Abstract
Backgroud
The role of maternal hypertensive disorders in pregnancy (HDP) in the development of retinopathy of prematurity (ROP) is unclear.
Methods
Studies were retrieved through literature searches in PubMed, EMBASE, Web of Science and the Cochrane Library up to May 5, 2016 without language restrictions. Cohort or case–control studies that reported the association of maternal hypertensive disorders and retinopathy of prematurity were eligible. Either a fixed- or a random-effects model was used to calculate the overall combined risk estimates.
Results
Thirteen cohort studies involving a total of 45082 individuals were included in the review. The pooled odds ratios of maternal hypertensive disorders in pregnancy for any stage and severe stages of ROP was 1.12 (95%CI: 0.90–1.40) and 0.80 (95%CI: 0.47–1.35), respectively. Sensitivity analyses confirmed that no single study qualitatively influenced the pooled OR. However, substantial heterogeneity and publication bias were observed in the meta-analysis.
Conclusions
Additional larger, prospective and well-adjusted studies are needed to determine the association between HDP and ROP, especially regarding the effects of different types of maternal hypertensive disorders in pregnancy on retinopathy of prematurity.
Introduction
Retinopathy of prematurity (ROP), a multiple-factor-induced disease of abnormal retinal vascular proliferation, often occurs in premature infants and low-birth-weight infants and may lead to blindness. With advances in neonatal care, increasing survival of premature infants has been achieved despite increased incidence of ROP. ROP has remained one of the leading causes of preventable childhood blindness, and occurs frequently in middle-income countries such as Latin America and some eastern European countries [1]. Also Preterm was found to be associated visual impairment and estimates of retinopathy of prematurity at regional and global levels for 2010 [2]. In addition to blindness, ROP can lead to eye or visual problems, such as visual impairment, strabismus, or major refractive error [3]. Infants with ROP have been found to be at risk of motor impairment, cognitive impairment, and severe hearing loss [4]. ROP pathogenesis has evolved over the past 50 years, yet the etiology is not fully understood. Some studies have proposed that antenatal or maternal risk factors might lead to ROP [5].
Hypertensive disorders in pregnancy (HDP) comprise a wide spectrum of disorders before or during pregnancy, which are generally characterized by a blood pressure above 140/90 mm Hg recorded with or without edema and proteinuria [6]. These disorders were clinically classified as chronic hypertension, gestational hypertension, preeclampsia, eclampsia, preeclampsia superimposed on chronic hypertension, and unspecified hypertension [7]. In total, 6% to 8% of pregnant women suffer from this complication [8]. Preeclampsia is characterized by the occurrence of hypertension and/or proteinuria after 20 weeks of gestation. The presence of HDP influences the intrauterine environment and contributes significantly to maternal and perinatal morbidity and mortality. [8–9]. In 1996, Gerd et al first described the relationship between preeclampsia and ROP [10]. Since then, opposing results have been observed in a series of studies, making the association of HDP with ROP inconclusive. Determining the association between HDP and ROP may have better understanding of ROP and clinical implications given the possibility that prevention and treatment of HDP might reduce the incidence of ROP. Thus, the objective of this systematic review is to determine the impact of HDP on ROP using cohort and case-control studies that assess the association between HDP and ROP published before 2016.
Methods
This article was followed the PRISMA and MOOSE Guidelines for meta-analysis.
Literature search
The PubMed, EMBASE, Web of Science and Cochrane Library databases were searched from initial to May 5 2016. Search terms consisted of a combination of Medical Subject Headings ("Pregnancy Complications" “Pre-Eclampsia”, “retinopathy of prematurity”) and key words (“maternal hypertension”, “gestational hypertension”, “retinopathy of prematurity”) without restriction to language. Potential articles were identified by reading titles and abstracts. If the titles and abstracts suggested that the study discussed risk factors for ROP, then two authors (TTZ and YQ) independently read the full text of the studies and decided whether the studies met the inclusion criteria. Disagreements were resolved by a third author (ZFY), who independently examined the studies, and a consensus was then reached. The reference lists of the included studies and relevant reviews were hand searched for further relevant articles.
