Abstract
Background
Asians have higher frequency of intracranial arterial stenosis. The present study aimed to compare the clinical features and outcomes of ischemic stroke patients with and without middle cerebral artery (MCA) stenosis, assessed by transcranial sonography (TCS), based on the Taiwan Stroke Registry (TSR).
Methods
Patients with acute ischemic stroke or transient ischemic attack registered in the TSR, and received both carotid duplex and TCS assessment were categorized into those with stenosis (≥50%) and without (<50%) in the extracranial internal carotid artery (ICA) and MCA, respectively. Logistic regression analysis, Kaplan-Meier method and Cox proportional hazard model were applied to assess relevant variables between groups.
Results
Of 6003 patients, 23.3% had MCA stenosis, 10.1% ICA stenosis, and 3.9% both MCA and ICA stenosis. Patients with MCA stenosis had greater initial NIHSS, higher likelihood of stroke-in-evolution, and more severe disability than those without (all p<0.001). Patients with MCA stenosis had higher prevalence of hypertension, diabetes and hypercholesterolemia. Patients with combined MCA and extracranial ICA stenosis had even higher NIHSS, worse functional outcome, higher risk of stroke recurrence or death (hazard ratio, 2.204; 95% confidence intervals, 1.440–3.374; p<0.001) at 3 months after stroke than those without MCA stenosis.
Conclusions
In conclusion, MCA stenosis was more prevalent than extracranial ICA stenosis in ischemic stroke patients in Taiwan. Patients with MCA stenosis, especially combined extracranial ICA stenosis, had more severe neurological deficit and worse outcome.
Introduction
Intracranial arterial stenosis (ICAS) is increasingly recognized as an important factor in defining stroke subtypes and in selecting preventive or therapeutic measures [1–6]. Patients with symptomatic ICAS may have a higher risk of recurrent stroke [1–7]. The prevalence of ICAS among stroke patients varies across ethnic groups [3,4,8–15]. Asians, Africans, and Hispanics have a greater preponderance for ICAS than Caucasians [11–17]. ICAS is noted in 5% to 10% of Caucasians patients with ischemic strokes [10,15–19], but in 28% to 54% in Asian counterparts [11–14]. In Chinese patients with ischemic stroke, the middle cerebral artery (MCA) was the most commonly identified location of ICAS [19].
The Taiwan Stroke Registry (TSR) is a prospective, multicenter registry of patients with stroke or transient ischemic attack (TIA) [20]. The aims of the TSR are to investigate the risk factors, stroke types, and outcome in a nation-wide stroke registry, and to assess the quality of stroke care. The present study aimed to compare the risk factors, clinical features and outcome between ischemic stroke patients with and without MCA stenosis, assessed by transcranial sonography (TCS), based on the Taiwan Stroke Registry (TSR) database. The impact of superimposed extracranial internal carotid artery (ICA) stenosis was also assessed.
Materials and methods
Patients
Patients with acute ischemic stroke or TIA who were admitted to 39 TSR hospitals constitute the TSR cohort [20]. The diagnosis of ischemic stroke and TIA was acute neurologic dysfunction of vascular origin lasting for more and less than 24 hours, respectively. All patients received examination including computed tomography (CT) or/and magnetic resonance imaging (MRI) for the index event. They were included in the present study if they met the following criteria: (1) acute ischemic stroke or TIA; (2) receiving both duplex and TCS assessment; and (3) having been followed for at least 3 months. This study was approved by the Institutional Review Board of Taipei Medical University. The TSR protocols and the consent procedure were approved by the Institutional Review Board of each participating hospital, and the registered stroke patients gave their written informed consent for follow-up. All clinical investigation conducted according to the principles expressed in the Declaration of Helsinki.
TSR provides a structured record of demographics, including risk factors, stroke types, stroke subtypes (TOAST classification), National Institute of Health Stroke Scale (NIHSS) on admission and discharge, neuroimaging and ultrasonographic examination of relevant arteries, in-hospital management and complications, and functional outcomes in follow-up [20].
Ultrasonographic study
Extracranial ICA and MCA stenosis were diagnosed by carotid duplex and TCS, respectively, and were categorized into <50% and ≥50% stenosis according to the following criteria. Extracranial ICA stenosis ≥50% was recorded if the peak systolic velocity of the ICA ≥125 cm/s, or the peak systolic velocity ratio of the ICA to the ipsilateral common carotid artery ≥2, or no Doppler flow signal detected in the ICA indicating total occlusion [21,22]. MCA stenosis ≥50% was recorded if the peak systolic velocity (PSV) ≥140 cm/s, mean flow velocity (MFV) ≥100 cm/s, only trickle flow signals, or no detectable Doppler flow signal in the MCA in patients with a good temporal window [19,23–27]. Patients with poor temporal bone windows were excluded. These criteria were determined by a panel of neurosonographic experts based on published criteria [23,24]. We have validated the PSV criteria in diagnosis of MCA stenosis ≥50% [25,26]. The accuracy of MFV criteria in diagnosis of MCA stenosis was reported [27], and further validated in recent studies in Asians [28]. All sonographic studies were reimbursed by National Health Insurance (NHI), the universal payer in Taiwan, under strict guidelines. Ultrasonographic assessment was conducted by trained neurosonographers who have gone through strict certification processes to be qualified for sonographic claims for NHI reimbursement.
Patient follow-up
All registered stroke patients were followed in clinics at 1 and 3 months poststroke. For those who could not attend the clinic, telephone interviews were made to assess functional status, and to check for stroke recurrence and survival status. In the present study, functional outcome 3 months after stroke was assessed using modified Rankin Scale (mRS) and Barthel Index. A mRS of 2 or greater at 3 months was considered an unfavorable outcome.
Statistical analysis
Categorical variables are presented as percentage and continuous or discrete variables mean ± standard deviation or median (25th-75th percentile). Student’s t-test, χ2 test, ANOVA and Kruskal-Wallis test were used for univariate analysis between groups with relevant variables as indicated. A logistic regression analysis was adapted to evaluate the odds ratio and 95% confidence intervals (CI) of predictors of MCA stenosis. The Kaplan-Meier method was used to estimate the probability of early stroke recurrence and mortality 3 months after stroke. The Cox proportional hazards model was used for the hazard ratio (HR) and 95% CI of predictors of early stroke recurrence and mortality. Statistical analysis was performed at an α level of 0.05 (two-tailed). SAS (SAS Institute, Cary, NC, release 9.1) was used for statistical analyses.
