Abstract
Objective:
To test whether higher global functional connectivity of the left frontal cortex (LFC) in Alzheimer disease (AD) is associated with more years of education (a proxy of cognitive reserve [CR]) and mitigates the association between AD-related fluorodeoxyglucose (FDG)-PET hypometabolism and episodic memory.
Methods:
Forty-four amyloid-PET–positive patients with amnestic mild cognitive impairment (MCI-Aβ+) and 24 amyloid-PET–negative healthy controls (HC) were included. Voxel-based linear regression analyses were used to test the association between years of education and FDG-PET in MCI-Aβ+, controlled for episodic memory performance. Global LFC (gLFC) connectivity was computed through seed-based resting-state fMRI correlations between the LFC (seed) and each voxel in the gray matter. In linear regression analyses, education as a predictor of gLFC connectivity and the interaction of gLFC connectivity × FDG-PET hypometabolism on episodic memory were tested.
Results:
FDG-PET metabolism in the precuneus was reduced in MCI-Aβ+ compared to HC (p = 0.028), with stronger reductions observed in MCI-Aβ+ with more years of education (p = 0.006). In MCI-Aβ+, higher gLFC connectivity was associated with more years of education (p = 0.021). At higher levels of gLFC connectivity, the association between precuneus FDG-PET hypometabolism and lower memory performance was attenuated (p = 0.027).
Conclusions:
Higher gLFC connectivity is a functional substrate of CR that helps to maintain episodic memory relatively well in the face of emerging FDG-PET hypometabolism in early-stage AD.
Cognitive reserve (CR) is defined as the ability to maintain cognition relatively well in the presence of brain pathology1 and is assessed mainly via proxies such as years of education or IQ. Neuroimaging studies in Alzheimer disease (AD) have shown that higher CR is associated with exacerbated temporoparietal fluorodeoxyglucose (FDG)-PET hypometabolism at a given level of cognitive performance,2–4 suggesting that CR allows the patient to better cope with AD pathology.1,5 The neural mechanisms underlying CR, however, are largely unknown. Previous resting-state fMRI studies suggest that global functional connectivity of the left frontal cortex (LFC) might support CR because it is associated with IQ (CR proxy) and cognitive performance in young individuals.6 As a major cortical hub within the cognitive control network, the LFC supports cognitive performance at a task-invariant level,7 which sets it apart from other task-specific changes that may relate to CR.8–12 Because the LFC is relatively spared in AD,13 we hypothesized that higher LFC functional integrity as measured by global LFC (gLFC) connectivity underlies CR in prodromal AD. In a first step, we aimed to confirm previous findings of the ability of patients with AD with higher CR (education) to maintain cognitive performance relatively well despite parietal FDG-PET hypometabolism.2,14 Second, we examined whether gLFC connectivity underlies CR, i.e., the increased ability to tolerate FDG-PET hypometabolism. Therefore, we tested whether in patients with prodromal AD higher gLFC connectivity is associated with more years of education (criterion of face validity) and attenuates the association between FDG-PET hypometabolism and memory impairment (criterion of cognitive benefit).
METHODS
Participants.
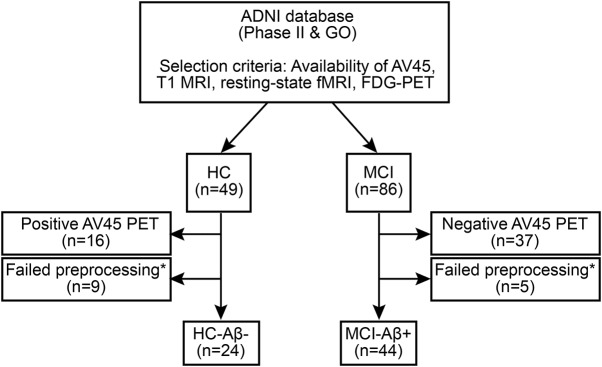
All participants were recruited within the Alzheimer's Disease Neuroimaging Initiative (ADNI; recruitment phases GO and II) and were selected on the basis of the availability of AV45-PET scans to assess β-amyloid (Aβ) levels, T1-weighted MRI, resting-state fMRI, FDG-PET, and cognitive testing. The final sample (see figure 1 for a flowchart) included 24 healthy controls (HC) and 44 patients with amnestic mild cognitive impairment (MCI) with elevated Aβ levels meeting research criteria for prodromal AD. MCI was diagnosed according to the Petersen15 criteria. With the use of pre-established cutoff values16 applied to the global AV45-PET standardized uptake value ratio (SUVR), participants were characterized as having abnormally high amyloid deposition (SUVR ≥ 1.11, MCI-Aβ+, n = 44). HC showed normal cognitive performance and normal AV45-PET SUVR values (SUVR < 1.11, n = 24). For details on PET acquisition, see appendix e-1 at Neurology.org.
Figure 1. Participant selection flowchart.
*Excluded because of excessive motion or failed segmentation, coregistration, or normalization. Aβ = β-amyloid; ADNI = Alzheimer's Disease Neuroimaging Initiative; FDG = fluorodeoxyglucose; HC = healthy controls; MCI = mild cognitive impairment.
Standard protocol approvals, registrations, and patient consents.
Ethics approval was obtained by the ADNI investigators. All study participants provided written informed consent.
Neuropsychological assessment and CR.
Episodic memory was measured through a previously described episodic memory composite score (ADNI-MEM) composite score.17 The ADNI-MEM score is a weighted factor score based on neuropsychological memory tests, including the Rey Auditory Verbal Learning Test, the Alzheimer's Disease Assessment Scale, the Wechsler Logical Memory I and II, and the word recall of the Mini-Mental State Examination. We expanded our analysis of gLFC connectivity on secondary nonmemory domains, including language function (Boston Naming Test [BNT]) and executive functions (Trail-Making Test B [TMT-B]). The TMT-B was chosen because we previously found this test to be affected early in AD.18 Consistent with our previous study2 and others (for a review, see reference 19), we used years of education as a CR proxy. Education is highly correlated with other CR proxies, including IQ20 or occupational attainment,21 and is to date the best established CR proxy.19
MRI acquisition.
