Abstract
Objective:
To examine moderators and predictors of response to behavior therapy for tics in children and adults with Tourette syndrome and chronic tic disorders.
Methods:
Data from 2 10-week, multisite studies (1 in children and 1 in adults; total n = 248) comparing comprehensive behavioral intervention for tics (CBIT) to psychoeducation and supportive therapy (PST) were combined for moderator analyses. Participants (177 male, 71 female) had a mean age of 21.5 ± 13.9 years (range 9–69). Demographic and clinical characteristics, baseline tic-suppressing medication, and co-occurring psychiatric disorders were tested as potential moderators for CBIT vs PST or predictors of outcome regardless of treatment assignment. Main outcomes measures were the Yale Global Tic Severity Scale Total Tic score and the Clinical Global Impression–Improvement score assessed by masked evaluators.
Results:
The presence of tic medication significantly moderated response to CBIT vs PST (p = 0.01). Participants showed tic reduction after CBIT regardless of tic medication status, but only participants receiving tic medication showed reduction of tics after PST. Co-occurring psychiatric disorders, age, sex, family functioning, tic characteristics, and treatment expectancy did not moderate response. Across both treatments, greater tic severity (p = 0.005) and positive participant expectancy (p = 0.01) predicted greater tic improvement. Anxiety disorders (p = 0.042) and premonitory urge severity (p = 0.005) predicted lower tic reduction.
Conclusions:
Presence of co-occurring attention-deficit/hyperactivity disorder, obsessive-compulsive disorder, or anxiety disorders did not moderate response to CBIT. Although participants on tic medication showed improvement after CBIT, the difference between CBIT and PST was greater for participants who were not on tic-suppressing medication.
ClinicalTrials.gov identifiers:
The child and adult CBIT studies are listed on clinical trials.gov (NCT00218777 and NCT00231985, respectively).
Classification of evidence:
This study provides Class I evidence that CBIT is effective in reducing tic severity across subgroups of patients with chronic tic disorders, although the difference between treatments was smaller for participants on tic-suppressing medications, suggesting reduced efficacy in this subgroup.
Chronic tic disorders (CTDs)—Tourette syndrome (TS) and chronic motor or vocal tic disorder—are characterized by childhood onset of motor or vocal tics affecting an estimated 14/1,000 children.1 Tics usually begin between 5 and 7 years of age and reach peak severity between 9 and 12 years, with gradual improvement through adolescence; a minority of patients have moderate to severe tics into adulthood.2 CTDs often co-occur with other psychiatric disorders, including attention-deficit/hyperactivity disorder (ADHD), obsessive-compulsive disorder (OCD), and other anxiety and mood disorders.3–5 Available treatments for CTDs focus on tic reduction or a co-occurring condition. Medications to reduce tic severity include dopamine receptor blocking agents (e.g., haloperidol and risperidone) and α2-adrenergic agonists (e.g., clonidine and guanfacine).6,7 Efficacy of Comprehensive Behavioral Intervention for Tics (CBIT)8 for reducing tic severity was demonstrated in 2 large, 10-week randomized controlled trials of youth (n = 126)9 and adults (n = 122).10 In both trials, CBIT was compared to psychoeducation and supportive therapy (PST). Given that the CBIT studies used the same design and outcome measures, we combined these datasets to identify baseline characteristics that differentially affected response to CBIT vs PST (moderators) or affected outcome equally across both treatments (predictors).11
METHODS
Primary research question.
The primary research question was to examine moderators and predictors of response to behavior therapy for tics in children and adults with CTDs. This study provides Class I evidence.
Design.
Participants were randomized (1:1) to CBIT or PST. The random assignment was within-site and stratified by the presence of tic medication.
Participants.
Children, adolescents, and adults with diagnosis of TS or CTD with a Clinical Global Impression of Severity (CGI-S)12 score of moderate (4) or greater were eligible to participate. Those with TS were required to have a Yale Global Tic Severity Scale (YGTSS)13 Total Tic score of ≥13 in the child study and ≥14 in the adult study. For participants with CTD only, the YGTSS total motor or vocal tic scores had to be ≥9 and ≥10 in the child and adult studies, respectively. Participants on psychotropic medication (including tic medication) were eligible if the medication was stable for at least 6 weeks before baseline with no planned changes during the 10-week trial. Individuals with IQ <80, current diagnosis of substance abuse or substance dependence, lifetime diagnosis of autism spectrum disorder or psychotic disorder, or previous treatment with 4 or more sessions of habit reversal training for tics were excluded. Those with co-occurring diagnoses (e.g., depression, anxiety, OCD, or ADHD) were eligible if the condition did not require immediate treatment or change in existing treatment.9,10
Three sites enrolled participants in the child study (Johns Hopkins University, University of California at Los Angeles, University of Wisconsin–Milwaukee) and 3 sites enrolled older adolescent and adult participants (Massachusetts General Hospital/Harvard Medical School, Health Sciences Center University of Texas at San Antonio, Yale University).
Standard protocol approvals, registrations, and patient consents.
The studies were approved by the institutional review boards at each site. Adult participants and parents of child participants provided consent; children provided assent.
The child and adult CBIT studies are listed on clinical trials.gov (NCT00218777 and NCT00231985, respectively).
Treatments.
