Abstract
Objectives
Tigecycline is a treatment option for infections caused by carbapenem-resistant Klebsiella pneumoniae (CRKP). Emerging tigecycline resistance in CRKP represents a growing threat. Knowledge of the clinical, microbiological, and molecular characteristics of tigecycline- and carbapenem-resistant Klebsiella pneumoniae (TCRKP) is limited.
Methods
Patients infected with TCRKP were identified from a Taiwanese national surveillance study. Clinical data were collected from medical records. We performed susceptibility tests, carbapenemase gene detection, pulsed-field gel electrophoresis (PFGE) and multilocus sequence typing (MLST). Furthermore, we performed quantitative real-time polymerase chain reaction (qRT-PCR) analyses to assess the expression levels of the efflux pump genes acrB and oqxB.
Results
We identified 16 patients infected with TCRKP, with urinary tract infection (UTI) being the most common type of infection (63%). The all-cause 30-day mortality rate was 44% in patients with TCRKP infection. Patients with a site of infection other than the urinary tract had a significantly higher mortality rate than patients with UTIs (83% vs. 20%, p = 0.035). PFGE and MLST revealed no dominant clone or sequence type. Using qRT-PCR, overexpression of both the acrB and oqxB genes was identified in seven isolates, and overexpression of the oqxB gene was observed in another seven. There was poor correlation between acrB or oqxB expression and tigecycline MICs (r = -0.038 and -0.166, respectively).
Conclusions
The mortality rate in patients infected with TCRKP in this study was 44% and this is an important subset of patients. The absence of a linear relationship between efflux pump genes expression and MICs indicates that tigecycline resistance may be mediated by other factors. Continuous monitoring of tigecycline resistance among CRKP isolates and resistance mechanisms are necessary.
Introduction
Klebsiella pneumoniae is an important pathogen that causes various infections including bacteremia, pneumonia, liver abscesses, and urinary tract infections [1]. The prevalence of carbapenem-resistant K. pneumoniae (CRKP) has been increasing globally, making antimicrobial treatment difficult and causing higher disease-related mortality rates [2,3]. Tigecycline is one of the few available choices for treating carbapenem-resistant bacterial infections [4]. Once CRKP develops resistance to tigecycline, the treatment options are much more limited.
To date, studies on the clinical characteristics and outcome of tigecycline resistance superimposed to CRKP are very limited [5–7]. The nonsusceptibility rate of tigecycline against CRKP was 9.1% in a surveillance study from Taiwan [8]. The resistance rate of tigecycline among carbapenemase-producing K. pneumoniae was reported to be 11.2% in China [9] and 14.5% in Korea [10]. In their study, van Duin et al. found that the rate of tigecycline resistance among patients with CRKP was 18% in a multicenter, prospective cohort of hospitalized patients in the USA [7]. The increasing prevalence of tigecycline resistance, after its launch in 2005, is a growing concern [11].
The mechanism of tigecycline resistance is complex and has not yet been fully elucidated [12]. Previous studies have reported that increased expression of efflux pumps such as AcrAB and OqxAB play an essential role in the tigecycline resistance mechanisms of K. pneumoniae [9,13]. The AcrAB efflux pump is a tripartite complex consisting of the large cytoplasmic membrane protein AcrB, the membrane fusion protein AcrA and a channel, TolC; this pump expels a variety of antibiotics including β-lactams, chloramphenicol, erythromycin fluoroquinolones, fusidic acid and tetracycline [14]. The OqxAB efflux pump is chromosomally located in K. pneumoniae [15] and requires a functional AcrAB efflux pump, although its natural function remains unknown [11].
In the present study, we collected clinical isolates of carbapenem non-susceptible K. pneumoniae (CnSKP) between January 2012 and December 2014 in a Taiwanese surveillance study and identified 16 isolates of tigecycline- and carbapenem-resistant K. pneumoniae (TCRKP). We investigated the clinical characteristics and outcome of patients with TCRKP infection, efflux pumps expression among the TCRKP isolates, and elucidated the possible role of AcrAB and OqxAB efflux pumps in tigecycline-resistant phenotypes.
Materials and methods
Hospital settings and bacterial isolates
Consecutive non-replicate clinical isolates of CnSKP were collected from 21 hospitals across Taiwan between January 2012 and December 2014, as part of the Study Group of Carbapenem Resistance in Klebsiella pneumoniae in Taiwan.
This study was approved by the Institutional Review Boards of all participating hospitals, including Taipei Veterans General Hospital (VGHIRB No.: 2011-11-001IC), Tri-Service General Hospital (IRB No.: 100-05-205), National Taiwan University Hospital (IRB No.: 201110043RB), Kaohsiung Medical University Chung-Ho Memorial Hospital (KMUH-IRB-20110328), Chang Gung Memorial Hospital (IRB No.: 1003399B), China Medical University Hospital (CMUH IRB No.: DMR100-IRB-214), Chi- Mei Medical Center (IRB No.: 10012–001) and Kaohsiung Armed Forces General Hospital (IRB No.: 100–076). The IRBs waived the need for informed consents (both written and verbal) from source patients of the enrolled bacterial isolates because this was an observational study and involved very minimal risk to the source patients; this waiver does not adversely affect the rights and welfare of the source patients.
Tigecycline resistance was interpreted according to the clinical breakpoints specified by the European Committee on Antimicrobial Susceptibility Testing (EUCAST) (a minimum inhibitory concentration (MIC) > 2.0 mg/L is resistant) [16]. Carbapenem resistance was defined by the Clinical and Laboratory Standards Institute (CLSI) M100-S25 interpretive breakpoints [17]. Clinical isolates of K. pneumoniae with dual resistance to tigecycline and carbapenem were studied.
The isolates collected from each hospital were sent to the National Health Research Institutes, Miaoli, Taiwan and stored at −70°C in 10% glycerol Luria-Bertani medium before analysis. Species confirmation was performed by standard biochemical methods, on a VITEK® 2 automated system (bioMérieux, Marcy l’Etoile, France). This study was approved by the Review Boards of each hospital.
