Abstract
Objectives
The aim of this study was to determine if early gadolinium enhancement (EGE) by cardiovascular magnetic resonance (CMR) imaging in a canine model of reperfused myocardial infarction depicts the area at risk (AAR) as determined by microsphere blood flow analysis.
Background
It remains controversial whether only the irreversibly injured myocardium enhances when performing CMR imaging in the setting of acute myocardial infarction. Recently, EGE has been proposed as a measure of the AAR in acute myocardial infarction as it correlates well with T2-weighted imaging of the AAR, but still requires pathological validation.
Methods
Eleven dogs underwent 2 hours of coronary artery occlusion and 48 hours of reperfusion prior to imaging at 1.5T. EGE imaging was performed 3 minutes after contrast administration with coverage of the entire left ventricle. Late gadolinium enhancement (LGE) imaging was performed between 10 and 15 minutes after contrast injection. AAR was defined as myocardium with blood flow (mL/min/g) <2SD from remote myocardium determined by microspheres during occlusion. The size of infarction was determined using triphenyltetrazolium chloride (TTC).
Results
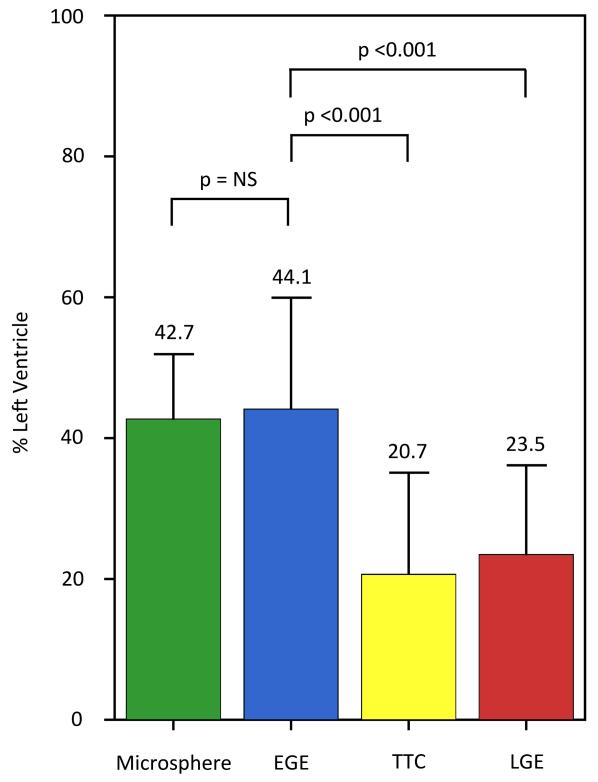
There was no significant difference in the size of enhancement by EGE compared to the size of AAR by microspheres (44.1± 15.8% vs. 42.7± 9.2%, p=0.61) with good correlation (r=0.88, p <0.001) and good agreement by Bland-Altman analysis (mean bias 1.4± 17.4%). There was no difference in the size of enhancement by EGE compared to enhancement on native T1 and T2 maps. The size of EGE was significantly greater than the infarct by TTC, (44.1± 15.8% vs. 20.7± 14.4%, p<0.001) and LGE (44.1± 15.8% vs. 23.5± 12.7%, p<0.001).
Conclusion
At three minutes post-contrast, EGE correlated well with the AAR by microspheres and CMR, and was greater than infarct size. Thus, EGE enhances both reversibly and irreversibly injured myocardium.
Keywords: Gadolinium enhancement, Area at risk, Acute myocardial infarction, Cardiovascular Magnetic Resonance Imaging
Introduction
Cardiovascular magnetic resonance (CMR) imaging with late gadolinium enhancement (LGE) is widely used in the determination of size and extent of myocardial infarction (1). However, it remains controversial whether gadolinium enhanced CMR only depicts infarcted tissue. Recently, two reports presented data in which early gadolinium enhancement(EGE) correlated well with T2-weighted images as a marker of area at risk(AAR) in a clinical setting (2,3). These findings are intriguing, and supported by previous work that found overestimation of infarct size by gadolinium enhanced imaging (4-6). To further validate the correlation between EGE and AAR, comparison of EGE to a pathological reference standard for the AAR is necessary. Furthermore, the previous studies of the correlations between EGE and T2-weighted AAR assessment were performed on a single slice per patient. How well EGE performs on a whole heart basis has yet to be determined.
The aim of this study was to examine the relationship between the size of EGE and pathological standards of AAR and infarct size using a phase sensitive inversion recovery(PSIR) sequence that images the entire left ventricle minutes after contrast administration in a canine model of acute myocardial infarction. How well the size of EGE compared to CMR measures of the AAR (quantitative native T1 and T2 maps) was also determined, as these modalities have shown good correlation to the pathological AAR (7).