MRI scans were performed on Philips 3T MRI scanners using an 8-channel coil. T1-weighted images were acquired with a 3-dimensional magnetization-prepared rapid gradient-echo sequence, with whole-brain coverage at a voxel resolution of 1 × 1 × 1.2 mm. Resting-state fMRI images were acquired with a single-shot T2*-weighted echo-planar imaging (EPI) sequence in transverse slice orientation (repetition time = 3,000 milliseconds, flip angle of 80°, 3.3-mm isotropic voxel resolution). Overall, 140 EPI volumes were acquired during which participants were instructed to keep their eyes open.
Preprocessing of FDG-PET data.
Preprocessing was conducted with SPM8 (Wellcome Trust Centre for Neuroimaging, University College London). Spatial normalization of FDG-PET images was performed with DARTEL,22 a nonlinear registration algorithm implemented in SPM8 (for details, see appendix e-2). All FDG-PET images were subsequently smoothed (8-mm full-width half-maximum gaussian kernel) and adjusted to the individual mean signal of the pons and cerebellar vermis to address interindividual differences.
Preprocessing of resting-state fMRI data.
The first 10 EPI volumes were discarded because of equilibration effects of the magnetic field. The remaining 130 volumes were realigned to the first volume, motion-corrected, registered to T1-weighted images, and smoothed (6-mm full-width half-maximum gaussian kernel). The DARTEL flow fields and affine transformation matrix were combined and applied to the registered EPI volumes for normalization to Montreal Neurological Institute space. To remove noise, all spatially normalized EPI images were detrended and band-pass filtered (0.01–0.08 Hz). Additionally, we regressed out the 6 motion parameters and the blood oxygen level–dependent signal averaged across the white matter and CSF.
Assessment of gLFC connectivity.
gLFC connectivity was determined through seed-based functional connectivity following a previously described protocol.6 The LFC seed (BA 6/44; Montreal Neurological Institute: x = −42, y = 6, z = 28) was determined as the peak coordinate of meta-analytically detected brain activation associated with cognitive control (for further details, see appendix e-3).23 We created an 8-mm sphere centered around the LFC coordinate (figure 2A), which was used as a seed for subsequent connectivity analyses. Using voxel-wise one-sample t tests, we explored the pattern of significant (p < 0.001) positive LFC functional connectivity in MCI-Aβ+, which was found preferentially, although not exclusively, to frontoparietal brain areas belonging to the cognitive control network (figure 2B). For the assessment of gLFC connectivity, the LFC region of interest (ROI) was superimposed onto the preprocessed and gray matter (GM)–masked resting-state scans to extract the mean LFC time series. Next, we calculated the Pearson-moment correlations between the LFC time series and each GM voxel. The resulting voxel-based correlations were Fisher z-transformed, and all positive voxel values were averaged to yield gLFC connectivity for each participant.6,24 The gLFC connectivity values were log-transformed and centered to achieve a gaussian distribution. Global connectivity was further assessed for 2 control ROIs (occipital pole [OP] and precuneus) to test the specificity of the LFC as a substrate of CR (for details, see appendix e-4).
Figure 2. Meta-analysis of brain activation related to cognitive control.
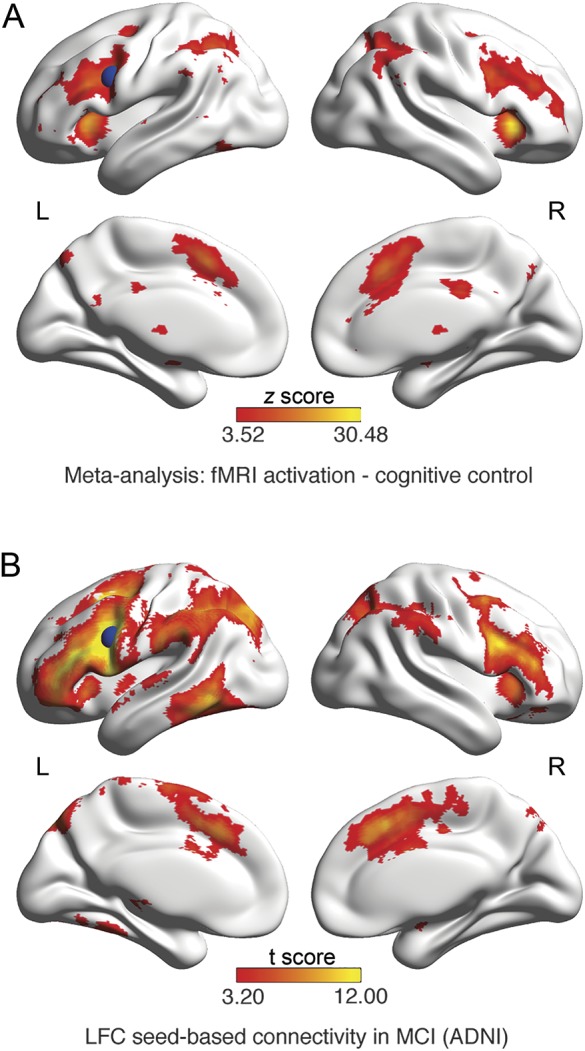
(A) Meta-analytical map of activation peaks across task fMRI studies that were associated with cognitive control (false discovery rate corrected at p < 0.01). Colors indicate z scores. Superimposed in blue is an 8-mm spherical region of interest (ROI) centered around the peak voxel of the left frontal cortex (LFC) cluster of the z map. The ROI was used as the seed region in the seed-based functional connectivity analysis to obtain global LFC connectivity in the resting-state fMRI scans. (B) Spatial pattern of positive LFC seed-based connectivity (t test against zero p < 0.001, family-wise error corrected at the cluster threshold p < 0.05). The analysis was restricted to voxels falling within the gray matter mask. ADNI = Alzheimer's Disease Neuroimaging Initiative; MCI = mild cognitive impairment.