Study selection
Original studies evaluating the association between ROP and HDP were included if: (i) case-control study or cohort study; (ii) The diagnosis of ROP was based on the results of ophthalmoscopy. The stage from 1 to 5 of ROP was classified according to the International Classification of ROP [11]. Stage 3, more than stage 3 and need for surgery for ROP were deemed to be severe ROP; (iii) The study involved human subjects; (iv) provided relative risk (RR) estimates such as risk ratios, incidence rate ratios, hazard ratios or odds ratios (OR) and 95%CIs (CIs) for HDP, or raw data from which these factors could be calculated. Two authors independently examined the studies for eligibility. Review articles, commentary articles, animal studies, letters, and case-series were excluded.
Data extraction
Using a form of data extraction, two authors independently collected data. The results were compared between collectors, and any uncertainties were resolved by consensus with a third author. The following characteristics of the study were recorded during data extraction: first author, year of publication, country, study design, inclusion population, number of study, gestational age, birth weight, diagnosis of ROP, impact of HDP on ROP, and confounders adjusted for. Studies without these information were excluded. We included the study with the largest number of participants if populations overlapped between studies.
Quality assessment and risk of bias
To assess the risk bias of the studies, two authors screened the study design, the size and representativeness of the study population, the validity of outcomes, and the quality of the statistical analysis. The Newcastle-Ottawa Quality Assessment Scale (NOS) was used [12]. This scale is widely applied to evaluate case-control or cohort studies with maximum scores of nine stars, which comprises eight items such as selection bias, comparability bias, exposure bias and outcome bias. Studies possessing five or more stars were deemed to be high quality studies. Any disagreement was settled as described above.
Statistical analysis
For the included studies, OR was used to evaluate the association between HDP and ROP. For one study that reported ORs separately for preeclampsia and gestational hypertension, we combined these 2 groups into a single group and calculated a combined OR using a fixed-effects model for the main analysis. Effect measures were weighted by log inverse variance. Combined results were performed by using random-effects or fixed-effects models. Heterogeneity was examined by the Q statistic (P< 0.1 was considered to represent significant) and the I2 test (values of > 50% was considered to represent significant). If significant heterogeneity was observed between the studies, the pooled OR was estimated using a random effects model. Otherwise, a fixed effects model was used. To assess the stability of the results, a sensitivity analysis was performed by removing each individual study in turn from the total, and re-analyzing the remaining studies. To find the source of the heterogeneity, we performed subgroup analyses combined with meta-regression according to the variance in the studies. Publication bias was evaluated by visual funnel plots and Begg's linear regression test. All statistical analyses were performed using Review Manager 5.3 and Stata 12.0.
