Abstract
Purpose of review
It is 20 years since the start of the combination antiretroviral therapy (cART) era and >10 years since cART scale-up began in resource-limited settings. We examined survival of vertically HIV-infected infants and children in the cART era.
Recent findings
Good survival has been achieved on cART in all settings with up to ten-fold mortality reductions compared to before cART availability. Although mortality risk remains high in the first few months after cART initiation in young children with severe disease, it drops rapidly thereafter even for those who started with advanced disease, and longer term mortality risk is low. However, suboptimal retention on cART in routine programs threatens good survival outcomes and even on treatment children continue to experience high comorbidity risk; infections remain the major cause of death. Interventions to address infection risk include co-trimoxazole prophylaxis, isoniazid preventive therapy, routine childhood and influenza immunization and improving maternal survival.
Summary
Pediatric survival has improved substantially with cART and HIV-infected children are aging into adulthood. It is important to ensure access to diagnosis and early cART, good program retention and optimal co-morbidity prophylaxis and treatment to achieve the best possible long-term survival and health outcomes for vertically infected children.
Keywords: combination antiretroviral therapy, survival, children, tuberculosis, malignancy
Introduction
HIV-related paediatric deaths in 2014 were impressively nearly 50% fewer than in 2004, largely due to reductions in new pediatric infections (58% decrease since 2000) through prevention of mother to child transmission (PMTCT) programs [1,2,3]. Nevertheless, HIV remains a leading cause of child deaths, especially among those >5 years old where the burden of children infected before good coverage of effective PMTCT is substantial [4]. Globally, HIV is the twelfth leading cause of child deaths and among the top five leading causes in high prevalence countries [4]. Optimizing survival of the 2.6 million children living with HIV therefore remains important [3].
Survival of children in the absence of combination antiretroviral therapy (cART)
In sub-Saharan Africa approximately half of perinatally infected and a quarter of infants infected through breastfeeding will die before their second birthday, compared to <5% infant mortality in HIV-exposed uninfected infants [5,6,7]. Mortality in untreated perinatally infected infants was so high that in South Africa it caused an increase in all-cause mortality at 2–3 months of age from 1997–2002 [8]. Pre-cART mortality was lower in USA/Europe; one-year mortality was 6.4–30% in 6-month old infants [9,10].
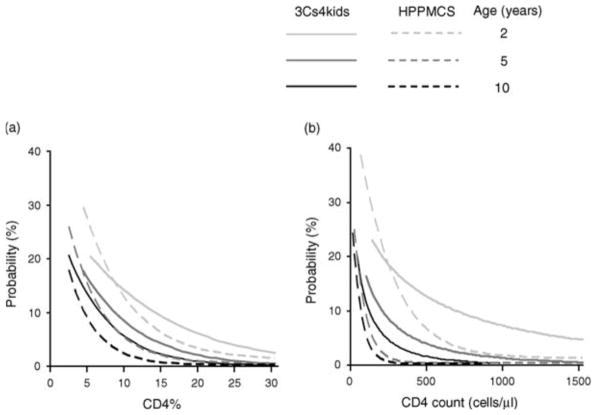
Due to survivor bias, mortality without cART decreases markedly in children surviving to two years. The UNAIDS Spectrum model estimates that survival probability to age 20 years without cART increases with timing of infection from 9% (infected at birth) to 24% (infected through breastfeeding), although data to inform assumptions for children aged >2.5 years is limited [11,12,13]. Annual mortality estimates without treatment in USA/Europe ranged from 1.2–12.0% in 2 year olds to 0.2–2.1% in children aged 10 years; CD4 was a poorer predictor of mortality in younger children [10]. In resource-limited settings (RLS), mortality is higher for equivalent age and CD4 values, and CD4 is less discriminatory for predicting mortality than in well-resourced countries; annual risk varied by CD4% from 2.6–32.5% in 1–2 year olds to 0.6–23.0% in children ≥7 years (Figure 1) [10,14]. Viral load was a much weaker predictor of mortality than CD4 count/percent [10].
Figure 1.
Estimated risk of death within 12-months in Cross Continents Collaboration for Kids (3Cs4kids) (low and middle-income countries) compared with HIV Paediatric Prognostic Collaborative Study (HPPMCS)(USA and Europe) according to age and: (a) CD4% (b) CD4 cell count. Estimates in 3Cs4kids apply to a child receiving co-trimoxazole prophylaxis. Curves are truncated at the 5th and 95th centiles for each age.
Source: Cross Continents Collaboration for Kids (3Cs4kids) Analysis and Writing Committee. Markers for predicting mortality in untreated HIV-infected children in resource-limited settings: a meta-analysis. AIDS. 2008; 22:97–105.
Impact of cART on survival
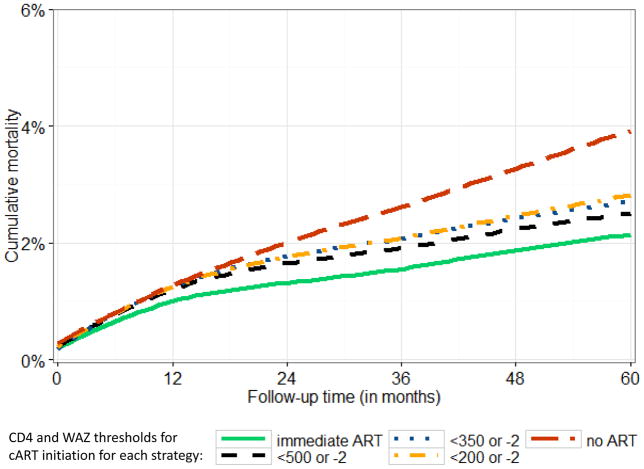
Developed country cohorts of mainly older children, initially demonstrated the survival benefit of cART [15]. Subsequently, studies have examined whether there is a survival benefit of immediate versus deferred therapy. The Children with HIV Early antiRetroviral (CHER) randomized controlled trial (RCT)showed 75% mortality reduction by 40 weeks in infants aged <3 months who started immediate early limited cART (until first or second birthday) compared to deferring until immunologic or clinical progression (defined as WHO 2006 cART initiation thresholds) [16]. However, a smaller RCT showed no mortality benefit of immediate cART at CD4 15–24% versus deferring until a CDC Stage C event or CD4 <15% in older Thai children (median age 6.4 years) [17]. Notably, the overall event rate was low and few children were in the younger age groups where the benefit of immediate cART is likely to be highest [17]. Causal modelling analyses that adjust for time-dependent confounding affected by prior treatment showed no mortality benefit of immediate cART 1–5 year old children, possibly because few children in routine care presented with CD4 count >750 cells/μl or CD4% >25% (WHO 2010 cART initiation threshold) and a high proportion were lost to follow-up (LTFU) with likely mortality under-ascertainment [18,19]. For children 5–10 years old, causal modelling showed a small but significant mortality benefit of 0.4% (95% Confidence IntervaI [CI]: 0.02–0.6%) by 5 years of follow-up when starting cART immediately versus deferring until CD4 <500 cells/μl (Figure 2) [20].
Figure 2.
Estimated mortality from enrolment into HIV care in children aged 5–10 years at enrolment with initial CD4 >500 cells/μl from Southern Africa, West Africa and Europe comparing different cART initiation strategies as follows: immediate cART; cART at CD4 < 500 or weight-for-age z-score (WAZ)<−2; CD4 <350/WAZ <−2; CD4 <200/WAZ <−2; no cART. The mortality difference between immediate cART (solid green line) and deferring cART to CD4 >500 cells/μl or WAZ<−2 is 0.4% (95%CI: 0.02–0.6%). Mortality is estimated from g-computation to adjust for time-dependent confounding affected by prior treatment of CD4 count, CD4 percent and weight-for-age z-score.
Survival on cART in routine care
Middle and high income countries
Long-term cohorts from USA and Europe report a ten-fold mortality rate decline over calendar years (7.2–8.2/100 child years (CY) in the late 1990s vs 0.6–0.8/100 CY in 2000–2006), with plateauing thereafter [21,22]. In these cohorts, 72–83% of children received cART [21,22] and 6–10 year survival probabilities during the cART era were >94–98% [22,23,24]. The European Pregnancy and Paediatric HIV Cohort Collaboration (EPPICC) reported nearly ten-fold higher mortality in the first 6 months on cART (2.5/100 PY vs 0.27/100 CY) compared to later. After 6 months, children <2 years old and adolescents ≥14 years had higher mortality versus 5–<14 year olds (aHR [95%CI] <2 years: 3.7 [1.2–10.8]; >14 years: 1.8 [0.9–3.7]). Mortality was 50% lower in high versus middle income (Thailand and Eastern Europe) countries [25]. Mortality was only 5% by nearly 6 years median follow-up in an EPPICC study of infants starting cART (median age <4 months) [26]. Despite overwhelming cART success, mortality remains nearly 30 times higher than in the general population [22].