Clinical data collection
Medical charts were reviewed to extract patient information, including demographic characteristics, underlying medical conditions, Acute Physiology and Chronic Health Evaluation II (APACHE II) scores at the time of TCRKP identification, and clinical outcomes. Types of infection were defined according to the standardized definitions of the Centers for Disease Control and Prevention/National Healthcare Safety Network [5]. Prior tigecycline treatment was considered significant and was included in our analysis only if: (1) the tigecycline had been administered for at least 3 consecutive days; and (2) the exposure had occurred within 30 days prior to the identification of TCRKP. Appropriate antibiotics treatment was defined as treatment with at least one agent for ≥ 48 h after the isolation of a clinical culture specimen to which the isolate was susceptible in vitro [18].
Clinical outcome was classified as success for patients who had resolution of signs and symptoms that defined the infection and as death for patients who died within 30 days of the onset of TCRKP infection.
Case-control study
For each TCRKP infection case, one control patient with tigecycline-susceptible CRKP infection was selected from CnSKP patients matched by age, sex and infection type as closely as possible. Clinical information on the TCRKP patients and matched controls were collected from medical records.
Antimicrobial susceptibility testing
The MICs for tigecycline were determined using the E-test (AB Biodisk, Solna, Sweden) on Mueller-Hinton media. MICs for carbapenems (ertapenem, imipenem, meropenem, and doripenem) and other antimicrobial agents were determined using the broth microdilution method (Sensititre, TREK Diagnostic Systems, Cleveland, OH, USA). Tigecycline and colistin susceptibility were interpreted according to the EUCAST clinical breakpoints [16]. The CLSI M100-S25 interpretive breakpoints were used to interpret the MIC results for all antimicrobial agents studied except tigecycline and colistin [17].
Detection of genes encoding carbapenemase
Carbapenemase genes (encoding Ambler class A families KPC, NMC, IMI, SME, and GES; Ambler class B families IMP, VIM, NDM, GIM, SPM, and SIM; and Ambler class D family OXA-48-type) were detected using polymerase chain reaction (PCR) analysis [19]. The primers used in this study are listed in S1 Table and the PCR analyses were performed under previously described conditions [8].
The amplicons were sequenced and the entire sequences were compared with those in the National Center for Biotechnology Information (NCBI) database at https://blast.ncbi.nlm.nih.gov/Blast.cgi to determine the molecular type.
Pulsed-field gel electrophoresis and multilocus sequence typing
Pulsed-field gel electrophoresis (PFGE) was performed for TCRKP isolates. In brief, bacterial chromosomal DNA was digested using XbaI (New England Biolabs, Beverly, MA, USA) [20]. Electrophoresis was carried out for 22 h at 14°C with pulse times ranging from 2 to 40 s at 6 V/cm with a Bio-Rad CHEF MAPPER® apparatus (Bio-Rad Laboratories, Richmond, CA, USA). A dendrogram based on the unweighted pair group was generated by previously described methods [21]. Isolates with PFGE profiles exhibiting more than 80% similarity were considered closely related strains.
Multilocus sequence typing (MLST) was performed on TR-CRKP isolates according to the protocol described on the K. pneumoniae MLST website (https://www.pasteur.fr/fr/recherche). The MLST results were typed using the international K. pneumoniae MLST database at the Pasteur Institute, Paris, France [22].
Quantitative real-time PCR
The mRNA expression levels of the efflux pump genes (acrB and oqxB) were examined using quantitative real-time PCR (qRT-PCR). The experiments were performed using a housekeeping gene (23S) as an internal control, and the fold change of each gene was calculated by dividing the mRNA expression level of the abovementioned genes by that of the 23S gene. cDNA (100 ng) was amplified by PCR with 40 cycles of denaturing (95°C, 15 s), annealing (55°C, 30 s), and extension (72°C, 45 s) using Fast SYBR® Green Master Mix (Applied Biosystems®). Quantitative analysis of the PCR products was carried out by a sequence detector (Model 7500 Fast, Applied Biosystems®) according to the manufacturer’s instructions. The experiments were performed in triplicate. The threshold cycle (Ct) value was defined as the cycle number at which the fluorescence generated within a reaction crossed the threshold value, and the relative Ct value of each target gene was compared to that of the tigecycline-susceptible strain (CG43) [23] as a control (expression = 1) to estimate the fold changes in relative mRNA expression among the samples.
Statistical analysis
Data analyses were conducted using the statistical package SPSS 13.0 (SPSS Inc., Chicago, Illinois). Univariate analysis was performed by a chi-square test or Fisher’s exact test for categorical variables and Student’s t test for continuous variables. A value of p < 0.05 was considered statistically significant. The Pearson product-moment correlation coefficient (r) was calculated to measure the linear relationship between two random variables. The plus or minus sign of the correlation coefficient denotes the direction of the relationship, ranging from a strong negative correlation (-1) to a strong positive correlation (+1).
Results
Hospital settings and bacterial isolates
Consecutive non-replicate clinical isolates (n = 1093) of CnSKP were collected from 21 hospitals across Taiwan from January 2012 through December 2014. Sixteen isolates of TCRKP were identified from eight participating hospitals, of which ten isolates were from northern Taiwan (Hospital A, D, E and H), three were from central Taiwan (Hospital C and F) and three isolates were from southern Taiwan (Hospital B and G).
Clinical characteristics of patients with TCRKP infections
The clinical features and outcomes of all patients are summarized in Table 1. The male-to-female ratio was 9:7. The mean age was 64.6 (range, 3–103). Ten patients had type 2 diabetes mellitus, which was the most frequent underlying disease among these TCRKP-infected patients. In general, the patients were critically ill, with a median APACHE II score of 22.3 (range, 7–37). Types of infection included urinary tract infections (9 [56%]), pneumonia (2 [13%]), biliary tract infections (2 [13%]), bacteremia (1 [6%]), and peritonitis (1 [6%]). The source of bacteremia was presumed to be catheter-related in one patient. Seven patients were in the ICU at the time of TCRKP isolation. Four patients had received tigecycline treatment prior to TCRKP isolation. Of these four patients, three received tigecycline treatment for antecedent urinary tract infections and one for an antecedent biliary tract infection.