Results
Search results and the characteristics of the included studies
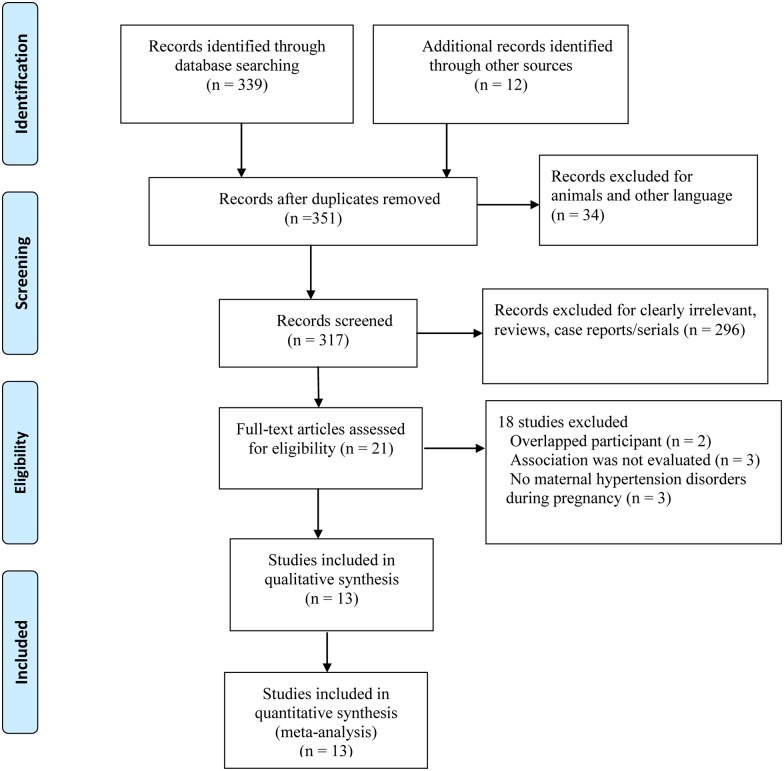
The literature search identified 351 studies based on our search strategy, of which, thirteen cohort studies with a total of 45082 babies were selected for analysis (Fig 1). Characteristics of included studies are summarized in Table 1 [5, 8–10, 13–21]. These studies were published between 1996 and 2016; two of them were conducted in the United States, three in Turkey, two in Italy, one in Germany, one in Sweden, two in China, one in Singapore, and one in Brazil. All participants were preterm or very low birth weight infants. For diagnosis of ROP, included studies measured or used register data based on ophthalmoscopy. The category of HDP was consistent among studies. Ten studies reported preeclampsia [5,10,13–18,20–21], two reported gestational hypertension [16,20], and two reported HDP [8–9]. Outcomes were categorized as 2 broad categories: “any stage ROP” and “severe ROP”.
Fig 1. Flow diagram of literature screen process.
Table 1. Characteristics of included studies.
| First Author (Year) | Country | Study Design | Population | GA or BW | Number/case | ROP Diagnosis | Impact of HDP on ROP | Adjustments | Risk of bias |
|---|---|---|---|---|---|---|---|---|---|
| Huang (2016)13 | China | Prospective/cohort | database of the Premature Baby Foundation of Taiwan | VLBW infant | 5718 /844 | Registatary data | Preeclampsia: any stage, OR = 1.00[0.84–1.20]; severe stage, OR = 0.89[0.83–1.25] | GA, BW, cesarean section, sex, Apgar score, transfusion, sepsis, distress syndrome with surfactant treatment, patent ductus arteriosus. | Only VLBL infants; NOS = 7 |
| Gagliardi (2014)9 | Italian | Prospective/cohort | 82 hospitals adhering to the Italian Neonatal Network | GA<30w; BW<1501g | 3606 | Registatary data | HDP: severe stage, OR = 1.48[1.02–2.15] | GA, antenatal steroids, gender, multiple pregnancies, inborn/outborn, mode of delivery | Only VLBL infants; NOS = 7 |
| Araz-Ersan (2013)14 | Turkey | Retrospective/ cohort | Istanbul Faculty of Medicine in Turkey | Preterm infant | 788 | Medical record | Preeclampsia: severe stage, OR = 0.21[0.09–0.52] | Sepsis, Male gender, Multiple gestation, BW<1500 | Only preterm infants; NOS = 7 |
| Gagliardi (2013)8 | Italian | Retrospective/cohort | the Accesso alle Cure e Terapie Intensive Ostetriche e Neonatali study in Italian | GA:23-31w | 2058/89 | Medical record | HPD: severe stage, OR = 2.0[1.0–4.0] | level of birth center, GA | Only preterm infants; NOS = 8 |
| Yu (2012)15 | US | Retrospective cohort | 12 clinical centers across 9 American College of Obstetricians and Gynecologists US districts | GA:23-36w | 25473/1053 | Medical record | Preeclampsia: any stage, OR = 0.66[0.50–0.87]; Gestational hypertension: any stage, OR = 0.85[0.54–1.34] | GA, mode of delivery, number of fetuses, race, BMI at delivery, BW, gender, intraventricular hemorrhage, blood transfusion, and congenital anomalies | Only preterm infants; NOS = 8 |
