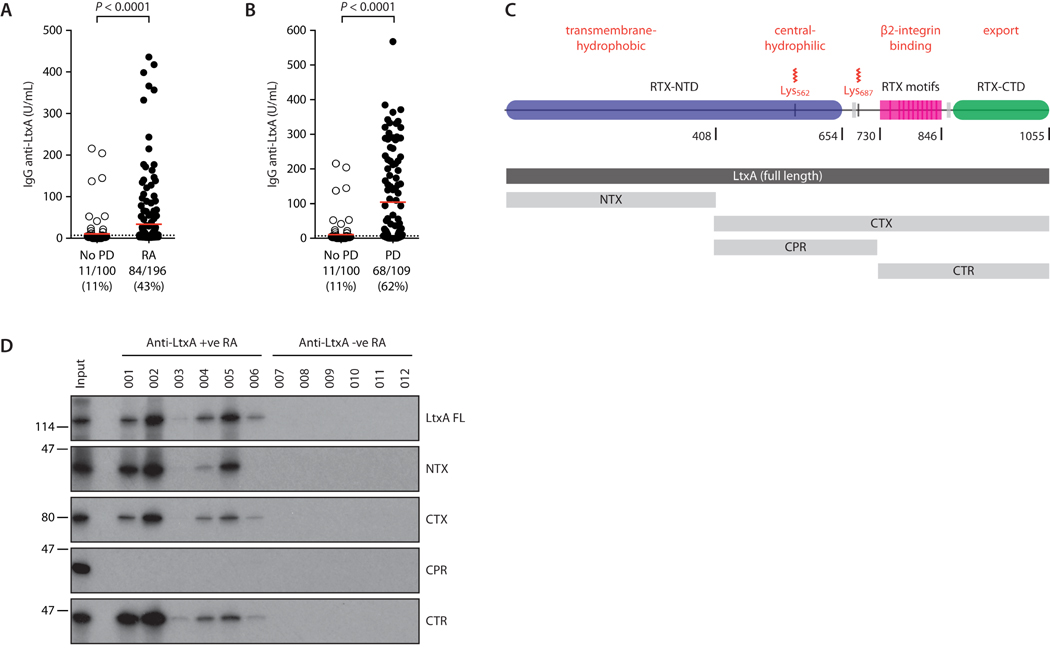
Clinical isolates of serotype b are highly enriched in leukotoxic strains of Aa, but substantial variation in leukotoxicity exists among distinct serotypes and strains (14). Detection of anti-LtxA antibodies may therefore provide a more direct estimate of immune exposure to leukotoxic strains independent of Aa serotype. In our cohort, 11% of controls without periodontitis (11/100) showed detectable antibodies against LtxA by ELISA, confirmed by immunoprecipitation (IP) of the radiolabelled in vitro transcribed–translated (IVTT) toxin (Fig. 6A and fig. S3C-D). This is consistent with the small population of healthy individuals that are infected with Aa, but have not developed periodontitis (21). Using the 100th percentile of unexposed controls as a cut-off for positivity, anti-LtxA antibodies were strikingly associated with RA (43% vs 11% positivity; p<0.0001), indicating infection with leukotoxic strains of Aa in a large subset of patients. Anti-LtxA antibodies showed considerable overlap with anti-Aa serotype b antibodies in RA (33/41; 80%), but identified a large additional patient subset that was negative by Aa serotype b ELISA (51 patients) (fig. S3E) and in whom alternative serotypes (non-b strains) may dominate (14). These data suggest that exposure to leukotoxic Aa in RA is not limited to serotype b strains commonly implicated in periodontitis and provide evidence of Aa infection in at least 47% (92/196) of patients with established RA.
Fig. 6. Antibodies to Aa and LtxA are enriched in patients with RA.
(A) Antibodies against LtxA in patients with RA (n=196) and healthy controls without periodontitis (No PD; n=100). Red line indicates mean IgG concentration; dotted line marks the cut-off for positivity as confirmed by IVTT-IP (fig. S3D). (B) Antibodies against LtxA in patients with periodontitis (PD; n=109) and No PD controls (n=100). Red line indicates mean IgG concentration; dotted line marks the cut-off for positivity. Mann-Whitney test was used for statistical comparison. (C) Truncated proteins in relation to functional domains of LtxA. N-terminal LtxA (NTX) (AA 1-408) comprises the transmembrane regions of the Repeat-in-toxin N-terminal domain (RTX-NTD). C-terminal LtxA (CTX) (409-1055) includes the central and C-terminal regions (CTR and CTS, respectively). CTR (409-729) contains the acylation sites. CTS (730-1055) contains the RTX motifs and the RTX C-terminal domain (RTX-CTD). (D) Characterization of immunodominant regions recognized by anti-LtxA antibodies in RA. Immunoprecipitation of IVTT-radiolabeled full length LtxA (LtxA FL) and truncated proteins (NTX, CTX, CTR, and CTS) using RA patient sera positive (n=6; 01-06) or negative (n=6; 07-12) for anti-LtxA by ELISA. Each lane corresponds to an individual patient’s serum. The same pattern of binding was observed in two independent experiments.
Although Aa has historically been linked to localized aggressive periodontitis (LAP) in adolescents (25), more recent evidence suggests a previously underappreciated role in chronic periodontitis (26-28). Indeed, we confirmed exposure to Aa LtxA in 62% (68/109) of individuals with chronic periodontitis by ELISA (62% vs 11% of controls; p<0.0001) (Fig. 6B). Anti-LtxA antibodies were significantly associated with both mild and severe periodontitis, with the strongest association observed in severe periodontal disease (71% vs 52%; p=0.012) (fig. S4A). In patients with periodontitis, amounts of anti-LtxA antibodies correlated with periodontal disease severity (multiple linear regression, beta=1.12; p<0.0001) (table S4 and fig. S4B). Together, these data support a strong clinical association of Aa with both RA and periodontal disease and suggest clinically relevant exposure to Aa in a subset of patients with established arthritis.
