Abstract
Objective:
To explore the association between postmenopausal hormone therapy (HT) and Alzheimer disease (AD).
Methods:
Twenty-year follow-up data from the Kuopio Osteoporosis Risk Factor and Prevention study cohort were used. Self-administered questionnaires were sent to all women aged 47–56 years, residing in Kuopio Province starting in 1989 until 2009, every 5th year. Register-based information on HT prescriptions was available since 1995. Probable AD cases, based on DSM-IV and National Institute of Neurological and Communicative Disorders and Stroke–Alzheimer's Disease and Related Disorders Association criteria, were identified from the special reimbursement register (1999–2009). The study population included 8,195 women (227 cases of incident AD).
Results:
Postmenopausal estrogen use was not associated with AD risk in register-based or self-reported data (hazard ratio/95% confidence interval 0.92/0.68–1.2, 0.99/0.75–1.3, respectively). Long-term self-reported postmenopausal HT was associated with reduced AD risk (0.53/0.31–0.91). Similar results were obtained with any dementia diagnosis in the hospital discharge register as an outcome.
Conclusions:
Our results do not provide strong evidence for a protective association between postmenopausal HT use and AD or dementia, although we observed a reduced AD risk among those with long-term self-reported HT use.
Alzheimer disease (AD), the most common form of dementia, accounts for 60%–80% of cases.1 Women have AD more often than men either due to their increased life expectancy2,3 or decline in sex steroid hormone levels around menopause,1,4 although some small studies have not detected this sex difference.5,6 Neuroprotective effects of estrogen have been observed in experimental animals,7–10 but clinical trials on postmenopausal hormone therapy (HT) use have not been successful,11–13 including the largest clinical trial to date, the Women's Health Initiative Memory Study (WHIMS).14 Observational studies support use of HT against AD if initiated around menopause in some15,16 but not all studies.17–20 Similarly, register-based studies have yielded conflicting results.18,20,21 Results from the Leisure World cohort revealed that the reduced risk of AD among HT users was a function of dose and duration.22,23
The aim of our study is to investigate the association of self-reported and register-recorded postmenopausal HT use with AD in a longitudinal prospective cohort, while taking into account various socioeconomic and lifestyle-related AD risk factors.
METHODS
Study population.
The present study is based on the 20-year follow-up of the population of the Kuopio Osteoporosis Risk Factor and Prevention study cohort. A self-administered baseline postal questionnaire was sent to all women aged 47–56 years who were residents of Kuopio Province, Eastern Finland (n = 14,220), in February 1989. A total of 13,100 (92.1%) women responded. The reasons for nonresponse were lack of address or residence not detected (n = 119) and death (n = 4). In addition, 947 women did not respond for other reasons and 50 returned a blank form. The 5-year follow-up in 1994, 10-year follow-up in 1999, 15-year follow-up in 2004, and 20-year follow-up in 2009 was mailed to the 13,100, 12,562, 12,075, and 11,420 women, respectively. A total of 11,954 (91.2%), 11,538 (91.8%), 10,926 (90.4%), and 8,195 (71.8%) women responded to the 5-, 10-, 15-, and 20-year follow-ups, respectively. The questionnaires were sent to women who had responded to the baseline inquiry, were alive, and had a valid postal address at that time.
The baseline questionnaire covered demographics, lifestyle, medical issues, and other characteristics such as age, height, weight, smoking, alcohol consumption, health disorders diagnosed by a physician, reproductive history, type and duration of HT use, operations, occupation, and physical activity. These questionnaires were repeated in the follow-up inquiries.
The present study included those 8,195 women who had complete data on confounders and self-reported exposure. Outcome data and register-based exposure data were available for all participants.
Outcomes.
The main outcome of our study was clinically verified AD diagnosis. These diagnoses from 1999–2009 were identified from the Finnish special reimbursement register maintained by the Social Insurance Institution (SII). The register contains information on the reimbursed drugs used for chronic illnesses such as AD. The Finnish special reimbursement register possesses high validity and positive predictive value for AD diagnosis.24 The Finnish Current Care Guideline recommends that all persons with AD are treated with antidementia drugs unless there is a specific contraindication (such as gastric ulcer/intestinal tract operation <6 months ago or severe asthma or chronic obstructive pulmonary disease for acetylcholinesterase inhibitors).25 In Finland, the diagnosis of probable AD is based on DSM-IV criteria for AD and National Institute of Neurologic and Communicative Disorders and Stroke–Alzheimer Disease and Related Disorders Association.26,27 The main diagnostic criteria were progressive decline in memory and cognition and exclusion of other reasons. Diagnosis of AD was supported by abnormal MRI or CSF biomarker findings typical for AD.