Resource limited settings
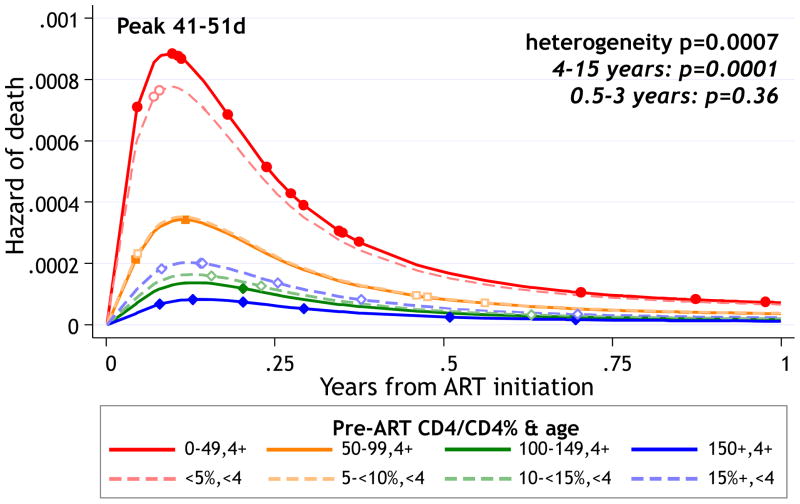
There are >80 publications of survival on cART from a range of RLS [27,28,29,30,31,32], including trials, cohorts and national programs, with increasing follow-up durations. Clinical trials with high retention and adherence demonstrate good survival on cART, especially after the first 6 months, even in peripheral clinics [16,33,34,35,36]. In the Antiretroviral Research for Watoto (ARROW) RCT of different monitoring strategies, one-year mortality on cART ranged from 1.3%–10.1% in children aged 4–15 years with CD4 ≥100 and <50 cells/μl respectively [36]. Corresponding values for children 4 months to 3 years were 9.1% (CD4% <5%) and 2.8% (CD4% >10%) (Figure 3) [36]. Although mortality was high in the first 3 month of cART, it declined rapidly to <5% by one year for all age and CD4 strata [36]. The CHAPAS 3 trial of different non-nucleoside reverse transcriptase inhibitor- based first-line cART regimens reported similar low mortality of 4.0% by 2.3 years median follow-up [35]. In CHER, mortality was 9% by median follow-up of 4.8 years in infants initiating immediate cART at <3 months old [33].
Figure 3.
Daily risk of death through 1 year on cART in children aged 4 months to 15 years in the ARROW RCT according to age and pre-cART CD4 count or percent using flexible parametric models on log-normal scale with 1 interior knot. Points show times when deaths occurred.
Source: Walker AS, et al. Mortality in the year following antiretroviral therapy initiation in HIV-infected adults and children in Uganda and Zimbabwe. Clinical Infectious Diseases. 2012; 55:1707–18.
Routine cART programmes in RLS suggest poorer and more variable survival than trials, partly due to lower retention and programmatic or adherence-related challenges, including drug stockouts. Indeed, better survival in children with greater adherence to placebo in co-trimoxazole prophylaxis trial suggests that good adherence may be a surrogate for overall better child care [37]. Mortality at ≥2 years on cART from routine programs in RLS ranges from 3.7 to 29% [38,39] and rates vary from 1.3–6.0/100 CY [40,41]. Children still initiate treatment with severe disease with mortality remaining higher in the first year (Figure 3)[42]. Young age and severe disease are consistently associated with higher mortality [28,31,43,44,45,46]. Prognostic models for RLS predict one-year mortality on cART ranging from <2% (children 5–10 years, no severe disease, CD4%>10%) to >45% (infants, severe disease, CD4<5%)[47,48]. Nevertheless, nearly 60% of children had characteristics predicting low (<5%) one-year mortality [47].
Importantly, there are concerns about survival on cART and interpreting outcomes of routine programs in RLS. First, routine cohorts frequently report high LTFU (>10%) with likely unascertained mortality [38,49,50,51]. In Malawi, mortality was 11% among children traced from three weeks after a missed visit [52]; using longer durations to define LTFU, mortality was nearly 40%among LTFU [38]. Second, many programs have high transfer rates, particularly in recent years with decentralisation of cART programmes [23,28,31,53,54,55,56,57,58]. While decentralization may contribute to better retention [54,59], limited ability to track post-transfer outcomes hampers long term survival assessment. Additionally, unrecorded transfers to another facility add to high LTFU rates with underestimation of survival at the program level [52]. Third, some programs report worsening outcomes [39,45,54]; from 2005–2008 in Cote d’Ivoire, one-year mortality and LTFU increased from 3% to 11% and 2% to 23% respectively [45]. In rural Mozambique mortality and LTFU were 16–24% and 25–70% respectively with substantial heterogeneity between districts [39]. Finally, infants remain vulnerable with poor outcomes in routine care in RLS despite WHO recommendations for universal cART since 2010 [60]. Although more infants have initiated cART with less severe disease [61,62,63], a substantial proportion still start treatment late [64,65]. In Southern Africa, despite significant improvements after 2010, one-year mortality (13%) and LTFU (19%)remained high overall [61]. While annual mortality on cART was lower in Botswana (4.6/100CY), only 60% of infants initiated treatment [56]; 39% of all HIV-infected infants died [56].
Infant survival requires early infant diagnosis (EID) with rapid cART initiation. In 2014, only 50% of HIV-exposed infants were tested by age 2 months; only a third of those diagnosed started cART promptly [66,67,68]. At best, most infants initiate treatment between 12–14 weeks, after the infant mortality peak [8,69]. Innovative approaches (point-of-care assays; adding birth EID) may accelerate diagnosis and cART initiation; but further implementation research is needed [69,70,71,72,73].
With increasing coverage of PMTCT, most new pediatric infections are in infants whose mothers did not access PMTCT; hence not identified through post-PMTCT EID programs. Identifying children missed by EID is critical, and remains challenging [74]. The proportion of children newly diagnosed through provider-initiated testing in hospitals or malnutrition centres is high (6%–22%). Testing strategies outside PMTCT programmes need to be strongly emphasized [72,75,76,77,78,79,80,81,82].
Models of care to optimize real world survival on cART
Given WHO 2015 recommendations to start everyone on cART [83] and UNAIDS targets (90% of HIV-infected people are diagnosed, 90% retained on cART, with 90% virologic suppression [84]), focus is shifting from “when to start” to achieving optimal treatment access and outcomes. Encouragingly, decentralization of pediatric HIV care has increased with good outcomes [85]. Survival and retention may be better for children treated nearer their homes, especially with community support [38,41,50,86]. Facilities with better quality of care [29]and high health worker morale report better outcomes, particularly lower LTFU [45]. Good outcomes in Rwanda (≤5% one-year LTFU or mortality) were ascribed to staff mentoring and decentralization [59].
Comorbidities that reduce survival in HIV-infected children
Even with cART, comorbidity incidence (including tuberculosis, childhood infections and malignancies) is higher in HIV-infected than uninfected children [87,88,89,90,91]; infections still predominate as causes of death [21,22,92,93]. AIDS-defining infections have declined, but non-AIDS defining conditions (sepsis; pneumonia) remain relatively stable [21,22,92,93,94]. The contribution of non-infectious conditions (metabolic syndrome, renal, liver and cardiovascular disease) in children is not clear.
Tuberculosis
In 2015, global estimates showed that up to 30% of HIV-infected child deaths may be tuberculosis-related [95]. HIV-infected children are more than twice as likely to die from tuberculosis compared to HIV-uninfected children [96,97,98]; within pediatric HIV cohorts tuberculosis increases mortality risk 2–4 times [99,100]. Infants are at the highest risk with nearly 30% mortality with bacteriologically confirmed tuberculosis [101]. While cART reduces tuberculosis-related mortality substantially [99,102], delays in cART initiation remain challenging [100,103]. Unlike in adults [104,105], there are no RCTs on the optimal timing of cART initiation in tuberculosis co-infected children. Observational studies showed better survival with starting cART within 2 months (or less) of tuberculosis therapy compared to later [106,107,108], but it is unclear whether early cART improves survival in co-infected children without severe immunocompromise.
Treatment outcomes for children with drug resistant tuberculosis (DR-TB) vary widely. A South African study of DR-TB (55% of children HIV-infected) found 20% overall mortality [109], while a systematic review found mortality was twice as high in HIV-infected compared to uninfected children (11.5% vs. 6%) [110].