To define immunodominant regions targeted by the anti-LtxA antibody response, we tested RA patient sera against full-length and truncated constructs of LtxA by IP (Fig. 6C-D). Sera positive for anti-LtxA antibodies by ELISA, but not sera from ELISA-negative RA patients, immunoprecipitated radiolabelled full-length IVTT-LtxA (Fig. 6D). Two major regions of LtxA were immunoprecipitated by patient sera, namely the N-terminal transmembrane region (NTX, amino acids 1-408) and a C-terminal region (730-1055) that contains the repeats-in-toxin (RTX) motifs (Fig. 6C-D) (12). No reactivity was observed against the central protein region (CTR, 409-729) (Fig. 6D).
Anti-LtxA antibodies are associated with ACPA and RF positivity in patients with RA
Because Aa is a potent inducer of cellular hypercitrullination, an association between ACPAs and infection with leukotoxic strains of Aa is expected in patients with RA who are able to present citrullinated peptides. Anti-LtxA positivity in RA was significantly associated with the presence of ACPAs (83% vs 67%; p=0.011 in anti-LtxA antibody positive vs negative RA, respectively) (table S5). Moreover, this association was similarly observed for rheumatoid factor (RF) positivity (77% vs 57%; p=0.003) (table S5). Exposure to Aa was enriched in African-Americans and significantly decreased in Caucasians (p=0.004) (table S5), as previously reported for LAP (29).
Analyses of individual ACPA fine specificities revealed that the association with anti-LtxA was driven by a specific subset of citrullinated autoantigens. ACPA fine specificities enriched in anti-LtxA antibody positive RA patients primarily recognized citrullinated autoantigens generated during LtxA-induced neutrophil hypercitrullination (Table 1 and Fig. 4). These included citrullinated hnRNP B1b (RA33), citrullinated vimentin, and citrullinated histone H2B, among others (Table 1). In contrast, antibodies against citrullinated extracellular proteins that are absent in neutrophils (such as biglycan, fibrinogen, and filaggrin) were not enriched in LtxA-positive RA (Table 1).
Table 1.
ACPA fine specificities by anti-LtxA antibody status in RA.
| No anti-LtxA (n=112) |
Anti-LtxA +ve (n=84) |
p | |
|---|---|---|---|
| Any ACPA, n (%)† | 75 (67) | 69 (83) | 0.011 |
| ACPA fine specificities (citrullinated antigens) | |||
| Apolipoprotein A1 | 106 (52-367) | 246 (84-890) | <0.001 |
| Apolipoprotein E | 289 (210-520) | 352 (243-1129) | 0.004 |
| Fibrinogen alpha | 70 (47-224) | 102 (62-242) | 0.066 |
| Histone H2B | 1198 (264-4621) | 2365 (715-5898) | 0.020 |
| Vimentin | 544 (128-2012) | 1403 (239-3150) | 0.018 |
| Apolipoprotein E 277-296, cyclic peptide | 707 (136-2615) | 1374 (338-3751) | 0.032 |
| Enolase alpha, cyclic peptide | 1114 (120-3932) | 1698 (405-5445) | 0.13 |
| Filaggrin 48-65, cyclic peptide | 1452 (147-5410) | 1479 (323-6716) | 0.36 |
| Biglycan 247-266, cyclic peptide | 1214 (132-5090) | 1380 (422-4713) | 0.23 |
| Clusterin 231-250, cyclic peptide | 1604 (154-5950) | 3444 (870-10370) | 0.020 |
| HnRNP B1b (citRA33), n (% positive) | 42 (38) | 44 (52) | 0.038 |
Anti-LtxA, anti-leukotoxin A antibodies; ACPA, anti-citrullinated protein antibodies,
any positivity by multiplex assay as previously defined (23); ACPA fine specificity was expressed as median fluorescence intensity (IQR) (63); antibodies against citrullinated RA33 (citRA33) were quantified by full-protein ELISA using citrullinated heterogeneous nuclear ribonucleoprotein B1b (hnRNP B1b) as previously described (9).
The association between LtxA positivity and RA autoantibodies was even more pronounced when patients were analyzed in the context of shared epitope (SE)-containing HLA-DRB1 alleles. SE alleles are the strongest genetic risk factor for RA and convey susceptibility to ACPA and RF production in this disease (30, 31). In our cohort, the association of SE alleles with ACPAs and RF was restricted to RA patients who had evidence of LtxA exposure, but not maintained in anti-LtxA antibody negative patients (p-values for interaction: p=0.022 for ACPA positivity; p=0.022 for anti-CCP2; p=0.012 for RF) (Table 2), suggesting that the effect of SE susceptibility alleles on ACPAs and RF positivity in RA may be conditioned on the exposure to the periodontal pathogen Aa. In contrast, when we studied exposure to P gingivalis among patients with RA in the same cohort (fig. S5A-B), we observed no significant association between this pathogen, HLA-DRB1 SE alleles, and the presence of autoantibodies (p-values for interaction: p=0.94 for ACPA positivity; p=0.55 for anti-CCP2; p=0.98 for RF) (table S6). Instead, SE alleles conveyed the highest effect on seropositivity in anti-P gingivalis negative patients (adjusted OR of 5.7; p<0.001) (table S6).
Table 2.
The association of shared epitope alleles with ACPAs and RF based on exposure to LtxA in patients with RA.