| Ozkan (2011)16 | Turkey | Prospective cohort | NICU of Uludag University School of Medicine in Turkey | GA<32w | 385/109 | Medical record | Preeclampsia: any stage, OR = 1.78[1.66–1.90] | GA, BW, Duration of mechanical ventilation, Duration of total oxygen | Only VLBW infants, NICU participant; NOS = 8 |
| Fortes (2011)17 | Brazil | Prospective / cohort | Hospital de Clínicas de Porto Alegre | GA≤32w BW≤1500g | 324/97 | Medical record | Preeclampsia: any stage, OR = 0.41[0.20–0.82]; severe stage, OR = 0.20[0.04–0.94] | GA, Antenatal steroid treatment, Essential hypertension, Any grade of intraventricular hemorrhage, Use of oxygen in mechanical ventilation, Use of indomethacin, Blood transfusion, Vaginal delivery Small for gestational age | Only VLBW infants; NOS = 8 |
| Mehmet (2011)18 | Turkey | Retrospective/ cohort | NICU of Dr Behcet Uz Children'Hospital in Turkey | GA<37w | 203/86 | Medical record | Preeclampsia: any stage, OR = 1.41[0.58–3.43] | - | Only preterm infants; NOS = 7 |
| Zayed (2010)19 | US | Retrospective/ cohort | Hospital databases and charts of all preterm inborn infants at the University of North Carolina | GA≤37w | 5143/323 | Medical record | new-onset gestational hypertension: any stage, OR = 1.32[1.01–1.72] | - | Only preterm infants; NOS = 7 |
| Yang (2010)20 | China | prospective/ cohort | NICU of Chang Gung Children’s Hospital in Taiwan | VLBW infant | 216/99 | Medical record | Preeclampsia: any stage, OR = 2.52[1.32–4.7]; severe stage, OR = 0.39[0.05–3.29] | Duration of mechanical ventilation, BW | Only VLBW infants, NICU participant; NOS = 7 |
| Shah (2005)21 | Singapore | Retrospective/ cohort | The Neonatal Department of the Singapore General Hospital | VLBW infant | 564/165 | Medical record | Preeclampsia: any stage, OR = 2.51[1.32–4.7] | BW, Duration of CPAP, Duration of mechanical ventilation, Pulmonary haemorrhage | Only VLBW infants; NOS = 7 |
| Seiberth (2000)5 | Germany | Retrospective/ cohort | University Children’s Hospital in Heidelberg and Mannheim and Worms City Hospital in Germany | BW≤1500g | 402/145 | Registatary data | Preeclampsia: any stage, OR = 0.52[0.32–0.86] | Only VLBW infants; NOS = 6 | |
| Gerd (1996)10 | Sweden | Retrospective/ cohort | population-based study in Sweden | BW≤ 1500g | 202/81 | Registatary data | Preeclampsia: any stage, OR = 0.69[0.37–1.30] | Only VLBW infants; NOS = 5 |
BW: birth weight; GA: gestational age; VLBW: very low birth weight; HDP: hypertensive disorder in pregnancy; mHTN: maternal new-onset gestational hypertension, NICU: neonatal intensive care unit; NOS: Newcastle-Ottawa scale scores.
Quality assessment
The studies were heterogeneous and various sizes; nine studies included between 100 and 1000 participants, and three studies had more than 1000 participants, with a range from 202 to 25473. More than half of the studies were retrospective and recruited consecutive patients. Most studies provided data on diagnosis of ROP in the at-risk population, and they reported the mortality rate of patients with ROP. Five studies adjusted for potential confounding factors. All included publications scored >5 assessed by the NOS S1 Table.