Methods
Animal model
Mongrel dogs weighing 15-20kg were studied after approval by the Animal Care and Use Committee of the National Heart, Lung and Blood Institute of the National Institutes of Health. Animals were pretreated with amiodarone(6mg/kg/day) for one week to minimize arrhythmia during ischemia. Anesthesia was induced with a mixture of intramuscular 0.4mg/kg midazolam and 0.1mg/kg hydromorphone, followed by intravenous propofol (2-6mg/kg). General anesthesia was maintained during surgery after intubation with 2-5% inhaled sevoflurane. Intravenous and arterial lines were established, as well as a permanent left atrial catheter for administration of microspheres. After thoracotomy at the left fifth or sixth intercostal space, the left anterior descending artery was isolated and a snare placed distal to the first diagonal branch. Collateral vessels were not tied off. During occlusion, approximately 5×106 fluorescent microspheres (IMT Laboratories, Irvine, CA, USA) were administrated with simultaneous reference blood sampling from an arterial line. The snare was released after 120 minutes of occlusion. After surgery, the animals were monitored and treated for pain control and hemodynamic stability by trained animal care personnel for 48 hours prior to CMR imaging. Animals were anesthetized prior to imaging as previously described. Immediately before CMR, 5×106 fluorescent microspheres of a different color were administrated to assess the quality of reperfusion. All animals were euthanized upon completion of imaging with an overdose of potassium chloride under general anesthesia.
CMR imaging
Imaging was performed on a 1.5T clinical scanner (Magnetom Avanto, Siemens Healthcare Sector, Erlangen, Germany) with an eight-channel phased array coil. Quantitative native T1 mapping was performed with a motion corrected Modified Look- Locker Inversion-Recovery sequence with image acquisition 5 seconds after the first inversion followed by a 3 second pause and 3 seconds of acquisition after the second inversion, and steady state free procession readout. The following typical imaging parameters were used: field of view: 280×154mm2, matrix: 192×80, slice thickness: 6mm, voxel size 1.9×1.5×6mm3 = 17μL/voxel, repetition time: 2.6ms, echo time 1.1ms, parallel imaging factor 2. Quantitative native T2 mapping was performed using a fast low angle shot readout sequence and T2 preparations at 5, 40, and 80ms. Typical parameters were: field of view: 280×154mm2, matrix: 192×80, slice thickness: 6mm, voxel size 1.9×1.5×6 mm3, repetition time 4ms, echo time 1.6ms, parallel imaging factor 2.
EGE imaging was performed three minutes after administration of a 0.2mmol/kg intravenous bolus of contrast (gadopentetate dimeglumine, Magnevist®, Bayer Healthcare Pharmaceuticals, Wayne, New Jersey). To achieve whole heart coverage at this time point, images were acquired every other heart beat with an ECG triggered, breath-held, PSIR single shot sequence and steady state free procession readout. Nine slices were obtained in 18 heart beats. Typical parameters were: field of view: 280mm×154mm, matrix: 192×80, voxel size 1.9×1.5×6 mm3, 50° flip angle, repetition time: 3ms, echo time: 1.5ms, parallel imaging factor 2, and inversion time 200ms.
Standard LGE images were acquired >10 minutes after contrast injection using an ECG gated, segmented, PSIR fast low angle shot readout sequence (8) with the following typical parameters: field of view 280mm×156mm, matrix 256×108, voxel size 1.5×1.1× 6mm3, 10 μL/voxel, 25° flip angle, repetition time: 8.5ms, echo time: 3.3ms, parallel imaging factor 2. The inversion time was manually adjusted to null normal myocardium.
Pathology and microsphere analysis
After explantation, hearts were set in 2% agarose gel and sliced on a commercial meat slicer in 3 mm thick slices and stained with 1% triphenyltetrazolium chloride(TTC) for infarct demarcation and subsequently photographed. TTC images were matched to CMR images using landmarks such as papillary muscles and the right ventricular insertion point blinded to the microsphere blood flow results. For whole heart coverage, a basal TTC slice and apical TTC slice were matched to corresponding CMR images, and all slices in that range were included in the analysis. The borders of endocardium, epicardium and non-stained areas were manually delineated to determine infarct size as a percentage of the left ventricle. Additionally, a slice-by-slice comparison of the AAR by microspheres and by EGE was done. In this comparison, only the slices that could be matched to an image were included. A representative example is depicted in Figure 1.
Figure 1. Slice-by-slice comparison from one animal of early and late gadolinium enhancement compared to infarct by pathology.
Short axis stack was aligned from base (left) to apex (right) for quantification of enhancement. The extent of EGE (upper row) exceeds that of LGE (middle row) and TTC (bottom row) in all slices. EGE= early gadolinium enhancement, LGE = late gadolinium enhancement, TTC = triphenyltetrazolium chloride.
Two consecutive TTC slices were combined for further transmural sectioning and microsphere analysis to achieve the same slice thickness as the CMR images. The myocardium of the left ventricle was sectioned into transmural radial sectors. Sectors in the infarcted area (defined by TTC) were further sectioned into an endocardial and epicardial section. This was done to account for the great deal of collateral circulation in dogs, which could result in residual blood flow (during occlusion) epicardial to the infarcted area. Doing this minimized partial volume effects. A few sectors of normal myocardium were also split into endocardial and epicardial subsections. Each myocardial sector weighed approximately 0.7g, to ensure a sufficient number of microspheres per sector for reliable analysis. Tissue samples were sent to an external laboratory for myocardial blood flow determination (IMT Laboratories, Irvine, CA, USA). Myocardial sectors with blood flow <2SD below blood flow in remote myocardium were defined as AAR. The summed weight of AAR sectors was divided by the total left ventricle mass.