Results
Of 29,195 TSR stroke patients [20], 6,757 received both duplex and TCS assessment, with 754 (11.2%) patients who had poor temporal bone window excluded from this study. Thus, 6,003 patients (male, 75.6%; average age, 65.3±12.9 years) fulfilled the selection criteria for the present study. Of the 6,003 patients, 5,430 (91.5%) were ischemic stroke and 573 (8.5%) were TIA. Of these, 1,397 (23.3%) had MCA stenosis ≥50%, 608 (10.1%) ICA stenosis ≥50%, and 236 (3.9%) both MCA and ICA stenosis ≥50%. The frequency of MCA stenosis was 2.3-fold higher than that of ICA stenosis. Of 608 patients with ICA stenosis, 183 (30.1%) had ipsilateral MCA stenosis, and 53 (8.7%) had MCA stenosis on the contralateral side.
Patients with MCA stenosis had significantly higher frequencies of hypertension (81.2% vs 76.6%), diabetes mellitus (DM) (46.1% vs. 35.6%), ischemic heart disease (13.7% vs 11.7%), hypercholesterolemia (44.6% vs. 38.4%) and hypertriglyceridemia (33.1% vs 26/7%) than those without MCA stenosis. Large-artery atherosclerosis (55.9%) was the most common stroke subtype and small-vessel occlusion (22.7%) was the second in those with MCA stenosis. However, among those without MCA stenosis, the most common stroke subtype was small-vessel occlusion (50.1%) and the second was large-artery atherosclerosis (23.2%). The distribution of cardioembolism, specific etiology, and undetermined etiology were similar between patients with and without MCA stenosis. The treatment of antiplatelets and carotid stenting were almost the same among patents with and without MCA stenosis. There was around 80% of patients with antiplatelets and only 0.2% with carotid stenting (Table 1). The multivariate analysis showed only DM (OR, 1.55; 95% CI, 1.33–1.81), hypercholesterolemia (OR, 1.22; 95% CI, 1.05–1.43) and family history of stroke (OR, 1.22; 95% CI, 1.04–1.44) were significantly related to MCA stenosis (Table 2).
Table 1. Comparison between patients with or without MCA stenosis.
| All | MCA <50% | MCA ≥50% | P value | |
|---|---|---|---|---|
| (n = 6003) | (n = 4606) | (n = 1397) | ||
| Male sex | 4537 (75.6%) | 3470 (75.3%) | 1067 (76.4%) | 0.427 |
| Mean age, y | 65.3±12.9 | 65.2±12.9 | 65.7±12.7 | 0.255 |
| Body mass index, kg/m2 | 24.6±3.7 | 24.6±3.7 | 24.6±3.7 | 0.755 |
| Risk factors | ||||
| Hypertension | 4663 (77.7%) | 3529 (76.6%) | 1134 (81.2%) | <0.0001 |
| Diabetes mellitus | 2286 (38.1%) | 1642 (35.6%) | 644 (46.1%) | <0.0001 |
| Ischemic heart disease | 732 (12.2%) | 540 (11.7%) | 192 (13.7%) | 0.043 |
| Atrial fibrillation | 656 (10.9%) | 510 (11.1%) | 146 (10.5%) | 0.514 |
| Hypercholesterolemia | 2394 (39.9%) | 1771 (38.4%) | 623 (44.6%) | <0.0001 |
| Hypertriglyceridemia | 1693 (28.2%) | 1230 (26.7%) | 463 (33.1%) | <0.0001 |
| Cigarette smoking | 3070 (51.1%) | 2367 (51.4%) | 703 (50.3%) | 0.484 |
| Alcohol drinking | 1098 (18.3%) | 852 (18.5%) | 246 (17.6%) | 0.452 |
| Family history of stroke | 1444 (24.1%) | 1082 (23.5%) | 362 (25.9%) | 0.064 |
| Treatment | ||||
| Antiplatelets | 4827 (80.4%) | 3711 (80.6%) | 1116 (79.9%) | 0.573 |
| Carotid stenting | 10 (0.2%) | 7 (0.2%) | 3 (0.2%) | 0.614 |
| Stroke types | 0.002 | |||
| Transient ischemic attack | 573 (8.5%) | 469 (10.2%) | 104 (7.4%) | |
| Ischemic stroke | 5430 (91.5%) | 4137 (89.8%) | 1293 (92.6%) | |
| Stroke subtype (TOAST) | < .0001 | |||
| LAA | 1681 (31.0%) | 958 (23.2%) | 723 (55.9%) | |
| SVO | 2366 (43.6%) | 2072 (50.1%) | 294 (22.7%) | |
| CE | 485 (8.9%) | 393 (9.5%) | 92 (7.1%) | |
| SE | 80 (1.5%) | 61 (1.5%) | 19 (1.5%) | |
| UE | 818 (15.1%) | 653 (15.8%) | 165 (12.8%) | |
| Clinical course | ||||
| Initial NIHSS | 4 (2–7) | 4 (2–7) | 5 (3–9) | <0.001 |
| Stroke in evolution | 413 (6.9%) | 289 (6.3%) | 124 (8.9%) | 0.001 |
| mRS at 3 m | 2 (1–3) | 1 (1–3) | 2 (1–4) | <0.001 |
| mRS ≥2 at 3 m | 3096 (51.6%) | 2247 (48.8%) | 849 (60.8%) | <0.001 |
Values are numbers with percentage in parenthesis for each category except for age and body mass index that are presented as mean ± standard deviation. Stroke subtypes were based on TOAST (Trial of Org 10172 in Acute Stroke Treatment) classification; LAA, large-artery atherosclerosis; SVO, small-vessel occlusion; CE, Cardioembolism; SE, specific etiology; UE, undetermined etiology.
