Abstract
Background
Physical exercise has well-characterized positive effects on depressive symptoms. The underlying biologic mechanisms are, however, far from established. A recently discovered mechanism has linked the enhanced conversion of kynurenine to kynurenic acid (KYNA) to an increased resilience toward stress-induced depression in mice. The aim of this study was to translate these findings to humans.
Materials and methods
Kynurenine and KYNA levels were measured by high-performance liquid chromatography in plasma samples from 117 patients affected by mild-to-moderate depression before and within a week after a 12-week training period at three different intensities. The patients were part of the Regassa study.
Results
No differences in plasma levels of kynurenine and KYNA or in their ratio could be detected between before and after training. No effect of the intensity group could be observed. No correlation with the improvement in cardiovascular fitness (Åstrand score) or the improvement in mood (Montgomery Åsberg Depression Rating Scale score) could be observed.
Limitations
As the Regassa study is based on an intention-to-treat protocol, the exact time and the exact intensity of the physical exercise are not known. Analyses of pulse data as well as personal interviews, however, were used to control the exercise protocols. Furthermore, the observations reflect chronic changes.
Conclusion
Physical exercise positively affects mood and cardiovascular fitness, but does not lead to long-lasting changes in plasma levels of kynurenine and KYNA in patients affected by mild-to-moderate depression.
Keywords: kynurenine pathway, kynurenine, kynurenic acid, depression, physical exercise
Introduction
Most people with unipolar depressive disorder never seek professional help and among those who do, adherence to the therapies is often low because of side effects, stigmatization or poor outcome. Physical exercise has well-characterized positive effects on depressive symptoms, and can therefore be considered as a good nonstigmatizing treatment alternative.1–3 The underlying biologic mechanisms are far from established; however, several models have been proposed, including changes in core body temperature, serotonin synthesis, hippocampal cell proliferation and reduced levels of proinflammatory cytokines.1,2 A recently discovered mechanism4 has provided a new biological link between the physical exercise and depressive behavior in mice through the kynurenine pathway (KP).
The KP (Figure 1A) is the main metabolic pathway by which the amino acid tryptophan is degraded. Its products include kynurenine, a metabolite able to cross the blood–brain barrier, as well as kynurenic acid (KYNA), a neuroactive metabolite that has been implicated in the pathophysiology of schizophrenia,5 depression6,7 and other psychiatric disorders.8
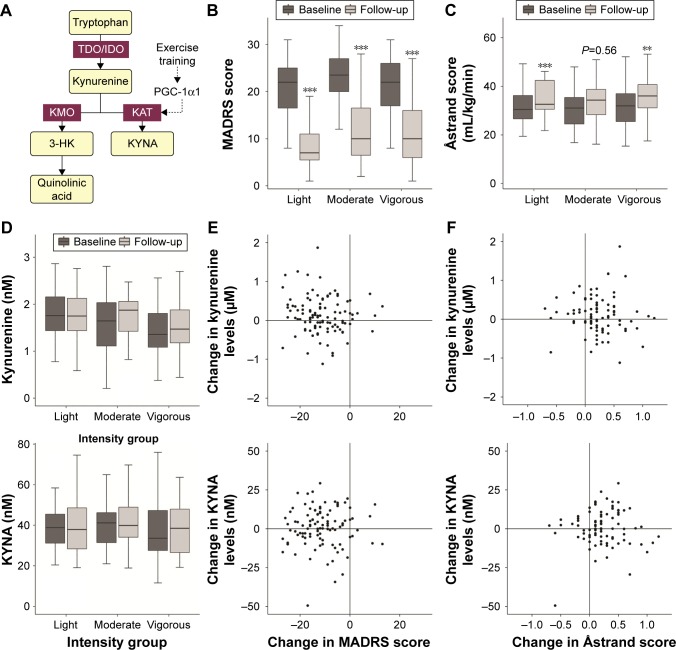
Figure 1.
Overview of results.
