Abstract
Objective:
To report the clinical features, comorbidities, receptor subunit targets, and outcome in patients with anti-GABAA receptor (GABAAR) encephalitis.
Methods:
Clinical study of 26 patients, including 17 new (April 2013–January 2016) and 9 previously reported patients. Antibodies to α1, β3, and γ2 subunits of the GABAAR were determined using reported techniques.
Results:
Patients' median age was 40.5 years (interquartile range 48.5 [13.75–62.35] years; the youngest 2.5 months old; 13 female). Symptoms included seizures (88%), alteration of cognition (67%), behavior (46%), consciousness (42%), or abnormal movements (35%). Comorbidities were identified in 11 (42%) patients, including 7 tumors (mostly thymomas), 2 herpesvirus encephalitis (herpes simplex virus 1, human herpesvirus 6; coexisting with NMDAR antibodies), and 2 myasthenia without thymoma. Brain MRI was abnormal in 23 (88%) patients, showing in 20 (77%) multifocal, asynchronous, cortical-subcortical T2/fluid-attenuated inversion recovery abnormalities predominantly involving temporal (95%) and frontal (65%) lobes, but also basal ganglia and other regions. Immunologic or tumor therapy resulted in substantial improvement in 18/21 (86%) assessable patients; the other 3 (14%) died (2 status epilepticus, 1 sepsis). Compared with adults, children were more likely to have generalized seizures (p = 0.007) and movement disorders (p = 0.01) and less likely to have a tumor (p = 0.01). The main epitope targets were in the α1/β3 subunits of the GABAAR.
Conclusions:
Anti-GABAAR encephalitis is characterized by frequent seizures and distinctive multifocal cortical-subcortical MRI abnormalities that provide an important clue to the diagnosis. The frequency of symptoms and comorbidities differ between children (more viral-related) and adults (more tumor-related). The disorder is severe but most patients respond to treatment.
The GABAA receptor (GABAAR) is a ligand-gated chloride channel that mediates fast inhibitory synaptic transmission in the CNS. At the synapse, most GABAARs contain 2 α subunits, 2 β subunits, and 1 γ subunit, arranged as γ-β-α-β-α. Pharmacologic or genetic alteration of this receptor causes seizures,1–7 and we recently reported that human autoantibodies to the α1 and β3 subunits associate with seizures and status epilepticus in the context of autoimmune encephalitis.8 Since then, the γ2 subunit was also found to be a target of autoantibodies in one patient,9 and subsequently confirmed in other cases.10 Recognition of anti-GABAAR encephalitis is important because the seizures may be refractory to antiepileptic drugs unless the autoimmune response is treated. It is unclear whether the clinical features associated with antibodies against the γ2 subunit are different from those associated with antibodies against the α1 and β3 subunits. Moreover, since anti-GABAAR encephalitis was described recently, the spectrum of symptoms has not been fully defined. We describe the clinical, MRI, and immunologic features of 17 newly identified patients and 9 previously reported but not investigated for antibodies against the γ2 subunit of the receptor.
METHODS
Patients, controls, clinical definitions, and sample collection.
Between April 2013 and January 2016, we investigated the sera or CSF of 2,914 patients with suspected autoimmune neurologic disorders. We included in the current study only those patients who fulfilled the following 3 criteria (figure e-1 at Neurology.org): (1) syndrome compatible with possible autoimmune encephalitis,11 (2) serum or CSF reactivity with neuropil of rat brain suggesting a cell surface or synaptic target antigen, and (3) reactivity in a specific GABAAR cell-based assay (CBA) using live HEK cells expressing α1β3 or α1β3γ2 subunits. In addition, we included 9 patients (1–6, 9, 11, and 12 from our original report describing the GABAAR antibodies) whose serum or CSF fulfilled the above criteria; the other 9 patients from that study were excluded because the antibodies did not show brain reactivity.8
Clinical information was obtained from questionnaires completed by physicians. The severity of symptoms was evaluated by the modified Rankin Scale.12 The outcome at the last follow-up was defined as complete recovery (able to return to all previous activities), partial recovery (objective improvement but unable to return to all activities), no improvement, or death. Controls (total 461) for tissue immunohistochemistry and CBA antibody studies included serum or CSF of 169 patients with autoimmune encephalitis (paraneoplastic or nonparaneoplastic), 114 with opsoclonus-myoclonus, 117 with stiff-person syndrome, 20 with neurodegenerative disorders, and 41 healthy blood donors.
Immunohistochemical studies.
All samples were screened for reactivity with rat brain sections using previously reported immunohistochemical methods.8,13 Specific neuronal surface targets were investigated with CBA that included 13 autoantigens (NMDAR, AMPAR, GABABR, GABAAR, LGI1, CASPR2, DPPX, GlyR, GAD65, IgLON5, mGluR5, Dopamine2R, neurexin-3α), as reported.13–18 The CBA for GABAAR antibodies is described in the supplemental material.
Standard protocol approvals, registrations, and patient consents.
All patients gave written informed consent for use of samples and clinical information. This study was approved by the Institutional Review Board of the Hospital Clinic in Barcelona, Spain.
Statistical analysis.
Comparisons between adults and children, and between patients with and without tumor, were assessed with the 2-tailed Fisher exact test and Mann-Whitney U test. Results <0.05 were regarded as statistically significant.