HDP and any stage of ROP
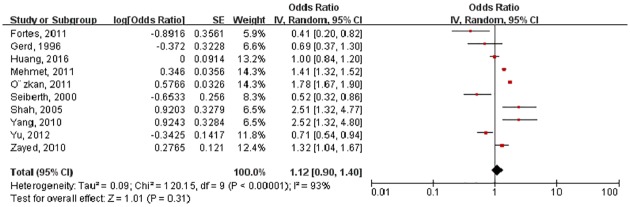
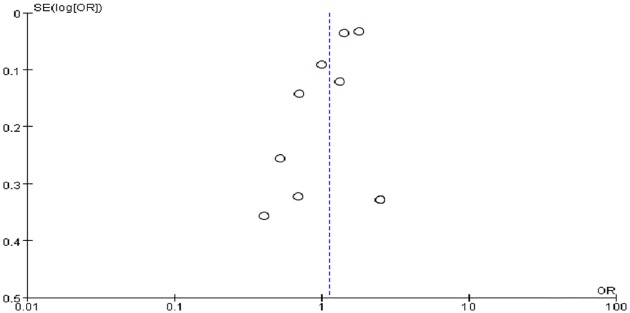
Ten ORs of any stage ROP were pooled in the meta-analysis. However, the ORs for the association varied from 0.20 to 2.52 across studies. The pooled OR from the random-effects model was 1.12 (95%CI: 0.90–1.40) (Fig 2). Substantial heterogeneity existed in this estimate (P < 0.01; I2 = 93%). Sensitivity analyses confirmed that no single study qualitatively influenced the pooled OR. Visual inspection of the funnel plot identified no substantial asymmetry for HDP and any stage (Fig 3). The modified Bgger’s test showed no publication bias (P = 1.00).
Fig 2. Forest plot showing the association between any stage retinopathy of prematurity and hypertensive disorders in pregnancy.
Fig 3. Funnel plot showing publication bias of any stage retinopathy of prematurity and hypertensive disorders in pregnancy.
HDP and the severe stage of ROP
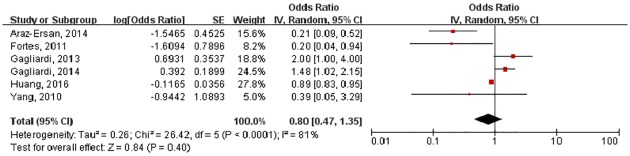
Six studies reported OR of severe ROP and were pooled in the meta-analysis. Fig 4 shows the combined results of severe stage of ROP from the random-effects model. The pooled OR was 0.80 (95%CI: 0.47–1.35). Substantial heterogeneity was observed (P < 0.01; I2 = 81%). Sensitivity analyses confirmed that no single study qualitatively influenced the pooled OR. The modified Begg’s test confirmed that there was no publication bias (P = 0.624).
Fig 4. Forest plot showing the association between severe stage retinopathy of prematurity and hypertensive disorders in pregnancy.
Subgroup analysis
Stratified meta-analyses in subgroups according to study design (retrospective cohort or prospective cohort), OR style and HDP classification revealed similar results. The results showed that control for duration of mechanical ventilation (Coef. = -0.95, p-value = 0.021) was a significant predictor of ROP, but study design, HDP classification, and adjusted OR or not measurements were not (p>0.09) (Table 2).
Table 2. Subgroup analysis.