Notes: (A) The kynurenine pathway. Tryptophan is converted into kynurenine by TDO and IDO, which in turn is converted into either 3-HK and quinolinic acid by KMO or KYNA by KATs. Exercise has been shown to increase PGC-1α1 in skeletal muscles, which in turn leads to an increase in KATs. (B) MADRS score at baseline and follow-up in each of the intensity groups. (C) Åstrand score at baseline and follow-up in each of the intensity groups. (D) Kynurenine and KYNA levels at baseline and follow-up in each of the intensity groups. (A–D) Horizontal bars indicate the median, the first, and the third quantiles. The whiskers extend from the hinges to the highest or lowest value that is within 1.5 times the interquartile range. **P<0.01; ***P<0.001. (E) Change in kynurenine and KYNA levels (level[follow-up] – level[baseline]) against the change in MADRS score (level[follow-up] – level[baseline]). (F) Change in kynurenine and KYNA levels (level[follow-up] – level[baseline]) against the change in Åstrand score (level[follow-up] – level[baseline]).
Abbreviations: 3-HK, 3-hydroxykynurenine; IDO, indoleamine 2,3-dioxygenase; KATs, kynurenine aminotransferases; KMO, kynurenine monooxygenase; KYNA, kynurenic acid; MADRS, Montgomery Åsberg Depression Rating Scale; TDO, tryptophan 2,3-dioxygenase.
The transcription coactivator PGC-1α1 (Peroxisome proliferator-activated receptor gamma coactivator 1-alpha1) is induced in skeletal muscle by physical activity and promotes muscle adaptation to exercise. Agudelo et al have recently shown that PGC-1α1 overexpression in skeletal muscle in mice induces the expression of kynurenine aminotransferases.4 This leads to an enhanced conversion of peripheral kynurenine to KYNA, thus reducing the levels of kynurenine in the central nervous system, as KYNA is not able to pass the blood–brain barrier. Mice with an overexpression of PGC-1α1 are thus protected against depressive behavior induced by either chronic mild stress or administration of kynurenine. Improvement of depressive symptoms through physical activity could therefore be mediated by a reduction of kynurenine reaching the central nervous system.
A few studies have focused on physical activity and kynurenine metabolites. The picture, however, is incomplete as none of them has reported both kynurenine and KYNA levels. Lewis et al reported increased KYNA plasma levels in 25 human healthy subjects after they ran a marathon.9 Another study found no difference in plasma kynurenine levels in patients with major depression (n=38) or healthy controls (n=48) after 1 week of daily moderate physical activity, although depressive symptoms in patients improved during the intervention.10 Schlittler et al explored the effects of intense physical activity on KYNA levels and muscular PGC-1α1 in healthy subjects.11 The authors showed that endurance exercise of 150 km road cycling increased the expression of PGC-1α1 and kynurenine aminotransferases in muscles and led to an increase of KYNA in peripheral blood which was observed 1 h after the exercise. Similar effects for KYNA were observed in another group of subjects who performed a half marathon race on a hilly course. However, no effects were seen in another group of subjects performing a less strenuous activity consisting of a series of 100 drop jumps. Finally, Strasser et al reported a 6% increase in serum kynurenine after an incremental cycle ergometer exercise test until exhaustion.12
Here, we address the question of whether a 12-week-long low-to-moderately intense physical exercise intervention influences peripheral levels of kynurenine and KYNA in patients with mild-to-moderate depression.
Materials and methods
Study population
This study was designed with material from the Regassa project (www.regassa.se), a randomized controlled trial that aimed at comparing the effectiveness of physical exercise, Internet-based cognitive behavioral therapy and treatment as usual in patients affected by mild-to-moderate depression. The study was performed between February 2011 and March 2013, with recruitment taking place from primary care centers in six different Swedish county councils. Patients scoring >9 on the Patient Health Questionnaire-9 were invited to participate. The exclusion criteria were severe somatic illness, a primary alcohol or drug use disorder, or a psychiatric diagnosis that required specialist treatment. In total, 946 patients were included, among whom 317 were randomized to the 12-week physical exercise intervention. Ethical approval was granted by the regional ethical review board of Stockholm (2010/1779–31). All participating patients signed a written informed consent.
The authors observed larger improvement in depressive symptoms in patients in the exercise group than in the treatment as usual group at both 3 and 12 months follow-up.2,13
Examination and physical exercise intervention
Before the intervention (at baseline), depression status was assessed using the Montgomery Åsberg Depression Rating Scale (MADRS) and the Åstrand physical fitness test (sub-maximal ergometry) was performed.14 Blood samples were collected before the test on the morning of the same day after a night fast using lithium heparin tubes, centrifuged at 1,700× g for 20 min at 4°C and stored at −20°C.