RESULTS
Seventeen newly identified and 9 previously reported patients fulfilled the indicated criteria for anti-GABAAR encephalitis. These 26 patients, but none of the 461 controls, had antibodies identified with rat brain immunostaining and live HEK CBA expressing α1β3 subunits of the GABAAR (figures 1 and e-2). Among the 16 patients with paired serum and CSF samples, 14 had antibodies in both and 2 only in serum. The samples of all 26 patients reacted with live neurons (data not shown) similarly to those previously reported. Parallel studies in all 461 controls with live HEK cells expressing α1β3γ2 subunits of the GABAAR (expression of individual subunits confirmed with commercial antibodies) did not reveal additional patients, indicating the absence of patients with isolated γ2 antibodies in our study. Indeed, all patients with γ2 antibodies (8/26, 31%) also had antibodies against the α1 or β3 subunits (see supplemental material and figure e-3).
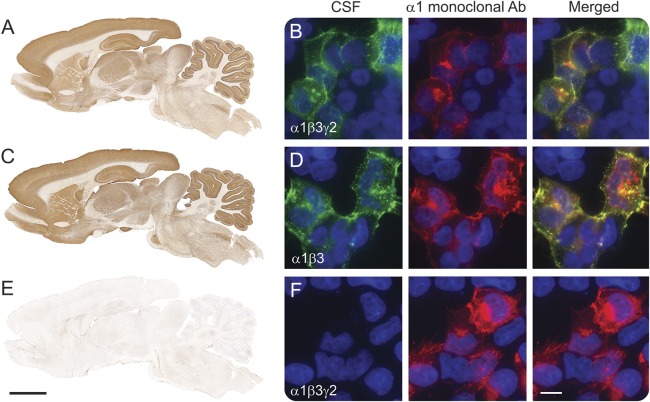
Figure 1. Reactivity of patient's antibodies with the GABAA receptor (GABAAR).