| Anti-LtxA negative RA (n=112) | Anti-LtxA positive RA (n=82) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| SE negative (n=33) |
SE positive (n=79) |
OR | P | SE negative (n=25) |
SE positive (n=57) |
OR | P |
P- interaction |
|
| Anti-CCP positivity, % | |||||||||
| Crude prevalence (95% CI) | 61 (43, 76) | 78 (68, 86) | 2.37 | 0.051 | 56 (37, 74) | 95 (85, 98) | 14.14 | <0.001 | 0.035 |
| Adjusted* prevalence (95% CI) | 66 (46, 81) | 83 (73, 90) | 2.52 | 0.064 | 53 (31, 73) | 96 (87, 99) | 21.43 | <0.001 | 0.022 |
| ACPA positivity, %† | |||||||||
| Crude prevalence (95% CI) | 64 (46, 78) | 68 (57, 78) | 1.23 | 0.63 | 64 (44, 80) | 93 (82, 97) | 7.31 | 0.002 | 0.025 |
| Adjusted* prevalence (95% CI) | 66 (47, 80) | 70 (58, 79) | 1.22 | 0.67 | 63 (41, 80) | 93 (83, 98) | 8.22 | 0.003 | 0.022 |
| RF positivity, % | |||||||||
| Crude prevalence (95% CI) | 67 (49, 80) | 72 (61, 81) | 1.30 | 0.56 | 68 (48, 83) | 96 (87, 99) | 12.94 | <0.001 | 0.015 |
| Adjusted* prevalence (95% CI) | 72 (53, 85) | 76 (65, 85) | 1.26 | 0.64 | 68 (45, 84) | 97 (89, 99) | 16.52 | 0.002 | 0.012 |
Any ACPA fine specificity (see Table 1)
Anti-LtxA, anti-leukotoxin A antibodies as determined by ELISA; anti-CCP, anti-cyclic citrullinated peptide antibody, cut-off for positivity >20 units; ACPA, anti-citrullinated protein antibodies, any positivity by multiplex assay as previously defined (23); RF, rheumatoid factor, cut-off for positivity >40 units; SE, HLA-DRB1 shared epitope allele
Adjusted for age, Caucasian race, current smoking, IL-6, pain severity, and biologic use
Discussion
A microbial etiology of the autoimmune disease RA has been hypothesized for more than a century (32, 33). The therapeutic use of gold salts in RA was introduced based on the now-rejected model that this disease was due to infection with Mycobacterium tuberculosis of low virulence (34). Beyond the postulates for causation by Henle and Koch (35, 36), a large body of work has implicated numerous bacterial and viral infectious agents in RA pathogenesis, albeit inconclusively (37-39).
The evolving understanding of antigenic determinants targeted in this autoimmune disease, specifically the discovery of ACPAs, has facilitated the interrogation of mechanism-based hypotheses (1). In particular, the association of periodontal disease and lung disease such as bronchiectasis with RA has shaped efforts to understand how environmental factors might initiate loss of tolerance to citrullinated proteins in this disease (4, 40). Despite advances in understanding the microbial composition of the oral, gut, and lung microbiomes in RA (41-44), no pathogen with the capacity to reproduce the spectrum of citrullinated autoantigens found in the RA joint has yet been identified.
In this study, we identify Aa as an organism able to (I) drive dysregulated protein citrullination in host immune cells (analogous to cellular hypercitrullination in the RA joint) (2), and (II) generate the known antigen repertoire targeted by autoantibodies in RA. In contrast, hypercitrullination was not seen with P gingivalis or any other bacterial species studied. Unlike candidate pathogens previously implicated in RA pathogenesis, Aa provides a mechanism that can account for the generation of citrullinated autoantigens independent of molecular mimicry or bacterial citrullinating enzymes.
The study of mucosal immunity has resulted in several distinct hypotheses to explain a role of microbial dysbiosis in the etiology of RA. The finding that the gut microbiome can shape antigen-specific mucosal and systemic immunity has broadened interest in searching for bacterial species that promote autoimmunity in human disease. In this regard, Prevotella copri has recently been identified as a putative bacterium that may enhance susceptibility to RA (particularly in HLA-DR shared epitope-negative patients) (43). Although colonization with these bacteria can increase sensitivity to chemically-induced colitis in mice (43), a role for P copri in RA still needs experimental demonstration. Mechanistic considerations of protein citrullination during mucosal infection have evoked hypotheses around the bacterial PAD of P gingivalis (PPAD) (6, 45, 46). In vitro, PPAD can citrullinate two RA autoantigens, α-enolase and fibrinogen, after cleavage with bacterial arginine gingipains, generating truncated peptides with a single C-terminal citrulline residue (6, 11). ACPAs recognize endocitrullinated proteins and peptides (1); however, evidence that ACPAs can also bind C-terminal mono-citrullinated peptides in a citrulline-dependent fashion is lacking. The presentation of peptides with a single C-terminal citrulline residue may indeed be disfavored in RA because shared epitope containing HLA-DRB1 variants preferentially accommodate endocitrulline within the P4 pocket (31). Finally, molecular mimicry between host proteins and citrullinated microbial products has been suggested as a potential driver of the ACPA response (45, 47, 48). However, evidence for molecular mimicry is inconclusive (46, 49), and the existence of citrullinated microbial proteins as generated by P gingivalis has not been demonstrated in vivo.
Aa, formerly Actinobacillus (Haemophilus) actinomycetemcomitans, is a Gram-negative, facultative anaerobe bacterium implicated in both LAP and chronic periodontitis (25-27, 29). In this study, 62% of chronic periodontitis cases were associated with Aa. In patients with periodontitis, anti-Aa LtxA antibody amounts directly correlated with periodontal disease severity, supporting the idea that serological surrogates of LtxA exposure are useful biomarkers of clinically relevant disease (19-21). This finding is highly relevant for studying exposure to leukotoxic Aa strains in large cohorts of patients, whose serum is stored but information regarding periodontal status is often lacking. Indeed, although the presence of periodontitis was not defined in the ESCAPE RA cohort, using antibodies against LtxA as a surrogate to indicate both past and current infection, 43% of patients with RA were identified to be exposed to Aa. Previous studies have also noted a similar association between Aa and RA (50, 51). However, the absence of mechanistic insight may have limited interest in pursuing these findings. Exposure to Aa was initially suggested by the presence of antibodies against Aa DnaJ (HSP40) in RA (50). Moreover, in a recent study that used commercial DNA probes (micro-Ident) to identify periodontitis-associated bacteria in GCF, Aa was the only bacterial species enriched in the periodontal pocket of patients with RA as compared to non-RA controls and was detected in 17/52 (33%) patients (51). Together, the data from these 3 independent studies using 3 different methods for Aa detection (DNA probes, anti-LtxA, and anti-DnaJ antibodies) strongly support an association of Aa with a subset of RA patients.