Participants were randomized to three different intensity groups: “light exercise” (yoga-like classes), “moderate exercise” (intermediate-level aerobics class) and “vigorous exercise” (high-intensity aerobics/strength training). Patients were recommended to train three times for 60 min per week during 12 weeks. Weekly meetings with a qualified personal trainer were used to assess adherence. If patients did not attend these meetings, they were contacted by phone or text message and encouraged to continue the intervention. The intensities of the classes were calibrated and the patients wore pulse watches when attending sessions in order to monitor the heart rate. The average heart rates, measured as percentages of the maximal heart rate (MHR; calculated as 220–age), over the session differed significantly between conditions (data shown as percentage of MHR [95% confidence interval]: light: 54.1 [52.2–56.0], moderate: 70.3 [68.1–72.3], vigorous: 76.2 [73.9–78.6]).15
Within 1 week after the 12-week intervention, the patients were invited to a follow-up assessment, in order to evaluate the MADRS score and the Åstrand score. The same day, a blood sample was drawn in the same conditions as in baseline.
We included all patients who had given blood before and after the intervention (N=117). MADRS data were available for all patients before the intervention and from 107 patients after the intervention. Åstrand data were available from 108 before the intervention and from 86 patients after the intervention. Characteristics of our cohort are given in Table 1. Regarding antidepressant medication, selective serotonin reuptake inhibitors were the most commonly used. The number of patients taking any kind of antidepressant medication dropped from 39 at baseline to 33 at follow-up.
Table 1.
Demographic and clinical characteristics of the cohort (N=117)
| Patient characteristics | Median (IQR) | Range |
|---|---|---|
| Age (years) | 45 (34–53) | 20–71 |
| BMI, kg/m2 | 25.3 (22.5–28.3) | 17.1–35.4 |
| n | % | |
|
| ||
| Gender | ||
| Male | 42 | 35.9 |
| Female | 75 | 64.1 |
| Smoking | ||
| Yes (every day or sometimes) | 99 | 84.6 |
| No (never or quit) | 18 | 15.4 |
| Alcohol – hazardous drinker (audit >7) | ||
| Yes | 20 | 17.1 |
| No | 95 | 81.2 |
| N/A | 2 | 1.7 |
| Comorbidities at baseline | ||
| Cardiovascular disorders including high blood pressure | 13 | 11.1 |
| Neurologic disorders | 4 | 3.4 |
| Musculoskeletal disorders | 20 | 17.1 |
| Endocrine disorders | 10 | 8.5 |
| Respiratory disorders | 5 | 4.3 |
| Gastrointestinal disorders | 7 | 6.0 |
| Renal disorders | 4 | 3.4 |
| Psychiatric medication at baseline | ||
| Antidepressants | 39 | 33.3 |
| Hypnotics | 5 | 4.3 |
| Anxiolytics | 4 | 3.4 |
| Antiepileptics | 2 | 1.7 |
| Antipsychotics | 1 | 0.9 |
Abbreviations: BMI, body mass index; IQR, interquartile range; N/A, not available.
Kynurenine and KYNA level measurements
Biochemical analyses were performed as described previously.16 Shortly, plasma samples were deproteinated with perchloric acid. Metabolite levels were measured using an isocratic reverse phase high-performance liquid chromatography system coupled to a spectrometer set to a wavelength of 360 nm for kynurenine determination, and a fluorescence detector set to an excitation wavelength of 344 nm and an emission wavelength of 398 nm for KYNA determination. The mobile phase (50 mM sodium acetate and 7% acetonitrile, pH 6.2) was pumped through a ReproSil-Pur C18 column. A mobile phase containing 0.5 M zinc acetate was added to the mobile phase downstream of the column. The retention times for kynurenine and KYNA were ~4 and 8 min, respectively. The metabolite concentrations were calculated through measurement of a standard mixture of kynurenine (0.08–10 µM) and KYNA (0.6–30 nM). In order to verify the reliability of the method, some samples were measured in duplicates; the intraindividual variation was <5%. In order to reduce interassay variation, corresponding patient samples were always measured together.