Table 1. Clinical features of patients with tigecycline- and carbapenem-resistant Klebsiella pneumoniae (TCRKP) infection and molecular types of the TCRKP isolates.
| Patient | Isolate | Hospital | Sex | Age | Underlying Diseases | APACHE Ⅱ Score | Type of Infection | Prior TGC Treatment | Treatment of TCRKP Infection | CarbapenemaseGene | MLST Result | Clinical Outcome | AppropriateAntibioticsTreatment |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P1 | TR1 | A | F | 74 | CVA, Dementia, DM, HTN | 33 | UTI | Yes, Combination therapy for UTI | AN, CL, TGC | ND | 15 | Success | Yes |
| P2 | TR2 | A | M | 88 | COPD, Dementia, DM, HTN | 14 | UTI | No | CL, DOR, GM, LVX | ND | 15 | Success | Yes |
| P3 | TR3 | B | F | 63 | CVA, DM, HTN | 18 | UTI | No | FOF | ND | 15 | Success | Yes |
| P4 | TR4 | C | M | 70 | Hepatoma | 28 | BTI | No | ERT, GM, PTZ | ND | 307 | Death | No |
| P5 | TR5 | D | F | 51 | Lung cancer | 8 | UTI | No | CAZ | ND | 231 | Success | No |
| P6 | TR6 | E | M | 61 | CHF, Esophageal cancer, DM, Uremia | 24 | Pneumonia | No | CL, MEM | ND | 1192 | Death | Yes |
| P7 | TR7 | F | M | 85 | DM, Prostate cancer | 37 | UTI | No | TGC | blaIMP-8 | 1087 | Success | No |
| P8 | TR8 | G | M | 72 | CVA, Cholangiocarcinoma | 15 | BTI | Yes, Combination therapy for BTI | CL, ERT, FEP | ND | 307 | Success | Yes |
| P9 | TR9 | A | F | 34 | Liver cirrhosis | 14 | UTI | Yes, Combination therapy for UTI | CL | ND | 1619 | Success | No |
| P10 | TR10 | E | M | 81 | CVA, COPD, DM, Prostate cancer | 24 | UTI | Yes, Monotherapy for UTI | TGC | blaKPC-2 | 11 | Death | No |
| P11 | TR11 | A | F | 32 | Dilated cardiomyopathy, DM | 26 | UTI | No | FEP, TGC | ND | 15 | Death | No |
| P12 | TR12 | G | M | 3 | Acute lymphoblastic leukemia | 15 | Bacteremia | No | AN, MEM, SXT | ND | 711 | Death | Yes |
| P13 | TR13 | D | M | 83 | DM, Uremia | 33 | UTI | No | AN, ETP, PTZ | ND | 11 | Success | Yes |
| P14 | TR14 | H | F | 45 | Bipolar disorder | 22 | UTI | No | CL, FEP | ND | 1644 | Success | Yes |
| P15 | TR15 | D | M | 88 | CAD, DM | 37 | Pneumonia | No | FEP | ND | 1253 | Death | Yes |
| P16 | TR16 | C | F | 103 | DM | 16 | Peritonitis | No | CL, TGC | blaIMP-8 | 1565 | Death | Yes |
AN, amikacin; APACHE II, Acute Physiology and Chronic Health Evaluation II; BTI, biliary tract infection; CAD, coronary artery disease; CAZ, ceftazidime; CHF, congestive heart failure; CL, colistin; COPD, chronic obstructive pulmonary disease; CVA, cerebrovascular accident; DM, diabetes mellitus; DOR, doripenem; ERT, ertapenem; F, female; FEP, cefepime; FOF, fosfomycin; GM, gentamicin; HTN, hypertension; LVX, levofloxacin; M, male; MEM, meropenem; MLST, multilocus sequence typing; ND, not detected; PTZ, piperacillin/tazobactam; SXT, trimethoprim-sulfamethoxazole; TCRKP, tigecycline- and carbapenem-resistant Klebsiella pneumoniae; TGC, tigecycline; UTI, urinary tract infection.
Of the 16 patients with TCRKP infection, seven received various colistin-based combination regimens. The 30-day mortality of patients with TCRKP infection was 44% (7/16). Among the patients with urinary tract infections, the mortality rate was relatively low (2/10, [20%]) compared with that of patients with pneumonia, biliary tract infection, bacteremia, or peritonitis. The mortality rate of patients with non-urinary tract infections was 83% (5/6).
To determine the variables associated with 30-day mortality among patients with TCRKP infection, a comparison of the clinical features between survivors and non-survivors 30 days after TCRKP infection is shown in Table 2. Age, sex, underlying diseases, APACHE II score, prior tigecycline treatment, appropriate antibiotics treatment, a MIC for meropenem > 4 mg/L, and a MIC for tigecycline > 4 mg/L were not associated with 30-day mortality. The infection type was reclassified into low-risk infection site (urinary tract infection) and high-risk infection site (all others) [24]. An infection site other than the urinary tract was significantly associated with 30-day mortality when compared with urinary tract infections (p = 0.035).
Table 2. Risk factors for 30-day mortality among 16 patients infected with tigecycline- and carbapenem-resistant Klebsiella pneumoniae.
| Variables | 30-Day Survivor (n = 9) | 30-Day Non-survivor (n = 7) | p value |
|---|---|---|---|
| Age (years; mean ± SD) | 66.1 ± 19.1 | 62.6 ± 34.6 | 0.452 |
| Sex | 0.286 | ||
| Female | 5 | 2 | |
| Male | 4 | 5 | |
| Underlying disease | |||
| DM | 5 | 5 | 0.633 |
| CVA | 3 | 1 | 0.585 |
| Solid tumor | 4 | 2 | 0.633 |
| Hematological malignancy | 0 | 1 | 0.438 |
| Uremia | 2 | 0 | 0.475 |
| Liver cirrhosis | 1 | 0 | 1.000 |
| Type of infection | 0.035 | ||
| UTI | 8 | 2 | |
| High-risk infection sitea (Non-UTI) | 1 | 5 | |
| APACHE II (mean ± SD) | 21.56 ± 10.33 | 24.29 ± 7.46 | 0.289 |
| Prior tigecycline treatment | 3 | 1 | 0.585 |
| Appropriate antibiotics treatment | 0.549 | ||
| Yes | 6 | 4 | |
| No | 3 | 3 | |
| MICs | |||
| Meropenem MIC > 4 mg/L | 1 | 3 | 0.262 |
| Tigecycline MIC > 4 mg/L | 7 | 3 | 0.302 |
APACHE II, Acute Physiology and Chronic Health Evaluation II, BTI, biliary tract infection; CVA, cerebrovascular accident; DM, diabetes mellitus; MIC, minimal inhibitory concentration; SD, standard deviation; UTI, urinary tract infection.
a High-risk infection types: any infection except urinary tract infection.