Table 2. Multivariate analysis of factors related to MCA stenosis.
| Odds ratio | 95% Confidence interval | p value | |
|---|---|---|---|
| Male sex | 1.08 | 0.88–1.32 | 0.459 |
| Mean age, y | 1.00 | 0.99–1.01 | 0.851 |
| Body mass index, kg/m2 | 1.00 | 0.98–1.02 | 0.815 |
| Hypertension | 1.10 | 0.91–1.34 | 0.318 |
| Diabetes mellitus | 1.55 | 1.33–1.81 | 0.001 |
| Ischemic heart disease | 1.16 | 0.94–1.44 | 0.174 |
| Atrial fibrillation | 1.04 | 0.81–1.32 | 0.772 |
| Hypercholesterolemia | 1.22 | 1.05–1.43 | 0.011 |
| Hypertriglyceridemia | 0.93 | 0.78–1.11 | 0.434 |
| Cigarette smoking | 0.97 | 0.82–1.16 | 0.758 |
| Alcohol drinking | 0.93 | 0.76–1.14 | 0.965 |
| Family history of stroke | 1.22 | 1.04–1.44 | 0.013 |
Patients with MCA stenosis had higher NIHSS on presentation, more likely to have stroke in evolution, worse functional status (Barthel Index and mRS) and higher mortality or stroke recurrence at 3 months after stroke than those without MCA stenosis. Unfavorable outcome with a mRS ≥2 at 3 months was higher in patients with MCA stenosis than those without (60.8% vs. 48.8%, p<0.0001) (Table 1). Patients with combined MCA and ICA stenosis had even higher NIHSS on presentation, worse functional outcome, and higher mortality or stroke recurrence at 3 months than those with MCA stenosis only (Table 3).
Table 3. Comparison among patients with or without stenosis of the MCA and ICA.
| MCA <50% | MCA ≥50% | P value | |||
|---|---|---|---|---|---|
| ICA<50% | ICA≥50% | ICA<50% | ICA≥50% | ||
| (n = 4234) | (n = 372) | (n = 1161) | (n = 236) | ||
| Male sex | 3151 (74.4%) | 319 (85.8%) | 870 (74.9%) | 197 (83.5%) | <0.001 |
| Mean age, y | 64.7±12.9 | 70.6±11.9 | 64.9±12.9 | 69.7±11.2 | <0.001 |
| Hypertension | 3225 (76.2%) | 304 (81.7%) | 947 (81.6%) | 187 (79.2%) | <0.001 |
| Diabetes mellitus | 1512 (35.7%) | 130 (34.9%) | 547 (47.1%) | 97 (41.1%) | <0.001 |
| Ischemic heart disease | 465 (11.0%) | 75 (20.2%) | 138 (11.9%) | 54 (22.9%) | <0.001 |
| Atrial fibrillation | 465 (11.5%) | 45 (12.1%) | 116 (10.0%) | 30 (12.7%) | 0.389 |
| Hypercholesterolemia | 1630 (38.5%) | 141 (37.9%) | 521 (44.9%) | 102 (43.2%) | <0.001 |
| Hypertriglyceridemia | 1001 (23.6%) | 68 (18.3%) | 309 (26.6%) | 48 (20.3%) | 0.005 |
| Cigarette smoking | 2125 (50.2%) | 242 (65.1%) | 564 (48.6%) | 139 (58.9%) | <0.001 |
| Alcohol drinking | 781 (18.4%) | 71 (19.1%) | 209 (18.0%) | 37 (15.7%) | 0.713 |
| Antiplatelets | 3419 (80.8%) | 292 (78.5%) | 930 (80.1%) | 186 (78.8%) | 0.653 |
| Carotid steting | 0 (0.0%) | 7 (1.9%) | 0 (0.0%) | 3 (1.3%) | < .001 |
| Initial NIHSS score | 4 (2–7) | 5 (3–10) | 5 (2–9) | 6 (3–12) | <0.001 |
| Stroke in evolution | 246 (5.8%) | 43 (11.6%) | 99 (8.5%) | 25 (10.6%) | <0.001 |
| mRS at 3 m | 1 (0–3) | 2 (1–4) | 2 (1–3) | 3 (1–4) | <0.001 |
| Barthel index at 3 m | 100 (80–100) | 95 (55–100) | 100 (70–100) | 90 (30–100) | <0.001 |
| Death or stroke recurrence at 3 m | 173 (4.1%) | 27 (7.3%) | 68 (5.9%) | 24 (10.2%) | <0.001 |
NIHSS, National Institute of Health Stroke Scale; mRS, modified Rankin Scale. Values are median (interquartile range) for NIHSS, Barthel index and mRS; and number (percentage) for other categories.
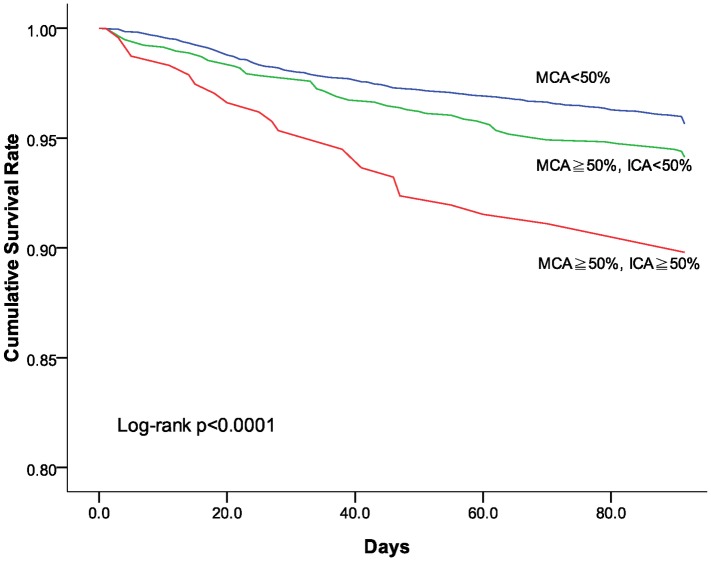
The Kaplan-Meier analysis based on stroke recurrence or mortality at 3 months shows patients without MCA stenosis fared better with lower rate of stroke recurrence or death than those with MCA stenosis (log rank p<0.001). Patients with both MCA and ICA had even worse outcome than those with MCA stenosis based on stroke recurrence rate and mortality at 3 months poststroke (Fig 1). The Cox multivariate regression model also demonstrated that ischemic stroke patients with both MCA and ICA stenosis had higher stroke recurrence or death than those without MCA stenosis (HR, 2.204; 95% CI, 1.440–3.374; p<0.001) (Table 4).