| Subgroup | Number of risk estimate | Heterogeneity | Pooled OR | Adjusted R2 | |
|---|---|---|---|---|---|
| Q | I2 | ||||
| Any stage of ROP | |||||
| -Study design | -12.72% | ||||
| retrospective | 6 | <0.001 | 89% | 1.03[0.73 1.46] | |
| prospective | 4 | <0.001 | 94% | 1.22[0.74 1.99] | |
| -Control for duration of mechanical ventilation | 54.98% | ||||
| yes | 3 | 0.34 | 8% | 1.84[1.59 2.12] | |
| no | 7 | <0.01 | 89% | 1.03[0.78 1.36] | |
| -HDP classification | -17.41% | ||||
| HDP | 1 | 0.71[0.54 0.94] | |||
| preeclampsia | 8 | <0.01 | 92% | 1.18[0.93 1.50] | |
| Gestational hypertension | 1 | 0.65 | 1.32[1.04 1.67] | ||
| Severe stage of ROP | |||||
| -Study design | -39.79% | ||||
| retrospective | 2 | <0.01 | 93% | 0.66[0.07 5.96] | |
| prospective | 4 | 0.01 | 73% | 0.91[0.55 1.51 | |
| -OR | -28.97% | ||||
| Adjusted OR | 1 | 0.39[0.05 3.29] | |||
| Crude OR | 5 | <0.01 | 85% | 0.82[0.48 1.43] | |
| -HDP classification | 19.08% | ||||
| HDP | 2 | 0.45 | 0% | 1.58[1.14 2.20] | |
| preeclampsia | 4 | 0.03 | 79% | 0.39[0.14 1.09] | |
| Gestational hypertension | 0 | ||||
Discussion
To our knowledge, this meta-analysis is the first of its kind that analyzes the effect of HDP on the development of ROP in infants. The meta-analysis of data extracted from included studies showed that the OR of developing ROP was no different between infants exposed to maternal hypertension and those who were not. Even though there seems to be an increased ROP rates in infants with exposure to maternal HDP in some studies [8–9], this has not been supported by findings across all studies. In the subgroup analyses, results and heterogeneity were similar to those of the pooled analysis. An odds ratio of 1.12 was found between HDP and ROP, but wide divergence in study results contributed to our confidence interval crossing unity. The non-significant association between AMD and stroke may arise from several sources.
The incomplete or various possible confounding factors not adjusted in studies may have influenced the results. Of the thirteen studies included, nine attempted to control for gestational age or birth weight of infants [8–9,13–14,16–19,21]; and three studies that adjusted for the duration of mechanical ventilation supported preeclampsia as a risk factor for developing ROP in offspring [14,17,19]. Few studies adjusted for the presence of antenatal use of steroids and oxygen. None of the included studies examined the role that other pregnancy disorders might have in played in influencing the association between HDP and ROP. Most studies recruited infants of < 1500 g birth weight, and some were from NICU with hospital control. It is possible that the meta-analysis results are representative of low birth weight subjects. In addition, few studies are focused on this topic, This caused the heterogeneity and the skewing of results when one study dominated in the weighting.
Although we found no overall differences in HDP, some category-specific differences were observed between infant exposure to preeclampsia and gestational hypertension. Yu et al investigated the impact of maternal gestational hypertension and preeclampsia on the occurrence of ROP in preterm infants and reported that preeclampsia, but not gestational hypertension, was associated with a significantly reduced risk of ROP [15]. It is well recognized that maternal HDP leads to low birth weight and small-for-gestational-age infants, which are important risk factors for ROP [16]. However, the potential protective effect on ROP cannot be ruled out.
As we know, overproduction of VEGF is an important mechanism in physiologic retinal vascular development and pathologic angiogenesis in the preterm infant retina [22]. sFlt1, a specific endogenous inhibitor of VEGF, can combine with VEGF and prevent it from mediating its biological activities through its receptors [23]. Using a murine oxygen-induced ischemic retinopathy (OIR) model, mice treated with lenti.sFlt-1 demonstrated a more marked reduction in neovascularization in their retinas than untreated mice [24]. Furthermore, treatment with intravitreal bevacizumab, an anti-VEGF agent, for retinopathy of prematurity resulted in regression of neovascularization [25]. Interestingly, elevated levels of antiangiogenic factors, such as sFlt-1 and PlGF, were detected in preeclampsia [26–27]. Recent studies demonstrated that serum levels of circulating sFlt1 and PIGF changed as HDP developed. Preeclampsia patients showed higher sFlt1 and lower PIGF levels than patients with pregnancy-induced-hypertension and gestational proteinuria. This change of angiogenic level was also evident in varying degrees of severity of preeclampsia [28–29]. The elevated antiangiogenic factors that are produced by the placenta and enter into the systemic circulation may also have effects in the fetus.