We attempted to compare urinary and non-urinary infection sources among the 16 patients with TCRKP infection (Table 3). The result also showed a difference in 30-day mortality (20% vs. 83%, p = 0.035). The other variables between the two groups were not significantly different.
Table 3. Comparison between urinary and non-urinary source among 16 patients with tigecycline- and carbapenem-resistant Klebsiella pneumoniae infection.
| Variables | Urinary source (n = 10) | Non-urinary source (n = 6) | p value |
|---|---|---|---|
| Age (years), mean ± SD | 63.6 ± 21.7 | 66.2 ± 34.3 | 0.856 |
| Male gender, no. (%) | 4 (40) | 5 (83) | 0.145 |
| APACHE II score, mean ± SD | 22.9 ± 9.5 | 22.5 ± 8.9 | 0.935 |
| Prior tigecycline treatment, no. (%) | 3 (30) | 1 (17) | 1.000 |
| Appropriate antibiotics treatment, no. (%) | 5 (50) | 5 (83) | 0.307 |
| 30-day mortality, no. (%) | 2 (20) | 5 (83) | 0.035 |
APACHE II, Acute Physiology and Chronic Health Evaluation II; SD, standard deviation.
Case-control study
A comparison of variables between patients in case and control group is shown in Table 4. The age, sex, infection type and APACHES II score between the two groups were not significantly different. The mortality for tigecycline-susceptible carbapenem-resistant K. pneumoniae infection was 31%. Compared with the case group, there was no significant difference in 30-day mortality between the two groups (p = 0.716). The 30-day mortality attributable to tigecycline resistance was 13%.
Table 4. Case-control analysis for 30-day mortality between patients with tigecycline-resistant and tigecycline-susceptible carbapenem-resistant Klebsiella pneumoniae infection.
| Variables | Tigecycline-resistant(n = 16) | Tigecycline-susceptible(n = 16) | p value |
|---|---|---|---|
| Age (years), mean ± SD | 64.6 ± 26.0 | 66.3 ± 20.1 | 0.833 |
| Male gender, no. (%) | 9 (56) | 9 (56) | 1.000 |
| Type of infection, no. (%) | 1.000 | ||
| UTI | 10 (63) | 10 (63) | |
| Non-UTI | 6 (38) | 6 (38) | |
| APACHE II score, mean ± SD | 22.8 ± 9.0 | 22.1 ± 11.2 | 0.863 |
| Appropriate antibiotics treatment, no. (%) | 10 (63) | 9 (56) | 1.000 |
| 30-day mortality, no. (%) | 7 (44) | 5 (31) | 0.716 |
APACHE II, Acute Physiology and Chronic Health Evaluation II; SD, standard deviation; UTI, urinary tract infection.
Antimicrobial susceptibility and carbapenemase-encoding genes
The MIC values of various antimicrobial agents against the TCRKP isolates are listed in S2 Table. The MICs of tigecycline against these isolates ranged from 4–32 mg/L. All isolates (16/16) were resistant to tigecycline, imipenem and ceftazidime. The susceptibility rates to ertapenem and meropenem were 13% (2/16). The susceptibility rates to doripenem and aztreonam were 25% (4/16). The susceptibility rate to cefepime was 19% (3/16). The susceptibility rates to gentamicin and amikacin were 38% (6/16) and 100% (16/16), respectively. The susceptibility rates to ciprofloxacin and levofloxacin were 13% (2/16). The susceptibility rate to trimethoprim-sulfamethoxazole was 13% (2/16). The susceptibility rate to colistin was 100% (16/16).
Carbapenemase genes were detected in 19% (3/16) of isolates collected, with blaKPC-2 found in one isolate and blaIMP-8 in two isolates.
PFGE analysis and MLST
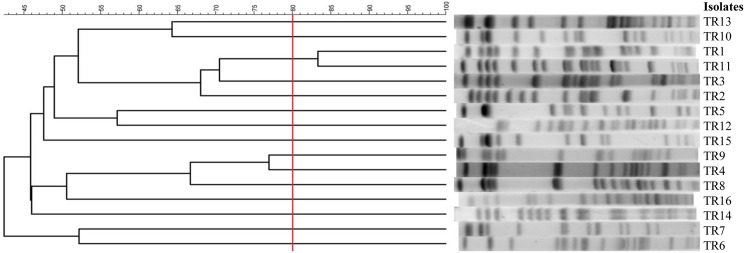
The PFGE patterns of the 16 isolates were clustered into 15 pulsotypes at the similarity level of >80%, with more than three bands different from each other, as shown in Fig 1. A wide diversity of PFGE patterns was found, with the exception of two isolates (TR1 and TR11) with the same PFGE band pattern.
Fig 1. Pulsed-field gel electrophoresis profiles and dendrogram of tigecycline- and carbapenem-resistant Klebsiella pneumoniae isolates.
Fifteen pulsotypes are shown, using 80% similarity as the cut-off, demonstrating substantial diversity.
Of the 16 isolates, 11 different sequence types were identified, 25% were sequence type ST15 (4/16), 13% were ST11 (2/16), and 13% were ST307 (2/16). Other ST types (ST231, 711, 1087, 1192, 1253, 1565, 1619, and 1644) related to only one isolate each (Table 1). ST1619 and ST1644 were newly-typed MLST types in this study, and the isolate record was sent to the K. pneumoniae MLST database.