Fig 1. Cumulative survival (free of recurrent stroke or death) among patients with or without MCA stenosis.
Table 4. Cox-model for stroke recurrence or death at 3 months after stroke.
| All patients | Ischemic stroke patients | |||
|---|---|---|---|---|
| (n = 6003) | (n = 5430) | |||
| HR (95% CI) | p value | HR (95% CI) | p value | |
| Male sex | 1.316 (0.984–1.759) | 0.064 | 1.380 (1.019–1.867) | 0.037 |
| Mean age, y | 1.028 (1.018–1.038) | <0.001 | 1.029 (1.019–1.040) | <0.001 |
| Diabetes mellitus | 1.258 (1.004–1.577) | 0.046 | 1.271 (1.008–1.602) | 0.043 |
| MCA <50% | 1.000 | — | 1.000 | — |
| MCA ≥50% | ||||
| ICA <50% | 1.344 (1.019–1.771) | 0.036 | 1.287 (0.966–1.713) | 0.085 |
| ICA ≥50% | 2.204 (1.440–3.374) | <0.001 | 2.154 (1.406–3.298) | <0.001 |
HR indicates hazard ratio; CI, confidence interval.
Discussion
This study was based on the TSR [20], a nation-wide, large-scale stroke registry with rigorous control of entry data to compare selective clinical features and outcomes between ischemic stroke patients with and without MCA stenosis. Results show that MCA stenosis was more frequently found than extracranial ICA stenosis in ischemic stroke patients. The MCA stenosis to ICA stenosis (23.3% vs. 10.1%) ratio of 2.3 is in agreement with studies in other Asian populations [12,13,15,19].
Coexisting ICA and MCA stenosis were found in 3.9% of patients in the present study. Among patients with extracramial ICA stenosis, 38.6% had MCA stenosis. In the North American Symptomatic Carotid Endarterectomy Trial, one-third of patients with symptomatic extracranial ICA stenosis also had ICAS [29]. In a Hong Kong study, 10% of patients with ischemic stroke had both intracranial and extracranial lesions [19]. ICAS was noted in 48% of patients with extracranial carotid stenosis in Korea [30]. The findings suggest that in patients with extracranial carotid stenosis, ICAS is a common comorbidity and should be searched for. This situation is especially important for Asian patients, who have higher prevalence rate of ICAS [11–16], and are considered for carotid endarterectomy or stenting. ICAS is known to unfavorably affect the outcome following surgical or stenting interventions on carotid artery stenosis [29,30].
The present study also shows that patients with MCA stenosis presented with higher NIHSS, which reflects more severe neurological deficit at stroke onset, were more likely to manifest stroke-in-evolution and to have unfavorable outcome and higher mortality at 3 months after stroke as compared to patients without MCA stenosis. Within the MCA stenosis group, those with superimposed ICA stenosis had even higher NIHSS on admission, worse outcome, and higher mortality than those without ICA stenosis. In a survival analysis, the endpoint of combined stroke recurrence or death was significantly increased in patients with both MCA and increased further among those with both MCA and ICA stenosis [31].
The Warfarin-Aspirin Symptomatic Intracranial Disease (WASID) study showed that the annual recurrence rates of stroke or TIA with symptomatic intracranial atherosclerosis were nearly 20%, and most subsequent strokes were in the same territory and nonlacunar [32]. Higher recurrence rates of ischemic events have also been reported in patients with symptomatic MCA disease compared to those who were asymptomatic [33]. In the subgroup of our patients with MCA≥50%/ICA<50%, symptomatic MCA stands for 45.9%. In the subgroup of our patients with MCA ≥50%/ICA ≥50%, symptomatic MCA stands for 58.7%. The percentage of symptomatic MCA in the index stroke was around 46%-59% in our study. Since symptomatic MCA stenosis predicts worse outcome, more rapid and rigorous preventive measures would seem warranted for this patient population. A recent study suggests that more rigorous interventions may be warranted in patients with symptomatic ICAS [5,6]. MCA stenosis may be asymptomatic to the index stroke. Asymptomatic ICAS is often relatively benign. Among the WASID patients, 27.3% had asymptomatic ICAS, and the 1-year risk of stroke in the asymptomatic ICAS territory was 3.5% [34].
Results from the present study show that there was a difference in risk factor profile between patients with and without MCA stenosis. Patients with MCA stenosis had significantly higher rates of DM and hypercholesterolemia. DM has a significant impact on the extent of ICAS [35,36]. In one study of asymptomatic high-risk patients in Hong Kong, DM was linked to increased risk of MCA stenosis [19]. The present study also demonstrates that hypercholesterolemia was more frequently noted in patients with MCA stenosis than those without. In the WASID study, hypercholesterolemia is a determinant of ICAS with an odds ratio of 1.62 [37].
The strengths of the present study include the prospective, multicenter stroke registry based on a well-designed registry protocol with rigorous quality control [20]. The large sample size provides useful profiles to broaden our view into subpopulations of patients with ischemic stroke. Results derived from the present study support the contention that MCA stenosis is a marker for more severe neurological deficit at stroke onset, worse functional outcome, higher stroke recurrence rate or mortality. Although TCS has been widely applied to stroke patients its benefit have yet to be fully established [38]. In patients with TIA, abnormal TCS findings were noted to be useful in predicting subsequent cardiovascular events [17,39]. The multispecialty panel of experts convened by the Clinical Practice Committee of the American Society of Neuroimaging has defined TCS for a number of clinical indications [40]. Validation of TCS with magnetic resonance angiography or digital subtraction angiography in ischemic stroke patients have been made [25–28]. The present study provides a preliminary piece of evidence supporting that TCS is helpful for identifying a subgroup of stroke patients with worse clinical course and poorer outcome, who may benefit from more rigorous preventive and therapeutic measures as has been shown in recent clinical trials [5,6].