To be eligible for reimbursed AD medication (acetylcholinesterase inhibitors or memantine), a medical statement of a clinically verified AD diagnosis needs to be submitted to SII. Thus, case identification is carried out in clinics but is reconfirmed by SII. Summary of anamnestic information from the patients and family, as well as findings, e.g., MRI/CT, laboratory tests, and Consortium to Establish a Registry for Alzheimer's Disease, are submitted to the SII, where a geriatrician/neurologist systematically evaluates the diagnostic evidence for each AD case and confirms whether the prespecified criteria are met excluding possible alternative diagnoses for memory impairment such as severe depression, metabolic disturbances, and other forms of dementia such as vascular dementia without AD-like symptoms.
We also performed sensitivity analyses with dementia as an outcome. These data were extracted from the National Hospital Discharge register using the following ICD-10 codes: F00–F03 (F00, dementia in Alzheimer disease; F01, vascular dementia; F02, dementia in other diseases classified elsewhere; F03, unspecified dementia) and G30 (Alzheimer disease, early/late onset). This register includes all inpatient admissions. Diagnosis codes for each admission are recorded by attending physician. All Finnish citizens/long-term residents are covered by tax-supported public health service.
Use of HT.
Self-reported HT use was recorded as lifetime use in years (and indication of use) at the baseline inquiry in 1989. In all follow-up questionnaires, numbers of months per year of estrogen use were reported and the duration of self-reported estrogen use was calculated on basis of these questionnaires. Current medication prescribed by a physician, duration and purpose of use, and name of tablets used were asked in all inquiries. To exclude the possibility of recall bias in the self-reported questionnaires, we accessed the prescription register data to ascertain HT use. Vaginal products were excluded. HT was defined from the registry as those preparations having systemic estrogenic properties belonging to the following codes of ATC classification: G03C (estrogens), G03F (estrogen and progesterone) excluding oral contraceptives. Self-reported use of estrogen was categorized into postmenopausal HT based on use after the onset of menopause.
Covariates.
Baseline menopause status was categorized to 5 categories: (1) menstruating, no HT; (2) true menopause; (3) menstruating, HT 1–30 years; (4) hysterectomy and HT, no oophorectomy; (5) unclear (hysterectomy, no data on oophorectomy or HT use). When categorizing the self-reported HT to postmenopausal use, a woman was considered postmenopausal if ≥12 months had passed since her natural menstrual cycle, if she had undergone surgical menopause through bilateral oophorectomy with or without hysterectomy, or if the time since menopause and the history of HT use could be clarified from the follow-up questionnaire. For the present study, we used data on natural menopause (i.e., date of last menstruation, collected through self-reports at baseline and 5-year follow-up assessments). Over 90% of women were postmenopausal at the second follow-up. Missing information was collected from participants by telephone. Body mass index (BMI) was calculated as the ratio of weight in kilograms to height in meters squared and was based on self-reporting.
Physical activity was inquired through self-reported data in 3 ways at baseline and in all follow-up surveys as follows: leisure time physical activity as well as asking about how physically demanding work was in the last year; ambulatory status as capability and extent of movement, need of aids in movement, and history of joint degeneration; and amount of physical activity, including winter and summer activities, amount of current regular physical activity, and its duration (hours per week). Of these 3 categories, the amount of physical activity was the most predictive of AD and thus was included in the analyses.
Data on education were obtained in self-reported data only from a subcohort undergoing bone marrow density measurement. Education was categorized into 4 groups: compulsory schooling, compulsory schooling + maximum 2 years of supplementary school or occupational training, high school + minimum 2 years of supplementary school or professional training, and university degree. To assess whether the adjustment for education affected the results, we conducted a separate sensitivity analyses in the subcohort for whom we had education data (n = 2,383). History of ever/never smoking was asked in all self-reported questionnaires along with regularity of smoking, number of years of smoking, and number of cigarettes smoked per day.
Data on self-reported occupation were gathered under 9 different categories (managers; professionals; technicians and associate professionals; clerical support workers; service and sales workers; skilled agricultural, fish, and forestry; crafts and related trades workers; plant and machine operators; other occupation; and housewife/pensioner/caregiver/no occupation) but converted into a dichotomous variable (employed and unemployed) for the analyses. Alcohol consumption was inquired in questionnaires as the amount of alcohol beverages consumed during a 1-month period and converted into grams of alcohol intake per month.
At baseline, women were asked in questionnaire about the age at menarche, age at menopause, number of pregnancies, number of live births, and abortions. Abortion was inquired in questionnaire as “Number of times interrupting pregnancies due to abortion/miscarriage.” A history of any gynecologic operations (caesarian sections and sterilizations) was also obtained, as well as what, if anything, was removed in these operations (uterus, ovary, part of both, cervix, or other parts of genitals).