Rat brain immunostaining with CSF of a patient with antibodies against the α1, β3, and γ2 subunits of the GABAAR (A), compared with that of another CSF sample containing antibodies against α1 and β3 subunits (C). Note the remarkable similarity of immunostaining of the samples of both patients. (B, D) Reactivity of the same patients' CSF samples with the corresponding HEK cells expressing the α1β3γ2 subunits (B), and HEK cells expressing the α1β3 subunits (D). For patients with antibodies against α1β3 subunits, adding the γ2 subunit did not increase the intensity of reactivity with α1β3 (data not shown). The CSF of a patient without these antibodies serves as control (E, F). Scale bar rat brain = 2 mm, scale bar HEK cells = 10 μm.
Detailed clinical features of the 17 newly identified patients are described in table 1, and of the 26 pooled cases in tables 2 and e-1. The median age of all patients was 40.5 years (interquartile range 48.5 [13.75–62.35] years), including 15 adults and 11 children; 13 were (50%) female. The median follow-up was 9 months (range 2 weeks–7 years), and the median duration of symptoms by the time of diagnosis was 2 months (range 1 week–5 years).
Table 1.
Main clinical features and antibody specificity in 17 new patients with anti-GABAA receptor (GABAAR) encephalitis
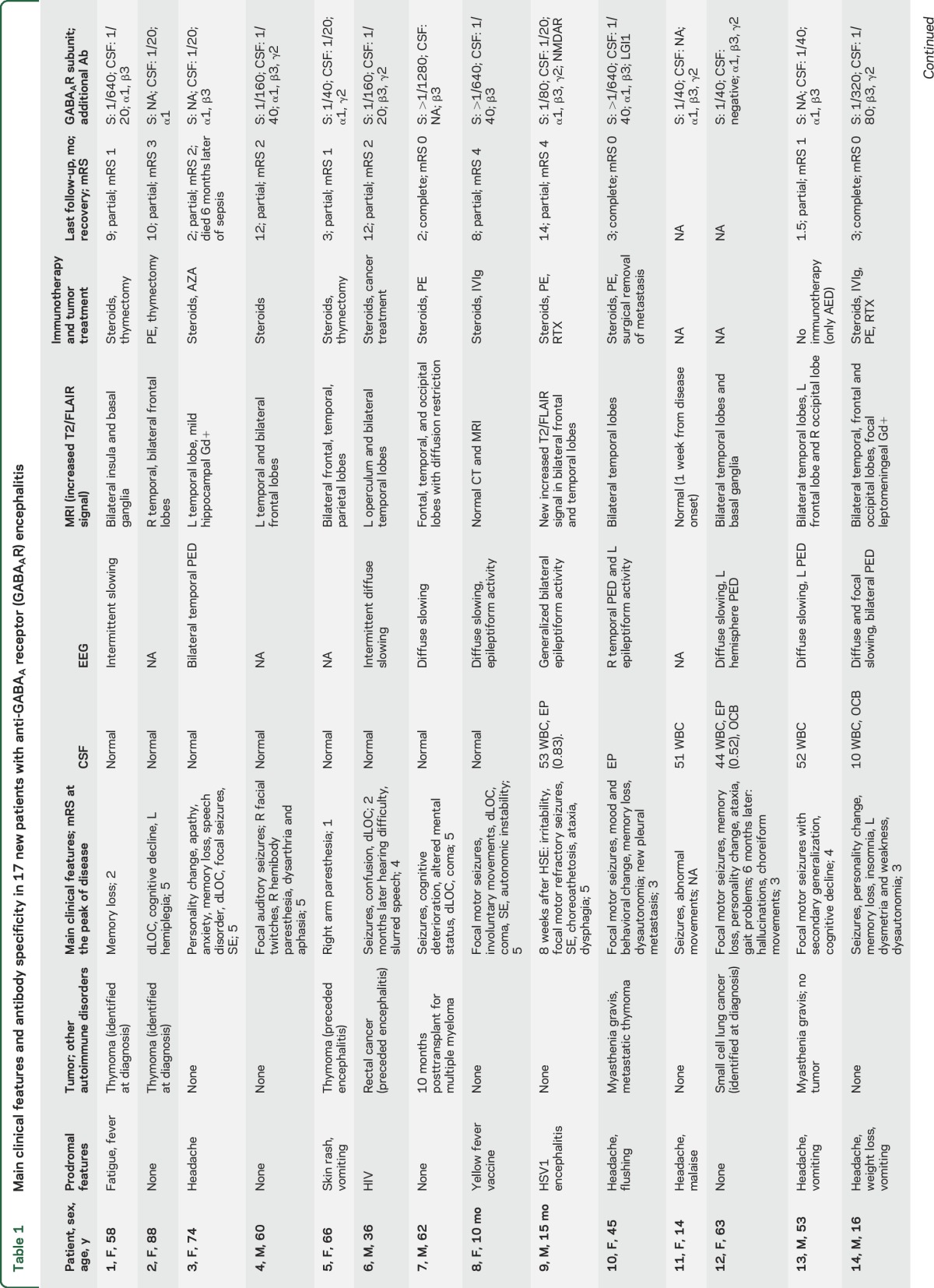
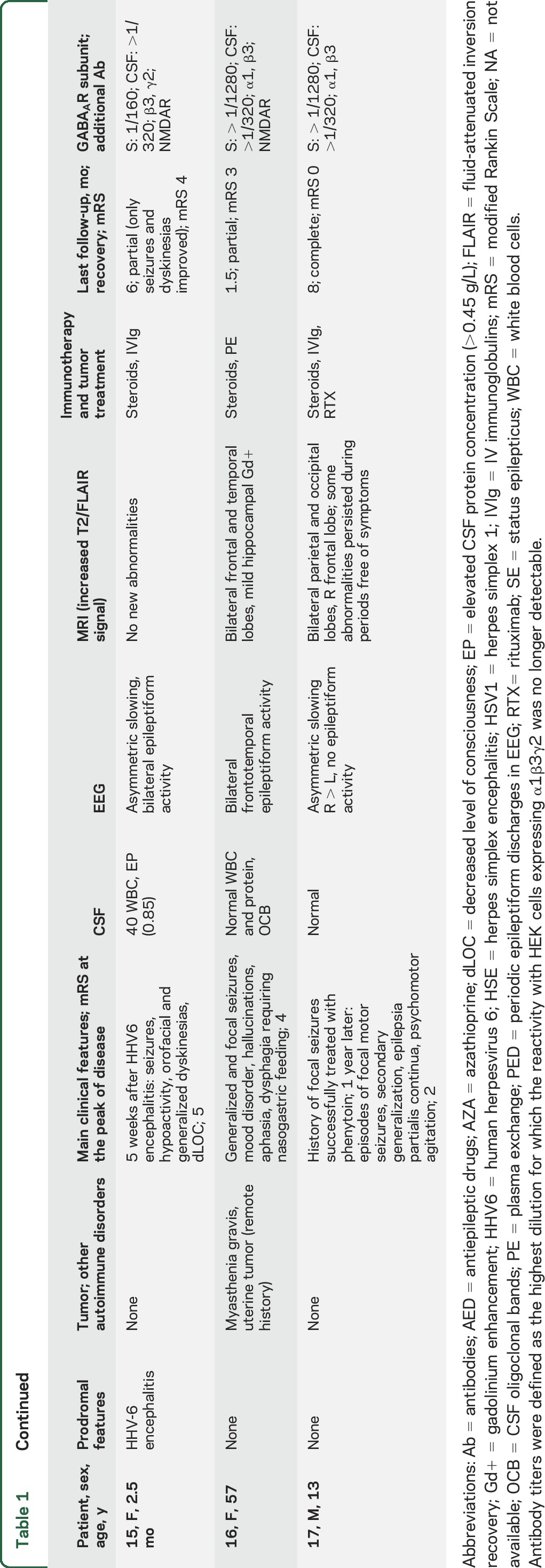
Table 2.