Malignancy
Cancer is ≥5–10 times more common in HIV-infected compared to uninfected children [87,91,111,112,113]. In the cART era, AIDS-defining cancers (ADCs) have reduced dramatically, but non-ADC rates remain unchanged or increased [87,91,114]. Treatment outcomes are worse in HIV-infected vs HIV-uninfected children [111]. In South Africa >80% of HIV-infected children with cancer presented with advanced disease; only a third survived. Survival was higher (57.8%) if treated with cART and chemotherapy [115]. In Uganda, half of HIV-infected children with cancer died or were LTFU; mortality was at least three-fold higher than the clinic crude mortality rate (33% vs 5–10%) [116]. The increasing rates of some non–ADCs in the cART era is likely due to increased life expectancy allowing development of these cancers, however the impact of long-term cART has been debated. With vertically infected children surviving into adulthood there is growing concern about malignancy risk and associated mortality.
Interventions other than ART to improve survival in HIV-infected children
Co-trimoxazole nearly halved all-cause mortality in cART-naïve Zambian children despite high levels of bacterial resistance [117,118]. The ARROW trial found fewer hospitalizations when continuing co-trimoxazole in Ugandan/Zimbabwean children after 2 years of cART, hence WHO recommends continuing prophylaxis until adulthood if prevalence of bacterial infections and malaria is high [119,120]. Co-trimoxazole appears protective against incident tuberculosis in HIV-infected adults and possibly children[89,121] and reduces mortality and morbidity in people with HIV/tuberculosis [122,123]. Adding co-trimoxazole to anti-tuberculosis treatment has been successfully implemented in high tuberculosis burden countries with 87% coverage in co-infected patients [95].
Evidence of survival benefit of isoniazid preventive treatment (IPT) in HIV-infected children is conflicting. IPT halved all-cause mortality in an RCT including mostly cART-naïve children [124]; a subsequent cohort study demonstrated additional benefit of IPT on tuberculosis incidence in children on cART [125]. Contrastingly, an RCT in infants showed no mortality or tuberculosis prevention benefit of pre-exposure IPT [126], however infants were screened for tuberculosis exposure at every visit, with prompt post-exposure IPT. While this approach is unlikely to be feasible in routine care where uptake of post-exposure IPT has been shown to be very low [127,128], pre-exposure IPT for children in HIV clinics can have high uptake and treatment completion [129]. Although WHO has recommended pre-exposure IPT since 2004, coverage remains disappointingly low [95]. Barriers to IPT implementation include exaggerated fear of resistance development [130], unavailability of single entity isoniazid formulations in RLS and concerns about increased pill burden affecting cART adherence [131]. A scored fixed dose combination of isoniazid, vitamin B6 and co-trimoxazole was highly acceptable to adults and children >5 years in the REALITY trial [132] and could help overcome universal IPT implementation barriers; a half-strength scored dispersible tablet is needed for younger children.
Since common childhood infections remain the leading causes of death in HIV-infected children, routine childhood immunisations may improve survival [94]. Haemophilus influenza, pneumococcal conjugate vaccines (PCV) and seasonal influenza vaccinations prevent severe infections in HIV-infected children [88,133]. Influenza and PCV may reduce tuberculosis-related mortality as influenza epidemics lead to increased hospitalizations for invasive pneumococcal disease and pulmonary tuberculosis [88]. Further, PCV appeared protective against tuberculosis[134]. The high prevalence of rotavirus gastroenteritis in HIV-infected infants in RLS indicates a potential survival benefit of vaccination [135,136]. Rotavirus vaccine efficacy is lower in high-mortality countries but disease burden is higher, hence the absolute benefit is large [137]. A package of preventive strategies addressing common co-morbidities should be considered to optimize survival.
Maternal health, socio-economic status and treatment of OI in adults
Several studies show links between maternal and infant survival - maternal HIV/AIDS or tuberculosis-related death reduce child survival [138,139,140]. Maternal cART protected against under-five mortality; death rates in children of HIV-infected mothers were reduced to levels of children of HIV-uninfected mothers [141]. Maternal health is therefore important and “Option B+” (lifelong cART for pregnant/breastfeeding women) offers key child survival benefit.
Conclusion
Pediatric survival has improved substantially with cART; with the additional success of PMTCT programs in preventing new infant infections, focus can start to shift from averting deaths to optimizing health for adolescence and adulthood. Nevertheless, there remain substantial survival gains to be made especially in RLS by strengthening health systems and improving models of decentralised HIV care to ensure access to diagnosis and early cART at all levels of the health system, continuous drug supplies, better adherence and retention, prophylaxis and treatment for co-morbidities and promoting maternal survival including maternal cART.
Key points.
Good survival has been achieved during the cART era in all settings with up to ten-fold mortality reductions compared to before cART availability.
Immediate cART has been shown to improve survival compared to deferring until clinical or immunological progression in infants in the CHER RCT, and in children aged 5–10 years in causal modelling analyses of cohort data.
Since mortality risk remains high in the first few months after cART initiation in young children with severe disease, it is important to improve access to early diagnosis and cART before disease progression; nevertheless mortality drops rapidly after the first 6 months on cART even for those who started with advanced disease, and longer term mortality risk is low in all settings
Routine programs providing pediatric cART in resource-limited settings frequently report high loss to follow-up which may include unascertained mortality and suboptimal retention on cART threatens potential good survival outcomes.
HIV-infected children continue to experience high comorbidity risk even when treated with cART with infections remaining the major cause of death; interventions shown to reduce infection risk and related mortality include co-trimoxazole prophylaxis, isoniazid preventive therapy, routine childhood and influenza immunization and improving maternal survival.
Acknowledgments
Financial support and sponsorship
MD receives support from the National Institutes of Health under Award Numbers U01AI069924 (International Epidemiologic Databases to Evaluate AIDS Southern Africa) and R01HD075156 (Closing the gaps in PMTCT program coverage, early infant diagnosis and treatment).
Abbreviations
- ADC
AIDS-defining cancer
- ARROW
Antiretroviral Research for Watoto
- cART
combination antiretroviral therapy
- CHER
Children with HIV Early antiRetroviral study
- CY
child years
- DR-TB
Drug resistant tuberculosis
- EID
early infant diagnosis
- EPPICC
European Pregnancy and Paediatric HIV Cohort Collaboration
- LTFU
loss to follow-up
- PCV
pneumococcal conjugate vaccine
- PMTCT
prevention of mother to child transmission
- RCT
randomized controlled trial
- RLS
resource-limited settings
Footnotes
Conflicts of interest
None
References and recommended reading
- 1.Johnson LF, Davies MA, Moultrie H, et al. The effect of early initiation of antiretroviral treatment in infants on pediatric AIDS mortality in South Africa: a model-based analysis. Pediatr Infect Dis J. 2012;31:474–480. doi: 10.1097/INF.0b013e3182456ba2. [DOI] [PubMed] [Google Scholar]
- 2.Johnson L, Stinson K, Newell ML, et al. The contribution of maternal HIV seroconversion during late pregnancy and breastfeeding to mother-to-child transmission of HIV. J Acquir Immune Defic Syndr. 2012;59:417–425. doi: 10.1097/QAI.0b013e3182432f27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.UNAIDS. HIV Factsheet 2015. 2015 http://www.unaids.org/sites/default/files/media_asset/20150714_FS_MDG6_Report_en.pdf.