Image analysis
Image analysis was performed using a custom in-house software program. Endocardial and epicardial borders were manually delineated. Hyperenhancement on EGE images, as well as native T1 and T2 maps, was defined as pixels with signal intensities >2SD from remote myocardium for semiautomatic quantification. Spurious, non–contiguous pixels were excluded. Hypoenhanced pixels within an area of hyperenhancement (regions of microvascular obstruction or hemorrhage) were included as infarcted pixels in the hyperenhanced area.
On LGE images, infarct was defined as areas of enhancement based on the feature analysis and combined thresholding computer algorithm, which has previously been validated by pathology (9). Enhancement on CMR images was presented as a percentage of the entire left ventricular myocardium. Whole heart coverage was used to minimize partial volume effects, potential mis-registration and ex vivo shrinking of the myocardium for comparisons to pathological infarct sizing by TTC.
Statistical analysis
Statistical analysis was performed using MedCalc (version 12.7.7, Ostend, Belgium). Normally distributed data is presented as mean± SD, non-normally distributed data is presented with medians and interquartile ranges(IQR). After confirmation of normality, paired t-tests were performed to compare means. The Wilcoxon signed-rank test was used for non-parametric data. Correlations were assessed using Pearson’s correlation coefficient r for normally distributed data, and Spearman’s rank correlation in other cases. Limits of agreement were performed with Bland-Altman analysis and presented as mean difference ± 2SD. Based on an a priori sample size calculation, a minimum of 10 experiments were required to detect a correlation of 0.9 between AAR at risk by EGE and microspheres with a power of 0.9 and alpha 0.01.
Results
Eleven animals underwent successful coronary artery occlusion and reperfusion before CMR imaging (Figure 1). Microsphere data was available for all animals (n=11) at occlusion. On average, the left ventricle of each animal was sliced into 91 sectors (range 66-131). Microsphere data was only available for nine animals 48 hours after reperfusion due to inadequate reference blood flow sampling. During vessel occlusion, blood flow in the AAR was significantly lower than remote myocardium, 0.177± 0.043 ml/min/g vs. 0.579± 0.17 ml/min/g, p<0.001. In the ischemic core, the myocardial blood flow during occlusion was 9.4% (IQR, 6.2%-8.7%) of the blood flow in remote myocardium. The myocardial sectors identified as infarcted by TTC during slicing had significantly lower blood flow than the epicardial sectors that were part of the AAR (0.067 ml/min/g (IQR 0.034-0.17 ml/min/g) vs. 0.16 ml/min/g (IQR 0.097-0.28 ml/min/g) p<0.001). The transmural gradient in the subdivided remote myocardial sectors was not statistically significant (remote endocardium 0.60± 0.20 ml/min/g vs. remote epicardium 0.57± 0.17 ml/min/g, p=0.25)
At the time of CMR imaging, there was no difference in the blood flow in the AAR as compared to remote (0.34± 0.21 ml/min/g vs. 0.31± 0.18ml/min/g, p=0.17), or in the ischemic core compared to remote (0.29± 0.20 ml/min/g vs. 0.31± 0.18 ml/min/g, p=0.58) indicating successful reperfusion. The size of the AAR by microspheres was greater than the size of infarct by TTC in all animals (42.7± 9.2% vs. 20.7± 14.4%, p<0.001) indicating significant salvage.
Early Gadolinium Enhancement for Area at Risk Assessment by Microsphere Analysis
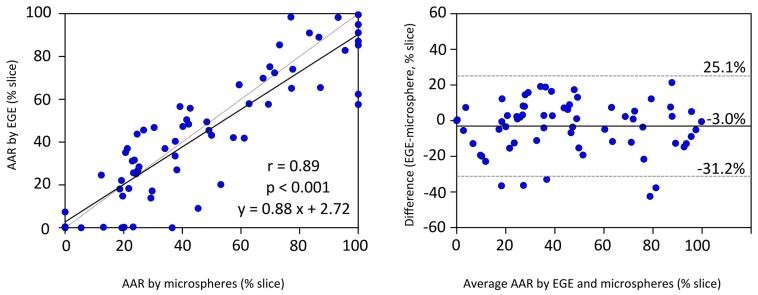
There was no significant difference in the size of enhancement on EGE images compared to the size of perfusion defect by microspheres (44.1± 15.8% vs. 42.7± 9.2%, p=0.61, Figure 2). The size of EGE correlated well to the size of the AAR by microspheres (r=0.88, p<0.001, Figure 3). Bland-Altman analysis revealed good agreement, with a mean bias of 1.4± 17.4% of the entire left ventricle, Figure 3. In a per slice comparison, the size of enhancement on EGE also correlated well with the microsphere AAR (r=0.89, p<0.001,), Figure 4..
Figure 2. AAR and infarct measures by pathology and CMR.
There was no difference in the size of AAR by microspheres compared to the size of EGE. EGE was greater than both CMR and pathological measures of infarction. EGE = early gadolinium enhancement, LGE= late gadolinium enhancement, TTC = triphenyltetrazolium chloride.
Figure 3. Per-animal comparison of EGE and AAR by microspheres.