Efflux pump expression in TCRKP isolates
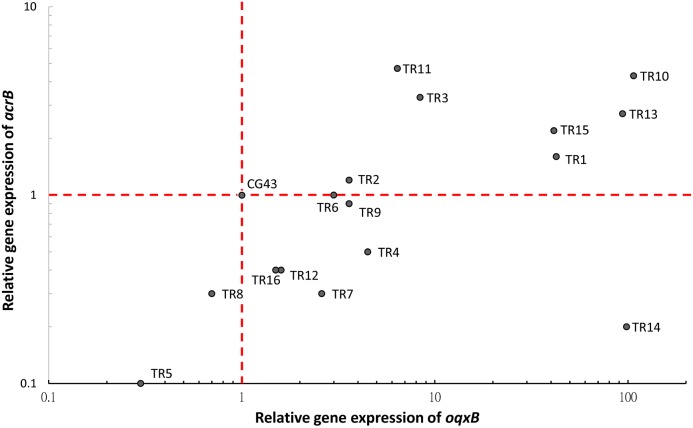
The expression of tigecycline resistance-related efflux pump genes such as acrB, and oqxB were altered in these isolates compared with the reference strain, CG43 (Fig 2). The expression of both acrB and oqxB increased in seven isolates (TR1, 2, 3, 10, 11, 13 and 15), while in another seven isolates (TR4, 6, 7, 9, 12, 14 and 16) only the expression of oqxB increased. These data indicated that tigecycline resistance in 14 isolates may have been caused by increased expression of acrB and/or oqxB. However, overexpression of acrB and/or oqxB could not be detected in two isolates (TR5 and TR8). Taken together, these data indicated that tigecycline resistance mechanisms were not limited to the overexpression of acrB and oqxB.
Fig 2. Relative gene expression of acrB and oqxB in tigecycline- and carbapenem-resistant Klebsiella pneumoniae isolates.
Overexpression of both acrB (1.6- to 4.7-fold) and oqxB (3.6- to 107.1-fold) was observed in 7 isolates. Overexpression of oqxB (1.5- to 98.5-fold) but not of acrB was observed in 7 isolates. No upregulation of acrB and oqxB in was observed in 2 isolates (TR5 and TR8). Relative gene expression compared with CG43 (expression = 1).
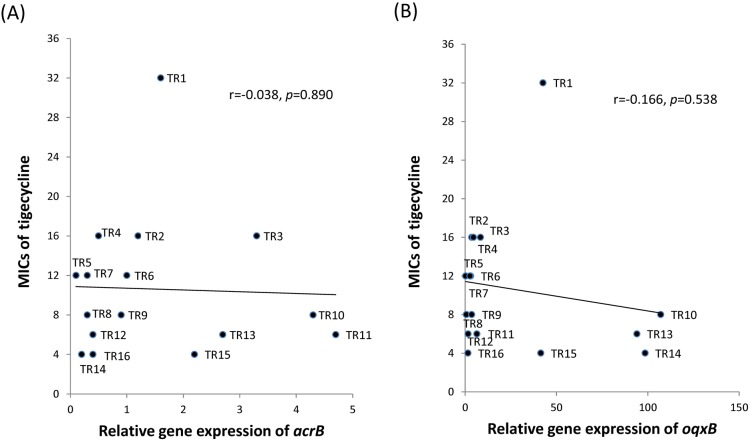
To evaluate the relationship between the MIC of tigecycline and the relative gene expression levels of acrB and oqxB, the Pearson product-moment correlation coefficient (r) was calculated. However, there was no positive correlation between tigecycline MIC and the gene expression of acrB (r = -0.038, p = 0.89) or oqxB (r = -0.166, p = 0.538) in theses TCRKP isolates (Fig 3).
Fig 3. Correlation of gene expression with the minimum inhibitory concentration (MIC) of tigecycline in tigecycline- and carbapenem-resistant Klebsiella pneumoniae isolates.
A scatter plot displays the poor correlation between tigecycline MIC values and the gene expression of acrB (A) and oqxB (B).
Discussion
To the best of our knowledge, the current case series is the first to analyze TCRKP infection with a focus on clinical characteristics and outcome in Taiwan. This study highlight the 30-day mortality rate among patients with TCRKP infection was 44%. Patients with an infection site other than the urinary tract were significantly associated with 30-day mortality when compared with urinary tract infection. The findings from this study are representative of the clinical and molecular characteristics of TCRKP in Taiwan. The emergence of tigecycline resistance in CRKP in Taiwan may be underestimated, as hospitals do not routinely perform tigecycline susceptibility testing against K. pneumoniae.
Since four patients in this study had previously been exposed to tigecycline before the isolation of TCRKP, this suggests that tigecycline therapy may be a risk factor for developing tigecycline resistance. Long-term tigecycline monotherapy may carry a higher risk for developing tigecycline resistance [11]. A nested case-control study had found that receipt of tigecycline was the independent predictor for subsequent isolation of a tigecycline-resistant K. pneumoniae isolates [25]. In their study, van Duin et al. described that the use of tigecycline in patients with CRKP bacteriuria was significantly associated with the subsequent development of tigecycline resistance [6]. In contrast, since 75% of the patients in this study were not exposed to tigecycline before TCRKP identification, this suggests that tigecycline resistance may occur without exposure to tigecycline. A previous report suggested that an elevated MIC of tigecycline might be attributed indirectly to the use of other antibiotics that are also effluxed through the AcrAB–TolC pump [26].
This study showed that the overall 30-day mortality rate of patients infected with TCRKP was 44%. Patients with TCRKP bacteremia, pneumonia and peritonitis had very high mortality rates. However, patients with urinary tract infections had a lower mortality rate. Multidrug-resistant K. pneumoniae infections have been reported to have high attributable mortality rates of up to 50% [27]. The source of infection, severity of underlying conditions, severity of sepsis and appropriateness of the definitive antibiotic therapy were identified as predictors of mortality in a previous study of infections caused by carbapenemase-producing Enterobacteriaceae [24]. Our study also demonstrated that the infection site is important for mortality prediction. However, other predictors could not be identified in our study, which may be due to the small sample size. A large-scale clinical study evaluating the risk factors of mortality and attributable mortality of TCRKP infections is needed. A recent study disclosed that a positive culture of CnSKP was associated with high in-hospital mortality, regardless of colonization or infection [28]. Patients with TCRKP colonization would also likely exhibit high mortality rates. It is difficult to distinguish between colonization and infection when TCRKP isolates are obtained from non-sterile sites in clinical practice. Patients with a positive TCRKP culture, regardless of colonization or infection, may be an emerging challenge for physicians.