Statistical analyses
The assumption of normality of distributions was assessed by Q–Q plots. Differences in metabolites were assessed by paired t-tests or Mann–Whitney U tests. Corrections for multiple testing are indicated by Padj. Correlations were calculated with Pearson’s correlation. Statistical analyses were performed using R version 3.2.3, and graphical representations were created using the ggplot2 package.
Results and discussion
As in the full study cohort,2 the severity of depression as measured by MADRS score dropped significantly over the 12-week physical exercise intervention period in the analyzed subgroup with blood samples (n=117; P<0.001; Figure 1B). The kynurenine and KYNA levels were similar to the levels reported in healthy individuals in previous papers (kynurenine: 1.59±0.58 µmol/L, KYNA: 40.0±14.7 nmol/L),16–18 but did not change over the intervention period in the whole study group or in any of the exercise intensity groups (Padj>0.05; Figure 1D).
The effects of smoking, heavy drinking, antidepressant medication, as well as the comorbidities listed in Table 1 were assessed, but none had an effect on the baseline levels of the metabolites or the changes over the intervention period (Padj>0.05).
No correlation could be found between the change in MADRS score and the change in levels of plasma kynurenine (r=−0.08, P=0.41) or plasma KYNA (r=0.03, P=0.77) between baseline and the 12-week follow-up (Figure 1E). Likewise, no differences could be observed in the KYNA/kynurenine ratio between baseline and follow-up (r=0.19, P=0.37).
As expected, the cardiovascular fitness of the patients improved significantly over the 12 weeks of exercise intervention, indicating that the patients were indeed exercising. In the whole group, the Åstrand score improved from 31.7 mL/kg/min (standard deviation [SD]: 8.6) at baseline to 35.1 mL/kg/min (SD: 8.8) at follow-up (paired t-test: P<0.001). Significant increases were also seen when analyzing the intensity groups separately, with a trend for increase seen in the moderate activity group, most probably due to small sample size (Figure 1C). These changes indicate an important improvement in metabolic and cardiovascular health of the patients and are in line with other studies published on 12-week intervention programs.19,20 Although it has not been tested in depressed patients before, data on patients with schizophrenia indicate that familiarization effects are minimal.21 The increase in fitness, however, was not correlated in any way to changes in kynurenine (r=−0.11, P=0.32), KYNA (r=−0.01, P=0.89) or the KYNA/kynurenine ratio (r=0.11, P=0.44; Figure 1E). There was also no correlation between change in fitness and change in MADRS score between baseline and follow-up (r=−0.14; P=0.20).
Although both an effect on fitness and an amelioration of depressive symptoms were observed, physical exercise did not lead to any long-lasting changes in kynurenine, KYNA or their ratio. These results are in accordance with the results in healthy individuals reported by Schlittler et al, which showed that KYNA levels increased 1 h after intense exercise, but returned to baseline levels as quickly as 5 h later.11 This showed a pattern similar to PGC-1α that increased rapidly after exercise, but returned to baseline within 6–24 h.22 As in our study the blood sampling was performed within 1 week after the end of the training period, our measures do not give any indication about the acute level changes following the training sessions. However, as the intervention lasted for 12 weeks, we were more interested in the long-lasting changes than in the acute effects of the last training session. As no changes in kynurenine or KYNA concentrations were observed after 1 week, we can conclude that physical activity does not lead to long-lasting effects on peripheral kynurenine and KYNA levels. Previous studies on plasma levels of KYNA in depressed patients are not uniform.7 While some studies showed similar KYNA levels in patients with major depression as in healthy controls,23,24 other studies pointed toward lower KYNA levels in patients.16,18 This difference could possibly be explained by patient selection and depression severity. Schwieler et al reported levels for treatment-resistant patients only,16 whereas patients from our cohort were those from primary ward with mild-to-moderate depression and overall showed a very good response toward physical activity treatment.
Limitations
Finally, it is also important to consider other limitations of the study. The Regassa study was designed on an intention-to-treat protocol in order to study the effects of prescription of physical exercise to mild-to-moderately depressed patients. The exact timing and the intensity of physical exercise for each patient are, therefore, not known. However, as quality control measures, patients were interviewed regularly and asked to wear pulse watches during the training sessions. This data indicates that overall the intended training protocol was followed. Also, the study design did not allow for muscle biopsies to analyze PGC-1α1. However, as no long-lasting differences could be seen in peripheral blood, no follow-up study including this analysis was planned. Finally, as no differences were seen, either in regard of the original hypothesis or in regard to antidepressant treatment, it was decided not to measure kynurenines in a control group.