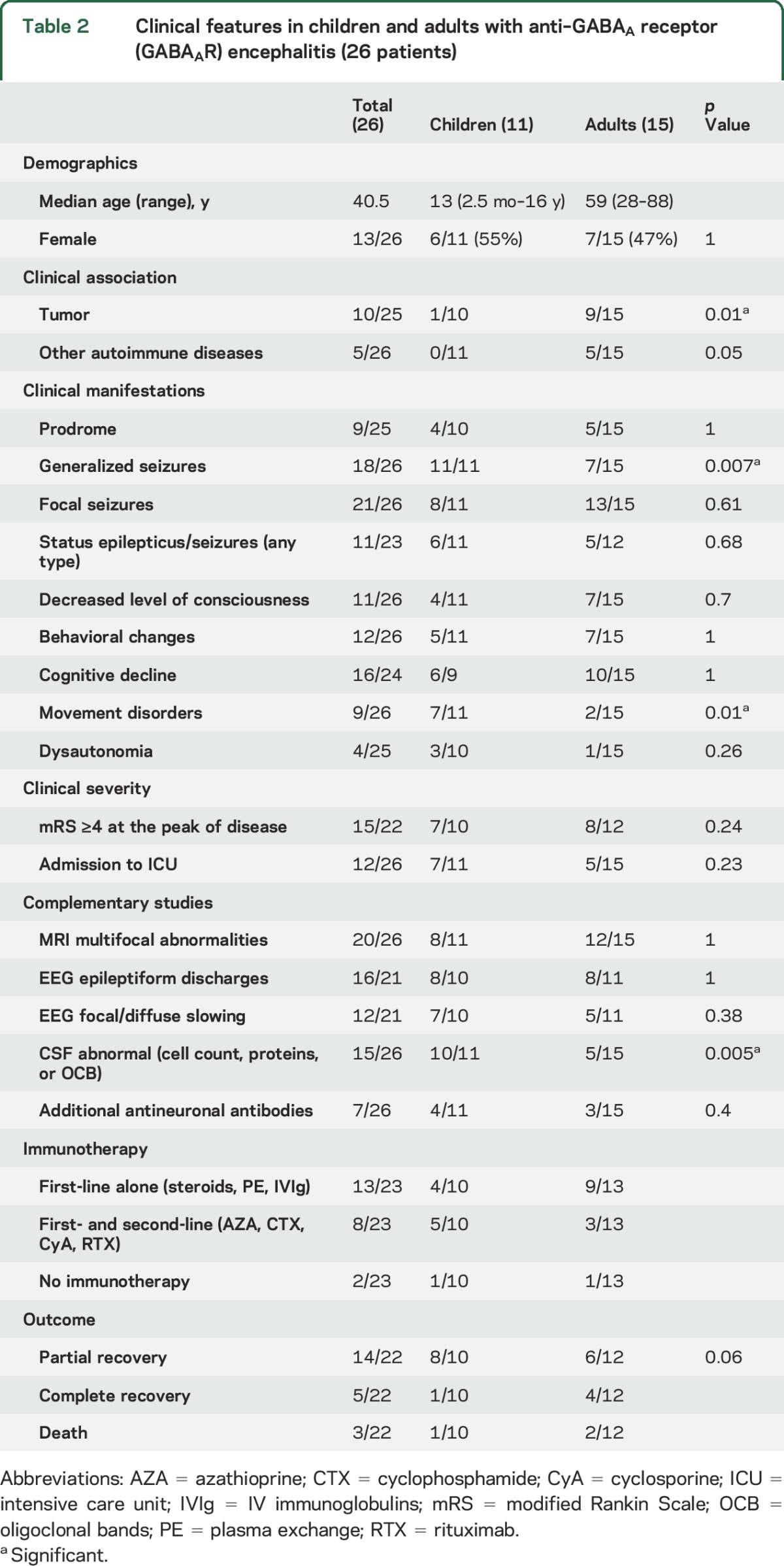
Clinical features in children and adults with anti–GABAA receptor (GABAAR) encephalitis (26 patients)
Tumor, viral, and other autoimmune associations occurred in 11 patients (table 1). Seven of these patients had an underlying tumor: 4 thymoma (1 with LGI1 antibodies, 1 with myasthenia), 1 small-cell lung cancer, 1 rectal cancer in association with HIV, and 1 multiple myeloma treated with autologous peripheral stem cell transplantation (PSCT) and lenalidomide (the autoimmune encephalitis developed 10 months after PSCT). Another 2 patients developed the autoimmune encephalitis a few weeks after viral encephalitis, one post herpes simplex 1 (HSV1) encephalitis and the other post human herpesvirus 6 (HHV6) encephalitis. By the time of the autoimmune encephalitis, both patients had GABAAR and NMDAR antibodies that were not present during the viral infection. Another 2 patients had myasthenia without thymoma. One of the patients with coexisting GABAAR and NMDAR antibodies is described in the supplemental material; the patient with thymoma and LGI1 antibodies has been reported previously.19
The most frequent (core) symptoms included seizures (23/26, 88%), cognitive impairment (n = 16/24, 67%, 2 babies excluded from analysis), altered behavior (12/26, 46%), decreased level of consciousness (11/26, 42%), and movement disorders (9/26, 35%). Status epilepticus occurred in 11 (48%) of the 23 patients with seizures, and 7 (64%) of them required pharmacologic coma. Seizures accompanied by at least another core symptom occurred in 23/26 (88%) patients and by at least 2 core symptoms in 14/26 (54%). Nine (35%) patients developed abnormal movements, including 7/11 (64%) children who showed orofacial dyskinesias, dystonic postures, or generalized choreoathetosis, and 2/15 (13%) adults, both showing facial twitches and cramps. Two of the 11 children developed the symptoms as part of a postviral encephalitis (coexisting with NMDAR antibodies) and 1 after vaccination for yellow fever (without NMDAR antibodies). The latter was a 10-month-old baby girl (patient 8, video) who developed a clinical picture that initially suggested anti-NMDAR encephalitis, including dysautonomia and orofacial and limb dyskinesias without NMDAR antibodies in serum and CSF.