Standard protocol approvals, registrations, and patient consents.
The study design was approved by the Ethics Committee of Kuopio University Hospital and written informed consent was obtained from all participants.
Statistical analysis.
Statistical analysis was carried out with Stata 12.0 (Stata Corp LP, College Station, TX). The characteristics of women with respect to AD incidence were compared using χ2 test for categorical variables and t test for continuous variables. Correlation between confounders and exposure were investigated with the Spearman correlation coefficient. As expected, hysterectomy and oophorectomy were strongly correlated (r = 0.561) and were thus combined into one variable (surgery). There were no other indications of collinearity (r < 0.4).
Cox proportional hazard models were used to evaluate the association between HT use and AD incidence. Separate analyses were carried out for different durations of use and different types of HT. Hazard ratios and 95% confidence intervals were estimated with age-adjusted model and model adjusted for age, BMI, alcohol, smoking, physical activity, occupation status, number of births, menopause status, any cancer, and surgery. Since the education data were available for only a subset of participants, sensitivity analyses were conducted in this group separately. Association between HT and dementia was studied similarly.
We studied whether the association between HT and AD was different among women who had undergone oophorectomy or hysterectomy by assessing the interaction of hysterectomy or oophorectomy and self-reported and register-recorded HT use. There was no evidence for effect modification by these surgeries (All p values for interaction were >0.6) and thus stratified analyses were not performed.
RESULTS
Average age at AD diagnosis was 72.3 years (range 59.2–78.6 years). Women who later developed AD were significantly older than women without AD. They also reported less physical activity and were more likely to be unemployed than women who were not diagnosed with AD during the follow-up. Number of childbirths, abortions, and pregnancies was similar between groups, but women who developed AD were more likely to be menopausal at baseline than those who were not diagnosed with AD. Register-reported HT or oophorectomy/hysterectomy was not associated with AD in these univariable analyses (table e-1 at Neurology.org).
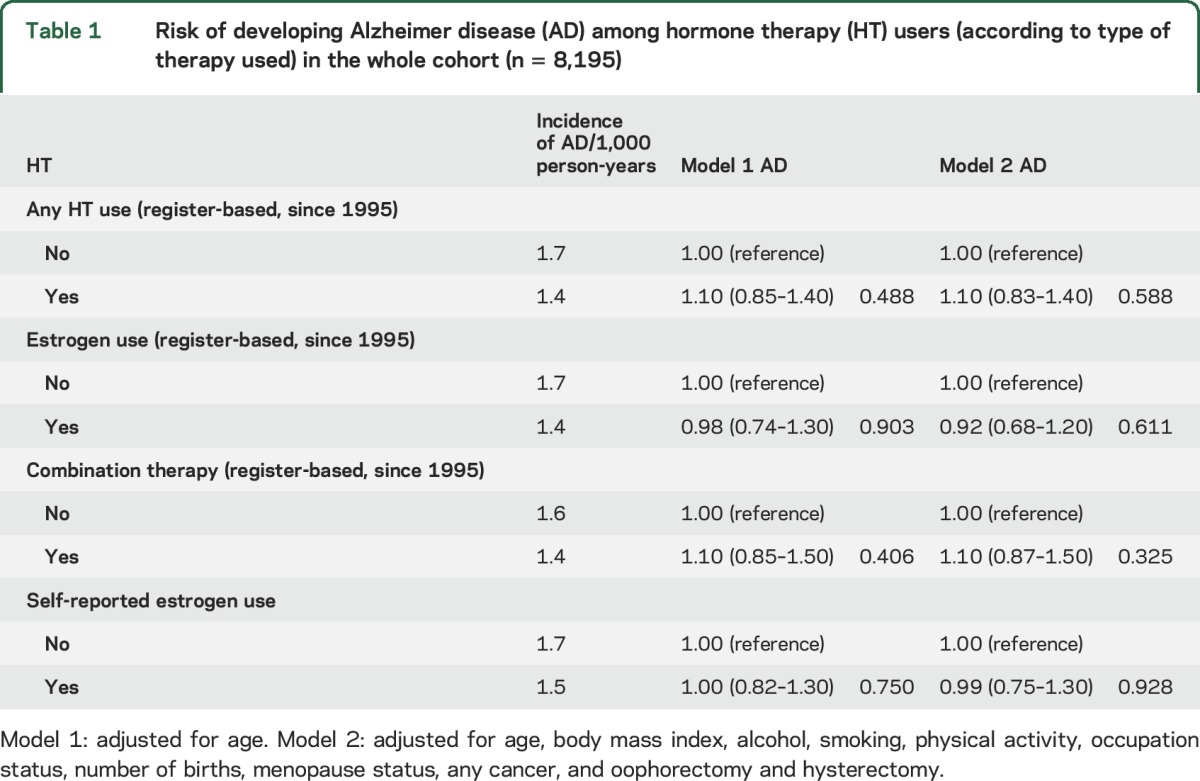
Table 1 shows the relative risk of AD according to both register-based and self-reported HT use (yes/no). Neither register-based nor self-reported HT use was associated with AD risk. The results were similar in the sensitivity analyses conducted among those with data on education (n = 2,383; tables e-2 and e-3), i.e., no association was detected between HT use (any HT, estrogen, or combination therapy; either register-based or self-reported) and AD.