Conclusion
In conclusion, physical exercise positively affects mood and cardiovascular fitness, but does not lead to long-lasting changes in plasma levels of kynurenine and KYNA in patients affected by mild-to-moderate depression.
Acknowledgments
This work was supported by grants from the Stockholm County Council, the Swedish Medical Research Council (www.vr.se; 2009–5546 YF, 2009–7053 SE, 2010–3631 CL, 2014–10171 CL, 2013–2838 SE), the Swedish Brain foundation, Petrus och Augusta Hedlunds Stiftelse and Karolinska Institutet and the regional agreement on medical training and clinical research between Stockholm County Council and Karolinska Institutet (SLL20110560 CL, SLL20090281 YF, SLL20140484 CL), as well as Stiftelsen Sigurd och Else Goljes Minne. Regassa was funded by REHSAM/Vårdal foundation. We kindly thank all patients for participating in the study.
Footnotes
Further information
Data will be made available to all interested researchers upon request. Requests should be submitted to the REGASSA Board. Contact person: Elisabet Erwall, elisabet.erwall@sll.se.
Author contributions
CL, VM and YF conceived and designed the study. CL and YF planned and organized the sample collection. All authors planned the experiments and the statistical analyses. VM carried out the measurements by high-performance liquid chromatography and the statistical analyses, and was the major contributor in writing the manuscript. All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Cooney GM, Dwan K, Greig CA, et al. Exercise for depression. Cochrane Database Syst Rev. 2013;9:CD004366. doi: 10.1002/14651858.CD004366.pub6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hallgren M, Kraepelien M, Ojehagen A, et al. Physical exercise and internet-based cognitive-behavioural therapy in the treatment of depression: randomised controlled trial. Br J Psychiatry. 2015;207(3):227–234. doi: 10.1192/bjp.bp.114.160101. [DOI] [PubMed] [Google Scholar]
- 3.Schuch FB, Vancampfort D, Richards J, Rosenbaum S, Ward PB, Stubbs B. Exercise as a treatment for depression: a meta-analysis adjusting for publication bias. J Psychiatr Res. 2016;77:42–51. doi: 10.1016/j.jpsychires.2016.02.023. [DOI] [PubMed] [Google Scholar]
- 4.Agudelo LZ, Femenia T, Orhan F, et al. Skeletal muscle PGC-1alpha1 modulates kynurenine metabolism and mediates resilience to stress-induced depression. Cell. 2014;159(1):33–45. doi: 10.1016/j.cell.2014.07.051. [DOI] [PubMed] [Google Scholar]
- 5.Linderholm KR, Skogh E, Olsson SK, et al. Increased levels of kynurenine and kynurenic acid in the CSF of patients with schizophrenia. Schizophr Bull. 2012;38(3):426–432. doi: 10.1093/schbul/sbq086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muller N, Schwarz MJ. The immune-mediated alteration of serotonin and glutamate: towards an integrated view of depression. Mol Psychiatry. 2007;12(11):988–1000. doi: 10.1038/sj.mp.4002006. [DOI] [PubMed] [Google Scholar]
- 7.Reus GZ, Jansen K, Titus S, Carvalho AF, Gabbay V, Quevedo J. Kynurenine pathway dysfunction in the pathophysiology and treatment of depression: evidences from animal and human studies. J Psychiatr Res. 2015;68:316–328. doi: 10.1016/j.jpsychires.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schwarcz R, Bruno JP, Muchowski PJ, Wu HQ. Kynurenines in the mammalian brain: when physiology meets pathology. Nat Rev Neurosci. 2012;13(7):465–477. doi: 10.1038/nrn3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lewis GD, Farrell L, Wood MJ, et al. Metabolic signatures of exercise in human plasma. Sci Transl Med. 2010;2(33):33–37. doi: 10.1126/scitranslmed.3001006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hennings A, Schwarz MJ, Riemer S, Stapf TM, Selberdinger VB, Rief W. Exercise affects symptom severity but not biological measures in depression and somatization – results on IL-6, neopterin, tryptophan, kynurenine and 5-HIAA. Psychiatry Res. 2013;210(3):925–933. doi: 10.1016/j.psychres.2013.09.018. [DOI] [PubMed] [Google Scholar]