CSF was abnormal in 15 of 26 (58%) patients including pleocytosis (6–154 leukocytes/mm3), increased protein concentration (0.52–0.85 g/L), or oligoclonal bands (table 2). EEG was available for 21 patients: 16 (76%) had epileptiform activity, mostly unilateral or bilateral periodic epileptiform discharges involving the temporal lobes (9 associated with focal or diffuse slow activity) and 5 (24%) had slow activity. Brain biopsy, performed in patient 15, demonstrated mild parenchymal and perivascular lymphocytic infiltrates without vessel wall involvement, and microglial activation (data not shown).
T2/fluid-attenuated inversion recovery (FLAIR) brain MRI abnormalities were identified in 23/26 (88%) patients. In 20 (77%), the abnormalities were multifocal, involving both gray and white matter in 2 or more of the following regions: temporal (95%, 16 bilateral, 3 unilateral), frontal (65%, 10 bilateral, 3 unilateral), parietal (25%, 3 bilateral, 2 unilateral), occipital (15%, 2 bilateral, 1 unilateral), basal ganglia (15%), cerebellum (10%), or brainstem (5%). Only 2 patients had isolated unilateral involvement of the temporal lobe, another patient had isolated unilateral parietal involvement, and 3 had normal MRI. The multifocal abnormalities involved cortical and subcortical regions (figure 2), without diffusion restriction (except for patient 7) and without gadolinium enhancement (except for patient 14, who had focal gyriform leptomeningeal enhancement, and 16, who had mild hippocampal enhancement). The multifocal T2/FLAIR changes were asynchronous, with some appearing while others were disappearing during the course of the disease. In one of the patients (case 17), the MRI abnormalities persisted during short periods (4–6 weeks) in which the patient was remarkably free of seizures or other symptoms.
Figure 2. MRI findings in 2 patients with anti–GABAA receptor (GABAAR) encephalitis.
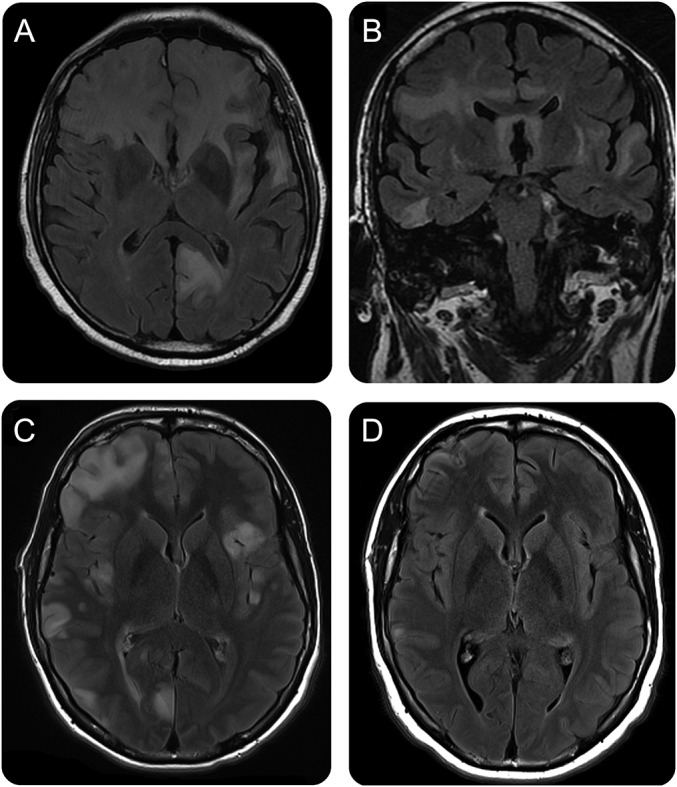
(A, B) MRI from patient 7, obtained on day 30 after symptom onset, shows extensive, confluent fluid-attenuated inversion recovery (FLAIR) abnormalities involving the left occipital lobe and the frontal and temporal regions, bilaterally, with moderate diffusion restriction (not shown). (C) MRI from patient 14, obtained on day 45 after symptom onset, shows extensive multiple cortical-subcortical FLAIR abnormalities involving bilateral frontal, temporal, and parietal-occipital lobes, without diffusion restriction, but mild gyriform leptomeningeal enhancement in the right temporal pole (not shown). Biopsy was performed in the right frontal region. (D) Follow-up MRI obtained 2.5 months later (4 months after symptom onset) shows substantial improvement and resolution of most abnormalities.
Treatment information was available for 23 patients: 13 (56%) received first-line immunotherapy (corticosteroids, plasma exchange, IV immunoglobulin), 8 (35%) first- and second-line immunotherapy (rituximab, azathioprine, cyclosporine, or cyclophosphamide), and 2 (9%) were only treated with antiepileptics. At the last follow-up, 18 of 21 (86%) patients treated with immunotherapy (and tumor removal when appropriate) had partial (n = 13, 72%) or complete (n = 5, 28%) recovery, and the other 3 (14%) patients died of sepsis, which in 2 was associated with status epilepticus; none of the patients who died had a tumor. One of the 2 patients who did not receive immunotherapy showed partial improvement (case 13), and the other was lost to follow-up (unknown outcome). At the last follow-up, none of the 26 patients has had a relapse. One patient (case 17) developed anti-GABAAR encephalitis 1 year after a first episode of seizures successfully treated with antiepileptic drugs. However, it is unclear whether the first episode was related to anti-GABAAR encephalitis given that no CSF, MRI, or antibody studies were obtained.