The size of enhancement on EGE images correlated very well to the size of AAR by microsphere analysis (scatterplot, left), with minimal systematic bias on the Bland-Altman plot (right). Dotted lines represent line of identity in scatterplot and mean± 2SD in Bland-Altman plot. AAR= area at risk, EGE = early gadolinium enhancement.
Figure 4. Slice-by-slice comparison of EGE and AAR by microspheres.
On a per-slice basis the correlation between EGE and pathological AAR (microsphere analysis) was very good (scatterplot, left). The bias by Bland-Altman (right) was only 3%. Data represents 68 slices from 11 animals. AAR= area at risk, EGE = early gadolinium enhancement.
Early Gadolinium Enhancement for Area at Risk Assessment by Quantitative Maps
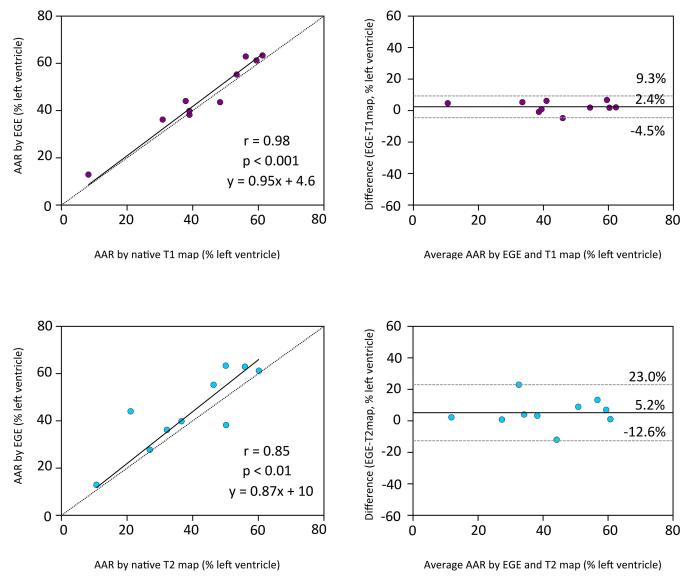
Compared to CMR measures of AAR (native T1 and T2 map data was available in 10 studies), there was no significant difference in the size of enhancement by EGE compared to the size of enhancement on native T1 maps (45.8± 15.6% vs 43.4± 16.1%, p=0.06). The correlation between EGE and T1 maps was excellent (r=0.98, p<0.001, with a mean bias of 2.4%± 6.9%. Similarly, there was no significant difference between the enhancement by EGE and native T2 maps (44.2± 16.7% vs. 39.0± 16.2%, p=0.11). There was good correlation (r=0.85, p<0.01,) between EGE and enhancement on T2 maps, with a slight overestimation by Bland-Altman analysis (5.2± 17.8%). The comparison of the size of EGE to the size of AAR defined by CMR measures is presented in Figure 5. The size of the AAR by both types of quantitative maps did not show any difference compared to the size of the AAR by microsphere analysis: 43.4± 16.1% vs. 43.2± 9.6%, p=0.95 for T1 maps vs. microspheres and 39.0± 16.2% vs. 43.2± 9.6%, p=0.27 for T2 maps vs. microspheres.
Figure 5. Comparison of EGE and CMR measures of AAR.
The size of enhancement by EGE also correlated very well to the size of the AAR by both CMR measures of AAR. Data for comparisons was available from 10 animals. Dotted lines represent line of identity in scatterplot and mean± 2SD in Bland-Altman plot (right panels). AAR= area at risk, EGE = early gadolinium enhancement.
Early Gadolinium Enhancement and Measures of Infarction
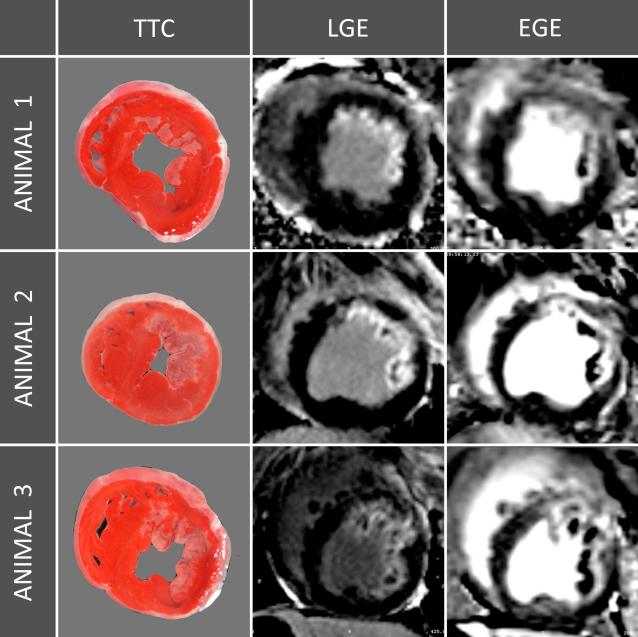
Three representative studies are depicted in Figure 6, where the EGE exceeds the infarction by LGE and TTC. In all animals, the size of enhancement by EGE was greater than the size of infarction by TTC, Figure 1 and 7. The size of EGE was significantly greater than the infarct by TTC, 44.1± 15.8% vs. 20.7± 14.4%, p<0.001). This was also the case for the size of enhancement by EGE compared to that of LGE (44.1± 15.8% vs. 23.5± 12.7%, p<0.001). Also, LGE showed excellent correlation to infarct size by TTC (r=0.95, p<0.001) and only very small systematic bias), see Figure 8.