There are a number of limitations in the present study. The study subjects were recruited from a multi-center stroke registry, the TSR [20]. Only patients with both extracranial and intracranial vascular sonographic studies were included in this analysis. It is possible that some patients with known etiologies, such as cardioembolism, or with very severe deficit early in clinical course might have been precluded from receiving TCS. Second, the TCS study requires a good acoustic window to identify the MCA. However, patients with older age, particularly in women, may impede the insonation of the intracranial vessels, including the MCA. Lower number of female patients in the present study may reflect a possible gender bias. Third, the vascular studies were often done immediately after the stroke. It is possible that the vascular process during the acute stage might have evolved later. Therefore, some of the patients considered to have MCA stenosis may have recanalized embolus. But the distribution of atrial fibrillation was insignificantly different between groups in Tables 1 and 3. Fourth, ICAS may involve arteries other than the MCA. TCS, focusing on the MCA, does not allow a more comprehensive assessment of different vascular territories. Finally, angiographic study was not widely used in this stroke population to allow confirmation of the validity of TCS results in the present study. Despite these limitations, results presented here are still of value for confirming the prognostic importance of MCA stenosis for a large population of ischemic stroke patients in Taiwan and other Asian countries, and suggest a role for TCS to identify this potentially important marker in patients with ischemic stroke for more assertive measures in stroke prevention.
In conclusion, the frequency of MCA stenosis was 2.3-fold higher than that of extracranial ICA stenosis in ischemic stroke patients in Taiwan. Patients with MCA stenosis were more likely to have DM and hypercholesterolemia and to present with severe neurological deficit with worse outcome. TCS is of practical value for identifying a subgroup of patients who are in need of more rigorous preventive and therapeutic measures. Regarding substantially high prevalence of MCA stenosis in Asian countries, applying TCS to acute ischemic stroke patients in clinical practice may be considered.
Acknowledgments
We would like to thank TSR investigators for their help of data collection from each hospital.
List of TSR investigators
National Taiwan University Hospital: Jiann-Shing Jeng (Principal Investigator), Sung-Chun Tang, Shin-Joe Yeh
Shin Kong WHS Memorial Hospital: Hou-Chang Chiu (Principal Investigator), Li-Ming Lien, Wei-Hung Chen, Chyi-Huey Bai, Tzu-Hsuan Huang, Chi-Ieong Lau, Ya-Ying Wu, Hsu-Ling Yeh, Anna Chang
Taipei Medical University Hospital: Rey-Yue Yuan (Principal Investigator), Jau-Jiuan Sheu, Jia-Ming Yu, Chun-Sum Ho
Taipei Medical University -Wan Fang Hospital: Wen-Ting Chung (Principal Investigator), Jia-Ying Sung, Chin-I Chen, Hsing-Yu Weng, Yu-Hsuan Han, Chun-Ping Huang
Chi Mei Medical Center: Huey-Juan Lin (Principal Investigator), Der-Shin Ke, Chia-Yu Chang, Poh-Shiow Yeh, Kao-Chang Lin, Tain-Junn Cheng, Chih-Ho Chou, Chun-Ming Yang, Hsiu-Chu Shen
Kaohsiung Veterans General Hospital: Yuk-Keung Lo (Principal Investigator), Cheng-Chang Yen, Ching-Hwung Lin
Tri-Service General Hospital: Jiann-Chyun Lin (Principal Investigator), Yaw-Don Hsu, Jong-Chyou Denq, Giia-Sheun Peng, Jiunn-Tay Lee, Chang-Hung Hsu, Chun-Chieh Lin, Che-Hung Yen, Chun-An Cheng, Yueh-Feng Sung, Yuan-Liang Chen, Ming-Tung Lien, Chung-Hsing Chou, Chia-Chen Liu, Fu-Chi Yang, Yi-ChungWu, An-Chen Tso, Yu- Hua Lai, Chun-I Chiang, Chia-Kuang Tsai, Meng-Ta Liu, Ying-Che Lin, Yu-Chuan Hsu
China Medical University Hospital: Chung-Hsiang Liu (Principal Investigator), Chon-Haw Tsai, Wei-Shih Huang, Chung-Ta Lu, Tzung-Chang Tsai, Chun-Hung Tseng, Kang-Hsu Lin, Woei-Cherng Shyn, Yu-Wan Yang, Yen-Liang Liu, Der-Yang Cho, Chun-Chung Chen
Kuang Tien General Hospital: Ming-Hui Sun (Principal Investigator), Li-Ying Ke
National Cheng Kung University Hospital: Ming-Liang Lai (Principal Investigator), Chih-Hung Chen, Pi-Shan Sung
Changhua Christian Hospital Yunlin Branch: Jiunn-Rong Chen (Principal Investigator), Song-Yen Tsai
Cathay General Hospital: Tsuey-Ru Chiang (Principal Investigator), Mei-Ching Lee, Pai-Hao Huang, Sian-King Lie, Pin-Wen Liao, Jen-Tse Chen