- 4*.Kyu HH, Pinho C, Wagner JA, et al. Global and National Burden of Diseases and Injuries Among Children and Adolescents Between 1990 and 2013: Findings From the Global Burden of Disease 2013 Study. JAMA Pediatr. 2016;170:267–287. doi: 10.1001/jamapediatrics.2015.4276. This paper examines global cause-specific mortality in children using data from >35620 epidemiological sources and describes the leading causes of death in children globally and in the 50 most populous countries by child and adolescent population. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marston M, Becquet R, Zaba B, et al. Net survival of perinatally and postnatally HIV-infected children: a pooled analysis of individual data from sub-Saharan Africa. Int J Epidemiol. 2011;40:385–396. doi: 10.1093/ije/dyq255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Becquet R, Marston M, Dabis F, et al. Children who acquire HIV infection perinatally are at higher risk of early death than those acquiring infection through breastmilk: a meta-analysis. PLoS One. 2012;7:e28510. doi: 10.1371/journal.pone.0028510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Newell ML, Coovadia H, Cortina-Borja M, et al. Mortality of infected and uninfected infants born to HIV-infected mothers in Africa: a pooled analysis. Lancet. 2004;364:1236–1243. doi: 10.1016/S0140-6736(04)17140-7. [DOI] [PubMed] [Google Scholar]
- 8.Bourne DE, Thompson M, Brody LL, et al. Emergence of a peak in early infant mortality due to HIV/AIDS in South Africa. AIDS. 2009;23:101–106. doi: 10.1097/qad.0b013e32831c54bd. [DOI] [PubMed] [Google Scholar]
- 9.Dunn D, Woodburn P, Duong T, et al. Current CD4 cell count and the short-term risk of AIDS and death before the availability of effective antiretroviral therapy in HIV-infected children and adults. J Infect Dis. 2008;197:398–404. doi: 10.1086/524686. [DOI] [PubMed] [Google Scholar]
- 10.Dunn D. Short-term risk of disease progression in HIV-1-infected children receiving no antiretroviral therapy or zidovudine monotherapy: a meta-analysis. Lancet. 2003;362:1605–1611. doi: 10.1016/s0140-6736(03)14793-9. [DOI] [PubMed] [Google Scholar]
- 11*.Penazzato M, Bendaud V, Nelson L, Stover J, Mahy M. Estimating future trends in paediatric HIV. AIDS. 2014;28(Suppl 4):S445–451. doi: 10.1097/QAD.0000000000000481. This paper describes the number of children <15 years expected to be living with HIV in 2020 based on projected reductions in new pediatric infections and projected survival of vertically infected children. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stover J, Andreev K, Slaymaker E, et al. Updates to the spectrum model to estimate key HIV indicators for adults and children. AIDS. 2014;28(Suppl 4):S427–434. doi: 10.1097/QAD.0000000000000483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stover J, Brown T, Marston M. Updates to the Spectrum/Estimation and Projection Package (EPP) model to estimate HIV trends for adults and children. Sex Transm Infect. 2012;88(Suppl 2):i11–16. doi: 10.1136/sextrans-2012-050640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cross Continents Collaboration for Kids (3Cs4kids) Analysis and Writing Committee. Markers for predicting mortality in untreated HIV-infected children in resource-limited settings: a meta-analysis. AIDS. 2008;22:97–105. doi: 10.1097/01.aids.0000302262.51286.a5. [DOI] [PubMed] [Google Scholar]
- 15.Gortmaker SG, Hughes M, Cervia JS, et al. Effect of combination therapy including protease inhibitors on mortality among children and adolescents infected with HIV-1. N Engl J Med. 2001;345:1522–1528. doi: 10.1056/NEJMoa011157. [DOI] [PubMed] [Google Scholar]
- 16.Violari A, Cotton MF, Gibb DM, et al. Early antiretroviral therapy and mortality among HIV-infected infants. N Engl J Med. 2008;359:2233–2244. doi: 10.1056/NEJMoa0800971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Puthanakit T, Saphonn V, Ananworanich J, et al. Early versus deferred antiretroviral therapy for children older than 1 year infected with HIV (PREDICT): a multicentre, randomised, open-label trial. Lancet Infect Dis. 2012;12:933–941. doi: 10.1016/S1473-3099(12)70242-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schomaker M, Egger M, Ndirangu J, et al. When to start antiretroviral therapy in children aged 2–5 years: a collaborative causal modelling analysis of cohort studies from southern Africa. PLoS Med. 2013;10:e1001555. doi: 10.1371/journal.pmed.1001555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schomaker M, Davies MA, Malateste K, et al. Growth and Mortality Outcomes for Different Antiretroviral Therapy Initiation Criteria in Children Ages 1–5 Years: A Causal Modeling Analysis. Epidemiology. 2016;27:237–246. doi: 10.1097/EDE.0000000000000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20**.Schomaker M, Leroy V, Wolfs TF, et al. Optimal timing of antiretroviral treatment initiation in HIV positive children and adolescents - A multiregional analysis from Southern Africa, West Africa and Europe. Int J Epidemiol. 2016 doi: 10.1093/ije/dyw097. In Press. This is an analysis of cohort data from West and Southern Africa as well as Europe examining mortality and growth differences for different treatment initiation strategies in children 5–15 years of age using g-computation to adjust for time-dependent confounding affected by prior treatment. It shows lower mortality and better growth with immediate cART vs deferring to CD4<500 in children 5–10 years of age, but differences are less clear for adolescents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Judd A, Doerholt K, Tookey PA, et al. Morbidity, mortality, and response to treatment by children in the United Kingdom and Ireland with perinatally acquired HIV infection during 1996–2006: planning for teenage and adult care. Clin InfectDis. 2007;45:918–924. doi: 10.1086/521167. [DOI] [PubMed] [Google Scholar]
- 22.Brady MT, Oleske J, Williams PL, et al. Declines in mortality rates and changes in causes of death in HIV-1-infected children during the HAART era. J Acquir Immune Defic Syndr. 2010;53:86–94. doi: 10.1097/QAI.0b013e3181b9869f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sturt AS, Halpern MS, Sullivan B, Maldonado YA. Timing of antiretroviral therapy initiation and its impact on disease progression in perinatal human immunodeficiency virus-1 infection. Pediatr Infect Dis J. 2012;31:53–60. doi: 10.1097/INF.0b013e31823515a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kapogiannis BG, Soe MM, Nesheim SR, et al. Mortality trends in the US Perinatal AIDS Collaborative Transmission Study (1986–2004) Clin Infect Dis. 2011;53:1024–1034. doi: 10.1093/cid/cir641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Judd A, Chappell E, Doerholt K, et al. Long-term trends in mortality and AIDS-defining events among perinatally HIV-infected children across Europe and Thailand. The 21st International AIDS Conference (AIDS 2016); 18–22 July 2016; Durban, South Africa. [Google Scholar]
- 26.Judd A for the European Pregnancy and Paediatric HIV Cohort Collaboration (EPPICC) study group in EuroCoord. Early antiretroviral therapy in HIV-1-infected infants, 1996–2008: treatment response and duration of first-line regimens. AIDS. 2011;25:2279–2287. doi: 10.1097/QAD.0b013e32834d614c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao Y, Li C, Sun X, et al. Mortality and treatment outcomes of China’s National Pediatric antiretroviral therapy program. Clin Infect Dis. 2013;56:735–744. doi: 10.1093/cid/cis941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ojeniran MA, Emokpae A, Mabogunje C, et al. How are children with HIV faring in Nigeria?--a 7 year retrospective study of children enrolled in HIV care. BMC Pediatr. 2015;15:87. doi: 10.1186/s12887-015-0405-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ojikutu B, Higgins-Biddle M, Greeson D, et al. The association between quality of HIV care, loss to follow-up and mortality in pediatric and adolescent patients receiving antiretroviral therapy in Nigeria. PLoS One. 2014;9:e100039. doi: 10.1371/journal.pone.0100039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anigilaje EA, Dabit OJ, Tyovenda RK, et al. Effects of leisure activities and psychosocial support on medication adherence and clinic attendance among children on antiretroviral therapy. HIV AIDS (Auckl) 2014;6:127–137. doi: 10.2147/HIV.S64964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gebremedhin A, Gebremariam S, Haile F, Weldearegawi B, Decotelli C. Predictors of mortality among HIV infected children on anti-retroviral therapy in Mekelle Hospital, Northern Ethiopia: a retrospective cohort study. BMC Public Health. 2013;13:1047. doi: 10.1186/1471-2458-13-1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alvarez-Uria G, Naik PK, Midde M, Pakam R. Predictors of loss to follow-up after engagement in care of HIV-infected children ineligible for antiretroviral therapy in an HIV cohort study in India. Germs. 2014;4:9–15. doi: 10.11599/germs.2014.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33**.Cotton MF, Violari A, Otwombe K, et al. Early time-limited antiretroviral therapy versus deferred therapy in South African infants infected with HIV: results from the children with HIV early antiretroviral (CHER) randomised trial. Lancet. 2013;382:1555–1563. doi: 10.1016/S0140-6736(13)61409-9. The long-term outcomes of the CHER trial are reported in this paper which shows low mortality overall for nearly 5 years of follow-up for infants initiating cART at <3 months of age before the onset of disease progression. Children who initiated treatment early and susbequently had planned treatment interruption after either 40 weeks or 96 weeks on cART had lower mortality and severe disease events and spent less total time on cART compared to those where initial therapy was deferred until clinical or immunological progression. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kekitiinwa A, Cook A, Nathoo K, et al. Routine versus clinically driven laboratory monitoring and first-line antiretroviral therapy strategies in African children with HIV (ARROW): a 5-year open-label randomised factorial trial. Lancet. 2013;381:1391–1403. doi: 10.1016/S0140-6736(12)62198-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35*.Mulenga V, Musiime V, Kekitiinwa A, et al. Abacavir, zidovudine, or stavudine as paediatric tablets for African HIV-infected children (CHAPAS-3): an open-label, parallel-group, randomised controlled trial. Lancet Infect Dis. 2016;16:169–179. doi: 10.1016/S1473-3099(15)00319-9. A RCT comparing stavudine, zidovudine, or abacavir as dual or triple fixed-dose-combination paediatric tablets with lamivudine and nevirapine or efavirenz as first-line therapy in children. Mortality was low overall and there was no difference in toxicity, clinical, immunological, and virological responses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Walker AS, Prendergast AJ, Mugyenyi P, et al. Mortality in the year following antiretroviral therapy initiation in HIV-infected adults and children in Uganda and Zimbabwe. Clin Infect Dis. 2012;55:1707–1718. doi: 10.1093/cid/cis797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Walker AS, Ford D, Mulenga V, et al. Adherence to Both Cotrimoxazole and Placebo is Associated with Improved Survival Among HIV-Infected Zambian Children. AIDS Behav. 2009;13:33–41. doi: 10.1007/s10461-008-9382-4. [DOI] [PubMed] [Google Scholar]