- 11.Schlittler M, Goiny M, Agudelo LZ, et al. Endurance exercise increases skeletal muscle kynurenine aminotransferases and plasma kynurenic acid in humans. Am J Physiol Cell Physiol. 2016;310(10):836–840. doi: 10.1152/ajpcell.00053.2016. [DOI] [PubMed] [Google Scholar]
- 12.Strasser B, Geiger D, Schauer M, Gatterer H, Burtscher M, Fuchs D. Effects of exhaustive aerobic exercise on tryptophan-kynurenine metabolism in trained athletes. PLoS One. 2016;11(4):e0153617. doi: 10.1371/journal.pone.0153617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hallgren M, Helgadottir B, Herring MP, et al. Exercise and internet-based cognitive-behavioural therapy for depression: multicentre randomised controlled trial with 12-month follow-up. Br J Psychiatry. 2016;209(5):414–420. doi: 10.1192/bjp.bp.115.177576. [DOI] [PubMed] [Google Scholar]
- 14.Astrand PO. Human physical fitness with special reference to sex and age. Physiol Rev. 1956;36(3):307–335. doi: 10.1152/physrev.1956.36.3.307. [DOI] [PubMed] [Google Scholar]
- 15.Helgadottir B, Hallgren M, Ekblom O, Forsell Y. Training fast or slow? Exercise for depression: a randomized controlled trial. Prev Med. 2016;91:123–131. doi: 10.1016/j.ypmed.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 16.Schwieler L, Samuelsson M, Frye MA, et al. Electroconvulsive therapy suppresses the neurotoxic branch of the kynurenine pathway in treatment-resistant depressed patients. J Neuroinflammation. 2016;13(1):51. doi: 10.1186/s12974-016-0517-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Capuron L, Neurauter G, Musselman DL, et al. Interferon-alpha-induced changes in tryptophan metabolism. Relationship to depression and paroxetine treatment. Biol Psychiatry. 2003;54(9):906–914. doi: 10.1016/s0006-3223(03)00173-2. [DOI] [PubMed] [Google Scholar]
- 18.Myint AM, Kim YK, Verkerk R, Scharpe S, Steinbusch H, Leonard B. Kynurenine pathway in major depression: evidence of impaired neuroprotection. J Affect Disord. 2007;98(1–2):143–151. doi: 10.1016/j.jad.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 19.Lee DC, Sui X, Artero EG, et al. Long-term effects of changes in cardiorespiratory fitness and body mass index on all-cause and cardiovascular disease mortality in men: the Aerobics Center Longitudinal Study. Circulation. 2011;124(23):2483–2490. doi: 10.1161/CIRCULATIONAHA.111.038422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stubbs B, Rosenbaum S, Vancampfort D, Ward PB, Schuch FB. Exercise improves cardiorespiratory fitness in people with depression: a meta-analysis of randomized control trials. J Affect Disord. 2016;190:249–253. doi: 10.1016/j.jad.2015.10.010. [DOI] [PubMed] [Google Scholar]
- 21.Vancampfort D, Guelinckx H, De Hert M, et al. Reliability and clinical correlates of the Astrand-Rhyming sub-maximal exercise test in patients with schizophrenia or schizoaffective disorder. Psychiatry Res. 2014;220(3):778–783. doi: 10.1016/j.psychres.2014.08.049. [DOI] [PubMed] [Google Scholar]
- 22.Pilegaard H, Saltin B, Neufer PD. Exercise induces transient transcriptional activation of the PGC-1alpha gene in human skeletal muscle. J Physiol. 2003;546(Pt 3):851–858. doi: 10.1113/jphysiol.2002.034850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meier TB, Drevets WC, Wurfel BE, et al. Relationship between neurotoxic kynurenine metabolites and reductions in right medial prefrontal cortical thickness in major depressive disorder. Brain Behav Immun. 2016;53:39–48. doi: 10.1016/j.bbi.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Savitz J, Dantzer R, Meier TB, et al. Activation of the kynurenine pathway is associated with striatal volume in major depressive disorder. Psychoneuroendocrinology. 2015;62:54–58. doi: 10.1016/j.psyneuen.2015.07.609. [DOI] [PMC free article] [PubMed] [Google Scholar]