Statistical analysis.
Continuous measures of demographics were compared between groups with 2-sample t tests; sex was compared with a χ2 test. To test whether more years of education allow the patient to cope better with FDG-PET hypometabolism in MCI-Aβ+,2 we applied voxel-wise regression including FDG-PET as the dependent variable and years of education (CR proxy) as the independent variable, controlling for age, sex, and ADNI-MEM. The voxel-wise analysis was restricted to the group-specific GM mask, and the resulting t statistics map was thresholded at the voxel level at α = 0.01 and corrected at the cluster level at α = 0.01. The FDG-PET value averaged across voxels within clusters of significant effects of education (a single cluster within the left precuneus; see Results) was computed for each participant and used as a marker of FDG-PET hypometabolism in MCI-Aβ+ in the subsequent analyses. In addition, the mean FDG-PET metabolism in the precuneus was extracted in HC. To test whether precuneus FDG-PET was decreased in MCI-Aβ+, we conducted an analysis of covariance, with group (HC vs MCI-Aβ+) as the predictor, controlling for age and sex. For testing our first hypothesis, i.e., that higher gLFC connectivity is associated with more years of education in MCI-Aβ+, we used linear regression analysis including gLFC connectivity as the dependent variable and education, age, and sex as independent variables. For testing our second hypothesis, i.e., that higher gLFC connectivity attenuates the association between FDG-PET hypometabolism and episodic memory impairment, we tested in a linear regression analysis the interaction of gLFC connectivity × FDG-PET (precuneus cluster) on ADNI-MEM, controlled for age and sex in MCI-Aβ+. Because our second hypothesis clearly specifies a directionality of the effects, i.e., detriments in memory performance associated with FDG-PET hypometabolism are worse at lower compared to higher levels of gLFC connectivity, we applied a one-tailed significance threshold to test this hypotheses (p < 0.05). Hypothesis 2 was also tested for secondary cognitive outcome measures, including BNT and TMT-B. To test the specificity of the gLFC connectivity as a substrate of CR, we conducted control analyses testing global connectivity of the OP and precuneus instead of the LFC. To this end, all regression analyses were repeated in an analogous way, this time replacing gLFC connectivity by global connectivity of the OP or precuneus. For each regression model, gaussianity of the distribution of the residuals was tested with the Shapiro-Wilk test, where none of the models showed significant deviation (α = 0.05). The variance inflation factor was <10 for all models tested, indicating that there was no multicollinearity among predictors.
Voxel-wise regression analyses were computed with SPM8. Further analyses were computed with the R statistical software package (r-project.org).25 Regression parameters of log-transformed regressors were back-transformed to ensure interpretability.
RESULTS
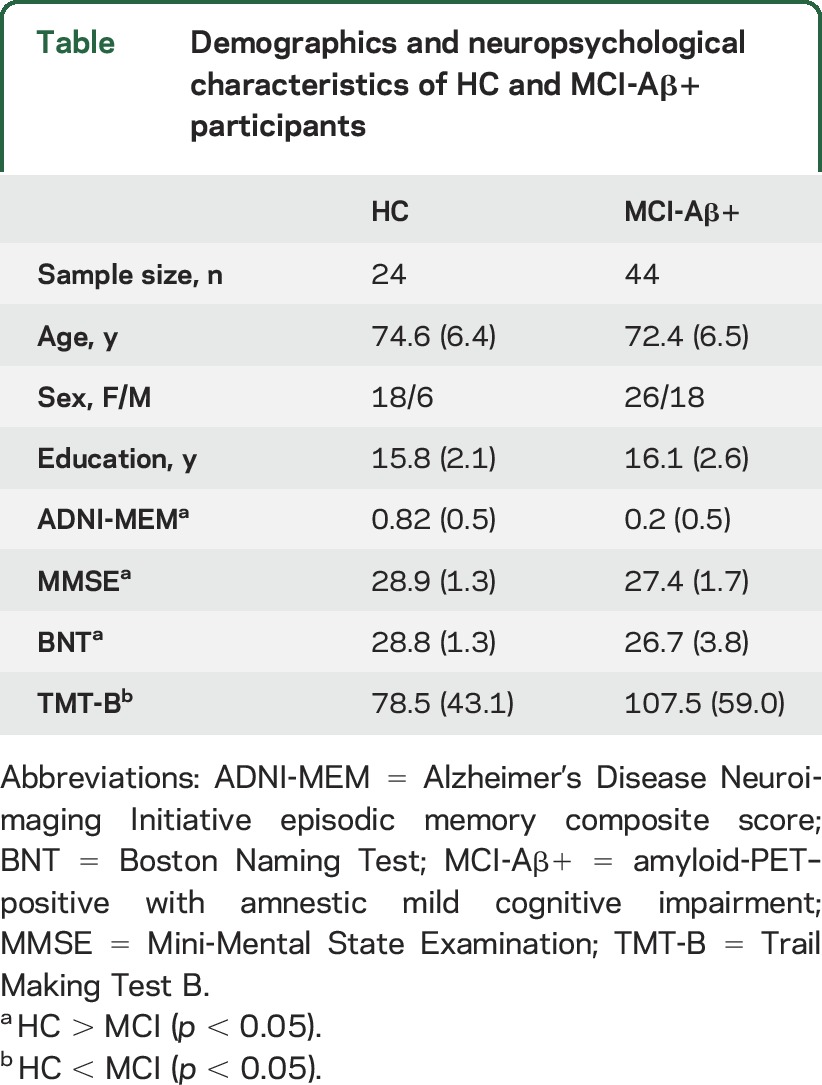
Descriptive statistics for each group are displayed in the table. Two-sample t tests showed that HC performed significantly better than MCI-Aβ+ on all cognitive measures.