Figure 6. Midventricular EGE, LGE and TTC slices from three representative animals.
The transmural extent of EGE is clearly greater than the infarction by TTC or LGE. EGE = early gadolinium enhancement, LGE = late gadolinium enhancement, TTC = triphenyltetrazolium chloride.
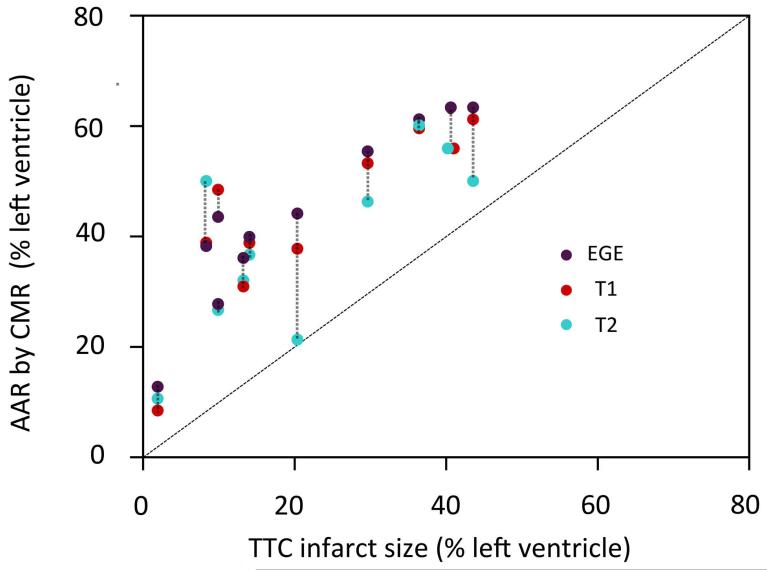
Figure 7. Comparison of AAR by CMR (native T1, native 2 and EGE) and infarct size by pathology.
In all animals, the size of enhancement by CMR was greater than the size of infarct by TTC. Dotted lines link CMR data from the same animal. Native T1 data was only available for 10 dogs and native T2 data was only available for 10 other dogs. The diagonal line indicates the line of identity. EGE = early gadolinium enhancement, TTC = triphenyltetrazolium chloride.
Figure 8. Infarct size comparisons.
The size of infarction by LGE showed excellent correlation to infarct size by pathology and minimal bias in the Bland-Altman plot (right). Dotted lines represent line of identity in scatterplot (left) and mean ± 2SD in Bland-Altman plot. LGE = late gadolinium enhancement, TTC = triphenyltetrazolium chloride.
Discussion
This study demonstrates that the AAR can be determined by EGE, as there is a good correlation between the size of EGE by CMR and the size of the AAR as determined by microspheres, the pathological reference standard. There was also a very good correlation between the size of hyperenhancement by EGE and other CMR measures of AAR in this study: native T1 and T2 maps. Furthermore, the size of gadolinium enhancement three minutes after contrast administration was clearly greater than the pathological standard of infarct size in this canine model of reperfused acute myocardial infarction. LGE correlates much more closely with infarct size. Also, a method is presented that can image early gadolinium enhancement of the entire left ventricle in about 18 heartbeats. Combining early and late gadolinium enhancement enabled evaluation of AAR and infarct size for the whole heart as Matsumoto et al previously suggested (2). An independent pathological standard of microspheres and TTC confirms this finding.
While the correlation between AAR by T2-weighted imaging and EGE has been demonstrated in recent clinical studies (2,3), previous preclinical studies have also reported a close correlation between gadolinium enhancement and the AAR by pathological standards (6,10). The findings in this study support the hypothesis of possible overestimation of infarct size by gadolinium enhancement in acute myocardial infarction, which has been reported by numerous authors throughout the years (4,5,11). The use of PSIR sequences mitigates the difficulties associated with choosing correct TI times, reducing the bias that may be due to imaging parameters and addressing technical variables that may have contributed to the disparate findings. Overestimation of infarct size by gadolinium enhancement was recently confirmed in a porcine study by Jablonowski et al using a PSIR sequence (12).
Contrast enhanced cine steady state free precession images have also been reported to correspond to the AAR both determined by SPECT and T2-weighted imaging in patients (13,14). While this technique relies on both the T1 and T2 properties of the myocardium, it has been proposed that part of the bright signal may be a result of the T1 shortening properties of gadolinium in the salvaged myocardium and not only in the infarct. Additionally, clinical studies have found functional recovery in areas of myocardium that display gadolinium enhancement, as well as reduction in the extent of enhancement in the following weeks to months, indicating also that irreversibly injured myocardium shows dynamic gadolinium enhancement(15-18).