On the basis of our findings, several questions arise. First, is the real association between HDP and ROP. Because of limited studies and variable study quality, the results of meta-analysis must be interpreted with caution, and the current article could not provide enough evidence to determine the relationship between HDP and ROP. Further studies should be considered and should include more representative participants, account for the different types of HDP, and adequately control for confounding factors. Second, if HDP increases the risk of ROP, can we treat HDP through drug intervention, lifestyle modification, and/or dietary therapy to protect against ROP? Third, the exact mechanisms underlying the relationship between HDP and ROP have yet to be elucidated. More studies are needed to increase understanding of this association and to provide convincing evidence for ROP prevention.
Supporting information
(DOC)
(DOC)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the National Science Foundation of China (No.81330016, 81630038, and 81270724), grants from the State Commission of Science Technology of China (2012BAI04B04), grants from the Ministry of Education of China (IRT0935), grants from the Science and Technology Bureau of Sichuan province (2014SZ0149), and a grant of clinical discipline program (neonatology) from the Ministry of Health of China (1311200003303. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Gilbert C, Awan H. Blindness in children.BMJ. 2003;327(7418):760–761. 10.1136/bmj.327.7418.760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blencowe H, Lawn JE, Vazquez T. Preterm-associated visual impairment and estimates of retinopathy of prematurity at regional and global levels for 2010. Pediatr Res 2013; 74:35–49. 10.1038/pr.2013.205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holmstro¨m G. & Larsson E. Outcome of Retinopathy of Prematurity. Clin Perinatol 2013; 40: 311–321. 10.1016/j.clp.2013.02.008 [DOI] [PubMed] [Google Scholar]
- 4.Schmidt B, Davis PG, Asztalos EV. Association Between Severe Retinopathy of Prematurity and Nonvisual Disabilities at Age 5 Years. JAMA 2014; 311:523–525. 10.1001/jama.2013.282153 [DOI] [PubMed] [Google Scholar]
- 5.Seiberth V, Linderkamp O. Risk factors in retinopathy of prematurity. a multivariate statistical analysis. Ophthalmologica 2000;214: 131–5. doi: 27482 [DOI] [PubMed] [Google Scholar]
- 6.ACOG Committee on Practice Bulletins—Obstetrics. ACOG practice bulletin. Diagnosis and management of preeclampsia and eclampsia. Obstet Gynecol. 2002;99(1):159–167. [DOI] [PubMed] [Google Scholar]
- 7.Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am J Obstet Gynecol. 2000 Jul;183(1):S1–S22. [PubMed]
- 8.Gagliardi L, Rusconi F, Da FM. Pregnancy disorders leading to very preterm birth influence neonatal outcomes: results of the population-based ACTION cohort study. Pediatr Res. 2013; 73:794–801. 10.1038/pr.2013.52 [DOI] [PubMed] [Google Scholar]
- 9.Gagliardi L, Rusconi F, Bellu R. Association of maternal hypertension and chorioamnionitis with preterm outcomes. Pediatrics. 2014;134:e154–e161. 10.1542/peds.2013-3898 [DOI] [PubMed] [Google Scholar]
- 10.Holmstrom G, Thomassen P, Broberger U. Maternal risk factors for retinopathy of prematurity—a population-based study. Acta Obstet Gynecol Scand. 1996;75(7): p. 628–35. [DOI] [PubMed] [Google Scholar]
- 11.An international classification of retinopathy of prematurity. The Committee for the Classification of Retinopathy of Prematurity. Arch Ophthalmol. 1984;102:1130–4 [DOI] [PubMed] [Google Scholar]
- 12.Wells GA. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-analyses. Ottawa: Ottawa Hospital Research Institute; http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed 2 Apr 2012. [Google Scholar]