Table 1.
Risk of developing Alzheimer disease (AD) among hormone therapy (HT) users (according to type of therapy used) in the whole cohort (n = 8,195)
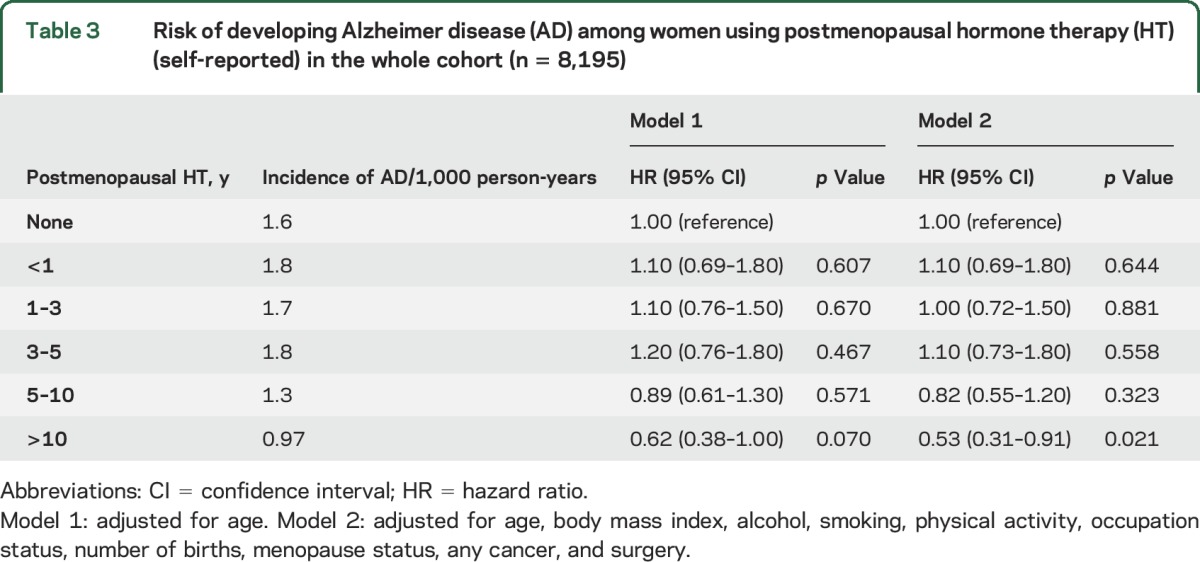
HT use when categorized according to duration register-based HT use was not associated with AD (table 2). However, when self-reported data were used, those with longest self-reported HT use >10 years had lower risk of AD in comparison to nonusers (table 3) also after adjusting for lifestyle and socioeconomic confounders and variables related to estrogen status. Sensitivity analyses with any dementia as an outcome produced similar results to those shown in tables 1–3, although the association between >10 years HT use and AD was attenuated and its confidence also included 1 in the sensitivity analyses (tables e-4 to e-6).
Table 2.