Table.
Demographics and neuropsychological characteristics of HC and MCI-Aβ+ participants
Association between years of education and FDG-PET in MCI-Aβ+.
Voxel-wise multiple regression analysis of FDG-PET data showed that more years of education were associated with lower FDG-PET metabolism within a single cluster within the left precuneus (B = −0.025, SE = 0.006, t [39] = 2.43, p = 0.006), controlled for ADNI-MEM, age, and sex (figure 3A). The association between education and the average FDG-PET within the precuneus cluster is plotted in figure 3B.
Figure 3. Voxel-wise regression for education predicting FDG-PET controlling for memory performance in MCI-Aβ+.
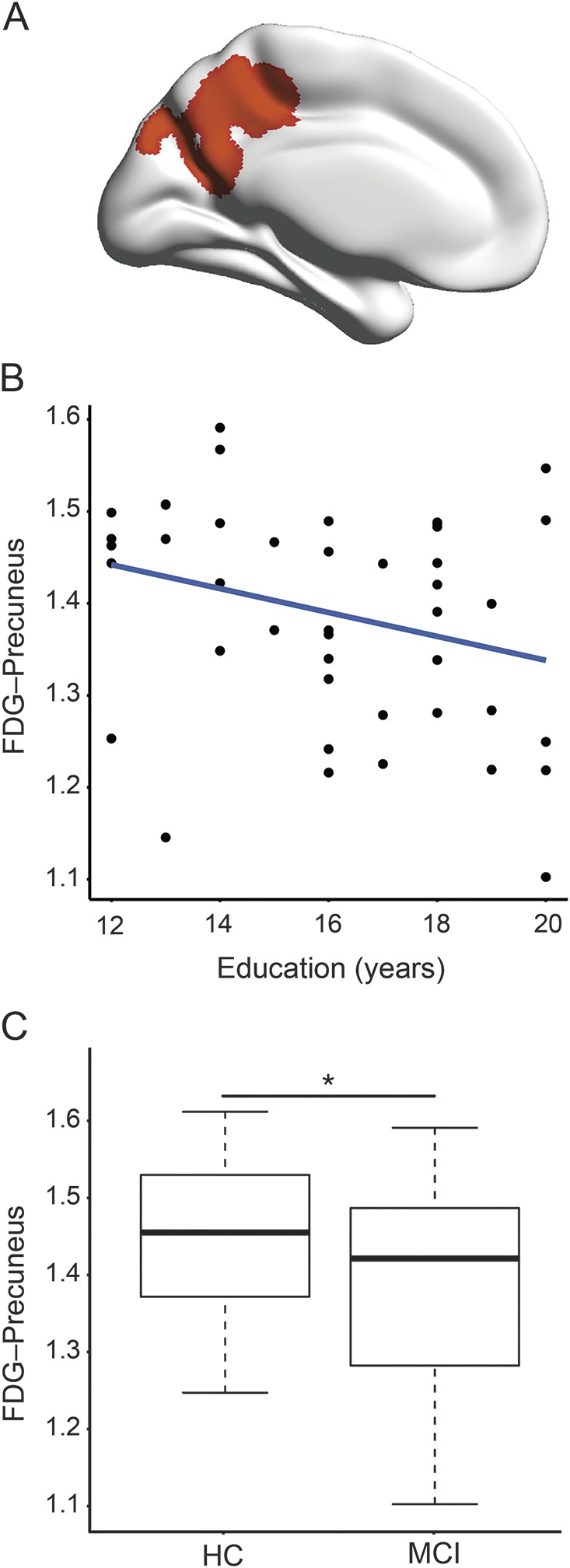
(A) Location of the precuneus cluster in the left hemisphere, where amyloid-PET–positive patients with amnestic mild cognitive impairment (MCI-Aβ+) with more years of education showed lower fluorodeoxyglucose (FDG)-PET metabolism when controlling for memory performance, age, and sex (p < 0.01 corrected at the cluster threshold at p < 0.01). (B) Scatterplot for the regression model of education on the average precuneus FDG-PET metabolism in MCI-Aβ+. (C) Box plot for the group comparison (MCI-Aβ+ vs healthy controls [HC]) in precuneus FDG-PET metabolism. Precuneus FDG-PET metabolism was significantly reduced in MCI-Aβ+, controlled for age and sex (p = 0.028).
Results of the analysis of covariance showed that FDG-PET within the precuneus cluster was significantly reduced in the MCI-Aβ+ compared to HC (F62 = 5.056, p = 0.028), controlled for age and sex (figure 3C), suggesting that precuneus FDG-PET was pathologically reduced in MCI-Aβ+.
Association among gLFC connectivity, education, and precuneus FDG-PET in MCI-Aβ+.
Next, we tested our hypothesis that gLFC connectivity is associated with more years of education (CR proxy) in MCI-Aβ+ (criterion of face validity). More years of education predicted higher gLFC connectivity, controlled for age and sex in the MCI-Aβ+ (B = 0.07, SE = 0.03, t [40] = 2.401, p = 0.021; figure 4A) but not in HC (p = 0.49). gLFC connectivity was not associated with precuneus FDG-PET metabolism in HC (p = 0.25) or MCI-Aβ+ participants (p = 0.12).
Figure 4. Scatterplot for the interaction of gLFC connectivity × precuneus FDG-PET metabolism on memory performance in MCI-Aβ+.