We demonstrate that Aa induces hypercitrullination in neutrophils through the activity of LtxA, the main virulence factor of LAP (12). LtxA is a member of the RTX family of bacterial protein toxins expressed and secreted by a diverse group of Gram-negative pathogenic bacteria (52). Cellular specificity of these toxins is determined by binding to target cell surface receptors. The cognate receptor for LtxA is β2-integrin (CD18), which accounts for the selective killing of human leukocytes (53). Binding of LtxA to β2-integrin initiates the process of toxin-receptor clustering within lipid rafts, membrane destabilization, influx of extracellular calcium, and ultimately cell death (13, 54). In this study, cytotoxic loss of membrane integrity mediated by LtxA resulted in rapid, calcium-dependent hypercitrullination of neutrophil proteins that were subsequently released into the extracellular space. Although this process seems morphologically similar to NETosis, it has distinct pathogenic implications. NETosis is a process that evolved to kill pathogens (16), whereas virulence factors such as LtxA are used by pathogens to kill immune cells and enhance bacterial invasion (12, 17, 52). The finding that NET-like structures induced by bacterial pore-forming toxins have limited bactericidal activity underscores major biological differences between NETosis and neutrophil lysis induced by virulence factors (17). Similarly, citrullination of histones decreases their bacterial killing activity (55), which may explain the antimicrobial differences between hypercitrullinated proteins released from neutrophils killed by bacterial cytolysins and NETosis, in which citrullination is limited (2, 17, 18). The morphological and biochemical changes induced by LtxA in neutrophils are consistent with cell death by leukotoxic hypercitrullination (LTH), a mechanism that distinguishes toxin-induced cytolytic changes from NETosis (17).
The finding that both immune-mediated membranolytic pathways in the RA joint (complement- and perforin-mediated pore formation) (2) and bacterial pore-forming toxins are prominent inducers of cellular hypercitrullination is unlikely coincidental and points to a central role of abnormal calcium influx into neutrophils in driving the generation of RA autoantigens. Membranolytic damage and cellular hypercitrullination may represent a unifying mechanism that initiates and sustains autoantigen production at various disease sites in RA. Infection with leukotoxic bacteria may thus provide the inciting inflammatory context driving immune events that trigger ACPA production. Of note, SE alleles were strongly associated with ACPAs in exposed, anti-LtxA positive RA patients, but not in patients that tested negative for LtxA exposure. As such, the HLA-DRB1 shared epitope effect on ACPA-positive disease may be contingent on infection with leukotoxic strains of Aa. In susceptible HLA-DRB1-SE+ individuals with periodontitis, the presentation of LtxA-induced (endo)citrullinated peptides may initiate an antibody response against citrullinated proteins that become autoimmune targets in the RA joint. Although bacterial hypercitrullination may be primarily relevant for disease initiation at mucosal surfaces, immune-mediated pore-forming pathways may ultimately create positive feedback loops that sustain inflammation and autoimmunity in established arthritis.
Although we identified Aa as a potent inducer of cellular hypercitrullination and citrullinated RA autoantigens, the studies have some limitations. First, direct demonstration that Aa can induce an ACPA response in vivo is still needed. LtxA is a toxin that is known to have activity against leukocytes in primates, with no toxicity on rodent cells (64). In vivo studies on Aa will therefore require the development of new strategies and animal models that are currently not available. Second, downstream pathways activated by LtxA in neutrophils need further experimental investigation. Third, although we identified patients with RA that have been exposed to leukotoxic strains of Aa, it would be of great interest to examine the longitudinal association between Aa infection, ACPA production, and onset of symptomatic disease.
Despite its distinct role among periodontitis-associated bacteria, we anticipate that Aa is not the only organism able to trigger cellular hypercitrullination in neutrophils. Pore-forming toxins with a broad range of target cell specificities are expressed by pathogenic bacteria that colonize and infect various mucosal surfaces implicated in RA, such as the oral, lung, and gut mucosa and urothelium. These include other members of the RTX family of cytolytic toxins, the large-pore-forming cholesterol-dependent cytolysins (CDCs), the α-toxins, and β-toxins among others, some of which have well-characterized specificities for neutrophils and other cells of the innate and adaptive immune system (52, 56). Of note, Staphylococcus aureus, a pathogen that can infect the urogenital tract in RA (57), secretes the pore-forming toxin Panton–Valentine leucocidin, which is a potent inducer of cellular hypercitrullination in neutrophils (17). Bacterial pore formation as a model may also explain the association of RA with smoking, bronchial inflammation, and bronchiectasis (40), and deserves dedicated consideration. A role of Aa and pore-forming toxins in generating RA autoantigens has critical implications for the development of both primary preventative and therapeutic strategies beyond immunosuppression in this chronic autoimmune disease.
Materials and Methods
Study design
The objective of this study was to determine mechanisms of protein citrullination during periodontal infection, a possible trigger of RA. Serum was obtained from 196 patients who met the 1987 revised criteria for RA (23). Disease activity and severity were assessed at baseline and at two additional time points, with the final visit occurring an average of 39±4 months after enrolment. Informed consent was obtained from all individuals as approved by the Johns Hopkins Institutional Review Board. Sera were also obtained from 109 patients with chronic periodontitis and 100 healthy controls without periodontitis according to the updated case definitions for population-based surveillance (58). In nine patients with chronic periodontitis and eight healthy controls without periodontitis, serum, GCF, and/or subgingival plaque were available for proteomic, PCR, and/or ELISA analysis. Samples from individuals with and without periodontitis were obtained from the National Institute of Dental and Craniofacial Research (NIDCR), National Institutes of Health (CNS Institutional Review Board of the National Institutes of Health NCT01568697), and from a multi-center clinical study funded by the NIDCR (NCT01489839). The clinical centers involved in study NCT01489839 were The Forsyth Institute (Cambridge, MA), New York University College of Dentistry (New York, NY), Southern Illinois University School of Dental Medicine (Alton, IL), and the University at Buffalo, State University of New York (Amherst, NY). The multi-center clinical study (NCT01489839) was approved by the Institutional Review Board from each of the 4 recruiting centers. SF was obtained after clinically indicated arthrocentesis under a Partners Healthcare IRB–approved protocol.
Statistical analysis
Statistical analysis of ELISA groups was performed using the Mann-Whitney test for unpaired groups. Statistical analyses were performed using GraphPad Prism 6. P values <0.05 were considered to be statistically significant. For the analyses of data from the ESCAPE RA cohort, the distributions of all variables were examined according to groups defined by antibody status. Differences in participant characteristics between the antibody-defined groups were compared using t-tests for normally distributed continuous variables, the Kruskal-Wallis test for non-normally distributed continuous variables, and the chi-square goodness-of-fit test or Fisher’s exact test, as appropriate, for categorical variables. Ordinary logistic regression was used to model the associations of the presence of any shared epitope alleles with autoantibody seropositivity in groups stratified by presence of anti-LtxA or anti-Pg, with differences modeled by introducing shared epitope × anti-LtxA or shared epitope × anti-Pg interaction terms into the models. Multivariable models were constructed by introducing covariates that were associated with seropositivity in univariate models at the p<0.20 level. Statistical calculations were performed using Intercooled Stata 12 (StataCorp). A two-tailed α of 0.05 was used throughout.