Risk of developing Alzheimer disease (AD) among hormone therapy (HT) users (register-based) with different durations of HT use in the whole cohort (n = 8,195)
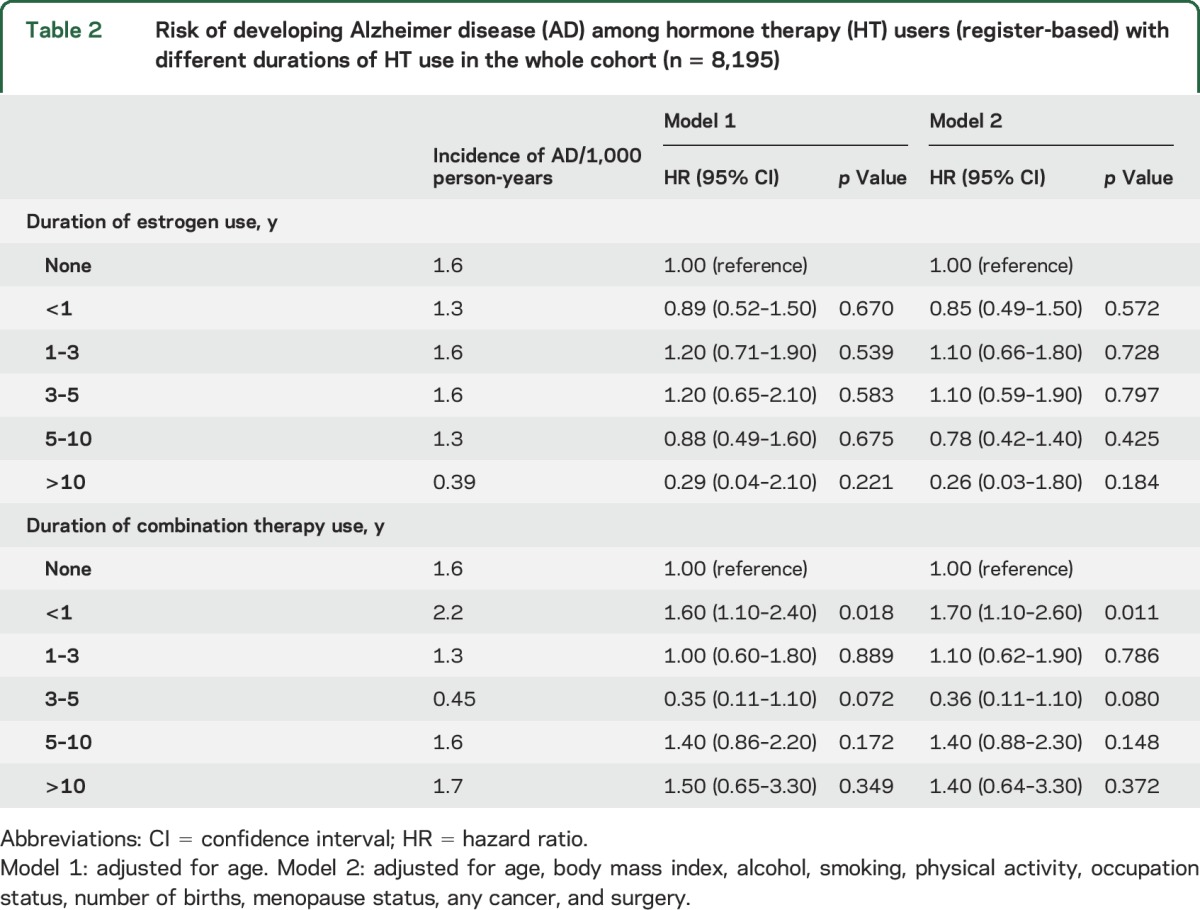
Table 3.
Risk of developing Alzheimer disease (AD) among women using postmenopausal hormone therapy (HT) (self-reported) in the whole cohort (n = 8,195)
DISCUSSION
The findings from our large prospective cohort study do not provide strong evidence for an association between postmenopausal HT use and AD or dementia, although a protective association between long-term (>10 years) self-reported use of HT and AD was observed. This finding indirectly favors the effectiveness of HT if started in the early postmenopausal period (critical window theory). Another explanation for this association can be reverse causation, i.e., AD (or more precisely, its preclinical symptoms) affecting the exposure to HT (or reporting of HT duration in the self-reported data), or a chance finding. The association with longer duration of HT use was only found in self-reported postmenopausal estrogen use, not for register-based HT use after 1995, and the association was less evident when all dementia diagnoses instead of clinically verified AD diagnosis were used as an outcome. One explanation for the different results with self-reported and register-based exposure could be different time period as register data were not available before 1995, which is 7 years after the baseline inquiry. Thus, the register-based data after 1995 would misclassify those who discontinued HT use before 1995 as nonusers. It is unlikely that this misclassification was differential in those who later developed AD and those who did not. Therefore it would lead to an underestimation of the association between HT use and AD with the register-based data after 1995. An alternative explanation is recall bias, but this is unlikely as previous validation study from the same cohort showed that a postal inquiry is a reliable method of recording long-term HT use in Finnish postmenopausal women.28
Our result is in line with the extended follow-up of the Cache County Study, which detected a reduced AD risk if HT was started within 5 years of menopause and continued for more than 10 years.15 The largest clinical trial on HT–all cause dementia association, i.e., WHIMS, assessed cognitive performance of women >65 years old, assigned to conjugated equine estrogen (CEE; unopposed therapy) vs placebo or continuous CEE + medroxyprogesterone acetate (CEE + MPA; opposed therapy) vs placebo. The use of opposed HT increased the dementia risk, but this did not occur with unopposed HT.14,29 In the WHIMS trial, women were randomized to receive opposed therapy as CEE combined with MPA. In Finland, estradiol in combination with norethisterone or levonorgestrel (not MPA) is used among women with intact uterus30 and only estradiol is used mainly among women undergoing hysterectomy.31 The difference between these 2 studies may be attributable to the differences in the effects exerted by the different estrogen preparations. In addition, the women in our cohort were around 10 years younger than women in the WHI trial, and the main outcome in our study was AD, while it was all-cause dementia in WHIMS.