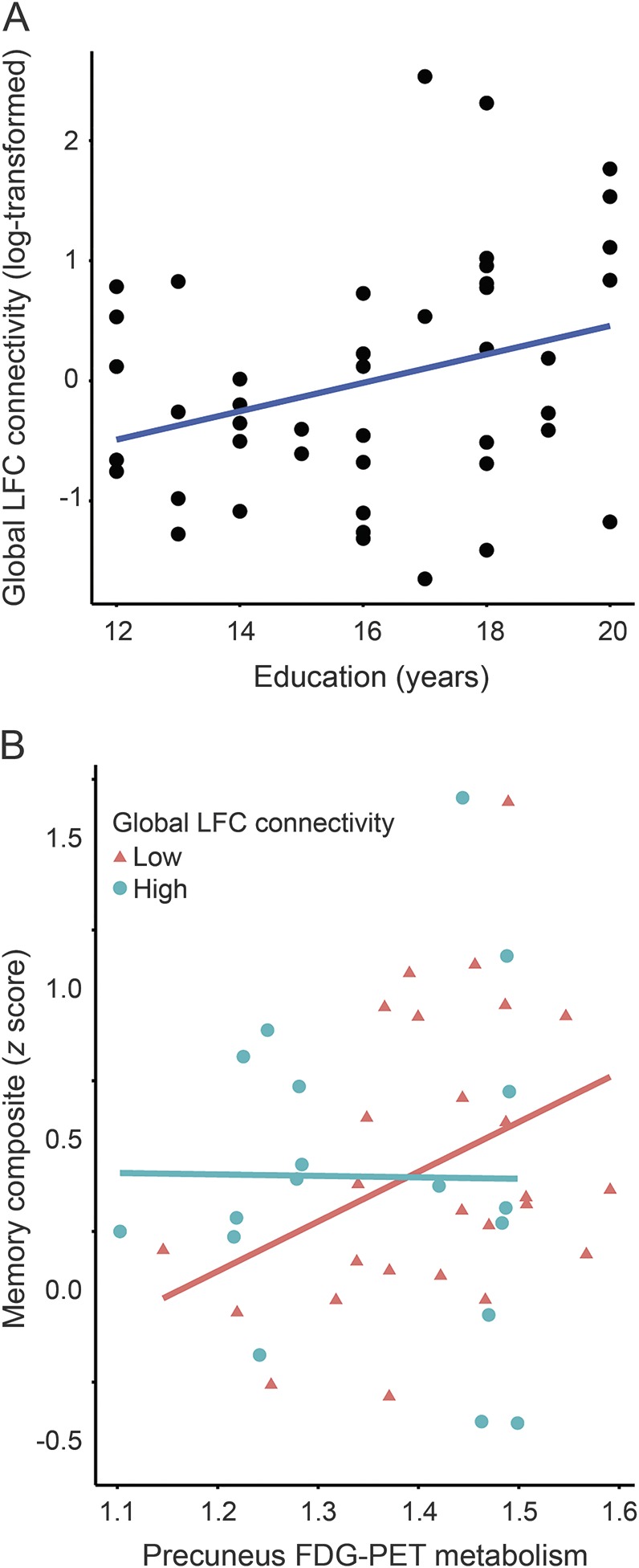
Association among education, global left frontal cortex (gLFC) connectivity, and precuneus fluorodeoxyglucose (FDG)-PET metabolism in amyloid-PET–positive patients with amnestic mild cognitive impairment (MCI-Aβ+). (A) Scatterplot of the association between years of education and gLFC connectivity. (B) Scatterplot for the interaction gLFC connectivity × precuneus FDG-PET metabolism on memory performance in MCI-Aβ+. Precuneus FDG-PET metabolism is plotted against the Alzheimer's Disease Neuroimaging Initiative memory score for participants with high and low gLFC connectivity. For illustrational purposes, groups of high and low gLFC connectivity (defined via median split) are plotted separately.
Addressing our main hypothesis, we assessed whether higher gLFC connectivity is associated with an attenuated effect of precuneus FDG-PET hypometabolism on memory in MCI-Aβ+ (criterion of cognitive benefit). The interaction effect gLFC connectivity × precuneus FDG-PET on ADNI-MEM was significant (B = −10.43, SE = 2.62, t [38] = −1.988, p = 0.027, hypothesis-free 2-tailed p = 0.054). Figure 4B shows that at low levels of gLFC connectivity, lower precuneus FDG-PET metabolism was associated with worse memory performance, whereas at higher levels of gLFC connectivity, the association between precuneus FDG-PET metabolism and ADNI-MEM was not observed. These results suggest that in the presence of higher gLFC connectivity, the detrimental effect of AD-related precuneus FDG-PET hypometabolism on memory is reduced. We detected no significant interaction effects on secondary cognitive measures, including BNT (p = 0.78) and TMT-B (p = 0.12). Control analyses including global connectivity of the OP or precuneus showed no significant associations with education (OP p = 0.34; precuneus p = 0.51) or precuneus FDG-PET metabolism (OP p = 0.33; precuneus p = 0.23). The interaction effect including ROI global connectivity × precuneus FDG-PET metabolism on ADNI-MEM was significant for none of the control ROIs (OP p = 0.58; precuneus p = 0.67).
DISCUSSION
Our major findings were that in prodromal AD, more years of education (CR proxy) allowed the patient to better cope with precuneus FDG-PET hypometabolism, higher gLFC connectivity was associated with more years of education (CR proxy; criterion of face validity), and higher gLFC connectivity was associated with milder effects of precuneus FDG-PET hypometabolism on memory performance (criterion of cognitive benefit). Together, these findings suggest that higher gLFC connectivity met major a priori defined criteria as a substrate of CR, including the association with a common CR proxy (education) and a beneficial effect that moderated the association between AD core pathology (precuneus FDG-PET hypometabolism) and the level of memory impairment. No association with education was found for global functional connectivity of 2 control ROIs (OP and precuneus), suggesting that our findings are specific to gLFC connectivity.