Significance of differences in mean clinical and demographic parameters between periodontally healthy and periodontitis subjects was tested using t-test. Significance of differences between these groups for median levels of Anti-LtxA antibodies was tested using Wilcoxon Rank-Sum test. Because there were statistically significant differences between clinical groups for age, gender, and race, and serum samples from the periodontally healthy group came from two distinct studies, we also applied a multiple linear regression model for Log Anti-LtxA as the outcome variable, adjusting for race, age, gender, and study. Statistical analyses were conducted using SAS software.
Supplementary Material
Acknowledgments
The authors would like to thank Lauren DeVine and Robert N O'Meally (Johns Hopkins University) for technical assistance, Joan M. Bathon (Columbia University) for access to samples and clinical data from the ESCAPE-RA cohort participants and Daniel H. Fine (Rutgers School of Dental Medicine) for providing Aa strain CU1000. Funding: F.A. was supported by The Jerome L. Greene Foundation, The Donald B. and Dorothy L. Stabler Foundation, and NIAMS/NIH grant R01AR069569. N.M.M. and R.J.P. were supported by the Intramural Research Program of the NIDCR/NIH. A.R. was supported by NIH/NIDCR grant R37 DE12354. R.P.T. was supported by NIDCR/NIH grant DE021127-01. P.A.N. was supported by the Fundación Bechara. J.S. was supported by the Rheumatology Research Foundation. ESCAPE-RA was supported by NIAMS/NIH AR050026-01.
Footnotes
Author contributions: M.F.K. conceptualized and planned the study, designed and performed all the experiments, analyzed/interpreted the data, and wrote the manuscript. L.A.R., R.J.P. N.M.M. and R.P.T. provided periodontitis and control patient samples, bacterial cultures, and advice in data analysis. J.R. provided purified LtxA and anti-LtxA antibody, and provided expertise relevant to Aa biology. K.S. participated in IP assays and ELISA. A.R. provided advice and participated in data analysis. P.A.N. provided patient samples. J.S. performed ACPA fine specificity assays. J.T.G. provided the ESCAPE RA cohort and performed statistical analyses. F.A. conceptualized and designed the study, participated in data analysis, directed the project, and wrote the manuscript. All authors contributed to the preparation of the final manuscript.
Competing interests: F.A. and M.F.K. submitted an Invention Disclosure (D14433) by the Johns Hopkins University that covers the use of antibodies to Aggregatibacter actinomycetemcomitans (Aa) leukotoxin A (LtxA) for the diagnosis and treatment of patients with Aa infection. F.A. received a grant from MedImmune. J.S. is now a full time employee of Abbvie. MedImmune and Abbvie had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Data and materials availability: All data relevant for this study have been included in the manuscript. Reagents derived from this project will be available upon request through material transfer agreements. Gingival crevicular fluid, subgingival plaque and serum from healthy controls and patients with periodontitis are available from N.M.M. under a material agreement with the NIDCR/NIH. Plasmids encoding LtxA full length and deletion mutants are available from F.A. under a material transfer agreement with The Johns Hopkins University.
Additional Materials and methods are provided as Supplementary Materials.
References and notes
- 1.Schellekens GA, de Jong BA, van den Hoogen FH, van de Putte LB, van Venrooij WJ. Citrulline is an essential constituent of antigenic determinants recognized by rheumatoid arthritis-specific autoantibodies. J. Clin. Invest. 1998;101:273–281. doi: 10.1172/JCI1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Romero V, Fert-Bober J, Nigrovic PA, Darrah E, Haque UJ, Lee DM, van EJ, Rosen A, Andrade F. Immune-mediated pore-forming pathways induce cellular hypercitrullination and generate citrullinated autoantigens in rheumatoid arthritis. Sci. Transl. Med. 2013;5:209ra150. doi: 10.1126/scitranslmed.3006869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brusca SB, Abramson SB, Scher JU. Microbiome and mucosal inflammation as extra-articular triggers for rheumatoid arthritis and autoimmunity. Curr. Opin. Rheumatol. 2014;26:101–107. doi: 10.1097/BOR.0000000000000008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Farquharson D, Butcher JP, Culshaw S. Periodontitis, Porphyromonas, and the pathogenesis of rheumatoid arthritis. Mucosal. Immunol. 2012;5:112–120. doi: 10.1038/mi.2011.66. [DOI] [PubMed] [Google Scholar]
- 5.Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RL., Jr. Microbial complexes in subgingival plaque. J Clin. Periodontol. 1998;25:134–144. doi: 10.1111/j.1600-051x.1998.tb02419.x. [DOI] [PubMed] [Google Scholar]
- 6.Wegner N, Wait R, Sroka A, Eick S, Nguyen KA, Lundberg K, Kinloch A, Culshaw S, Potempa J, Venables PJ. Peptidylarginine deiminase from Porphyromonas gingivalis citrullinates human fibrinogen and alpha-enolase: implications for autoimmunity in rheumatoid arthritis. Arthritis Rheum. 2010;62:2662–2672. doi: 10.1002/art.27552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Delima AJ, Van Dyke TE. Origin and function of the cellular components in gingival crevice fluid. Periodontol. 2000. 2003;31:55–76. doi: 10.1034/j.1600-0757.2003.03105.x. [DOI] [PubMed] [Google Scholar]