Arheden et al demonstrated in rats that the distributional volume of gadolinium contrast was greater in myocardium that had undergone 20 minutes of ischemia, but did not display infarction by TTC when compared to normal myocardium, although it was less than that of infarcted myocardium (19). Recently published data also showed that the salvaged myocardium in a porcine model of ischemia and reperfusion had an increased ECV compared to normal myocardium both one day and seven days after ischemia (12). This provides a basis for the understanding of accumulation of gadolinium, an extracellular contrast agent, not only in the irreversibly damaged myocardium, but also in reversibly damaged myocardium. The expansion of the extracellular space and interstitial edema as a response to ischemia in reversibly injured myocardium, in addition to intracellular edema, has been reported previously in pathoanatomical studies (19-21). Expansion of the ECV in reversibly injured myocardium may be a result of several features of ischemic injury: shifts in electrolytes from the intracellular to the extracellular space, increased microvascular permeability resulting in protein leakage from the intravascular compartment, and structural alterations in the extracellular matrix (21-25). Conversely, the greater ECV in irreversibly injured myocardium is also due to loss of membrane integrity in the injured cells and passive diffusion of gadolinium into the intracellular space (26), why this ECV is greater than in the normal and reversibly injured myocardium. Klein et al showed different wash-in/washout kinetics in infarcted and remote myocardium in a clinical study (27). Differences in contrast kinetics must also be important in reversibly damaged myocardium, as enhancement of the salvaged myocardium is not as pronounced in LGE acquisitions. The findings in this study therefore support the current literature and adds important new insight into the concept that gadolinium not only depicts irreversibly injured myocardium, but also transiently enhances reversibly injured myocardium.
While EGE for determination of the myocardial AAR is exciting, and easily interleaved in the clinical workflow, more work is necessary to unveil its clinical usefulness. It remains to be determined how long after a myocardial infarction this technique can reliably be applied. In a canine model, a substantial decrease of T2 values in the salvaged myocardium (and thus edema) was evident within the first 48 hours after infarction (28), and a recent porcine study suggests a bimodal pattern of edema within the first days of reperfusion (29). These may be species specific effects as the size of AAR by T2-weighted imaging in clinical studies has been shown to be stable within the first 7 days (30). Furthermore, the optimal timing for EGE image acquisition after contrast administration requires further elucidation. In patients imaged up to 5 days after acute myocardial infarction, Matsumoto et al found the optimal timing for demarcation of EGE to be 2 minutes after contrast administration, when compared to T2 weighted enhancement as an indicator of the AAR(3).
This preclinical validation study has some limitations. A canine model of reperfused infarction, may exhibit different contrast kinetics compared to humans. Animals were imaged only once after 48 hours of reperfusion. Studying EGE after varying durations of reperfusion is necessary in order to understand the dynamic processes involved in the regression of edema and the effect this has on the accuracy of EGE in quantifying the AAR. The AAR by EGE (or other CMR measures) may have a higher signal intensity compared to the remote in animals scanned after only a few hours of reperfusion, and thus a higher contrast-to-noise ratio compared to after 2 days, assuming that some of the initial expansion of the ECV resolves by 2 days of reperfusion. Additionally, this study was performed in a tightly controlled setting with healthy animals. The influence of factors such as preconditioning, varying amounts of collateral flow, adequacy of reperfusion, and differences in contrast elimination may have an influence on the these findings in the clinical setting.
In conclusion, EGE correlates well with the size of the AAR and thus enhances both reversibly and irreversibly injured myocardium. LGE accurately images myocardial infarction in the same animals.
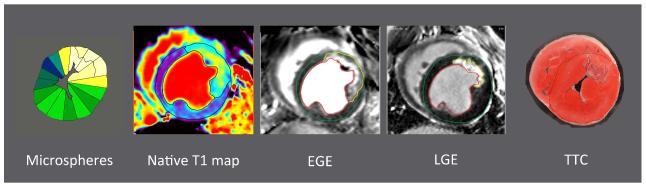
Figure 9. Pathology and CMR images of the same midventricular short axis slice.
The AAR determined by abnormal microsphere blood flow during occlusion was not significantly different than the size of hyperenhancement on native T1 maps, native T2 maps, or EGE. The size of enhancement by EGE 3 minutes after contrast administration was significantly greater than the size of enhancement by LGE and the pathological infarct size determined by TTC. EGE = early gadolinium enhancement, LGE = late gadolinium enhancement, TTC = triphenyltetrazolium chloride.
Perspectives.
Competency in Medical Knowledge
In acute reperfused MI, gadolinium enhancement of the myocardium in the first minutes after contrast administration is a novel CMR measure of the AAR that correlates well with established CMR measures and a histopathological defined size of the AAR.
Translational Outlook
This study demonstrates gadolinium enhancement of both reversible and irreversibly damaged myocardium in acute MI. Further study of the optimal timing of early gadolinium enhancement after contrast administration for AAR determination in clinical studies is warranted.
Acknowledgments
The authors thank Katherine Lucas for animal care and technical support.
Funding
This work was supported by the Intramural Research Program of the National Heart, Lung, and Blood Institute, National Institutes of Health, USA [Z01 HL006136-04 and HL004607-16]. Dr. Arai is a principal investigator on a US government Cooperative Research and Development Agreement (CRADA) with Siemens Medical Solutions (HL-CR-05-010).
Abbreviations
- AAR
area at risk
- CMR
cardiovascular magnetic resonance
- ECG
electrocardiography
- EGE
early gadolinium enhancement
- IQR
inter quartile range
- LGE
late gadolinium enhancement
- PSIR
phase sensitive inversion recovery
- ROI
region of interest
- SD
standard deviation
- TTC
triphenyltetrazolium chloride
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest:
Other coauthors have no conflicts of interest to declare.