First, we showed that in MCI-Aβ+ more years of education were associated with stronger precuneus FDG-PET hypometabolism when controlling for memory performance. These findings are consistent with results of previous studies showing an association between higher CR proxies and lower parietal FDG-PET or perfusion in AD,2,5,26,27 providing indirect evidence for an enhanced ability in patients with AD with greater education to cope better with brain pathology than those patients with lower education.
Next, we tested in a series of analyses whether gLFC connectivity may subserve protective effects in MCI-Aβ+. First, we showed an association between years of education and gLFC connectivity (criterion of face validity). This is in agreement with previous studies in healthy individuals in which more years of education were associated with increased frontal lobe function, i.e., higher anterior cingulate FDG-PET metabolism and fMRI functional connectivity.28 Similarly, more years of education are associated with higher frontal FDG-PET metabolism in prodromal AD.3 We found that higher gLFC connectivity was associated with more years of education in MCI-Aβ+ but not in HC. Higher LFC connectivity is most likely a functional difference associated with higher education that existed before the onset of MCI, in line with the idea of neural reserve.29 Ceiling effects, reduced interindividual variability of gLFC connectivity in HC, and a small sample size of 24 may have reduced the power to detect an association between education and gLFC connectivity. We caution that the primary focus of the current study was on CR in MCI. gLFC connectivity as a putative substrate of CR in elderly HC participants should be assessed in larger future studies.
For our second a priori defined criterion of CR-related brain differences, we postulated that any putative brain substrate of CR should be associated with higher levels of cognitive performance in the face of brain pathology (criterion of cognitive benefit).30,31 Consistent with this hypothesis, we showed that gLFC connectivity not only was associated with a CR proxy (education) but also was beneficial with regard to memory performance in the presence of brain pathology (precuneus FDG-PET hypometabolism). Our finding is reminiscent of a previous study in AD showing that at higher levels of CR (as measured by IQ), the association between precuneus Aβ deposition and memory impairment was attenuated.32 Our results suggest that gLFC connectivity may underlie such compensatory effects. Here, we found an interaction between gLFC connectivity and precuneus FDG-PET only on memory but not on secondary cognitive measures, including BNT and TMT-B. A possible explanation is that deficits in BNT or TMT-B are related to frontal and temporal FDG-PET hypometabolism rather than precuneus FDG-PET hypometabolism, as shown previously.33,34 Thus, it is possible that FDG-PET hypometabolism in areas other than the precuneus may have shown an interaction with gLFC connectivity on these nonmemory domains.
It is unclear how gLFC connectivity may support cognition in prodromal AD. One possibility is that because of its widespread connectedness, the LFC interacts with and regulates the activity of functional networks. According to the flexible-hub theory, the LFC shows adaptive functional connectivity to other brain regions during cognitive processes.35 A recent fMRI study testing different cognitive tasks showed that the LFC dynamically shifts connectivity to different networks across cognitive tasks.36 Previous resting-state fMRI studies reported that the LFC is characterized by a high participation coefficient with regard to its connectivity to major networks in the brain.37 At the cognitive level, increased gLFC connectivity was shown to be associated with increased cognitive control, higher IQ, and higher cognitive performance in young individuals.6 Together, these studies suggest that the LFC, possessing high global connectivity, may flexibly orchestrate the activation of specific networks during cognitive tasks and may thus enhance cognitive performance. Applied to the current findings, this means that high gLFC connectivity may be linked to greater flexibility in neural network activation to compensate for local neurodegeneration (i.e., precuneus FDG-PET hypometabolism) in patients with prodromal AD. Another possibility is that the LFC exerts a beneficial effect on cognitive performance particularly through its association with the frontoparietal control network, also called the task-positive network.38 Our spatial mapping of significant LFC connectivity supports particularly high functional connectivity with frontoparietal brain areas belonging to the control network, consistent with previous findings.6,7
In cognitively normal elderly with abnormal levels of amyloid PET, increased activation of the control network during memory encoding was associated with increased memory performance.39 These results suggest that increased frontoparietal activation may play a compensatory and beneficial role during memory performance in the early stage of AD. Future studies may investigate whether, in patients with AD with high CR, increased gLFC connectivity may support such increased frontoparietal activation and thus support cognitive function. While the exact compensatory mechanism of the LFC needs to be confirmed in future studies, the current results suggest that the LFC is associated with CR and may moderate the association between AD pathology and cognitive impairment.
For the interpretation of the current results, several caveats should be considered. First, we examined gLFC connectivity during resting-state fMRI, which may not necessarily translate into increased connectivity or activation during cognitive tasks because the association between connectivity and task-related brain activation can be complex.40 However, a previous study showed that during working memory task fMRI, the LFC connectivity increased in a task-related manner, suggesting a direct role of LFC connectivity during task performance.7 We specifically chose resting-state gLFC connectivity as a candidate neural marker of CR that can be readily assessed in patients with cognitive deficits and does not depend on a particular cognitive task. However, future studies should assess to what extent gLFC connectivity supports compensatory task-related brain activation in AD. Second, we examined only MCI-Aβ+ patients in the stage of prodromal AD. Thus, the generalization to other stages of AD and cognitive functions remains open. Because in AD the frontal lobe function is relatively spared until a late stage, the gLFC connectivity may be a prime candidate to show a generalized cognitive performance-enhancing function in AD. Overall, gLFC connectivity is a promising candidate marker of CR in AD that may play a compensatory and cognitively beneficial role when AD pathology emerges. The identification of the locus of functional brain mechanisms related to CR opens up the possibility to specifically train and stimulate such brain mechanisms through neurofeedback, transcranial direct current stimulation, or drugs to enhance compensatory brain mechanisms and to slow down the cognitive decline in AD.