- 8.Tutturen AE, Fleckenstein B, de Souza GA. Assessing the citrullinome in rheumatoid arthritis synovial fluid with and without enrichment of citrullinated peptides. J Proteome. Res. 2014;13:2867–2873. doi: 10.1021/pr500030x. [DOI] [PubMed] [Google Scholar]
- 9.Konig MF, Giles JT, Nigrovic PA, Andrade F. Antibodies to native and citrullinated RA33 (hnRNP A2/B1) challenge citrullination as the inciting principle underlying loss of tolerance in rheumatoid arthritis. Ann. Rheum. Dis. 2016;75:2022–2028. doi: 10.1136/annrheumdis-2015-208529. [DOI] [PubMed] [Google Scholar]
- 10.van Beers JJ, Schwarte CM, Stammen-Vogelzangs J, Oosterink E, Bozic B, Pruijn GJ. The rheumatoid arthritis synovial fluid citrullinome reveals novel citrullinated epitopes in apolipoprotein E, myeloid nuclear differentiation antigen, and beta-actin. Arthritis Rheum. 2013;65:69–80. doi: 10.1002/art.37720. [DOI] [PubMed] [Google Scholar]
- 11.Goulas T, Mizgalska D, Garcia-Ferrer I, Kantyka T, Guevara T, Szmigielski B, Sroka A, Millan C, Uson I, Veillard F, Potempa B, Mydel P, Sola M, Potempa J, Gomis-Ruth FX. Structure and mechanism of a bacterial host-protein citrullinating virulence factor, Porphyromonas gingivalis peptidylarginine deiminase. Sci. Rep. 2015;5:11969. doi: 10.1038/srep11969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johansson A. Aggregatibacter actinomycetemcomitans leukotoxin: a powerful tool with capacity to cause imbalance in the host inflammatory response. Toxins. (Basel) 2011;3:242–259. doi: 10.3390/toxins3030242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taichman NS, Iwase M, Lally ET, Shattil SJ, Cunningham ME, Korchak HM. Early changes in cytosolic calcium and membrane potential induced by Actinobacillus actinomycetemcomitans leukotoxin in susceptible and resistant target cells. J Immunol. 1991;147:3587–3594. [PubMed] [Google Scholar]
- 14.Hoglund AC, Haubek D, Kwamin F, Johansson A, Claesson R. Leukotoxic activity of Aggregatibacter actinomycetemcomitans and periodontal attachment loss. PLoS. One. 2014;9:e104095. doi: 10.1371/journal.pone.0104095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rabiei M, Masooleh IS, Leyli EK, Nikoukar LR. Salivary calcium concentration as a screening tool for postmenopausal osteoporosis. Int. J Rheum. Dis. 2013;16:198–202. doi: 10.1111/1756-185X.12003. [DOI] [PubMed] [Google Scholar]
- 16.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 17.Konig MF, Andrade F. A critical reappraisal of neutrophil extracellular traps (NETs) and NETosis mimics based on differential requirements for protein citrullination. Front Immunol. 2016;7:461. doi: 10.3389/fimmu.2016.00461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Douda DN, Khan MA, Grasemann H, Palaniyar N. SK3 channel and mitochondrial ROS mediate NADPH oxidase-independent NETosis induced by calcium influx. Proc. Natl. Acad. Sci. U. S. A. 2015;112:2817–2822. doi: 10.1073/pnas.1414055112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lakio L, Antinheimo J, Paju S, Buhlin K, Pussinen PJ, Alfthan G. Tracking of plasma antibodies against Aggregatibacter actinomycetemcomitans and Porphyromonas gingivalis during 15 years. J Oral Microbiol. 2009;1 doi: 10.3402/jom.v1i0.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Papapanou PN, Neiderud AM, Disick E, Lalla E, Miller GC, Dahlen G. Longitudinal stability of serum immunoglobulin G responses to periodontal bacteria. J Clin. Periodontol. 2004;31:985–990. doi: 10.1111/j.1600-051X.2004.00599.x. [DOI] [PubMed] [Google Scholar]
- 21.Rylev M, Abduljabar AB, Reinholdt J, Ennibi OK, Haubek D, Birkelund S, Kilian M. Proteomic and immunoproteomic analysis of Aggregatibacter actinomycetemcomitans JP2 clone strain HK1651. J Proteomics. 2011;74:2972–2985. doi: 10.1016/j.jprot.2011.07.022. [DOI] [PubMed] [Google Scholar]
- 22.Liljestrand JM, Gursoy UK, Hyvarinen K, Sorsa T, Suominen AL, Kononen E, Pussinen PJ. Combining salivary pathogen and serum antibody levels improves their diagnostic ability in detection of periodontitis. J Periodontol. 2014;85:123–131. doi: 10.1902/jop.2013.130030. [DOI] [PubMed] [Google Scholar]
- 23.Giles JT, Danoff SK, Sokolove J, Wagner CA, Winchester R, Pappas DA, Siegelman S, Connors G, Robinson WH, Bathon JM. Association of fine specificity and repertoire expansion of anticitrullinated peptide antibodies with rheumatoid arthritis associated interstitial lung disease. Ann. Rheum. Dis. 2014;73:1487–1494. doi: 10.1136/annrheumdis-2012-203160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takada K, Saito M, Tsuzukibashi O, Kawashima Y, Ishida S, Hirasawa M. Characterization of a new serotype g isolate of Aggregatibacter actinomycetemcomitans. Mol. Oral Microbiol. 2010;25:200–206. doi: 10.1111/j.2041-1014.2010.00572.x. [DOI] [PubMed] [Google Scholar]
- 25.Haubek D. The highly leukotoxic JP2 clone of Aggregatibacter actinomycetemcomitans: evolutionary aspects, epidemiology and etiological role in aggressive periodontitis. APMIS Suppl. 2010:1–53. doi: 10.1111/j.1600-0463.2010.02665.x. [DOI] [PubMed] [Google Scholar]
- 26.Chen C, Wang T, Chen W. Occurrence of Aggregatibacter actinomycetemcomitans serotypes in subgingival plaque from United States subjects. Mol. Oral Microbiol. 2010;25:207–214. doi: 10.1111/j.2041-1014.2010.00567.x. [DOI] [PubMed] [Google Scholar]