One cohort study reported that an early initiation of HT around menopause was protective against AD.32 Similar findings were reported from the Multi-Institutional Research in Alzheimer Genetic Epidemiology study, where HT use in the youngest age tertiles (50–63 years) protected against AD in comparison to those in the older age tertiles.33
The lack of association in our cohort between any HT use (all users of HT, not taking into account either the time of initiation or the duration of therapy) and AD/dementia is consistent with previous observational studies where HT was categorized as former or current use and lifetime estrogen exposure was taken into account.34,35 Postmenopausal estrogen therapy was not protective against AD in 2 of the case-control studies.18,20 This discrepancy in observational studies is explained by differences in outcome measurement, number of observations, duration, type, and the time of initiation of HT use.
The strengths of our study include its large sample size, long follow-up time, relatively homogenous population, clinically verified AD cases, prescription-based validation of HT use, and control for various important comorbidities. Confounders and exposures were measured with the same questions during the follow-up. In addition, the positive predictive value of AD diagnosis in the Special Reimbursement Register is high.24 However, it is likely that this register does not capture all AD cases, or persons with other forms of dementia. Therefore we conducted sensitivity analysis with any dementia as an outcome. Despite these strengths, our study has some limitations: first, information was collected by self-reported postal inquiries, which may be subject to recall bias; second, the effect of healthy user bias among the long-term users cannot be overlooked; third, register data on HT use and AD diagnosis were available from 1995 to 1999 onwards, respectively; fourth, we cannot eliminate the chance of a survival bias contributing to the protective association observed among the longest HT users; fifth, we were not able to control the results for genetic predisposition to AD (APOE status and familial AD). However, it is unlikely that these genetic factors would influence start of HT use and thus it is unlikely that the results are confounded by genetic background.
Misclassification of outcome (i.e., undiagnosed AD or dementia cases) is possible and this would dilute our estimates towards the null. However, the proportion of misclassified persons is likely to be small. Due to our outcome data source in sensitivity analyses for dementia, we captured only those persons with dementia who required inpatient admission (due to dementia or other disease/accident). The diagnostic process for dementia often begins in an outpatient setting, but these settings were not included in the hospital discharge register. Therefore, those persons with dementia who did not have any hospital admissions or antidementia medications were misclassified.
Our study examined a homogenous population (residents of Kuopio Province, all accessed via the national social security system), thus decreasing the chances of selection bias or confounding by socioeconomic-related factors. Some previous observational studies carried out in sociodemographic homogenous populations have observed decreased risk of AD among HT users.23,36,37 Estrogen replacement therapy was protective against AD in these studies23,37 and sociodemographic heterogeneity may account for the contrasting results obtained in the 2 observational studies.18,20
Our results based on 2 decades of follow-up of a large homogenous population did not provide strong evidence for a protective association between postmenopausal HT use and AD or dementia.
Supplementary Material
ACKNOWLEDGMENT
The authors thank Seija Oinonen for technical help regarding data extraction.
GLOSSARY
- AD
Alzheimer disease
- BMI
body mass index
- CEE
conjugated equine estrogen
- DSM-IV
Diagnostic and Statistical Manual of Mental Disorders, 4th edition
- HT
hormone therapy
- ICD-10
International Classification of Diseases–10
- MPA
medroxyprogesterone acetate
- SII
Social Insurance Institution
- WHIMS
Women's Health Initiative Memory Study
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Dr. Imtiaz: planned the research project, drafted the first version of the manuscript, performed statistical analyses, acts as guarantor, accepted the submitted version of the manuscript, and made important intellectual contribution to draft versions. Dr. Tuppurainen: planned the research project, participated in data collection, accepted the submitted version of the manuscript, and made important intellectual contribution to draft versions. Dr. Rikkonen: participated in data analysis, accepted the submitted version of the manuscript, and made important intellectual contribution to draft versions. Prof. Kivipelto: accepted the submitted version of the manuscript and made important intellectual contribution to draft versions. Prof. Soininen: planned the research project, accepted the submitted version of the manuscript, and made important intellectual contribution to draft versions. Prof. Kröger: planned the research project, participated in data collection, accepted the submitted version of the manuscript, and made important intellectual contribution to draft versions. Dr. Tolppanen: planned the research project, supervised data analyses and the research project, accepted the submitted version of the manuscript, and made important intellectual contribution to draft versions.