- 38.Grimwood A, Fatti G, Mothibi E, et al. Community adherence support improves programme retention in children on antiretroviral treatment: a multicentre cohort study in South Africa. J Int AIDS Soc. 2012;15:17381. doi: 10.7448/IAS.15.2.17381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vermund SH, Blevins M, Moon TD, et al. Poor clinical outcomes for HIV infected children on antiretroviral therapy in rural Mozambique: need for program quality improvement and community engagement. PLoS One. 2014;9:e110116. doi: 10.1371/journal.pone.0110116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Phongsamart W, Hansudewechakul R, Bunupuradah T, et al. Long-term outcomes of HIV-infected children in Thailand: the Thailand Pediatric HIV Observational Database. Int J Infect Dis. 2014;22:19–24. doi: 10.1016/j.ijid.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 41.Fayorsey RN, Saito S, Carter RJ, et al. Decentralization of pediatric HIV care and treatment in five sub-Saharan African countries. J Acquir Immune Defic Syndr. 2013;62:e124–130. doi: 10.1097/QAI.0b013e3182869558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koller M, Patel K, Chi BH, et al. Immunodeficiency in children starting antiretroviral therapy in low-, middle-, and high-income countries. J Acquir Immune Defic Syndr. 2015;68:62–72. doi: 10.1097/QAI.0000000000000380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peacock-Villada E, Richardson BA, John-Stewart GC. Post-HAART outcomes in pediatric populations: comparison of resource-limited and developed countries. Pediatrics. 2011;127:e423–441. doi: 10.1542/peds.2009-2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ciaranello A, Chang YL, Margulis AV, et al. Effectiveness of pediatric antiretroviral therapy in resource-limited settings: a systematic review and meta-analysis. Clin Infect Dis. 2009;15:1915–1927. doi: 10.1086/648079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Auld AF, Tuho MZ, Ekra KA, et al. Temporal trends in mortality and loss to follow-up among children enrolled in Cote d’Ivoire’s national antiretroviral therapy program. Pediatr Infect Dis J. 2014;33:1134–1140. doi: 10.1097/INF.0000000000000457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kabue MM, Buck WC, Wanless SR, et al. Mortality and clinical outcomes in HIV-infected children on antiretroviral therapy in Malawi, Lesotho, and Swaziland. Pediatrics. 2012;130:e591–599. doi: 10.1542/peds.2011-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47*.Davies M, May M, Bolton-Moore C, et al. Prognosis of children with HIV-1 infection starting antiretroviral therapy in Southern Africa: a collaborative analysis of treatment programs. Pediatr Infect Dis J. 2014;33:608–616. doi: 10.1097/INF.0000000000000214. The externally validated prognostic model provides mortality estimates by 3, 6 and 12 months on cART for different combinations of prognostic variables for children starting cART in Southern Africa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nugent J, Edmonds A, Lusiama J, Thompson D, Behets F. Predicting mortality in HIV-infected children initiating highly active antiretroviral therapy in a resource-deprived setting. Pediatr Infect Dis J. 2014;33:1148–1155. doi: 10.1097/INF.0000000000000454. [DOI] [PubMed] [Google Scholar]
- 49.Fenner L, Brinkhof M, Keiser O, et al. Early mortality and loss to follow-up in HIV-infected children starting antiretroviral therapy in Southern Africa. J Acquir Immune Defic Syndr. 2010;54:524–532. doi: 10.1097/QAI.0b013e3181e0c4cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fatti G, Bock P, Eley B, Mothibi E, Grimwood A. Temporal trends in baseline characteristics and treatment outcomes of children starting antiretroviral treatment: an analysis in four provinces in South Africa, 2004–2009. J Acquir Immune Defic Syndr. 2011;58:e60–67. doi: 10.1097/QAI.0b013e3182303c7e. [DOI] [PubMed] [Google Scholar]
- 51.Braitstein P, Songok J, Vreeman RC, et al. “Wamepotea” (they have become lost): outcomes of HIV-positive and HIV-exposed children lost to follow-up from a large HIV treatment program in western Kenya. J Acquir Immune Defic Syndr. 2011;57:e40–46. doi: 10.1097/QAI.0b013e3182167f0d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52*.Ardura-Garcia C, Feldacker C, Tweya H, et al. Implementation and Operational Research: Early Tracing of Children Lost to Follow-Up From Antiretroviral Treatment: True Outcomes and Future Risks. J Acquir Immune Defic Syndr. 2015;70:e160–167. doi: 10.1097/QAI.0000000000000772. One of the few tracing studies of children LTFU after cART initiation in RLS, which shows that among children more than 3 weeks late for a clinic visit, 79% could be traced, 11% had died, about a quarter of children each had either stopped treatment or transferred to another facility and 37% were still on cART. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zanoni BC, Phungula T, Zanoni HM, France H, Feeney ME. Risk factors associated with increased mortality among HIV infected children initiating antiretroviral therapy (ART) in South Africa. PLoS One. 2011;6:e22706. doi: 10.1371/journal.pone.0022706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McNairy ML, Lamb MR, Carter RJ, et al. Retention of HIV-infected children on antiretroviral treatment in HIV care and treatment programs in Kenya, Mozambique, Rwanda, and Tanzania. J Acquir Immune Defic Syndr. 2013;62:e70–81. doi: 10.1097/QAI.0b013e318278bcb0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hansudewechakul R, Naiwatanakul T, Katana A, et al. Successful clinical outcomes following decentralization of tertiary paediatric HIV care to a community-based paediatric antiretroviral treatment network, Chiangrai, Thailand, 2002 to 2008. J Int AIDS Soc. 2012;15:17358. doi: 10.7448/IAS.15.2.17358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56*.Motswere-Chirwa C, Voetsch A, Lu L, et al. Follow-up of infants diagnosed with HIV - Early Infant Diagnosis Program, Francistown, Botswana, 2005–2012. MMWR Morb Mortal Wkly Rep. 2014;63:158–160. A cohort study that reports low mortality in infants who initiate cART in Botswana, but a low proportion of infants diagnosed initiated cART with high overall mortality. [PMC free article] [PubMed] [Google Scholar]
- 57.Eley B, Nuttall J. Antiretroviral therapy for children: challenges and opportunities. Annals of Tropical Paediatrics. 2007;27:1–10. doi: 10.1179/146532807X170448. [DOI] [PubMed] [Google Scholar]
- 58.Davies M, Keiser O, Technau K, et al. Outcomes of the South African National Antiretroviral Treatment (ART) programme for children - The IeDEA Southern Africa Collaboration. S Afr Med J. 2009;99:730–737. [PMC free article] [PubMed] [Google Scholar]
- 59.Tene G, Lahuerta M, Teasdale C, et al. High retention among HIV-infected children in Rwanda during scale-up and decentralization of HIV care and treatment programs, 2004 to 2010. Pediatr Infect Dis J. 2013;32:e341–347. doi: 10.1097/INF.0b013e31828c2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.WHO. Antiretroviral therapy for HIV infection in infants and children: Towards universal access. Recommendations for a public health approach: 2010 revision. 2010 http://www.who.int/hiv/pub/paediatric/infants2010/en/index.html. [PubMed]
- 61*.Porter M, Davies MA, Mapani MK, et al. Outcomes of Infants Starting Antiretroviral Therapy in Southern Africa, 2004–2012. J Acquir Immune Defic Syndr. 2015;69:593–601. doi: 10.1097/QAI.0000000000000683. This large study of nearly 5000 infants initiating cART in Southern Africa from 2004–2012 shows high early mortality and LTFU on cART despite some improvements in proportion of infants initiating treatment with severe disease and outcomes since 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nuwagaba-Biribonwoha H, Wang C, Kilama B, et al. Implementation of antiretroviral therapy guidelines for under-five children in Tanzania: translating recommendations into practice. J Int AIDS Soc. 2015;18:20303. doi: 10.7448/IAS.18.1.20303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Davies M, Phiri S, Wood R, et al. Temporal trends in the characteristics of children at antiretroviral therapy initiation in Southern Africa: The International epidemiologic Databases to Evaluate AIDS Southern Africa (IeDEA-SA) Collaboration. PLoS One. 2013;8:e81037. doi: 10.1371/journal.pone.0081037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Innes S, Lazarus E, Otwombe K, et al. Early severe HIV disease precedes early antiretroviral therapy in infants: Are we too late? J Int AIDS Soc. 2014;17:18914. doi: 10.7448/IAS.17.1.18914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Purchase SE, Van der Linden DJ, McKerrow NH. Feasibility and effectiveness of early initiation of combination antiretroviral therapy in HIV-infected infants in a government clinic of Kwazulu-Natal, South Africa. J Trop Pediatr. 2012;58:114–119. doi: 10.1093/tropej/fmr053. [DOI] [PubMed] [Google Scholar]
- 66.Chatterjee A, Tripathi S, Gass R, et al. Implementing services for Early Infant Diagnosis (EID) of HIV: a comparative descriptive analysis of national programs in four countries. BMC Public Health. 2011;11:53. doi: 10.1186/1471-2458-11-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chamla D, Mbori-Ngacha D, Newman M, et al. Evidence from the field: missed opportunities for identifying and linking HIV-infected children for early initiation of ART. AIDS. 2013;27(Suppl 2):S139–146. doi: 10.1097/QAD.0000000000000101. [DOI] [PubMed] [Google Scholar]
- 68.WHO. What’s new in infant diagnosis? 2015 http://apps.who.int/iris/bitstream/10665/204346/1/WHO_HIV_2015.43_eng.pdf.