Compared to adults (table 2), children were more likely to develop generalized seizures (11/11, 100% vs 7/15, 47%, p = 0.007), movement disorders (7/11, 64% vs 2/15, 13%, p = 0.01), and CSF abnormalities (10/11, 91% vs 5/15, 33%, p = 0.005), and less likely to have a tumor (1/10, 10% vs 9/15, 60%, p = 0.01). Despite these findings, the outcome was not significantly different between the age groups (p = 0.06).
Compared to patients without tumor (table e-1), those with tumor were older (median 56.5 vs 16 years in patients without tumor, p = 0.006) and less frequently had generalized seizures (4/10, 40% vs 13/15, 87%, p = 0.02); the outcome, however, was similar (p = 0.14).
Detection of antibodies against the γ2 subunit, which in all cases occurred in association with antibodies against the α1 or β3 subunits, did not segregate with symptoms or paraclinical findings different from those in patients without γ2 subunit antibodies (data not shown).
DISCUSSION
We report 17 new patients with anti-GABAAR encephalitis and review 9 previously reported cases providing the main clinical and radiologic clues that assist in the differential diagnosis of this disorder in children and adults, tumor and viral associations, and the main subunit targets of the antibodies, the α1 and β3 subunits of the GABAAR.
Our current findings confirm that seizures are the most frequent clinical manifestation of this disorder. Combined with data from our previous study, up to 88% of the patients had seizures, usually at symptom presentation, and frequently accompanied by status epilepticus that often required pharmacologically induced coma. Status epilepticus (along with sepsis) may have contributed to the death of 2 patients. In all patients, the seizures were accompanied by at least one of the following core symptoms: cognitive impairment, decreased level of consciousness, altered behavior, or movement disorders. Interestingly, children were more likely to have generalized seizures and movement disorders and less likely to have an underlying tumor than adults. These age-related symptoms may result from the combination of specific antibody effects on synaptic circuits (e.g., antibody-mediated decrease of receptors) and increased vulnerability of some areas of the developing brain (hippocampus, basal ganglia) to inflammatory disorders. For example, other inflammation-related epileptic conditions (e.g., febrile infection-related epilepsy syndromes20) and movement disorders (e.g., postinfectious Sydenham chorea21) occur almost exclusively in children. In addition, in children, GABAAR antibodies may develop as postviral encephalitis and coexist with NMDAR antibodies, in which case the resulting symptoms (seizures, dyskinesias, choreoathetosis) are likely a manifestation of the combined presence of NMDAR antibody–mediated mechanisms.
Considering the clinical similarities among many forms of autoimmune encephalitis, an important finding of our study is the association of anti-GABAAR encephalitis with multifocal unilateral or bilateral cortical-subcortical T2/FLAIR MRI abnormalities. These T2/FLAIR abnormalities can manifest asynchronously during the course of the disease (some appear while others disappear), sometimes with limited correlation with the patient's symptoms, and rarely enhance with contrast. These MRI findings are important not only because they are frequent (77% of the patients) but also because they rarely occur in other autoimmune encephalitis, providing a valuable clue to the clinical recognition of GABAAR autoimmunity.
The experience gained from this and our previous study suggests that 40% of patients with anti-GABAAR encephalitis have tumors, mostly thymomas, and less commonly, other neoplasms (e.g., Hodgkin lymphoma, multiple myeloma) that may cause alterations of the immunologic system, perhaps leading to autoimmunity. Patients with a tumor were older (only 1 of 11 pediatric patients had a tumor; Hodgkin lymphoma in a 16-year-old patient) and less likely to have seizures than those without tumor, probably due to the general predisposition of children with inflammatory brain disorders to have seizures. Four adult patients had thymoma: 1 of them, previously reported,19 had a history of several tumor relapses without development of encephalitis until the last relapse, which also associated with LGI1 antibodies. Interestingly, the thymoma of this patient expressed both LGI1 and GABAAR proteins, and the clinical picture appeared to be driven by the GABAAR immune response showing widespread multifocal (not hippocampal limited) T2/FLAIR abnormalities, which are highly unusual in anti-LGI1 encephalitis. This clinical case resembled 2 reported patients with anti-GABAAR encephalitis in association with invasive thymoma, coexisting antibodies (LGI1 or Caspr2), myasthenia gravis (in one), and multifocal T2/FLAIR MRI abnormalities.9 We did not find another predominant tumor type among our patients with malignancies, as reported in previous studies.9,10 It is unclear whether the history of HIV, vaccination against yellow fever, or a peripheral stem cell transplant for multiple myeloma in 3 of our patients played a role in triggering the GABAAR immune response. However, it is interesting to note that posttransplant immunosuppressed patients can develop autoimmune encephalitis, as has been shown in patients with anti-NMDAR and anti-LGI1 encephalitis.22,23
The development of anti-GABAAR encephalitis as postviral encephalitis (HSV1 and HHV6) expands the number of receptors that can be involved as targets of postviral brain autoimmunity. This and previous findings24 support the concept of autoimmunity triggered by extensive antigen release by infected neurons undergoing degeneration. A mechanism of viral mimicry is less likely because the 2 patients with this complication developed de novo synthesis of antibodies against 2 different targets (GABAAR and NMDAR) during the weeks following the viral encephalitis.