STUDY FUNDING
Supported by Kuopio Osteoporosis Risk Factor and Prevention (OSTPRE) grant 250707. A.M.T. acknowledges funding from European Regional Development Fund (regional council of Pohjois Savo), M.K. is a recipient of Academy of Finland and Center for Innovative Medicine (CIMED), and H.S. received funding from the University of Eastern Finland for UEF Brain. B.I. acknowledges The Finnish Cultural Foundation (central fund) and funding by Doctoral Program of Molecular Medicine, University of Eastern Finland.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Alzheimer's Association. 2016 Alzheimer's disease facts and figures. Alzheimers Dement 2016;12. [DOI] [PubMed] [Google Scholar]
- 2.Hebert LE, Scherr PA, McCann JJ, Beckett LA, Evans DA. Is the risk of developing Alzheimer's disease greater for women than for men? Am J Epidemiol 2001;153:132–136. [DOI] [PubMed] [Google Scholar]
- 3.Seshadri S, Wolf PA, Beiser A, et al. Lifetime risk of dementia and Alzheimer's disease: the impact of mortality on risk estimates in the Framingham Study. Neurology 1997;49:1498–1504. [DOI] [PubMed] [Google Scholar]
- 4.Vest RS, Pike CJ. Gender, sex steroid hormones, and Alzheimer's disease. Horm Behav 2013;63:301–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yoshitake T, Kiyohara Y, Kato I, et al. Incidence and risk factors of vascular dementia and Alzheimer's disease in a defined elderly Japanese population: the Hisayama Study. Neurology 1995;45:1161–1168. [DOI] [PubMed] [Google Scholar]
- 6.Nilsson LV. Incidence of severe dementia in an urban sample followed from 70 to 79 years of age. Acta Psychiatr Scand 1984;70:478–486. [DOI] [PubMed] [Google Scholar]
- 7.Brinton RD, Chen S, Montoya M, Hsieh D, Minaya J. The estrogen replacement therapy of the Women's Health Initiative promotes the cellular mechanisms of memory and neuronal survival in neurons vulnerable to Alzheimer's disease. Maturitas 2000;34(suppl 2):S35–S52. [DOI] [PubMed] [Google Scholar]
- 8.Gibbs RB. Estrogen therapy and cognition: a review of the cholinergic hypothesis. Endocr Rev 2010;31:224–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tanapat P, Hastings NB, Reeves AJ, Gould E. Estrogen stimulates a transient increase in the number of new neurons in the dentate gyrus of the adult female rat. J Neurosci 1999;19:5792–5801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Behl C. Oestrogen as a neuroprotective hormone. Nat Rev Neurosci 2002;3:433–442. [DOI] [PubMed] [Google Scholar]
- 11.Henderson VW, Paganini-Hill A, Miller BL, et al. Estrogen for Alzheimer's disease in women: randomized, double-blind, placebo-controlled trial. Neurology 2000;54:295–301. [DOI] [PubMed] [Google Scholar]
- 12.Mulnard RA, Cotman CW, Kawas C, et al. Estrogen replacement therapy for treatment of mild to moderate Alzheimer disease: a randomized controlled trial: Alzheimer's Disease Cooperative Study. JAMA 2000;283:1007–1015. [DOI] [PubMed] [Google Scholar]
- 13.Wang PN, Liao SQ, Liu RS, et al. Effects of estrogen on cognition, mood, and cerebral blood flow in AD: a controlled study. Neurology 2000;54:2061–2066. [DOI] [PubMed] [Google Scholar]
- 14.Shumaker SA, Legault C, Rapp SR, et al. ; WHIMS Investigators. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women's Health Initiative Memory Study: a randomized controlled trial. JAMA 2003;289:2651–2662. [DOI] [PubMed] [Google Scholar]
- 15.Shao H, Breitner JC, Whitmer RA, et al. ; Cache County Investigators. Hormone therapy and Alzheimer disease dementia: new findings from the Cache County Study. Neurology 2012;79:1846–1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Whitmer RA, Quesenberry CP, Zhou J, Yaffe K. Timing of hormone therapy and dementia: the critical window theory revisited. Ann Neurol 2011;69:163–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barnes LL, Wilson RS, Schneider JA, Bienias JL, Evans DA, Bennett DA. Gender, cognitive decline, and risk of AD in older persons. Neurology 2003;60:1777–1781. [DOI] [PubMed] [Google Scholar]
- 18.Brenner DE, Kukull WA, Stergachis A, et al. Postmenopausal estrogen replacement therapy and the risk of Alzheimer's disease: a population-based case-control study. Am J Epidemiol 1994;140:262–267. [DOI] [PubMed] [Google Scholar]