- 13.Hsin-Chung Huang, Hwai-I Yang, Hung-Chieh Chou. Preeclampsia and Retinopathy of Prematurity in Very-Low-Birth-Weight Infants: A Population-Based Study [DOI] [PMC free article] [PubMed]
- 14.Araz-Ersan B, Kir N, Akarcay K. Epidemiological analysis of retinopathy of prematurity in a referral centre in Turkey. Br J Ophthalmol. 2013;97(1): p. 15–7. 10.1136/bjophthalmol-2011-301411 [DOI] [PubMed] [Google Scholar]
- 15.Yu XD, Branch DW, Karumanchi SA. Preeclampsia and retinopathy of prematurity in preterm births. Pediatrics. 2012;130:e101–e107. 10.1542/peds.2011-3881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ozkan Hilal, Cetinkaya Merih, Koksal Nilgun. Maternal preeclampsia is associated with an increased risk of retinopathy of prematurity. J. Perinat. Med. 2011;39:523–527 10.1515/JPM.2011.071 [DOI] [PubMed] [Google Scholar]
- 17.Fortes FJ, Costa MC, Eckert GU. Maternal preeclampsia protects preterm infants against severe retinopathy of prematurity. J Pediatr. 2011;158:372–376. 10.1016/j.jpeds.2010.08.051 [DOI] [PubMed] [Google Scholar]
- 18.Mehmet S, Fusun A, Sebnem C. One-year experience in the retinopathy of prematurity: frequency and risk factors, short-term results and follow-up. Int J Ophthalmol. 2011;4:634–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zayed MA, Uppal A, Hartnett ME. New-onset maternal gestational hypertension and risk of retinopathy of prematurity. Invest Ophthalmol Vis Sci. 2010;51:4983–4988. 10.1167/iovs.10-5283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang CY, Lien R, Yang PH. Analysis of incidence and risk factors of retinopathy of prematurity among very-low-birth-weight infants in North Taiwan. Pediatr Neonatol. 2011;52:321–326. 10.1016/j.pedneo.2011.08.004 [DOI] [PubMed] [Google Scholar]
- 21.Shah VA, Yeo CL, Ling YL. Incidence, risk factors of retinopathy of prematurity among very low birth weight infants in Singapore. Ann Acad Med Singapore. 2005;34:169–178. [PubMed] [Google Scholar]
- 22.Zeng G, Taylor SM, McColm JR. Orientation of endothelial cell division is regulated by VEGF signaling during blood vessel formation. Blood. 2007;109:1345–52. 10.1182/blood-2006-07-037952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roberts JM, Rajakumar A. Preeclampsiaand soluble fms-like tyrosine kinase 1. J Clin Endocrinol Metab. 2009;94(7):2252–2254 10.1210/jc.2009-0945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Min Z, Qiang W, Benwen S. Lentivirus-mediated sFlt-1 gene fragment transfer suppresses retinal neovascularization. Curr Eye Res. 2009;34:401–410. 10.1080/02713680902862971 [DOI] [PubMed] [Google Scholar]
- 25.Martinez-Castellanos MA, Schwartz S, Hernandez-Rojas ML. Long-term effect of antiangiogenic therapy for retinopathy of prematurity up to 5 years of follow-up. Retina. 2013;33:329–338. 10.1097/IAE.0b013e318275394a [DOI] [PubMed] [Google Scholar]
- 26.Sahay AS, Patil VV, Sundrani DP. A longitudinal study of circulating angiogenic and antiangiogenic factors and AT1-AA levels in preeclampsia. Hypertens Res. 2014;37:753–758. 10.1038/hr.2014.71 [DOI] [PubMed] [Google Scholar]
- 27.Rana S, Hacker MR, Modest AM. Circulating angiogenic factors and risk of adverse maternal and perinatal outcomes in twin pregnancies with suspected preeclampsia. Hypertension 2012;60:451–458. 10.1161/HYPERTENSIONAHA.112.195065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Engels T, Pape J, Schoofs K. Automated measurement of sFlt1, PlGF and sFlt1/PlGF ratio in differential diagnosis of hypertensive pregnancy disorders. Hypertens Pregnancy. 2013;32:459–473 10.3109/10641955.2013.827205 [DOI] [PubMed] [Google Scholar]
- 29.Schaarschmidt W, Rana S, Stepan H. The course of angiogenic factors in early- vs. late-onset preeclampsia and HELLP syndrome. J Perinat Med. 2013;41:511–516. 10.1515/jpm-2012-0248 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.