- 69.Lilian RR, Kalk E, Bhowan K, et al. Early diagnosis of in utero and intrapartum HIV infection in infants prior to 6 weeks of age. J Clin Microbiol. 2012;50:2373–2377. doi: 10.1128/JCM.00431-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lilian RR, Johnson LF, Moolla H, Sherman GG. A mathematical model evaluating the timing of early diagnostic testing in HIV-exposed infants in South Africa. J Acquir Immune Defic Syndr. 2014;67:341–348. doi: 10.1097/QAI.0000000000000307. [DOI] [PubMed] [Google Scholar]
- 71*.Collins IJ, Cairns J, Ngo-Giang-Huong N, et al. Cost-effectiveness of early infant HIV diagnosis of HIV-exposed infants and immediate antiretroviral therapy in HIV-infected children under 24 months in Thailand. PLoS One. 2014;9:e91004. doi: 10.1371/journal.pone.0091004. This cost-effectiveness analysis compared EID using with immediate ART or EID with deferred ART based on immune/clinical criteria with later clinical/serology based diagnosis and deferred ART. EID with immediate ART was more cost-effective than EID with deferred ART with a cost-effectiveness ratio of USD2615/life year gained compared to later clinical/serology based diagnosis and deferred ART and would lead to major survival benefits. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72**.Penazzato M, Revill P, Prendergast AJ, et al. Early infant diagnosis of HIV infection in low-income and middle-income countries: does one size fit all? Lancet Infect Dis. 2014;14:650–655. doi: 10.1016/S1473-3099(13)70262-7. A recent review of evidence for different EID strategies that highlights the need for different context-specific policies. [DOI] [PubMed] [Google Scholar]
- 73.WHO. March 2014 supplement to the 2013 consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. Recommendations for a public health approach. 2014 www.who.int. [PubMed]
- 74.Ahmed S, Kim MH, Sugandhi N, et al. Beyond early infant diagnosis: case finding strategies for identification of HIV-infected infants and children. AIDS. 2013;27(Suppl 2):S235–245. doi: 10.1097/QAD.0000000000000099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Weigel R, Kamthunzi P, Mwansambo C, Phiri S, Kazembe PN. Effect of provider-initiated testing and counselling and integration of ART services on access to HIV diagnosis and treatment for children in Lilongwe, Malawi: a pre- post comparison. BMC Pediatr. 2009;9:80. doi: 10.1186/1471-2431-9-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Topp SM, Li MS, Chipukuma JM, et al. Does provider-initiated counselling and testing (PITC) strengthen early diagnosis and treatment initiation? Results from an analysis of an urban cohort of HIV-positive patients in Lusaka, Zambia. J Int AIDS Soc. 2012;15:17352. doi: 10.7448/IAS.15.2.17352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mutanga JN, Raymond J, Towle MS, et al. Institutionalizing provider-initiated HIV testing and counselling for children: an observational case study from Zambia. PLoS One. 2012;7:e29656. doi: 10.1371/journal.pone.0029656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.McCollum ED, Preidis GA, Golitko CL, et al. Routine inpatient human immunodeficiency virus testing system increases access to pediatric human immunodeficiency virus care in sub-Saharan Africa. Pediatr Infect Dis J. 2011;30:e75–81. doi: 10.1097/INF.0b013e3182103f8a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79*.Kranzer K, Meghji J, Bandason T, et al. Barriers to provider-initiated testing and counselling for children in a high HIV prevalence setting: a mixed methods study. PLoS Med. 2014;11:e1001649. doi: 10.1371/journal.pmed.1001649. This study conducted in Zimbabwe found that only three-quarters of children/guardians were offered provider-initiated HIV testsing and counselling in primary care clinics, of whom nearly half refused; the main reasons health care workers gave for not offering HIV testing were the perceived unsuitability of the accompanying guardian to provide consent for HIV testing on behalf of the child and lack of availability of staff or HIV testing kits. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Asafo-Agyei SB, Antwi S, Nguah SB. HIV infection in severely malnourished children in Kumasi, Ghana: a cross-sectional prospective study. BMC Pediatr. 2013;13:181. doi: 10.1186/1471-2431-13-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Preidis GA, McCollum ED, Kamiyango W, et al. Routine inpatient provider-initiated HIV testing in Malawi, compared with client-initiated community-based testing, identifies younger children at higher risk of early mortality. J Acquir Immune Defic Syndr. 2013;63:e16–22. doi: 10.1097/QAI.0b013e318288aad6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Whitehouse K, Cohn J. To define the benefits of targeting pediatric HIV diagnosis in the following specific settings: pediatric inpatient, pediatric outpatient, nutrition centers, essential programme for immunization (EPI) centers in lower or middle income countries. PROSPERO 2014. 2014 CRD42014014372. http://www.crd.york.ac.uk/PROSPERO_REBRANDING/display_record.asp?ID=CRD42014014372.
- 83.World Health Organization. Guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV. 2015 http://who.int/hiv/en/ [PubMed]
- 84.UNAIDS. 90-90-90 An ambitious treatment target to help end the AIDS epidemic. 2014 http://www.unaids.org/sites/default/files/media_asset/90-90-90_en_0.pdf.