References
- 1.Kramer CM, Barkhausen J, Flamm SD, Kim RJ, Nagel E. Standardized cardiovascular magnetic resonance (CMR) protocols 2013 update. J Cardiovasc Magn Reson. 2013;15:91. doi: 10.1186/1532-429X-15-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matsumoto H, Matsuda T, Miyamoto K, Shimada T, Mikuri M, Hiraoka Y. Peri-infarct zone on early contrast-enhanced CMR imaging in patients with acute myocardial infarction. JACC Cardiovasc Imaging. 2011;4:610–8. doi: 10.1016/j.jcmg.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 3.Matsumoto H, Matsuda T, Miyamoto K, et al. Temporal change of enhancement after gadolinium injection on contrast-enhanced CMR in reperfused acute myocardial infarction. J Cardiol. 2015;65:76–81. doi: 10.1016/j.jjcc.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 4.Oshinski JN, Yang Z, Jones JR, Mata JF, French BA. Imaging time after Gd-DTPA injection is critical in using delayed enhancement to determine infarct size accurately with magnetic resonance imaging. Circulation. 2001;104:2838–42. doi: 10.1161/hc4801.100351. [DOI] [PubMed] [Google Scholar]
- 5.Saeed M, Lund G, Wendland MF, Bremerich J, Weinmann H, Higgins CB. Magnetic resonance characterization of the peri-infarction zone of reperfused myocardial infarction with necrosis-specific and extracellular nonspecific contrast media. Circulation. 2001;103:871–6. doi: 10.1161/01.cir.103.6.871. [DOI] [PubMed] [Google Scholar]
- 6.Saeed M, Bremerich J, Wendland MF, Wyttenbach R, Weinmann HJ, Higgins CB. Reperfused myocardial infarction as seen with use of necrosis-specific versus standard extracellular MR contrast media in rats. Radiology. 1999;213:247–57. doi: 10.1148/radiology.213.1.r99se30247. [DOI] [PubMed] [Google Scholar]
- 7.Ugander M, Bagi PS, Oki AJ, et al. Myocardial edema as detected by pre-contrast T1 and T2 CMR delineates area at risk associated with acute myocardial infarction. JACC Cardiovasc Imaging. 2012;5:596–603. doi: 10.1016/j.jcmg.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kellman P, Arai AE, McVeigh ER, Aletras AH. Phase-sensitive inversion recovery for detecting myocardial infarction using gadolinium-delayed hyperenhancement. Magn Reson Med. 2002;47:372–83. doi: 10.1002/mrm.10051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hsu LY, Natanzon A, Kellman P, Hirsch GA, Aletras AH, Arai AE. Quantitative myocardial infarction on delayed enhancement MRI. Part I: Animal validation of an automated feature analysis and combined thresholding infarct sizing algorithm. J Magn Reson Imaging. 2006;23:298–308. doi: 10.1002/jmri.20496. [DOI] [PubMed] [Google Scholar]
- 10.Schaefer S, Malloy CR, Katz J, et al. Gadolinium-DTPA-enhanced nuclear magnetic resonance imaging of reperfused myocardium: identification of the myocardial bed at risk. J Am Coll Cardiol. 1988;12:1064–72. doi: 10.1016/0735-1097(88)90477-9. [DOI] [PubMed] [Google Scholar]
- 11.Judd RM, Lugo-Olivieri CH, Arai M, et al. Physiological basis of myocardial contrast enhancement in fast magnetic resonance images of 2-day-old reperfused canine infarcts. Circulation. 1995;92:1902–10. doi: 10.1161/01.cir.92.7.1902. [DOI] [PubMed] [Google Scholar]
- 12.Jablonowski R, Engblom H, Kanski M, et al. Contrast-Enhanced CMR Overestimates Early Myocardial Infarct Size: Mechanistic Insights Using ECV Measurements on Day 1 and Day 7. JACC Cardiovasc Imaging. 2015;8:1379–89. doi: 10.1016/j.jcmg.2015.08.015. [DOI] [PubMed] [Google Scholar]
- 13.Sorensson P, Heiberg E, Saleh N, et al. Assessment of myocardium at risk with contrast enhanced steady-state free precession cine cardiovascular magnetic resonance compared to single-photon emission computed tomography. J Cardiovasc Magn Reson. 2010;12:25. doi: 10.1186/1532-429X-12-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ubachs JF, Sorensson P, Engblom H, et al. Myocardium at risk by magnetic resonance imaging: head-to-head comparison of T2-weighted imaging and contrast-enhanced steady-state free precession. Eur Heart J Cardiovasc Imaging. 2012;13:1008–15. doi: 10.1093/ehjci/jes091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dall'Armellina E, Karia N, Lindsay AC, et al. Dynamic changes of edema and late gadolinium enhancement after acute myocardial infarction and their relationship to functional recovery and salvage index. Circ Cardiovasc Imaging. 2011;4:228–36. doi: 10.1161/CIRCIMAGING.111.963421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O'Regan DP, Ariff B, Baksi AJ, Gordon F, Durighel G, Cook SA. Salvage assessment with cardiac MRI following acute myocardial infarction underestimates potential for recovery of systolic strain. Eur Radiol. 2013;23:1210–7. doi: 10.1007/s00330-012-2715-8. [DOI] [PubMed] [Google Scholar]