All patients' serum or CSF antibodies recognized the α1 or β3 subunits of the GABAAR, with 31% of the cases showing coexisting antibodies against the γ2 subunit. None of the patients had isolated antibodies against the γ2 subunit, and the presence of these antibodies along with antibodies to α1 or β3 subunits did not reveal a specific subphenotype (data not shown), suggesting that CBA expressing α1β3 or α1β3γ2 can be used for comprehensive antibody testing. In a previous study in which the clinical and paraclinical information (CSF, MRI, or EEG) were limited or not available for many patients, antibodies directed only against the γ2 subunit were identified in 5 cases.10 Each of these patients had a different syndrome or suspected etiology (celiac disease, psychological disorder, mild cognitive impairment, pathologically confirmed Huntington disease, focal epilepsy) and only 1 received immunotherapy, suggesting a low index of conviction among the treating physicians for an autoimmune cause. In contrast, our current clinical findings associated with brain tissue reacting antibodies and α1β3 subunit specificity (irrespective of γ2 subunit antibodies) show a more homogeneous clinical and radiologic syndrome, and 21 of 23 patients received immunotherapy.
It is premature to indicate whether serum or CSF should preferentially be tested. We have identified GABAAR antibodies in serum, but not CSF, of patients with other disorders such as stiff-person syndrome or opsoclonus-myoclonus.8 Interestingly, the samples of those patients do not react with brain tissue or cultured neurons, suggesting other epitope targets of unclear clinical relevance. This finding and the possible coexistence of CSF or serum antibodies against other synaptic or cell surface proteins8–10 suggests caution in selecting only serum or CSF for antibody testing, and for these reasons we prefer determining antibodies in both samples. A comprehensive approach to antibody testing using CBA with both serum and CSF or if only serum is available confirming the results with brain tissue immunohistochemistry has been recommended for most antibodies against cell surface or synaptic proteins.11 In our experience with this and other autoantibodies, the parallel demonstration of antibody reactivity using brain tissue and CBA has more clinical value than CBA alone (irrespective of the titers of this assay), as shown here in some cases.
Despite the limitations of being a retrospective study and that the disease is infrequent, the findings of this study are important in helping to recognize this potentially lethal disorder. Current experience suggests that anti-GABAAR encephalitis should be suspected in patients with encephalitis predominantly manifesting with seizures and multifocal cortical-subcortical T2/FLAIR MRI abnormalities that usually involve the temporal lobes (95% of cases). The disorder can affect very young children (the youngest in this study was 2.5 months) and adults. In younger patients, the disorder may be confused with anti-NMDAR encephalitis due to the common presence of dyskinesias, although it is important to keep in mind that both disorders may overlap in the context of postviral autoimmune encephalitis. Compared with other autoimmune encephalitis, anti-GABAAR encephalitis seems to be less responsive to treatment than NMDAR encephalitis. Although 86% of the patients showed responses to treatment (first- and second-line therapies and tumor treatment if needed), only 28% had full recovery (and only one of them was a child), and the other 14% died, emphasizing the need for prompt recognition and treatment of the disorder. Future studies should focus on clarifying the frequency of GABAAR antibodies in patients with postviral autoimmune encephalitis, the preferential occurrence of these antibodies in serum or CSF, and whether prompt treatment improves the degree of neurologic recovery.
Supplementary Material
ACKNOWLEDGMENT
The authors thank the physicians who provided clinical information, the patients and families for their contribution to research, and E. Aguilar and J. Planagumà for technical support.
GLOSSARY
- CBA
cell-based assay
- FLAIR
fluid-attenuated inversion recovery
- GABAAR
GABAA receptor
- HHV6
human herpesvirus 6
- HSV1
herpes simplex 1
- PSCT
peripheral stem cell transplantation
Footnotes
Editorial, page 1010
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Design/conceptualization of the study, analysis and interpretation of the data: M.S., M.P.P., and J.D. Data collection: M.S., M.P.P., M.M.S., T.A., C.F.J., M.I.B.A., M.R.J.B., L.B., M.G., A.F., R.L.C.O., M.R.R., F.G., J.D. Statistical analysis: M.S., T.A., J.D. Figure/video development: M.S., M.P.P., M.M.S., L.B., J.D. Drafting of the manuscript: M.S., M.R.R., F.G., and J.D.
STUDY FUNDING
This study was supported in part by Instituto Carlos III/FEDER (CM14/00081, T.A.; FIS PI15/00377, F.G.; FIS PI14/00203, J.D.), CIBERER (CB15/00010, J.D.), NIH RO1NS077851 (J.D.), Agaur SGR93 (J.D.), Fédération pour la Recherche sur le Cerveau, FCR-2013-01 (J.D.), Fundación Mutua Madrileña (T.A.), Fondation Pierre Mercier pour la Science and Société Académique Vaudoise (Lausanne, Switzerland) (M.S.), Dodot Procter & Gamble (DN040579, T.A.), and Fundació CELLEX (J.D.).