- 27.Pahumunto N, Ruangsri P, Wongsuwanlert M, Piwat S, Dahlen G, Teanpaisan R. Aggregatibacter actinomycetemcomitans serotypes and DGGE subtypes in Thai adults with chronic periodontitis. Arch. Oral Biol. 2015;60:1789–1796. doi: 10.1016/j.archoralbio.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 28.Minguez M, Pousa X, Herrera D, Blasi A, Sanchez MC, Leon R, Sanz M. Characterization and serotype distribution of Aggregatibacter actinomycetemcomitans isolated from a population of periodontitis patients in Spain. Arch. Oral Biol. 2014;59:1359–1367. doi: 10.1016/j.archoralbio.2014.07.021. [DOI] [PubMed] [Google Scholar]
- 29.Henderson B, Ward JM, Ready D. Aggregatibacter (Actinobacillus) actinomycetemcomitans: a triple A* periodontopathogen? Periodontol. 2000;54:78–105. doi: 10.1111/j.1600-0757.2009.00331.x. 2010. [DOI] [PubMed] [Google Scholar]
- 30.Morgan AW, Thomson W, Martin SG, Carter AM, Erlich HA, Barton A, Hocking L, Reid DM, Harrison P, Wordsworth P, Steer S, Worthington J, Emery P, Wilson AG, Barrett JH. Reevaluation of the interaction between HLA-DRB1 shared epitope alleles, PTPN22, and smoking in determining susceptibility to autoantibody-positive and autoantibody-negative rheumatoid arthritis in a large UK Caucasian population. Arthritis Rheum. 2009;60:2565–2576. doi: 10.1002/art.24752. [DOI] [PubMed] [Google Scholar]
- 31.Scally SW, Petersen J, Law SC, Dudek NL, Nel HJ, Loh KL, Wijeyewickrema LC, Eckle SB, van HJ, Pike RN, McCluskey J, Toes RE, La Gruta NL, Purcell AW, Reid HH, Thomas R, Rossjohn J. A molecular basis for the association of the HLA-DRB1 locus, citrullination, and rheumatoid arthritis. J Exp. Med. 2013;210:2569–2582. doi: 10.1084/jem.20131241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mantle A. The etiology of rheumatism considered from a bacterial point of view. Br Med J. 1887;1:1381–1384. doi: 10.1136/bmj.1.1382.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bannatyne GA, Wohlmann AS, Blaxall FR. Rheumatoid arthritis: Its clinical history, etiology, and pathology. Lancet. 1896;147:1120–1125. [Google Scholar]
- 34.Kean TA. Rheumatoid Arthritis and Gold Salts Therapy. Ulster Med. J. 1934;3:284–289. [PMC free article] [PubMed] [Google Scholar]
- 35.Koch R. Verhandlungen des X. Internationalen Medicinischen Congresses, Berlin. Vol. 1. August Hirschwald Verlag; Berlin: 1890. [Google Scholar]
- 36.Henle J. Von den Miasmen und Kontagien und von den miasmatisch-kontagiösen Krankheiten (1840) Verlag von J. A. Barth; 1910. reprint. [Google Scholar]
- 37.Bland JH, Phillips CA. Etiology and pathogenesis of rheumatoid arthritis and related multisystem diseases. Semin. Arthritis Rheum. 1972;1:339–359. doi: 10.1016/0049-0172(78)90009-4. [DOI] [PubMed] [Google Scholar]
- 38.Lossius A, Johansen JN, Torkildsen O, Vartdal F, Holmoy T. Epstein-Barr virus in systemic lupus erythematosus, rheumatoid arthritis and multiple sclerosis-association and causation. Viruses. 2012;4:3701–3730. doi: 10.3390/v4123701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wilder RL. Hypothesis for retroviral causation of rheumatoid arthritis. Curr. Opin. Rheumatol. 1994;6:295–299. doi: 10.1097/00002281-199405000-00009. [DOI] [PubMed] [Google Scholar]
- 40.Catrina AI, Ytterberg AJ, Reynisdottir G, Malmstrom V, Klareskog L. Lungs, joints and immunity against citrullinated proteins in rheumatoid arthritis. Nat. Rev. Rheumatol. 2014;10:645–653. doi: 10.1038/nrrheum.2014.115. [DOI] [PubMed] [Google Scholar]
- 41.Scher JU, Ubeda C, Equinda M, Khanin R, Buischi Y, Viale A, Lipuma L, Attur M, Pillinger MH, Weissmann G, Littman DR, Pamer EG, Bretz WA, Abramson SB. Periodontal disease and the oral microbiota in new-onset rheumatoid arthritis. Arthritis Rheum. 2012;64:3083–3094. doi: 10.1002/art.34539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang X, Zhang D, Jia H, Feng Q, Wang D, Liang D, Wu X, Li J, Tang L, Li Y, Lan Z, Chen B, Li Y, Zhong H, Xie H, Jie Z, Chen W, Tang S, Xu X, Wang X, Cai X, Liu S, Xia Y, Li J, Qiao X, Al-Aama JY, Chen H, Wang L, Wu QJ, Zhang F, Zheng W, Li Y, Zhang M, Luo G, Xue W, Xiao L, Li J, Chen W, Xu X, Yin Y, Yang H, Wang J, Kristiansen K, Li T, Huang Q, Li Y, Wang J. The oral and gut microbiomes are perturbed in rheumatoid arthritis and partly normalized after treatment. Nat. Med. 2015;21:895–905. doi: 10.1038/nm.3914. [DOI] [PubMed] [Google Scholar]
- 43.Scher JU, Sczesnak A, Longman RS, Segata N, Ubeda C, Bielski C, Rostron T, Cerundolo V, Pamer EG, Abramson SB, Huttenhower C, Littman DR. Expansion of intestinal Prevotella copri correlates with enhanced susceptibility to arthritis. Elife. 2013;2:e01202. doi: 10.7554/eLife.01202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scher JU, Joshua V, Ubeda C, Artacho, Segal, Catrina AI. The Lung Microbiome in Rheumatoid Arthritis and Associated Local/Systemic Autoimmunity [abstract] Arthritis Rheumatol. 2015;67(Suppl 10) [Google Scholar]
- 45.Quirke AM, Lugli EB, Wegner N, Hamilton BC, Charles P, Chowdhury M, Ytterberg AJ, Zubarev RA, Potempa J, Culshaw S, Guo Y, Fisher BA, Thiele G, Mikuls TR, Venables PJ. Heightened immune response to autocitrullinated Porphyromonas gingivalis peptidylarginine deiminase: a potential mechanism for breaching immunologic tolerance in rheumatoid arthritis. Ann. Rheum. Dis. 2014;73:263–269. doi: 10.1136/annrheumdis-2012-202726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Konig MF, Bingham CO, III, Andrade F. PPAD is not targeted as a citrullinated protein in rheumatoid arthritis, but remains a candidate for inducing autoimmunity. Ann. Rheum. Dis. 2015;74:e8. doi: 10.1136/annrheumdis-2014-206681. [DOI] [PubMed] [Google Scholar]