- 85*.Penazzato M, Davies MA, Apollo T, Negussie E, Ford N. Task shifting for the delivery of pediatric antiretroviral treatment: a systematic review. J Acquir Immune Defic Syndr. 2014;65:414–422. doi: 10.1097/QAI.0000000000000024. This recent systematic review of 8 studies found that mortality and LTFU in task-shifting programs were comparable to those reported by programs providing doctor- or specialist-led care. [DOI] [PubMed] [Google Scholar]
- 86.Massavon W, Barlow-Mosha L, Mugenyi L, et al. Factors Determining Survival and Retention among HIV-Infected Children and Adolescents in a Community Home-Based Care and a Facility-Based Family-Centred Approach in Kampala, Uganda: A Cohort Study. ISRN AIDS. 2014;2014:852489. doi: 10.1155/2014/852489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chiappini E, Galli L, Tovo PA, et al. Cancer rates after year 2000 significantly decrease in children with perinatal HIV infection: a study by the Italian Register for HIV Infection in Children. J Clin Oncol. 2007;25:97–101. doi: 10.1200/JCO.2006.06.6506. [DOI] [PubMed] [Google Scholar]
- 88*.Dangor Z, Izu A, Moore DP, et al. Temporal association in hospitalizations for tuberculosis, invasive pneumococcal disease and influenza virus illness in South African children. PLoS One. 2014;9:e91464. doi: 10.1371/journal.pone.0091464. This retrospective study showed that influenza epidemics were followed by increase in the hospitalisations for confirmed pulmonary tuberculosis and invasive pneumococcal disease, particularly in HIV-infected children. The authors discuss possible interactions between the host and these infectious agents and suggest that vaccination against influenza virus or pneumococcal disease may alter the epidemiology of hospitalizations in children in the similar settings. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89**.Crook AM, Turkova A, Musiime V, et al. Tuberculosis incidence is high in HIV-infected African children but is reduced by co-trimoxazole and time on antiretroviral therapy. BMC Med. 2016;14:50. doi: 10.1186/s12916-016-0593-7. This paper assessed the incidence of and the risk factors for tuberculosis, and the protective effect of continuing co-trimoxazole prophylaxis in HIV-infected children on ART in the ARROW trial. The incidence of tuberculosis was markedly higher in HIV-infected children in the trial than those reported in the general pediatric population in the same countries; the risk was particularly high in the first 3 months of ART initiation; continuation of co-trimoxazole led to significant reductions in incident tuberculosis strikingly, despite good immune reconstitution after 2 years of ART, suggesting an additional important role for prophylaxtic co-trimoxazole prophylaxis in settings of high tuberculosis burden. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90*.Turkova A, Chappell E, Judd A, et al. Prevalence, incidence, and associated risk factors of tuberculosis in children with HIV living in the UK and Ireland (CHIPS): a cohort study. Lancet HIV. 2015;2:e530–539. doi: 10.1016/S2352-3018(15)00200-3. This study of tuberculosis in the national cohort of HIV-infected children in the UK and Ireland showed that tuberculosis incidence was markedly higher than the reported in the general paediatric population, highlighting the need to evaluate the screening and preventive practices in this setting. [DOI] [PubMed] [Google Scholar]
- 91.Alvaro-Meca A, Micheloud D, Jensen J, et al. Epidemiologic trends of cancer diagnoses among HIV-infected children in Spain from 1997 to 2008. Pediatr Infect Dis J. 2011;30:764–768. doi: 10.1097/INF.0b013e31821ba148. [DOI] [PubMed] [Google Scholar]
- 92.Palladino C, Climent FJ, Jose MI, et al. Causes of death in pediatric patients vertically infected by the human immunodeficiency virus type 1 in Madrid, Spain, from 1982 to mid-2009. Pediatr Infect Dis J. 2011;30:495–500. doi: 10.1097/INF.0b013e318211399f. [DOI] [PubMed] [Google Scholar]
- 93.Chiappini E, Galli L, Tovo PA, et al. Changing patterns of clinical events in perinatally HIV-1-infected children during the era of HAART. AIDS. 2007;21:1607–1615. doi: 10.1097/QAD.0b013e32823ecf5b. [DOI] [PubMed] [Google Scholar]
- 94.Ford N, Shubber Z, Meintjes G, et al. Causes of hospital admission among people living with HIV worldwide: a systematic review and meta-analysis. Lancet HIV. 2015;2:e438–444. doi: 10.1016/S2352-3018(15)00137-X. [DOI] [PubMed] [Google Scholar]
- 95.WHO. Global Tuberculosis Report. (20) 2015 http://apps.who.int/iris/bitstream/10665/191102/1/9789241565059_eng.pdf?ua=1.
- 96.Russell GK, Merle CS, Cooke GS, et al. Towards the WHO target of zero childhood tuberculosis deaths: an analysis of mortality in 13 locations in Africa and Asia. Int J Tuberc Lung Dis. 2013;17:1518–1523. doi: 10.5588/ijtld.13.0238. [DOI] [PubMed] [Google Scholar]
- 97.Hailu D, Abegaz WE, MB Childhood tuberculosis and its treatment outcomes in Addis Ababa: a 5-years retrospective study. BMC Pediatr. 2014;14:61. doi: 10.1186/1471-2431-14-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cavanaugh J, Genga K, Marigu I, et al. Tuberculosis among children in Kenya: epidemiology and impact of HIV in two provinces. J Trop Pediatr. 2012;58:292–296. doi: 10.1093/tropej/fmr098. [DOI] [PubMed] [Google Scholar]
- 99.Bakeera-Kitaka S, Conesa-Botella A, Dhabangi A, et al. Tuberculosis in human immunodeficiency virus infected Ugandan children starting on antiretroviral therapy. Int J Tuberc Lung Dis. 2011;15:1082–1086. doi: 10.5588/ijtld.10.0538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chamla D, Asadu C, Davies A, et al. Patching the Gaps towards the 90-90-90 targets: outcomes of children receiving antiretroviral treatment co-infected with Tuberculosis in Nigeria. Journal of the International AIDS Society. 2015;18(Suppl 6):20251. doi: 10.7448/IAS.18.7.20251. http://www.jiasociety.org/index.php/jias/article/view/20251 | http://dx.doi.org/10.7448/IAS.18.7.20251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wiseman CA, Schaaf HS, Cotton MF, et al. Bacteriologically confirmed tuberculosis in HIV-infected infants: disease spectrum and survival. Int J Tuberc Lung Dis. 2011;15:770–775. doi: 10.5588/ijtld.10.0501. [DOI] [PubMed] [Google Scholar]
- 102.Walters E, Cotton MF, Rabie H, et al. Clinical presentation and outcome of tuberculosis in human immunodeficiency virus infected children on anti-retroviral therapy. BMC Pediatr. 2008;8:1. doi: 10.1186/1471-2431-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dahourou DL, Amorissani-Folquet M, Coulibaly M, et al. Missed opportunities of inclusion in a cohort of HIV-infected children to initiate antiretroviral treatment before the age of two in West Africa, 2011 to 2013. J Int AIDS Soc. 2016;19:20601. doi: 10.7448/IAS.19.1.20601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mfinanga SG, Kirenga BJ, Chanda DM, et al. Early versus delayed initiation of highly active antiretroviral therapy for HIV-positive adults with newly diagnosed pulmonary tuberculosis (TB-HAART): a prospective, international, randomised, placebo-controlled trial. Lancet Infect Dis. 2014;14:563–571. doi: 10.1016/S1473-3099(14)70733-9. [DOI] [PubMed] [Google Scholar]
- 105.Uthman OA, Okwundu C, Gbenga K, et al. Optimal timing of antiretroviral therapy initiation for HIV-infected adults with newly diagnosed pulmonary tuberculosis: a systematic review and meta-analysis. Ann Intern Med. 2015;163:32–39. doi: 10.7326/M14-2979. [DOI] [PubMed] [Google Scholar]
- 106.Yotebieng M, Van RA, Moultrie H, et al. Effect on mortality and virological response of delaying antiretroviral therapy initiation in children receiving tuberculosis treatment. AIDS. 2010;24:1341–1349. doi: 10.1097/QAD.0b013e328339e576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pensi T, Hemal A, Banerjee T. Simultaneous HAART improves survival in children coinfected with HIV and TB. Trop Med Int Health. 2012;17:52–58. doi: 10.1111/j.1365-3156.2011.02884.x. [DOI] [PubMed] [Google Scholar]
- 108.Buck WC, Olson D, Kabue MM, et al. Risk factors for mortality in Malawian children with human immunodeficiency virus and tuberculosis co-infection. Int J Tuberc Lung Dis. 2013;17:1389–1395. doi: 10.5588/ijtld.13.0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Moore BK, Anyalechi E, van der Walt M, et al. Epidemiology of drug-resistant tuberculosis among children and adolescents in South Africa, 2005–2010. Int J Tuberc Lung Dis. 2015;19:663–669. doi: 10.5588/ijtld.14.0879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110**.Isaakidis P, Casas EC, Das M, et al. Treatment outcomes for HIV and MDR-TB co-infected adults and children: systematic review and meta-analysis. Int J Tuberc Lung Dis. 2015;19:969–978. doi: 10.5588/ijtld.15.0123. This systematic review and meta-analysis showed that mortality in patients with multi-drug resistant tuberculosis was higher among HIV-infected individuals, highlighting the need for early diagnosis and more effective treatment regimens. Children had higher rates of successful treatment outcomes and lower mortality. [DOI] [PubMed] [Google Scholar]
- 111**.Stefan DC. Effect of HIV infection on the outcome of cancer therapy in children. Lancet Oncol. 2014;15:e562–567. doi: 10.1016/S1470-2045(14)70313-4. This paper reviews studies on cancer in HIV-infected children over the period of 1990–2013. The paper highlights the higher rates in HIV-infected compared to uninfected children, outlines changes in cancer epidemiology with the increased ART coverage in African and non-African settings, and discusses the reasons for the worse treatment outcomes in children with HIV. [DOI] [PubMed] [Google Scholar]