According to the susceptibility results of our study, colistin and amikacin have good in vitro activity against TCRKP, with susceptibility rates of 100%. Other classes of effective antibiotics should be considered according to individual susceptibility results. Combination treatment with two or more drugs with in vitro activity against K. pneumoniae carbapenemase (KPC) -producing K. pneumoniae isolates has been suggested to improve survival and may be more effective than monotherapy [27]. Combination treatment for patients infected with TCRKP in a high-risk site seems reasonable considering the high mortality rate under this circumstance. A therapeutic strategy leading to a better outcome for patients with TCRKP infection requires further study.
Of the 16 isolates in our study, only two isolates (TR1 and TR11) exhibited the same PFGE band pattern. Though they originated from the same hospital (hospital A), these two infected patients did not have overlapping periods of hospitalization. Intra-hospital spread of TCRKP in hospital A is improbable. Furthermore, as the PFGE patterns in the strains from the other 14 patients were all different, there is little evidence of inter-hospital spread. In summary, we hypothesize that tigecycline resistance among CRKP predominately arises from sporadic cases, and no horizontal spread occurred in this study period. A study conducted in Shanghai, China identified 24 distinct pulsotypes among 26 tigecycline-nonsusceptible K. pneumoniae clinical isolates [5], which was similar to the results of our study. To prevent the spread of TCRKP, infection control strategies such as standard and contact precautions should be enhanced. Prudent use of tigecycline and other antibiotics that might cause efflux-mediated resistance is also important to prevent the development of tigecycline resistance in K. pneumoniae.
Our MLST results revealed that the genetic background of TCRKP is heterogeneous in Taiwan. Among the 16 isolates, the strain with the highest tigecycline MIC belonged to ST15, and the predominant ST11 type was not found. KPC-producing K. pneumoniae ST11 has been reported as the predominant clone of carbapenem-resistant K. pneumoniae in China [29], Singapore [30], and Taiwan [8]. Our findings suggest that the development of tigecycline resistance in CRKP is unrelated to the local, epidemic ST11 that produces KPC.
The expression levels of tigecycline resistance-related efflux pump genes were determined, and we found that tigecycline resistance could be associated with overexpression of the acrB and/or oqxB genes in most isolates. A correlation analysis between the expression of efflux pump genes (acrB and oqxB) and tigecycline MIC indicated a poor correlation. Our findings have suggested that an alternative pathway other than AcrAB or OqxAB regulating tigecycline resistance in these strains. A literature review showed that reported mechanisms in tigecycline resistance of K. pneumoniae include overexpression of ramA and acrAB [9, 31, 32], deletions, insertions and point mutations in ramR [33, 34], overexpression of rarA and oqxB [9, 32, 35], a point mutation in the repressor oqxR and overexpression of the OqxAB efflux pump [13], IS5 element integration in a putative efflux pump, KpgABC [23], overexpression of marA and acrAB, and structural alteration of the ribosomal protein S10 [34]. The detailed role of each mechanism in conferring tigecycline resistance requires further investigation.
There are some limitations of our study. Our data were obtained from multiple centers in Taiwan; hence, the findings may reflect the status of Taiwanese patients, but cannot be generalized to patients in other countries. The patients with TCRKP infections had higher 30-day mortality than those with tigecycline-susceptible CRKP infections. However, there was no statistical significance. Our cases and controls numbers were relatively small to achieve enough statistical power. We need continue to collect data and sample from TCRKP and CnSKP patients for use in future studies. Thus, our investigation of the mechanisms of tigecycline resistance is limited to acrB and oqxB, which account for few elements of tigecycline resistance, but not all mechanisms of resistance. Transcriptional regulators e.g., RamA, MarA, SoxS and RarA, directly regulate genes linked to efflux pump-mediated resistance to tigecycline, and modulate genes linked to virulence [35–38]. Further study is mandatory to assess these regulators to determine their possible regulatory roles in the microbial response to antimicrobial challenges.
TCRKP that exhibits multidrug resistance reduces therapeutic options and may lead to untreatable infections. Therefore, efforts are urgently needed to improve the knowledge of the epidemiological status of tigecycline resistance accompanied by carbapenem resistance in order to tackle its spread.