- 47.Lundberg K, Kinloch A, Fisher BA, Wegner N, Wait R, Charles P, Mikuls TR, Venables PJ. Antibodies to citrullinated alpha-enolase peptide 1 are specific for rheumatoid arthritis and cross-react with bacterial enolase. Arthritis Rheum. 2008;58:3009–3019. doi: 10.1002/art.23936. [DOI] [PubMed] [Google Scholar]
- 48.Pratesi F, Tommasi C, Anzilotti C, Chimenti D, Migliorini P. Deiminated Epstein-Barr virus nuclear antigen 1 is a target of anti-citrullinated protein antibodies in rheumatoid arthritis. Arthritis Rheum. 2006;54:733–741. doi: 10.1002/art.21629. [DOI] [PubMed] [Google Scholar]
- 49.Konig MF, Paracha AS, Moni M, Bingham C, III, Andrade F. Defining the role of Porphyromonas gingivalis peptidylarginine deiminase (PPAD) in rheumatoid arthritis through the study of PPAD biology. Ann. Rheum. Dis. 2015;74:2054–2061. doi: 10.1136/annrheumdis-2014-205385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yoshida A, Nakano Y, Yamashita Y, Oho T, Ito H, Kondo M, Ohishi M, Koga T. Immunodominant region of Actinobacillus actinomycetemcomitans 40-kilodalton heat shock protein in patients with rheumatoid arthritis. J Dent. Res. 2001;80:346–350. doi: 10.1177/00220345010800010901. [DOI] [PubMed] [Google Scholar]
- 51.Laugisch O, Wong A, Sroka A, Kantyka T, Koziel J, Neuhaus K, Sculean A, Venables PJ, Potempa J, Moller B, Eick S. Citrullination in the periodontium-a possible link between periodontitis and rheumatoid arthritis. Clin. Oral Investig. 2015;20:675–83. doi: 10.1007/s00784-015-1556-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Linhartova I, Bumba L, Masin J, Basler M, Osicka R, Kamanova J, Prochazkova K, Adkins I, Hejnova-Holubova J, Sadilkova L, Morova J, Sebo P. RTX proteins: a highly diverse family secreted by a common mechanism. FEMS Microbiol. Rev. 2010;34:1076–1112. doi: 10.1111/j.1574-6976.2010.00231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reinholdt J, Poulsen K, Brinkmann CR, Hoffmann SV, Stapulionis R, Enghild JJ, Jensen UB, Boesen T, Vorup-Jensen T. Monodisperse and LPS-free Aggregatibacter actinomycetemcomitans leukotoxin: interactions with human beta2 integrins and erythrocytes. Biochim. Biophys. Acta. 2013;1834:546–558. doi: 10.1016/j.bbapap.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 54.Fong KP, Pacheco CM, Otis LL, Baranwal S, Kieba IR, Harrison G, Hersh EV, Boesze-Battaglia K, Lally ET. Actinobacillus actinomycetemcomitans leukotoxin requires lipid microdomains for target cell cytotoxicity. Cell Microbiol. 2006;8:1753–1767. doi: 10.1111/j.1462-5822.2006.00746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li P, Li M, Lindberg MR, Kennett MJ, Xiong N, Wang Y. PAD4 is essential for antibacterial innate immunity mediated by neutrophil extracellular traps. J. Exp. Med. 2010;207:1853–1862. doi: 10.1084/jem.20100239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Los FC, Randis TM, Aroian RV, Ratner AJ. Role of pore-forming toxins in bacterial infectious diseases. Microbiol. Mol. Biol. Rev. 2013;77:173–207. doi: 10.1128/MMBR.00052-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Grace LE, Bukhari M, Lauder RM, Bishop LA, Taylor AM. The Presence of Staphylococcal Toxins in The Urine of Patients with Rheumatoid Arthritis. Ann Rheum Dis. 2016;75:930. [Google Scholar]
- 58.Eke PI, Page RC, Wei L, Thornton-Evans G, Genco RJ. Update of the case definitions for population-based surveillance of periodontitis. J Periodontol. 2012;83:1449–1454. doi: 10.1902/jop.2012.110664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Karched M, Paul-Satyaseela M, Asikainen S. A simple viability-maintaining method produces homogenic cell suspensions of autoaggregating wild-type Actinobacillus actinomycetemcomitans. J Microbiol. Methods. 2007;68:46–51. doi: 10.1016/j.mimet.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 60.Senshu T, Akiyama K, Kan S, Asaga H, Ishigami A, Manabe M. Detection of deiminated proteins in rat skin: probing with a monospecific antibody after modification of citrulline residues. J. Invest Dermatol. 1995;105:163–169. doi: 10.1111/1523-1747.ep12317070. [DOI] [PubMed] [Google Scholar]
- 61.Moutsopoulos NM, Chalmers NI, Barb J, Abusleme L, Greenwell-Wild T, Dutzan N, Paster BJ, Munson PJ, Fine DH, Uzel G, Holland SM. Subgingival microbial communities in Leukocyte Adhesion Deficiency and their relationship with local immunopathology. PLoS. Pathog. 2015;11:e1004698. doi: 10.1371/journal.ppat.1004698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Poulsen K, Ennibi OK, Haubek D. Improved PCR for detection of the highly leukotoxic JP2 clone of Actinobacillus actinomycetemcomitans in subgingival plaque samples. J Clin. Microbiol. 2003;41:4829–4832. doi: 10.1128/JCM.41.10.4829-4832.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sokolove J, Johnson DS, Lahey LJ, Wagner CA, Cheng D, Thiele GM, Michaud K, Sayles H, Reimold AM, Caplan L, Cannon GW, Kerr G, Mikuls TR, Robinson WH. Rheumatoid factor as a potentiator of anti-citrullinated protein antibody-mediated inflammation in rheumatoid arthritis. Arthritis Rheumatol. 2014;66:813–821. doi: 10.1002/art.38307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Taichman NS, Shenker BJ, Tsai CC, Glickman LT, Baehni PC, Stevens R, Hammond BF. Cytopathic effects of Actinobacillus actinomycetemcomitans on monkey blood leukocytes. J Periodontal Res. 1984;19:133–145. doi: 10.1111/j.1600-0765.1984.tb00802.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.