- 112.Kest H, Brogly S, McSherry G, et al. Malignancy in perinatally human immunodeficiency virus-infected children in the United States. Pediatr Infect Dis J. 2005;24:237–242. doi: 10.1097/01.inf.0000154324.59426.8d. [DOI] [PubMed] [Google Scholar]
- 113.Evans JA, Gibb DM, Holland FJ, et al. Malignancies in UK children with HIV infection acquired from mother to child transmission. Arch Dis Child. 1997;76:330–333. doi: 10.1136/adc.76.4.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Simard EP, Shiels MS, Bhatia K, Engels EA. Long-term cancer risk among people diagnosed with AIDS during childhood. Cancer Epidemiol Biomarkers Prev. 2012;21:148–154. doi: 10.1158/1055-9965.EPI-11-0823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Davidson A, Wainwright RD, Stones DK, et al. Malignancies in South African children with HIV. J Pediatr Hematol Oncol. 2014;36:111–117. doi: 10.1097/MPH.0b013e31829cdd49. [DOI] [PubMed] [Google Scholar]
- 116.Tukei VJ, Kekitiinwa A, Beasley RP. Prevalence and outcome of HIV-associated malignancies among children. AIDS. 2011;25:1789–1793. doi: 10.1097/QAD.0b013e3283498115. [DOI] [PubMed] [Google Scholar]
- 117.Suthar AB, Granich R, Mermin J, Van Rie A. Effect of cotrimoxazole on mortality in HIV-infected adults on antiretroviral therapy: a systematic review and meta-analysis. Bull World Health Organ. 2012;90:128C–138C. doi: 10.2471/BLT.11.093260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chintu C, Bhat GJ, Walker AS, et al. Co-trimoxazole as prophylaxis against opportunistic infections in HIV-infected Zambian children (CHAP): a double-blind randomised placebo-controlled trial. Lancet. 2004;364:1865–1871. doi: 10.1016/S0140-6736(04)17442-4. [DOI] [PubMed] [Google Scholar]
- 119.WHO. Guidelines on post-exposure prophylaxis for HIV and the use of cotrimoxazole prophylaxis for HIV-related infections among adults, adolescents, and children. Recommendations for a public health approach. 2014 http://www.who.int/hiv/pub/guidelines/arv2013/arvs2013upplement_dec2014/en/ [PubMed]
- 120**.Bwakura-Dangarembizi M, Kendall L, Bakeera-Kitaka S, et al. A randomized trial of prolonged co-trimoxazole in HIV-infected children in Africa. N Engl J Med. 2014;370:41–53. doi: 10.1056/NEJMoa1214901. This paper reported on the results of the second randomisation in the ARROW trial of stopping versus continuing co-trimoxazole in children and adolescents in Uganda and Zimbabwe. The study showed higher rates of hospitalization or death in the participants who stopped co-trimoxazole; most hospitalisations were due to malaria and severe bacterial infections. Interestingly, the participants who stopped co-trimoxazole had higher rate of grade 4 adverse events with anaemia being the most common. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hasse B, Walker AS, Fehr J, et al. Co-trimoxazole prophylaxis is associated with reduced risk of incident tuberculosis in participants in the Swiss HIV Cohort Study. Antimicrob Agents Chemother. 2014;58:2363–2368. doi: 10.1128/AAC.01868-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wiktor SZ, Sassan-Morokro M, Grant AD, et al. Efficacy of trimethoprim-sulphamethoxazole prophylaxis to decrease morbidity and mortality in HIV-1-infected patients with tuberculosis in Abidjan, Côte d’Ivoire: a randomised controlled trial. Lancet. 1999;353:1469–1475. doi: 10.1016/s0140-6736(99)03465-0. [DOI] [PubMed] [Google Scholar]
- 123.Nunn A, Mwaba P, Chintu C, et al. Role of co-trimoxazole prophylaxis in reducing mortality in HIV infected adults being treated for tuberculosis: randomised clinical trial. BMJ. 2008;10:a2757. doi: 10.1136/bmj.a257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zar HJ, Cotton MF, Strauss S, et al. Effect of isoniazid prophylaxis on mortality and incidence of tuberculosis in children with HIV: randomised controlled trial. BMJ. 2007;334:136. doi: 10.1136/bmj.39000.486400.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Frigati LJ, Kranzer K, Cotton MF, et al. The impact of isoniazid preventive therapy and antiretroviral therapy on tuberculosis in children infected with HIV in a high tuberculosis incidence setting. Thorax. 2011;66:496–501. doi: 10.1136/thx.2010.156752. [DOI] [PubMed] [Google Scholar]
- 126.Madhi SA, Nachman S, Violari A, et al. Primary isoniazid prophylaxis against tuberculosis in HIV-exposed children. N Engl J Med. 2011;365:21–31. doi: 10.1056/NEJMoa1011214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Van Wyk SS, Hamade H, Hesseling AC, et al. Recording isoniazid preventive therapy delivery to children: operational challenges. Int J Tuberc Lung Dis. 2010;14:650–653. [PubMed] [Google Scholar]
- 128.Van Wyk SS, Reid A, Mandalakas AM, et al. Operational challenges in managing Isoniazid Preventive Therapy in child contacts: a high-burden setting perspective. BMC Public Health. 2011;11:544. doi: 10.1186/1471-2458-11-544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Masini EO, Sitienei J, Weyeinga H. Outcomes of isoniazid prophylaxis among HIV-infected children attending routine HIV care in Kenya. Public Health Action. 2013;3:204–208. doi: 10.5588/pha.13.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130*.Akolo C, Bada F, Okpokoro E, et al. Debunking the myths perpetuating low implementation of isoniazid preventive therapy amongst human immunodeficiency virus-infected persons. World J Virol. 2015;4:105–112. doi: 10.5501/wjv.v4.i2.105. This review article summarises the evidence in support of universal IPT in HIV-infected adults and children and discusses the barriers for the IPT implementation and the ways to overcome them. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131*.Harries AD, Lawn SD, Suthar AB, Granich R. Benefits of combined preventive therapy with co-trimoxazole and isoniazid in adults living with HIV: time to consider a fixed-dose, single tablet coformulation. Lancet Infect Dis. 2015;15:1492–1496. doi: 10.1016/S1473-3099(15)00242-X. This is an opinion paper which highlights the need of a fixed dose combination of co-trimoxazole, isoniazid and vitamin B6, which is likely to help with individual uptake and national scale-up of both preventive therapies. [DOI] [PubMed] [Google Scholar]
- 132.Gibb D, Bwakura-Dangarembizi M, Abhyankar D, et al. Sulfamethoxazole/trimethoprim/isoniazid/pyridoxine scored tablets are bioequivalent to individual products and are acceptable to patients with advanced HIV infection in the REALITY trial. 46th World Conference on Lung Health of the International Union Against tuberculosis and lung disease (The Union); 2–6 December 2015; Cape Town, South Africa. [Google Scholar]
- 133.Bliss SJ, O’Brien KL, Janoff EN, et al. The evidence for using conjugate vaccines to protect HIV-infected children against pneumococcal disease. Lancet Infect Dis. 2008;8:67–80. doi: 10.1016/S1473-3099(07)70242-6. [DOI] [PubMed] [Google Scholar]
- 134.Moore DP, Klugman KP, Madhi SA. Role of Streptococcus pneumoniae in hospitalization for acute community-acquired pneumonia associated with culture-confirmed Mycobacterium tuberculosis in children: a pneumococcal conjugate vaccine probe study. Pediatr Infect Dis J. 2010;29:1099–1004. doi: 10.1097/inf.0b013e3181eaefff. [DOI] [PubMed] [Google Scholar]
- 135.Groome MJ, Madhi SA. Five-year cohort study on the burden of hospitalisation for acute diarrhoeal disease in African HIV-infected and HIV-uninfected children: potential benefits of rotavirus vaccine. Vaccine. 2012;30(Suppl 1):A173–178. doi: 10.1016/j.vaccine.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 136.Steele AD, Cunliffe N, Tumbo J, Madhi SA. A review of rotavirus infection in and vaccination of Human Immunodeficiency Virus-infected children. J Infect Dis. 2009;200:S57–62. doi: 10.1086/605027. [DOI] [PubMed] [Google Scholar]
- 137.Soares-Weiser K, Maclehose H, Bergman H, et al. Vaccines for preventing rotavirus diarrhoea: vaccines in use. Cochrane Database Syst Rev. 2012;11:CD008521. doi: 10.1002/14651858.CD008521.pub3. [DOI] [PubMed] [Google Scholar]
- 138.Clark SJ, Kahn K, Houle B, et al. Young children’s probability of dying before and after their mother’s death: a rural South African population-based surveillance study. PLoS Med. 2013;10:e1001409. doi: 10.1371/journal.pmed.1001409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Musenge E, Vounatsou P, Collinson M, Tollman S, Kahn K. The contribution of spatial analysis to understanding HIV/TB mortality in children: a structural equation modelling approach. Glob Health Action. 2013;6:19266. doi: 10.3402/gha.v6i0.19266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Houle B, Clark SJ, Kahn K, Tollman S, Yamin A. The impacts of maternal mortality and cause of death on children’s risk of dying in rural South Africa: evidence from a population based surveillance study (1992–2013) Reprod Health. 2015;12:S7. doi: 10.1186/1742-4755-1112-S1181-S1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ndirangu J, Newell ML, Thorne C, Bland R. Treating HIV-infected mothers reduces under 5 years of age mortality rates to levels seen in children of HIV-uninfected mothers in rural South Africa. Antivir Ther. 2012;17:81–90. doi: 10.3851/IMP1991. [DOI] [PMC free article] [PubMed] [Google Scholar]