- 19.Roberts RO, Cha RH, Knopman DS, Petersen RC, Rocca WA. Postmenopausal estrogen therapy and Alzheimer disease: overall negative findings. Alzheimer Dis Assoc Disord 2006;20:141–146. [DOI] [PubMed] [Google Scholar]
- 20.Seshadri S, Zornberg GL, Derby LE, Myers MW, Jick H, Drachman DA. Postmenopausal estrogen replacement therapy and the risk of Alzheimer disease. Arch Neurol 2001;58:435–440. [DOI] [PubMed] [Google Scholar]
- 21.Waring SC, Rocca WA, Petersen RC, O'Brien PC, Tangalos EG, Kokmen E. Postmenopausal estrogen replacement therapy and risk of AD: a population-based study. Neurology 1999;52:965–970. [DOI] [PubMed] [Google Scholar]
- 22.Paganini-Hill A, Henderson VW. Estrogen deficiency and risk of Alzheimer's disease in women. Am J Epidemiol 1994;140:256–261. [DOI] [PubMed] [Google Scholar]
- 23.Paganini-Hill A, Henderson VW. Estrogen replacement therapy and risk of Alzheimer disease. Arch Intern Med 1996;156:2213–2217. [PubMed] [Google Scholar]
- 24.Solomon A, Ngandu T, Soininen H, Hallikainen MM, Kivipelto M, Laatikainen T. Validity of dementia and Alzheimer's disease diagnoses in Finnish national registers. Alzheimers Dement 2014;10:303–309. [DOI] [PubMed] [Google Scholar]
- 25.Suhonen J, Pirttila T, Erkinjuntti T, et al. Suomalaisen laakariseuran duodecimin, societas gerontologica Fennican, suomen neurologisen yhdistyksen, suomen psykogeriatrisen yhdistyksen, suomen yleislaaketieteen yhdistyksen asettama tyoryhma, update on current care guidelines: the diagnosis and medical treatment of memory disorders. Duodecim 2010;126:2167–2168. [PubMed] [Google Scholar]
- 26.Quick Reference to the Diagnostic Criteria From DSM-IV-TR. Washington, DC:American Psychiatric Association; 2000. [Google Scholar]
- 27.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 1984;34:939–944. [DOI] [PubMed] [Google Scholar]
- 28.Sandini L, Pentti K, Tuppurainen M, Kroger H, Honkanen R. Agreement of self-reported estrogen use with prescription data: an analysis of women from the Kuopio Osteoporosis Risk Factor and Prevention Study. Menopause 2008;15:282–289. [DOI] [PubMed] [Google Scholar]
- 29.Shumaker SA, Legault C, Kuller L, et al. ; Women's Health Initiative Memory Study. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women's Health Initiative Memory Study. JAMA 2004;291:2947–2958. [DOI] [PubMed] [Google Scholar]
- 30.Pentti K, Tuppurainen MT, Honkanen R, et al. Hormone therapy protects from diabetes: the Kuopio osteoporosis risk factor and prevention study. Eur J Endocrinol 2009;160:979–983. [DOI] [PubMed] [Google Scholar]
- 31.Pentti K, Honkanen R, Tuppurainen MT, Sandini L, Kroger H, Saarikoski S. Hormone replacement therapy and mortality in 52- to 70-year-old women: the Kuopio osteoporosis risk factor and prevention study. Eur J Endocrinol 2006;154:101–107. [DOI] [PubMed] [Google Scholar]
- 32.Bagger YZ, Tanko LB, Alexandersen P, Qin G, Christiansen C; PERF Study Group. Early postmenopausal hormone therapy may prevent cognitive impairment later in life. Menopause 2005;12:12–17. [DOI] [PubMed] [Google Scholar]
- 33.Henderson VW, Benke KS, Green RC, Cupples LA, Farrer LA; MIRAGE Study Group. Postmenopausal hormone therapy and Alzheimer's disease risk: interaction with age. J Neurol Neurosurg Psychiatry 2005;76:103–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ryan J, Carriere I, Scali J, et al. Characteristics of hormone therapy, cognitive function, and dementia: the prospective 3C Study. Neurology 2009;73:1729–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ryan J, Carriere I, Scali J, Ritchie K, Ancelin ML. Lifetime estrogen exposure and cognitive functioning in later life. Psychoneuroendocrinology 2009;34:287–298. [DOI] [PubMed] [Google Scholar]
- 36.Zandi PP, Carlson MC, Plassman BL, et al. ; Cache County Memory Study Investigators. Hormone replacement therapy and incidence of Alzheimer disease in older women: the Cache County Study. JAMA 2002;288:2123–2129. [DOI] [PubMed] [Google Scholar]
- 37.Kawas C, Resnick S, Morrison A, et al. A prospective study of estrogen replacement therapy and the risk of developing Alzheimer's disease: the Baltimore Longitudinal Study of Aging. Neurology 1997;48:1517–1521. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