DISCLOSURE
M. Spatola, M. Petit-Pedrol, M.M. Simabukuro, T. Armangue, F.J. Castro, M.I. Barcelo Artigues, M.R. Julià Benique, L. Benson, M. Gorman, A. Felipe, and R.L. Caparó Oblitas report no disclosures relevant to the manuscript. M.R. Rosenfeld receives royalties from Athena Diagnostics for the use of Ma2 as an autoantibody test and from Euroimmun for the use of NMDA receptor as an autoantibody test. F. Graus received a licensing fee from Euroimmun for the use of IgLON5 as an autoantibody test. J. Dalmau receives royalties from Athena Diagnostics for the use of Ma2 as an autoantibody test and from Euroimmun for the use of NMDA, GABAB receptor, GABAA receptor, DPPX, and IgLON5 as autoantibody tests; he has received an unrestricted research grant from Euroimmun. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Baulac S, Huberfeld G, Gourfinkel-An I, et al. First genetic evidence of GABA(A) receptor dysfunction in epilepsy: a mutation in the gamma2-subunit gene. Nat Genet 2001;28:46–48. [DOI] [PubMed] [Google Scholar]
- 2.Wallace RH, Marini C, Petrou S, et al. Mutant GABAA receptor γ2-subunit in childhood absence epilepsy and febrile seizures. Nat Genet 2001;28:49–52. [DOI] [PubMed] [Google Scholar]
- 3.Maljevic S, Krampfl K, Cobilanschi J, et al. A mutation in the GABA(A) receptor alpha(1)-subunit is associated with absence epilepsy. Ann Neurol 2006;59:983–987. [DOI] [PubMed] [Google Scholar]
- 4.Lachance-Touchette P, Martin C, Poulin C, Gravel M, Carmant L, Cossette P. Screening of GABRB3 in French-Canadian families with idiopathic generalized epilepsy. Epilepsia 2010;51:1894–1897. [DOI] [PubMed] [Google Scholar]
- 5.González MI, Grabenstatter HL, Cea-Del Rio CA, et al. Seizure-related regulation of GABAA receptors in spontaneously epileptic rats. Neurobiol Dis 2015;77:246–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greenfield LJ. Molecular mechanisms of antiseizure drug activity at GABAA receptors. Seizure 2013;22:589–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun Y, Wu Z, Kong S, et al. Regulation of epileptiform activity by two distinct subtypes of extrasynaptic GABAA receptors. Mol Brain 2013;6:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Petit-Pedrol M, Armangue T, Peng X, et al. Encephalitis with refractory seizures, status epilepticus, and antibodies to the GABAA receptor: a case series, characterisation of the antigen, and analysis of the effects of antibodies. Lancet Neurol 2014;13:276–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ohkawa T, Satake S, Yokoi N, et al. Identification and characterization of GABA(A) receptor autoantibodies in autoimmune encephalitis. J Neurosci 2014;34:8151–8163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pettingill P, Kramer HB, Coebergh JA, et al. Antibodies to GABAA receptor α1 and γ2 subunits: clinical and serologic characterization. Neurology 2015;84:1233–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Graus F, Titulaer MJ, Balu R, et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol 2016;15:391–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sulter G, Steen C. Use of the Barthel Index and modified Rankin Scale in acute stroke trials. Stroke 1999;30:1538–1541. [DOI] [PubMed] [Google Scholar]
- 13.Lai M, Hughes EG, Peng X, et al. AMPA receptor antibodies in limbic encephalitis alter synaptic receptor location. Ann Neurol 2009;65:424–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gresa-Arribas N, Planagumà J, Petit-Pedrol M, et al. Human neurexin-3α antibodies associate with encephalitis and alter synapse development. Neurology 2016;86:2235–2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dalmau J, Gleichman AJ, Hughes EG, et al. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol 2008;7:1091–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lai M, Huijbers MG, Lancaster E, et al. Investigation of LGI1 as the antigen in limbic encephalitis previously attributed to potassium channels: a case series. Lancet Neurol 2010;9:776–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lancaster E, Huijbers MGM, Bar V, et al. Investigations of caspr2, an autoantigen of encephalitis and neuromyotonia. Ann Neurol 2011;69:303–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lancaster E, Lai M, Peng X, et al. Antibodies to the GABA(B) receptor in limbic encephalitis with seizures: case series and characterisation of the antigen. Lancet Neurol 2010;9:67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Simabukuro MM, Petit-Pedrol M, Castro LH, et al. GABAA receptor and LGI1 antibody encephalitis in a patient with thymoma. Neurol Neuroimmunol Neuroinflammation 2015;2:e73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kramer U, Chi C-S, Lin K-L, et al. Febrile infection-related epilepsy syndrome (FIRES): pathogenesis, treatment, and outcome: a multicenter study on 77 children. Epilepsia 2011;52:1956–1965. [DOI] [PubMed] [Google Scholar]
- 21.Williams KA, Swedo SE. Post-infectious autoimmune disorders: Sydenham's chorea, PANDAS and beyond. Brain Res 2015;1617:144–154. [DOI] [PubMed] [Google Scholar]
- 22.Rathore GS, Leung KS, Muscal E. Autoimmune encephalitis following bone marrow transplantation. Pediatr Neurol 2015;53:253–256. [DOI] [PubMed] [Google Scholar]
- 23.Zhao CZ, Erickson J, Dalmau J. Clinical reasoning: agitation and psychosis in a patient after renal transplantation. Neurology 2012;79:e41–e44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Armangue T, Leypoldt F, Málaga I, et al. Herpes simplex virus encephalitis is a trigger of brain autoimmunity. Ann Neurol 2014;75:317–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.