Buddhist Tzu Chi General Hospital Taipei Branch: Shinn-Kuang Lin (Principal Investigator), Yuan-Fu Tseng, Jing-Er Lee, Cheng-Lun Hsiao, Jen-Feng Liang
Chung Shan Medical University Hospital: Chiu-Mei Chen (Principal Investigator),Chao-HsinWu, Shih-Jei Tsai, Tsong-Ming Lu, Sheng-Ling Kung, Mei-Ju Lee, An-Chih Cheng, Hsi-Hsien Chou
Landseed Hospital: Yu-Wei Chen (Principal Investigator), Kuo-Ying Lee, Yun-Yu Lin, Chen-Hua Li, Eric E. Smith, Hui-Fen Tsai, Chuan-Fa Hsieh, Chih-Dong Yang, Shiumn-Jen Liaw, How-Chin Liao
Taipei Veterans General Hospital: Han-Hwa Hu (Principal Investigator), Wen-JangWong, Yun-On Luk, Chang-Ming Chern, Li-Chi Hsu, Chih-Ping Chung
Changhua Christian Hospital: Mu-Chien Sun (Principal Investigator), Tien-Pao Lai, Wei-Liang Chen, Yen-Chun Chen, Ta-Cheng Chen, Wen-Fu Wang, Kwo-Whei Lee, Chen-Shu Chang, Chien-Hsu Lai, Siao-Ya Shih, Chieh-Sen Chuang, Yen-Yu Chen, Kai-Ming Jhang, Chun-Hsiang Lin, Chien-Min Chen
Lotung Poh Ai Hospital: Hung-Pin Tseng (Principal Investigator), Vai-Hong Fong, Chin-Hsiung Liu, Chun-Liang Lin, Hung-Chih Lin
Cheng Hsin General Hospital: Ta-Chang Lai (Principal Investigator), Jiu-Haw Yin, Chung-JenWang, Kai-ChenWang, Li-Mei Chen, Jong-Chyou Denq
Far Eastern Memorial Hospital: Lung Chan (Principal Investigator), Siu-Pak Lee
En Chu Kong Hospital: Yu Sun (Principal Investigator), Chieh-Cheng Huang, Chang-Hsiu Liu, Cheng-Huai Lin, Chien-Jung Lu
Cheng Ching General Hospital: Shoou-Jeng Yeh (Principal Investigator), Ling-Li Wu, Liang-Po Hsieh, Yong-Hui Lee, Chung-Wen Chen
Taichung Veterans General Hospital: Po-Lin Chen (Principal Investigator), Yu-Shan Lee, Shu-Yi Chen
E-Da Hospital / I-Shou University: Shih-Pin Hsu (Principal Investigator), Han-Jung Chen, Cheng-Sen Chang, Kan Lu, Hung-Chang Kuo, Huan-Wen Tsui, Jung-Chi Tsou, Wei-Pang Chen, Yan-Tang Wang, Po-Chou Liliang, Yu-Duan Tsai, Cheng-Loong Liang, Kuo-Wei Wang, Hao-Kuang Wang, Jui-Sheng Chen, Te-Yuan Chen, Po-Yuan Chen, Kuo-Hsuan Lin, Wei-Jie Tzeng, Cien-Le Chye, Pei-Hua Wu
Buddhist Tzu Chi General Hospital: Yue-Loong Hsin (Principal Investigator), Ray-Yen Yu
National Taiwan University Hospital Hsin-Chu Branch: Bak-Sau Yip (Principal Investigator), Pei-Chun Tsai,Ping-Chen Chou, Tsam-Ming Kuo, Yi-Chen Lee, Yi-Pin Chiu, Kun-Chang Tsai
Kaohsiung Medical University Chung-Ho Memorial Hospital: Ruey-Tay Lin (Principal Investigator), Chun-Hung Chen, Gim-Thean Khor, A-Ching Chao, Hsiu-Fen Lin
Min Sheng General Hospital: Chun-Yuan Chang (Principal Investigator)
Taipei City Hospital Ren Ai Branch: Sui-Hing Yan (Principal Investigator), Yi-Chun Lin, Pei-Yun Chen, Sheng-Huang Hsiao
National Taiwan University Hospital Yunlin Branch: Li-Kai Tsai (Principal Investigator)
Lin Shin Hospital: Ping-Kun Chen (Principal Investigator), Pai-Yi Chiu
Cardinal Tien Hospital: Ping-Keung Yip (Principal Investigator), Vinchi Wang, Kaw-ChenWang, Chung-Fen Tsai, Chao-Ching Chen, Chih-Hao Chen, Yi-Chien Liu
Show Chwan Memorial Hospital: Chung-Hsin Yeh (Principal Investigator),
Chou-Hsiung Pan, Shin-Yi Jih, Po-Chi Chan, Min-Hsien Hsu, Hai-Ming Shoung, Yi-Chen Lo, Fu-HwaWang
Chang Bing Show Chwan Memorial Hospital: Cheng-Yu Wei (Principal Investigator), Yu-Ching Huang, Chao-Nan Yang
Wei Gong Memorial Hospital: Ryh-Huei Lin (Principal Investigator), Ching-Hua Chu
Taipei Medical University—Shuang Ho Hospital: Chaur-Jong Hu (Principal Investigator), Nai-Fang Chi
Buddhist Dalin Tzu Chi General Hospital: Ming-Chin Hsu (Principal Investigator)
China Medical University Beigang Hospital: Kuan-Fei Chen (Principal Investigator), Hao-Yu Zhuang, Yan-Hong Pan, Shin-An Shih
Sin-Lau Hospital, Tainan, the Presbyterian Church in Taiwan: Tsang-Shan Chen(Principal Investigator)
Chi Mei Medical Center, Liouying: Ming-Hisu Wu (Principal Investigator), Jun-Bin Zhang, Shi-Cheng Chen, Szu-Yi Chiang
Ditmanson Medical Foundation Chia-Yi Christian Hospital: Sheng-Feng Sung (Principal Investigator)
Taichung Hospital Department of Health: Yi-Sheng Liao (Principal Investigator)
Yumin Medical Corporation Yumin Hospital: Shey-Lin Wu (Principal Investigator)
Kuang Tien General Hospital Dajia Division: Shih-Chieh Yu (Principal Investigator)
Ton Yen General Hospital: Shi-Ming Chen (Principal Investigator)
Kaohsiung Municipal Hsiao-Kang Hospital: Ching-Kuan Liu (Principal Investigator)
Data Availability
All relevant data are within the paper.