Supplementary Material
GLOSSARY
- Aβ
β-amyloid
- AD
Alzheimer disease
- ADNI
Alzheimer's Disease Neuroimaging Initiative
- BNT
Boston Naming Test
- CR
cognitive reserve
- EPI
echo-planar imaging
- FDG
fluorodeoxyglucose
- gLFC
global left frontal cortex
- GM
gray matter
- HC
healthy controls
- LFC
left frontal cortex
- MCI
mild cognitive impairment
- MEM
episodic memory composite score
- OP
occipital pole
- ROI
region of interest
- SUVR
standardized uptake value ratio
- TMT-B
Trail-Making Test B
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
N. Franzmeier: study concept and design, statistical analysis, interpretation of the data, drafting the manuscript. M. Duering: interpretation of the data, revising the manuscript. M. Weiner and M. Dichgans: revising the manuscript. M. Ewers: study concept and design, interpretation of the data, drafting the manuscript.
STUDY FUNDING
The study was funded by an European Research Council career integration grant (PCIG12-GA-2012-334259 to M.E.), LMUexcellent (to M.E.), and Alzheimer Forschung Initiative (to M. Dichgans and M.E.). Data collection and sharing for this project was funded by the ADNI (NIH grant U01 AG024904) and Department of Defense ADNI (Department of Defense award W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, by the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer's Association; Alzheimer's Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc; Biogen; Bristol-Myers Squibb Co; CereSpir, Inc; Eisai Inc; Elan Pharmaceuticals, Inc; Eli Lilly and Co; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc; Fujirebio; GE Healthcare; IXICO Ltd; Janssen Alzheimer Immunotherapy Research & Development, LLC; Johnson & Johnson Pharmaceutical Research & Development LLC; Lumosity; Lundbeck; Merck & Co, Inc; Meso Scale Diagnostics, LLC; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corp; Pfizer Inc; Piramal Imaging; Servier; Takeda Pharmaceutical Co; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer's Disease Cooperative Study at the University of California, San Diego. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
DISCLOSURE
N. Franzmeier received travel funding from the Alzheimer's Association. M. Düring received travel expenses for lectures from the United Leukodystrophy Foundation, the European Stroke Conference, and Kenes International. He receives research support from the German Center for Neurodegenerative Diseases. M. Weiner reports grants from General Electric, grants from NIH/National Institute of Mental Health/National Institute on Aging, grants from the Department of Defense, grants from the California Department of Public Health, grants from The Drew Foundation, grants from Rosenberg Alzheimer's Project, personal fees from Synarc, personal fees from Pfizer, personal fees from Janssen, personal fees from Merck, personal fees from Eli Lilly, personal fees from Alzheimer's Drug Discovery Foundation, personal fees from Neurotrope Bioscience, personal fees from Alzheon, Inc, personal fees from Avid Radiopharmaceuticals, personal fees from Clearview Healthcare Partners, personal fees from Bioclinica, personal fees from Araclon, personal fees from Genentech, outside the submitted work, and he is employed by the University of California San Francisco and the Veterans Administration Medical Center. M. Dichgans receives research support from the German Research Foundation, German Federal Ministry of Education and Research, the European Union (EU FP6 and FP7), Vascular Dementia Research Foundation, Corona Foundation, and Jackstädt Foundation. He received travel expenses and honoraria for lectures from Bayer Vital, Boehringer Ingelheim Pharma, Heel, Bristol-Meyer Squibb, Lundeck, Sanofi-Aventis, Ever Pharma, and Shire, as well as for educational activities not funded by industry; received honoraria for articles for Thieme, UpToDate, and Kohlhammer; serves as an editor for Stroke, the International Journal of Stroke, and the Journal of Neurochemistry and as an editorial board member of Cerebrovascular Diseases; and serves as a consultant for Bayer Vital, Boehringer Ingelheim, Bristol-Meyer Squibb, Ever Pharma, and Heel. M. Ewers received funding from LMUexcellent, European Research Council, and the Alzheimer Forschung Initiative, and honoraria from the Deutsche Gesellschaft für Neurologie. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Stern Y. Cognitive reserve and Alzheimer disease. Alzheimer Dis Assoc Disord 2006;20:S69–S74. [DOI] [PubMed] [Google Scholar]
- 2.Ewers M, Insel PS, Stern Y, Weiner MW, Alzheimer's Disease Neuroimaging I: cognitive reserve associated with FDG-PET in preclinical Alzheimer disease. Neurology 2013;80:1194–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morbelli S, Perneczky R, Drzezga A, et al. Metabolic networks underlying cognitive reserve in prodromal Alzheimer disease: a European Alzheimer disease consortium project. J Nucl Med 2013;54:894–902. [DOI] [PubMed] [Google Scholar]
- 4.Scarmeas N, Zarahn E, Anderson KE, et al. Association of life activities with cerebral blood flow in Alzheimer disease: implications for the cognitive reserve hypothesis. Arch Neurol 2003;60:359–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boots EA, Schultz SA, Almeida RP, et al. Occupational complexity and cognitive reserve in a middle-aged cohort at risk for Alzheimer's disease. Arch Clin Neuropsychol 2015;30:634–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cole MW, Yarkoni T, Repovs G, Anticevic A, Braver TS. Global connectivity of prefrontal cortex predicts cognitive control and intelligence. J Neurosci 2012;32:8988–8999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cole MW, Reynolds JR, Power JD, Repovs G, Anticevic A, Braver TS. Multi-task connectivity reveals flexible hubs for adaptive task control. Nat Neurosci 2013;16:1348–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scarmeas N, Zarahn E, Anderson KE, et al. Cognitive reserve-mediated modulation of positron emission tomographic activations during memory tasks in Alzheimer disease. Arch Neurol 2004;61:73–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bosch B, Bartres-Faz D, Rami L, et al. Cognitive reserve modulates task-induced activations and deactivations in healthy elders, amnestic mild cognitive impairment and mild Alzheimer's disease. Cortex 2010;46:451–461. [DOI] [PubMed] [Google Scholar]