- 17.Beek AM, Kuhl HP, Bondarenko O, et al. Delayed contrast-enhanced magnetic resonance imaging for the prediction of regional functional improvement after acute myocardial infarction. J Am Coll Cardiol. 2003;42:895–901. doi: 10.1016/s0735-1097(03)00835-0. [DOI] [PubMed] [Google Scholar]
- 18.Engblom H, Hedstrom E, Heiberg E, Wagner GS, Pahlm O, Arheden H. Rapid initial reduction of hyperenhanced myocardium after reperfused first myocardial infarction suggests recovery of the peri-infarction zone: one-year follow-up by MRI. Circ Cardiovasc Imaging. 2009;2:47–55. doi: 10.1161/CIRCIMAGING.108.802199. [DOI] [PubMed] [Google Scholar]
- 19.Arheden H, Saeed M, Higgins CB, et al. Reperfused rat myocardium subjected to various durations of ischemia: estimation of the distribution volume of contrast material with echo-planar MR imaging. Radiology. 2000;215:520–8. doi: 10.1148/radiology.215.2.r00ma38520. [DOI] [PubMed] [Google Scholar]
- 20.Garcia-Dorado D, Oliveras J, Gili J, et al. Analysis of myocardial oedema by magnetic resonance imaging early after coronary artery occlusion with or without reperfusion. Cardiovasc Res. 1993;27:1462–9. doi: 10.1093/cvr/27.8.1462. [DOI] [PubMed] [Google Scholar]
- 21.Kloner RA, Rude RE, Carlson N, Maroko PR, DeBoer LW, Braunwald E. Ultrastructural evidence of microvascular damage and myocardial cell injury after coronary artery occlusion: which comes first? Circulation. 1980;62:945–52. doi: 10.1161/01.cir.62.5.945. [DOI] [PubMed] [Google Scholar]
- 22.Jennings RB, Schaper J, Hill ML, Steenbergen C, Jr., Reimer KA. Effect of reperfusion late in the phase of reversible ischemic injury. Changes in cell volume, electrolytes, metabolites, and ultrastructure. Circ Res. 1985;56:262–78. doi: 10.1161/01.res.56.2.262. [DOI] [PubMed] [Google Scholar]
- 23.Dauber IM, VanBenthuysen KM, McMurtry IF, et al. Functional coronary microvascular injury evident as increased permeability due to brief ischemia and reperfusion. Circ Res. 1990;66:986–98. doi: 10.1161/01.res.66.4.986. [DOI] [PubMed] [Google Scholar]
- 24.Zhao MJ, Zhang H, Robinson TF, Factor SM, Sonnenblick EH, Eng C. Profound structural alterations of the extracellular collagen matrix in postischemic dysfunctional (“stunned”) but viable myocardium. J Am Coll Cardiol. 1987;10:1322–34. doi: 10.1016/s0735-1097(87)80137-7. [DOI] [PubMed] [Google Scholar]
- 25.Friedrich MG. Myocardial edema--a new clinical entity? Nat Rev Cardiol. 2010;7:292–6. doi: 10.1038/nrcardio.2010.28. [DOI] [PubMed] [Google Scholar]
- 26.Arheden H, Saeed M, Higgins CB, et al. Measurement of the distribution volume of gadopentetate dimeglumine at echo-planar MR imaging to quantify myocardial infarction: comparison with 99mTc-DTPA autoradiography in rats. Radiology. 1999;211:698–708. doi: 10.1148/radiology.211.3.r99jn41698. [DOI] [PubMed] [Google Scholar]
- 27.Klein C, Schmal TR, Nekolla SG, Schnackenburg B, Fleck E, Nagel E. Mechanism of late gadolinium enhancement in patients with acute myocardial infarction. J Cardiovasc Magn Reson. 2007;9:653–8. doi: 10.1080/10976640601105614. [DOI] [PubMed] [Google Scholar]
- 28.Hammer-Hansen S, Ugander M, Hsu LY, et al. Distinction of salvaged and infarcted myocardium within the ischaemic area-at-risk with T2 mapping. Eur Heart J Cardiovasc Imaging. 2014;15:1048–53. doi: 10.1093/ehjci/jeu073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fernandez-Jimenez R, Sanchez-Gonzalez J, Aguero J, et al. Myocardial Edema After Ischemia/Reperfusion Is Not Stable and Follows a Bimodal Pattern: Advanced Imaging and Histological Tissue Characterization. J Am Coll Cardiol. 2015;65:315–23. doi: 10.1016/j.jacc.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 30.Carlsson M, Ubachs JF, Hedstrom E, Heiberg E, Jovinge S, Arheden H. Myocardium at risk after acute infarction in humans on cardiac magnetic resonance: quantitative assessment during follow-up and validation with single-photon emission computed tomography. JACC Cardiovasc Imaging. 2009;2:569–76. doi: 10.1016/j.jcmg.2008.11.018. [DOI] [PubMed] [Google Scholar]