Supporting information
(DOC)
(DOC)
Acknowledgments
The authors would like to thank all collaborating laboratories for their participation in the Study Group of Carbapenem Resistance in Klebsiella pneumoniae in Taiwan. Partial results of this study were presented as a poster at the 7th International Congress of the Asia Pacific Society of Infection Control (APSIC. 2015) in Taipei, Taiwan.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The work was supported by research grants from the Centers for Disease Control, Taiwan (MOHW104-CDC-C-114-144406; http://www.cdc.gov.tw/english/index.aspx; JCL) and Tri-Service General Hospital (TSGH-C103-124; http://www.tsgh.ndmctsgh.edu.tw/; SKC). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Siu LK, Yeh KM, Lin JC, Fung CP, Chang FY. Klebsiella pneumoniae liver abscess: a new invasive syndrome. The Lancet Infectious diseases. 2012;12(11):881–7. doi: 10.1016/S1473-3099(12)70205-0 [DOI] [PubMed] [Google Scholar]
- 2.Nordmann P, Cuzon G, Naas T. The real threat of Klebsiella pneumoniae carbapenemase-producing bacteria. The Lancet Infectious diseases. 2009;9(4):228–36. Epub 2009/03/28. doi: 10.1016/S1473-3099(09)70054-4 [DOI] [PubMed] [Google Scholar]
- 3.Neuner EA, Yeh JY, Hall GS, Sekeres J, Endimiani A, Bonomo RA, et al. Treatment and outcomes in carbapenem-resistant Klebsiella pneumoniae bloodstream infections. Diagn Microbiol Infect Dis. 2011;69(4):357–62. Epub 2011/03/15. PubMed Central PMCID: PMC3058153. doi: 10.1016/j.diagmicrobio.2010.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee CR, Lee JH, Park KS, Kim YB, Jeong BC, Lee SH. Global Dissemination of Carbapenemase-Producing Klebsiella pneumoniae: Epidemiology, Genetic Context, Treatment Options, and Detection Methods. Frontiers in microbiology. 2016;7:895 PubMed Central PMCID: PMC4904035. doi: 10.3389/fmicb.2016.00895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sheng ZK, Wang W, Guo Q, Xu X, Wang M, Yang Y, et al. Emergence of tigecycline- and carbapenem-nonsusceptible Klebsiella pneumoniae ST11 clone in patients without exposure to tigecycline. Journal of microbiology, immunology, and infection. 2016;49(6):962–8. doi: 10.1016/j.jmii.2015.10.014 [DOI] [PubMed] [Google Scholar]
- 6.van Duin D, Cober ED, Richter SS, Perez F, Cline M, Kaye KS, et al. Tigecycline therapy for carbapenem-resistant Klebsiella pneumoniae (CRKP) bacteriuria leads to tigecycline resistance. Clinical microbiology and infection: the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2014;20(12):O1117–20. PubMed Central PMCID: PMC4265572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Duin D, Cober E, Richter SS, Perez F, Kalayjian RC, Salata RA, et al. Residence in Skilled Nursing Facilities Is Associated with Tigecycline Nonsusceptibility in Carbapenem-Resistant Klebsiella pneumoniae. Infect Control Hosp Epidemiol. 2015;36(8):942–8. PubMed Central PMCID: PMCPMC4642723. doi: 10.1017/ice.2015.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chiu SK, Wu TL, Chuang YC, Lin JC, Fung CP, Lu PL, et al. National surveillance study on carbapenem non-susceptible Klebsiella pneumoniae in Taiwan: the emergence and rapid dissemination of KPC-2 carbapenemase. PloS one. 2013;8(7):e69428 PubMed Central PMCID: PMC3722148. doi: 10.1371/journal.pone.0069428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sheng ZK, Hu F, Wang W, Guo Q, Chen Z, Xu X, et al. Mechanisms of tigecycline resistance among Klebsiella pneumoniae clinical isolates. Antimicrobial agents and chemotherapy. 2014;58(11):6982–5. PubMed Central PMCID: PMCPMC4249433. doi: 10.1128/AAC.03808-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jeong SH, Kim HS, Kim JS, Shin DH, Kim HS, Park MJ, et al. Prevalence and Molecular Characteristics of Carbapenemase-Producing Enterobacteriaceae From Five Hospitals in Korea. Annals of laboratory medicine. 2016;36(6):529–35. PubMed Central PMCID: PMC5011105. doi: 10.3343/alm.2016.36.6.529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun Y, Cai Y, Liu X, Bai N, Liang B, Wang R. The emergence of clinical resistance to tigecycline. International journal of antimicrobial agents. 2013;41(2):110–6. doi: 10.1016/j.ijantimicag.2012.09.005 [DOI] [PubMed] [Google Scholar]
- 12.Pournaras S, Koumaki V, Spanakis N, Gennimata V, Tsakris A. Current perspectives on tigecycline resistance in Enterobacteriaceae: susceptibility testing issues and mechanisms of resistance. International journal of antimicrobial agents. 2016;48(1):11–8. doi: 10.1016/j.ijantimicag.2016.04.017 [DOI] [PubMed] [Google Scholar]
- 13.Bialek-Davenet S, Lavigne JP, Guyot K, Mayer N, Tournebize R, Brisse S, et al. Differential contribution of AcrAB and OqxAB efflux pumps to multidrug resistance and virulence in Klebsiella pneumoniae. The Journal of antimicrobial chemotherapy. 2015;70(1):81–8. doi: 10.1093/jac/dku340 [DOI] [PubMed] [Google Scholar]
- 14.Elkins CA, Nikaido H. Substrate specificity of the RND-type multidrug efflux pumps AcrB and AcrD of Escherichia coli is determined predominantly by two large periplasmic loops. Journal of bacteriology. 2002;184(23):6490–8. PubMed Central PMCID: PMC135441. doi: 10.1128/JB.184.23.6490-6498.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim HB, Wang M, Park CH, Kim EC, Jacoby GA, Hooper DC. oqxAB encoding a multidrug efflux pump in human clinical isolates of Enterobacteriaceae. Antimicrobial agents and chemotherapy. 2009;53(8):3582–4. PubMed Central PMCID: PMC2715617. doi: 10.1128/AAC.01574-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Breakpoint tables for interpretation of MICs and zone diameters [Internet]. European Committee on Antimicrobial Susceptibility Testing. 2016. Available from: http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_6.0_Breakpoint_table.pdf. Accessed 2016 Dec 12.
- 17.Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing; 25th informational supplement; 100-S252015.