- 10.Solé-Padullés C, Bartrés-Faz D, Junqué C, et al. Brain structure and function related to cognitive reserve variables in normal aging, mild cognitive impairment and Alzheimer's disease. Neurobiol Aging 2009;30:1114–1124. [DOI] [PubMed] [Google Scholar]
- 11.Stern Y, Habeck C, Moeller J, et al. Brain networks associated with cognitive reserve in healthy young and old adults. Cereb Cortex 2005;15:394–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stern Y, Zarahn E, Habeck C, et al. A common neural network for cognitive reserve in verbal and object working memory in young but not old. Cereb Cortex 2008;18:959–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boccia M, Acierno M, Piccardi L. Neuroanatomy of Alzheimer's disease and late-life depression: a coordinate-based meta-analysis of MRI studies. J Alzheimers Dis 2015;46:963–970. [DOI] [PubMed] [Google Scholar]
- 14.Morbelli S, Nobili F. Cognitive reserve and clinical expression of Alzheimer's disease: evidence and implications for brain PET imaging. Am J Nucl Med Mol Imaging 2014;4:239–247. [PMC free article] [PubMed] [Google Scholar]
- 15.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med 2004;256:183–194. [DOI] [PubMed] [Google Scholar]
- 16.Landau SM, Breault C, Joshi AD, et al. Amyloid-beta imaging with Pittsburgh compound B and florbetapir: comparing radiotracers and quantification methods. J Nucl Med 2013;54:70–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crane PK, Carle A, Gibbons LE, et al. Development and assessment of a composite score for memory in the Alzheimer's Disease Neuroimaging Initiative (ADNI). Brain Imaging Behav 2012;6:502–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ewers M, Brendel M, Rizk-Jackson A, et al. Reduced FDG-PET brain metabolism and executive function predict clinical progression in elderly healthy subjects. Neuroimage Clin 2014;4:45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stern Y. Cognitive reserve in ageing and Alzheimer's disease. Lancet Neurol 2012;11:1006–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deary IJ, Johnson W. Intelligence and education: causal perceptions drive analytic processes and therefore conclusions. Int J Epidemiol 2010;39:1362–1369. [DOI] [PubMed] [Google Scholar]
- 21.Nucci M, Mapelli D, Mondini S. Cognitive Reserve Index questionnaire (CRIq): a new instrument for measuring cognitive reserve. Aging Clin Exp Res 2012;24:218–226. [DOI] [PubMed] [Google Scholar]
- 22.Ashburner J. A fast diffeomorphic image registration algorithm. NeuroImage 2007;38:95–113. [DOI] [PubMed] [Google Scholar]
- 23.Yarkoni T, Poldrack RA, Nichols TE, Van Essen DC, Wager TD. Large-scale automated synthesis of human functional neuroimaging data. Nat Methods 2011;8:665–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cole MW, Pathak S, Schneider W. Identifying the brain's most globally connected regions. NeuroImage 2010;49:3132–3148. [DOI] [PubMed] [Google Scholar]
- 25.R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2014. [Google Scholar]
- 26.Stern Y, Alexander GE, Prohovnik I, et al. Relationship between lifetime occupation and parietal flow: implications for a reserve against Alzheimer's disease pathology. Neurology 1995;45:55–60. [DOI] [PubMed] [Google Scholar]
- 27.Bastin C, Yakushev I, Bahri MA, et al. Cognitive reserve impacts on inter-individual variability in resting-state cerebral metabolism in normal aging. Neuroimage 2012;63:713–722. [DOI] [PubMed] [Google Scholar]
- 28.Arenaza-Urquijo EM, Landeau B, La Joie R, et al. Relationships between years of education and gray matter volume, metabolism and functional connectivity in healthy elders. NeuroImage 2013;83:450–457. [DOI] [PubMed] [Google Scholar]
- 29.Barulli D, Stern Y. Efficiency, capacity, compensation, maintenance, plasticity: emerging concepts in cognitive reserve. Trends Cogn Sci 2013;17:502–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vemuri P, Weigand SD, Przybelski SA, et al. Cognitive reserve and Alzheimer's disease biomarkers are independent determinants of cognition. Brain 2011;134:1479–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vemuri P, Lesnick TG, Przybelski SA, et al. Vascular and amyloid pathologies are independent predictors of cognitive decline in normal elderly. Brain 2015;138:761–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rentz DM, Locascio JJ, Becker JA, et al. Cognition, reserve, and amyloid deposition in normal aging. Ann Neurol 2010;67:353–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Melrose RJ, Campa OM, Harwood DG, Osato S, Mandelkern MA, Sultzer DL. The neural correlates of naming and fluency deficits in Alzheimer's disease: an FDG-PET study. Int J Geriatr Psychiatry 2009;24:885–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hunt A, Haberkorn U, Schröder J, Schönknecht P. Neural correlates of executive dysfunction in prodromal and manifest Alzheimer's disease. GeroPsych 2011;24:77–81. [Google Scholar]
- 35.Cole MW, Repovs G, Anticevic A. The frontoparietal control system: a central role in mental health. Neuroscientist 2014;20:652–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cole MW, Bassett DS, Power JD, Braver TS, Petersen SE. Intrinsic and task-evoked network architectures of the human brain. Neuron 2014;83:238–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cole MW, Ito T, Braver TS. Lateral prefrontal cortex contributes to fluid intelligence through multinetwork connectivity. Brain Connect 2015;5:497–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA 2005;102:9673–9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Elman JA, Oh H, Madison CM, et al. Neural compensation in older people with brain amyloid-beta deposition. Nat Neurosci 2014;17:1316–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jacobs HI, Radua J, Luckmann HC, Sack AT. Meta-analysis of functional network alterations in Alzheimer's disease: toward a network biomarker. Neurosci Biobehav Rev 2013;37:753–765. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.