Funding Statement
This project was funded by the Bureau of Health Promotion, Department of Health Taiwan Stroke Registry grant (DOH-95~98-HP-1102); Department of Health Clinical Trial and Research Center of Excellence (DOH100-TD-B-111-004), Center of Excellence for Clinical Trial and Research in Neuroscience (DOH102-TD-B-111-003), Taiwan; the Ministry of Health and Welfare (MOHW105-TDU-B-212-133018), Taiwan; Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW104-TDU-B-212-113002), China Medical University Hospital, Academia Sinica Taiwan Biobank, Stroke Biosignature Project (BM104010092), NRPB Stroke Clinical Trial Consortium (MOST 103-2325-B-039 -006); Taiwan Ministry of Science and Technology (MOST 105-2314-B-341-004); Shin Kong Wu Ho-Su Memorial Hospital (SKH-8302-103-DR-17); Dr. Chi-Chin Huang Stroke Research Center, Taipei, Taiwan; Tseng-Lien Lin Foundation, Taichung, Taiwan; Taiwan Brain Disease Foundation and Katsuzo and Kiyo Aoshima Memorial Funds, Japan. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Wong KS. Global burden of intracranial atherosclerosis. International journal of stroke. 2006; 1(3): 158–9. 10.1111/j.1747-4949.2006.00045.x [DOI] [PubMed] [Google Scholar]
- 2.Battistella V, Elkind M. Intracranial atherosclerotic disease. European journal of neurology. 2014; 21(7):956–62. 10.1111/ene.12385 [DOI] [PubMed] [Google Scholar]
- 3.Qureshi AI, Caplan LR. Intracranial atherosclerosis. Lancet. 2014; 383(9921): 984–98. 10.1016/S0140-6736(13)61088-0 [DOI] [PubMed] [Google Scholar]
- 4.Jeng JS, Tang SC, Liu HM. Epidemiology, diagnosis and management of intracranial atherosclerotic disease. Expert review of cardiovascular therapy. 2010; 8(10): 1423–32. 10.1586/erc.10.129 [DOI] [PubMed] [Google Scholar]
- 5.Chimowitz MI, Lynn MJ, Howlett-Smith H, Stern BJ, Hertzberg VS, Frankel MR, et al. Comparison of warfarin and aspirin for symptomatic intracranial arterial stenosis. The New England journal of medicine. 2005; 352(13): 1305–16. 10.1056/NEJMoa043033 [DOI] [PubMed] [Google Scholar]
- 6.Derdeyn CP, Chimowitz MI, Lynn MJ, Fiorella D, Turan TN, Janis LS, et al. Aggressive medical treatment with or without stenting in high-risk patients with intracranial artery stenosis (SAMMPRIS): the final results of a randomised trial. Lancet. 2014; 383(9914): 333–41. 10.1016/S0140-6736(13)62038-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wong KS, Chen C, Fu J, Chang HM, Suwanwela NC, Huang YN, et al. Clopidogrel plus aspirin versus aspirin alone for reducing embolisation in patients with acute symptomatic cerebral or carotid artery stenosis (CLAIR study): a randomised, open-label, blinded-endpoint trial. The Lancet. Neurology. 2010; 9(5): 489–97. [DOI] [PubMed] [Google Scholar]
- 8.Caplan LR, Gorelick PB, Hier DB. Race, sex and occlusive cerebrovascular disease: a review. Stroke. 1986; 17(4): 648–55. [DOI] [PubMed] [Google Scholar]
- 9.Feldmann E, Daneault N, Kwan E, Ho KJ, Pessin MS, Langenberg P, et al. Chinese-white differences in the distribution of occlusive cerebrovascular disease. Neurology. 1990; 40(10): 1541–5. [DOI] [PubMed] [Google Scholar]
- 10.Sacco RL, Kargman DE, Gu Q, Zamanillo MC. Race-ethnicity and determinants of intracranial atherosclerotic cerebral infarction. The Northern Manhattan Stroke Study. Stroke. 1995; 26(1): 14–20. [DOI] [PubMed] [Google Scholar]
- 11.Liu HM, Tu YK, Yip PK, Su CT. Evaluation of intracranial and extracranial carotid steno-occlusive diseases in Taiwan Chinese patients with MR angiography: preliminary experience. Stroke. 1996; 27(4): 650–3. [DOI] [PubMed] [Google Scholar]
- 12.Huang YN, Gao S, Li SW, Huang Y, Li JF, Wong KS, et al. Vascular lesions in Chinese patients with transient ischemic attacks. Neurology. 1997; 48(2): 524–5. [DOI] [PubMed] [Google Scholar]
- 13.De Silva DA, Woon FP, Lee MP, Chen CP, Chang HM, Wong MC. South Asian patients with ischemic stroke: intracranial large arteries are the predominant site of disease. Stroke. 2007; 38(9): 2592–4. 10.1161/STROKEAHA.107.484584 [DOI] [PubMed] [Google Scholar]
- 14.Wang Y, Zhao X, Liu L, Soo YO, Pu Y, Pan Y, et al. Prevalence and outcomes of symptomatic intracranial large artery stenoses and occlusions in China: the Chinese Intracranial Atherosclerosis (CICAS) Study. Stroke. 2014; 45(3): 663–9. 10.1161/STROKEAHA.113.003508 [DOI] [PubMed] [Google Scholar]
- 15.Bang OY, Kim JW, Lee JH, Lee MA, Lee PH, Joo IS, et al. Association of the metabolic syndrome with intracranial atherosclerotic stroke. Neurology. 2005; 65(2): 296–8. 10.1212/01.wnl.0000168862.09764.9f [DOI] [PubMed] [Google Scholar]
- 16.White H, Boden-Albala B, Wang C, Elkind MS, Rundek T, Wright CB, et al. Ischemic stroke subtype incidence among whites, blacks, and Hispanics: the Northern Manhattan Study. Circulation. 2005; 111(10): 1327–31. 10.1161/01.CIR.0000157736.19739.D0 [DOI] [PubMed] [Google Scholar]
- 17.Meseguer E, Lavallee PC, Mazighi M, Labreuche J, Cabrejo L, Olivot JM, et al. Yield of systematic transcranial Doppler in patients with transient ischemic attack. Annals of neurology. 2010; 68(1): 9–17. 10.1002/ana.21921 [DOI] [PubMed] [Google Scholar]
- 18.Weimar C, Goertler M, Harms L, Diener HC. Distribution and outcome of symptomatic stenoses and occlusions in patients with acute cerebral ischemia. Archives of neurology. 2006; 63(9): 1287–91. 10.1001/archneur.63.9.1287 [DOI] [PubMed] [Google Scholar]
- 19.Wong KS, Li H, Chan YL, Ahuja A, Lam WW, Wong A, et al. Use of transcranial Doppler ultrasound to predict outcome in patients with intracranial large-artery occlusive disease. Stroke. 2000; 31(11): 2641–7. [DOI] [PubMed] [Google Scholar]