- 18.Zarkotou O, Pournaras S, Tselioti P, Dragoumanos V, Pitiriga V, Ranellou K, et al. Predictors of mortality in patients with bloodstream infections caused by KPC-producing Klebsiella pneumoniae and impact of appropriate antimicrobial treatment. Clinical microbiology and infection: the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2011;17(12):1798–803. [DOI] [PubMed] [Google Scholar]
- 19.Queenan AM, Bush K. Carbapenemases: the versatile beta-lactamases. Clinical microbiology reviews. 2007;20(3):440–58, table of contents. PubMed Central PMCID: PMC1932750. doi: 10.1128/CMR.00001-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.D'Agata EM, Gerrits MM, Tang YW, Samore M, Kusters JG. Comparison of pulsed-field gel electrophoresis and amplified fragment-length polymorphism for epidemiological investigations of common nosocomial pathogens. Infect Control Hosp Epidemiol. 2001;22(9):550–4. Epub 2001/12/06. doi: 10.1086/501950 [DOI] [PubMed] [Google Scholar]
- 21.Tenover FC, Arbeit RD, Goering RV, Mickelsen PA, Murray BE, Persing DH, et al. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. Journal of clinical microbiology. 1995;33(9):2233–9. Epub 1995/09/01. PubMed Central PMCID: PMC228385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Diancourt L, Passet V, Verhoef J, Grimont PA, Brisse S. Multilocus sequence typing of Klebsiella pneumoniae nosocomial isolates. Journal of clinical microbiology. 2005;43(8):4178–82. Epub 2005/08/06. PubMed Central PMCID: PMC1233940. doi: 10.1128/JCM.43.8.4178-4182.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nielsen LE, Snesrud EC, Onmus-Leone F, Kwak YI, Aviles R, Steele ED, et al. IS5 element integration, a novel mechanism for rapid in vivo emergence of tigecycline nonsusceptibility in Klebsiella pneumoniae. Antimicrobial agents and chemotherapy. 2014;58(10):6151–6. PubMed Central PMCID: PMC4187979. doi: 10.1128/AAC.03053-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Palacios-Baena ZR, Oteo J, Conejo C, Larrosa MN, Bou G, Fernandez-Martinez M, et al. Comprehensive clinical and epidemiological assessment of colonisation and infection due to carbapenemase-producing Enterobacteriaceae in Spain. The Journal of infection. 2016;72(2):152–60. doi: 10.1016/j.jinf.2015.10.008 [DOI] [PubMed] [Google Scholar]
- 25.Nigo M, Cevallos CS, Woods K, Flores VM, Francis G, Perlman DC, et al. Nested case-control study of the emergence of tigecycline resistance in multidrug-resistant Klebsiella pneumoniae. Antimicrobial agents and chemotherapy. 2013;57(11):5743–6. PubMed Central PMCID: PMCPMC3811295. doi: 10.1128/AAC.00827-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roy S, Datta S, Viswanathan R, Singh AK, Basu S. Tigecycline susceptibility in Klebsiella pneumoniae and Escherichia coli causing neonatal septicaemia (2007–10) and role of an efflux pump in tigecycline non-susceptibility. The Journal of antimicrobial chemotherapy. 2013;68(5):1036–42. doi: 10.1093/jac/dks535 [DOI] [PubMed] [Google Scholar]
- 27.Tumbarello M, Viale P, Viscoli C, Trecarichi EM, Tumietto F, Marchese A, et al. Predictors of mortality in bloodstream infections caused by Klebsiella pneumoniae carbapenemase-producing K. pneumoniae: importance of combination therapy. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2012;55(7):943–50. [DOI] [PubMed] [Google Scholar]
- 28.Wu PF, Chuang C, Su CF, Lin YT, Chan YJ, Wang FD, et al. High minimum inhibitory concentration of imipenem as a predictor of fatal outcome in patients with carbapenem non-susceptible Klebsiella pneumoniae. Sci Rep. 2016;6:32665 PubMed Central PMCID: PMC 5009345. doi: 10.1038/srep32665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qi Y, Wei Z, Ji S, Du X, Shen P, Yu Y. ST11, the dominant clone of KPC-producing Klebsiella pneumoniae in China. The Journal of antimicrobial chemotherapy. 2011;66(2):307–12. doi: 10.1093/jac/dkq431 [DOI] [PubMed] [Google Scholar]
- 30.Balm MN, Ngan G, Jureen R, Lin RT, Teo J. Molecular characterization of newly emerged blaKPC-2-producing Klebsiella pneumoniae in Singapore. Journal of clinical microbiology. 2012;50(2):475–6. PubMed Central PMCID: PMC3264177. doi: 10.1128/JCM.05914-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosenblum R, Khan E, Gonzalez G, Hasan R, Schneiders T. Genetic regulation of the ramA locus and its expression in clinical isolates of Klebsiella pneumoniae. International journal of antimicrobial agents. 2011;38(1):39–45. PubMed Central PMCID: PMC3117140. doi: 10.1016/j.ijantimicag.2011.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Juan CH, Huang YW, Lin YT, Yang TC, Wang F-D. Risk Factors, Outcomes, and Mechanisms of Tigecycline-Nonsusceptible Klebsiella pneumoniae Bacteremia. Antimicrobial agents and chemotherapy. 2016;60:7357–63. doi: 10.1128/AAC.01503-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hentschke M, Wolters M, Sobottka I, Rohde H, Aepfelbacher M. ramR mutations in clinical isolates of Klebsiella pneumoniae with reduced susceptibility to tigecycline. Antimicrobial agents and chemotherapy. 2010;54(6):2720–3. PubMed Central PMCID: PMC2876394. doi: 10.1128/AAC.00085-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Villa L, Feudi C, Fortini D, Garcia-Fernandez A, Carattoli A. Genomics of KPC-producing Klebsiella pneumoniae sequence type 512 clone highlights the role of RamR and ribosomal S10 protein mutations in conferring tigecycline resistance. Antimicrobial agents and chemotherapy. 2014;58(3):1707–12. PubMed Central PMCID: PMC3957836. doi: 10.1128/AAC.01803-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Veleba M, Higgins PG, Gonzalez G, Seifert H, Schneiders T. Characterization of RarA, a novel AraC family multidrug resistance regulator in Klebsiella pneumoniae. Antimicrobial agents and chemotherapy. 2012;56(8):4450–8. PubMed Central PMCID: PMC3421627. doi: 10.1128/AAC.00456-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.De Majumdar S, Yu J, Fookes M, McAteer SP, Llobet E, Finn S, et al. Elucidation of the RamA regulon in Klebsiella pneumoniae reveals a role in LPS regulation. PLoS Pathog. 2015;11(1):e1004627 PubMed Central PMCID: PMCPMC4310594. doi: 10.1371/journal.ppat.1004627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.De Majumdar S, Veleba M, Finn S, Fanning S, Schneiders T. Elucidating the regulon of multidrug resistance regulator RarA in Klebsiella pneumoniae. Antimicrobial agents and chemotherapy. 2013;57(4):1603–9. PubMed Central PMCID: PMCPMC3623357. doi: 10.1128/AAC.01998-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bratu S, Landman D, George A, Salvani J, Quale J. Correlation of the expression of acrB and the regulatory genes marA, soxS and ramA with antimicrobial resistance in clinical isolates of Klebsiella pneumoniae endemic to New York City. The Journal of antimicrobial chemotherapy. 2009;64(2):278–83. PubMed Central PMCID: PMC2707265. doi: 10.1093/jac/dkp186 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.