- 20.Hsieh FI, Lien LM, Chen ST, Bai CH, Sun MC, Tseng HP, et al. Get With the Guidelines-Stroke performance indicators: surveillance of stroke care in the Taiwan Stroke Registry: Get With the Guidelines-Stroke in Taiwan. Circulation. 2010; 122(11): 1116–23. 10.1161/CIRCULATIONAHA.110.936526 [DOI] [PubMed] [Google Scholar]
- 21.Jeng JS, Chung MY, Yip PK, Hwang BS, Chang YC. Extracranial carotid atherosclerosis and vascular risk factors in different types of ischemic stroke in Taiwan. Stroke. 1994; 25(10): 1989–93. [DOI] [PubMed] [Google Scholar]
- 22.Grant EG, Benson CB, Moneta GL, Alexandrov AV, Baker JD, Bluth EI, et al. Carotid artery stenosis: gray-scale and Doppler US diagnosis—Society of Radiologists in Ultrasound Consensus Conference. Radiology. 2003; 229(2): 340–6. [DOI] [PubMed]
- 23.Röther J, Schwartz A, Wentz KU, Rautenberg W, Hennerici M. Middle cerebral artery stenoses: assessment by magnetic resonance angiography and transcranial Doppler ultrasound. Cerebrovascular diseases 1994; 4(4):273–9. [Google Scholar]
- 24.Gao S, Lam WW, Chan YL, Liu JY, Wong KS. Optimal values of flow velocity on transcranial Doppler in grading middle cerebral artery stenosis in comparison with magnetic resonance angiography. Journal of neuroimaging. 2002; 12(3): 213–8. [PubMed] [Google Scholar]
- 25.Tang SC, Jeng JS, Yip PK, Lu CJ, Hwang BS, Lin WH, et al. Transcranial color-coded sonography for the detection of middle cerebral artery stenosis. Journal of ultrasound in medicine. 2005; 24(4): 451–7. [DOI] [PubMed] [Google Scholar]
- 26.Hao Q, Gao S, Leung TW, Guo MH, You Y, Wong KS. Pilot study of new diagnostic criteria for middle cerebral artery stenosis by transcranial Doppler. Journal of neuroimaging. 2010; 20(2): 122–9. 10.1111/j.1552-6569.2008.00337.x [DOI] [PubMed] [Google Scholar]
- 27.Navarro JC, Lao AY, Sharma VK, Tsivgoulis G, Alexandrov AV. The accuracy of transcranial Doppler in the diagnosis of middle cerebral artery stenosis. Cerebrovascular diseases. 2007; 23(5–6): 325–30. 10.1159/000099130 [DOI] [PubMed] [Google Scholar]
- 28.Zhao L, Barlinn K, Sharma VK, Tsivgoulis G, Cava LF, Vasdekis SN, et al. Velocity criteria for intracranial stenosis revisited: an international multicenter study of transcranial Doppler and digital subtraction angiography. Stroke. 2011; 42(12): 3429–34. 10.1161/STROKEAHA.111.621235 [DOI] [PubMed] [Google Scholar]
- 29.Kappelle LJ, Eliasziw M, Fox AJ, Sharpe BL, Barnett HJ. Importance of intracranial atherosclerotic disease in patients with symptomatic stenosis of the internal carotid artery. The North American Symptomatic Carotid Endarterectomy Trail. Stroke. 1999; 30(2): 282–6. [DOI] [PubMed] [Google Scholar]
- 30.Lee SJ, Cho SJ, Moon HS, Shon YM, Lee KH, Kim DI, et al. Combined extracranial and intracranial atherosclerosis in Korean patients. Archives of neurology. 2003; 60(11): 1561–4. 10.1001/archneur.60.11.1561 [DOI] [PubMed] [Google Scholar]
- 31.Stelagowski M, Bogusiak K, Kasielska A, Lysakowski M, Kazmierski P, Szostek M. Intracranial occlusions and internal carotid artery stenoses: clinical implications. Annals of vascular surgery. 2010; 24(6): 786–93. 10.1016/j.avsg.2010.02.033 [DOI] [PubMed] [Google Scholar]
- 32.Famakin BM, Chimowitz MI, Lynn MJ, Stern BJ, George MG. Causes and severity of ischemic stroke in patients with symptomatic intracranial arterial stenosis. Stroke. 2009; 40(6): 1999–2003. 10.1161/STROKEAHA.108.546150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kern R, Steinke W, Daffertshofer M, Prager R, Hennerici M. Stroke recurrences in patients with symptomatic vs asymptomatic middle cerebral artery disease. Neurology. 2005; 65(6): 859–64. 10.1212/01.wnl.0000175983.76110.59 [DOI] [PubMed] [Google Scholar]
- 34.Nahab F, Cotsonis G, Lynn M, Feldmann E, Chaturvedi S, Hemphill JC, et al. Prevalence and prognosis of coexistent asymptomatic intracranial stenosis. Stroke. 2008; 39(3): 1039–41. 10.1161/STROKEAHA.107.499475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rincon F, Sacco RL, Kranwinkel G, Xu Q, Paik MC, Boden-Albala B, et al. Incidence and risk factors of intracranial atherosclerotic stroke: the Northern Manhattan Stroke Study. Cerebrovascular diseases. 2009; 28(1): 65–71. 10.1159/000219299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ovbiagele B, Saver JL, Lynn MJ, Chimowitz M. Impact of metabolic syndrome on prognosis of symptomatic intracranial atherostenosis. Neurology. 2006; 66(9): 1344–9. 10.1212/01.wnl.0000210530.46058.5c [DOI] [PubMed] [Google Scholar]
- 37.Turan TN, Makki AA, Tsappidi S, Cotsonis G, Lynn MJ, Cloft HJ, et al. Risk factors associated with severity and location of intracranial arterial stenosis. Stroke. 2010; 41(8): 1636–40. 10.1161/STROKEAHA.110.584672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sloan MA, Alexandrov AV, Tegeler CH, Spencer MP, Caplan LR, Feldmann E, et al. Assessment: transcranial Doppler ultrasonography: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2004; 62(9): 1468–81. [DOI] [PubMed] [Google Scholar]
- 39.Holzer K, Sadikovic S, Esposito L, Bockelbrink A, Sander D, Hemmer B, et al. Transcranial Doppler ultrasonography predicts cardiovascular events after TIA. BMC medical imaging. 2009; 9: 13 10.1186/1471-2342-9-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alexandrov AV, Sloan MA, Tegeler CH, Newell DN, Lumsden A, Garami Z, et al. Practice standards for transcranial Doppler (TCD) ultrasound. Part II. Clinical indications and expected outcomes. Journal of neuroimaging. 2012; 22(3): 215–24. 10.1111/j.1552-6569.2010.00523.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.