CBIT and PST consisted of 8 individually delivered 60- to 90-minute sessions administered over 10 weeks. Caretakers of children and significant others of adults were invited to participate. CBIT provides education about tic disorders and relaxation training and teaches patients to inhibit tics with a competing motor movement. CBIT also addresses situational antecedents and consequences of tics as contextual factors that may inadvertently occasion or reinforce tics. PST provided developmentally relevant information about CTDs and controlled for time and therapist attention, but did not include tic management strategies. Therapists with at least a master's degree were trained to reliability on both treatments and received weekly supervision at each site. Sessions were recorded on video and a 13%–16% randomly selected set of therapy sessions was independently reviewed for fidelity.9,10
Procedures.
Developmentally appropriate structured diagnostic interviews assessed for psychiatric diagnoses. The Anxiety Disorders Interview Schedule for DSM-IV: Child Version14 supplemented with a tic disorders module was administered separately to parents and youths in the child study. The Structured Clinical Interview for DSM-IV15 with tic disorder and adult ADHD modules was used in the adult trial. Symptom severity of ADHD was assessed with parent ratings and self-reports of the Attention Deficit Hyperactivity Disorder Rating Scale (ADHD-RS).16 Full-scale IQ was assessed by the Wechsler Abbreviated Scale of Intelligence17 in children and by the Wechsler Test of Adult Reading18 in adults. An experienced clinician at each site confirmed the presence and stability of tic medication.
Outcome measures.
The YGTSS13 is a semi-structured interview that measures tic severity over the prior week. Motor and phonic tics are separately rated on a 0–5 scale for number, frequency, intensity, complexity, and interference. The YGTSS Total Tic score was the first primary outcome measure. The second primary outcome measure was the Clinical Global Impression–Improvement (CGI-I)12 with scores ranging from very much improved (1) to very much worse (7); scores of much improved (1) or very much improved (2) defined positive response. Trained raters, who were blind to treatment assignment, administered the YGTSS and CGI-I at baseline, midpoint (week 5), and endpoint (week 10).
Moderator and predictor variables.
We selected baseline characteristics as moderators that could influence differential response to CBIT vs PST based on studies of behavioral interventions for other neurodevelopmental disorders19–21 and on consensus of CBIT investigators. The same variables were examined as predictors, which are characteristics affecting response regardless of treatment assignment.22 Selected variables included presence of baseline tic medication, tic phenomenology (age at onset, current severity, complexity, and premonitory urges), age, sex, family functioning, treatment expectancy, and co-occurring ADHD, OCD, and anxiety disorders.
Tic medication.
Seventy-seven (31%) participants reported taking 1 or more tic medications: 32 antipsychotics, 44 α2 agonists, 5 mood stabilizers, and 4 benzodiazepines. Tic medication status was initially coded 0 (on a medication) or 1 (no tic medication). To test the effects of the most common tic medications in this sample, we created a 4-level variable: 1 = no tic medications, 2 = antipsychotics only, 3 = α2 agonists only, and 4 = both.
Tic phenomenology.
We considered age at onset, tic complexity (cutoff greater than 3 on the YGTSS complexity item),23 overall severity (CGI-S of moderate vs marked or greater), and premonitory urge based on the 9-item self-report Premonitory Urges for Tics Scale.24
Demographic and family characteristics.
Age was examined as a continuous variable. In addition, we dichotomized age into 9–15 years and ≥16 years. This division reflects the replicated observation that tics tend to decline throughout adolescence. Thus, study participants age 16 years and older may have a more persistent form of CTDs. Family functioning was measured by the Brief Family Assessment Measure III (BFAM),25 which reflects family communication, problem-solving, and affective expression. The BFAM was completed by caregivers and participants in the child study and by adult participants. A z score was created based on the average of child and parent BFAM scores and the raw scores from adult participants.
Treatment expectations.
After the first CBIT or PST session, we collected a 3-item questionnaire from participants, parents, and therapists in the child study and from participants and therapists in the adult study. Items such as “I expect to get control over my tics though this treatment” were rated on a scale from 1 = strongly disagree to 5 = strongly agree. Thus, higher scores indicated greater expectancy for treatment benefits. z Scores were derived from the average of child and parent ratings in the child study and self-reports in the adult study and then merged. We also created a z score for therapist treatment expectation across both trials.
Data analysis.
The effect of treatment on the YGTSS Total Tic score was tested with mixed-model repeated-measures analyses, adjusted for baseline scores.26 These analyses were conducted on the modified intention-to-treat population (all participants with at least 1 postrandomization assessment visit [n = 232]). The model included fixed effects for treatment (2 levels), time (5 and 10 weeks), site (6 sites), interaction of treatment with time, a random effect for participant, and 3- and 2-way interactions of time, treatment, and moderator variable. Treatment-by-site interactions were not significant for any of the outcome variables and were excluded from the models. Three-way interactions (treatment × time × moderator) were not significant and were dropped from all models. In the absence of any significant 3-way interactions, we examined 2-way interactions (moderator × treatment) on the assumption that treatment differences between levels of the moderator were the same at weeks 5 and 10. To identify predictors of change on the YGTSS Total Tic score, we checked for significant main effects for baseline characteristics regardless of treatment assignment.22 Given the exploratory nature of these moderator and predictor analyses, we did not correct for multiple comparisons.26
Dichotomously coded moderator variables were entered in the mixed model repeated-measures analysis with adjustment for baseline YGTSS scores. Continuous variables were centered prior to analysis by subtracting the variable mean from the individual scores. Baseline and endpoint adjusted least squares means and SD for the YGTSS Total Tic score for the CBIT and PST groups stratified by the moderator variables are presented. Effect sizes were calculated by subtracting the change in YGTSS scores in PST from the change in the CBIT divided by the SD for the full sample at baseline.
RESULTS
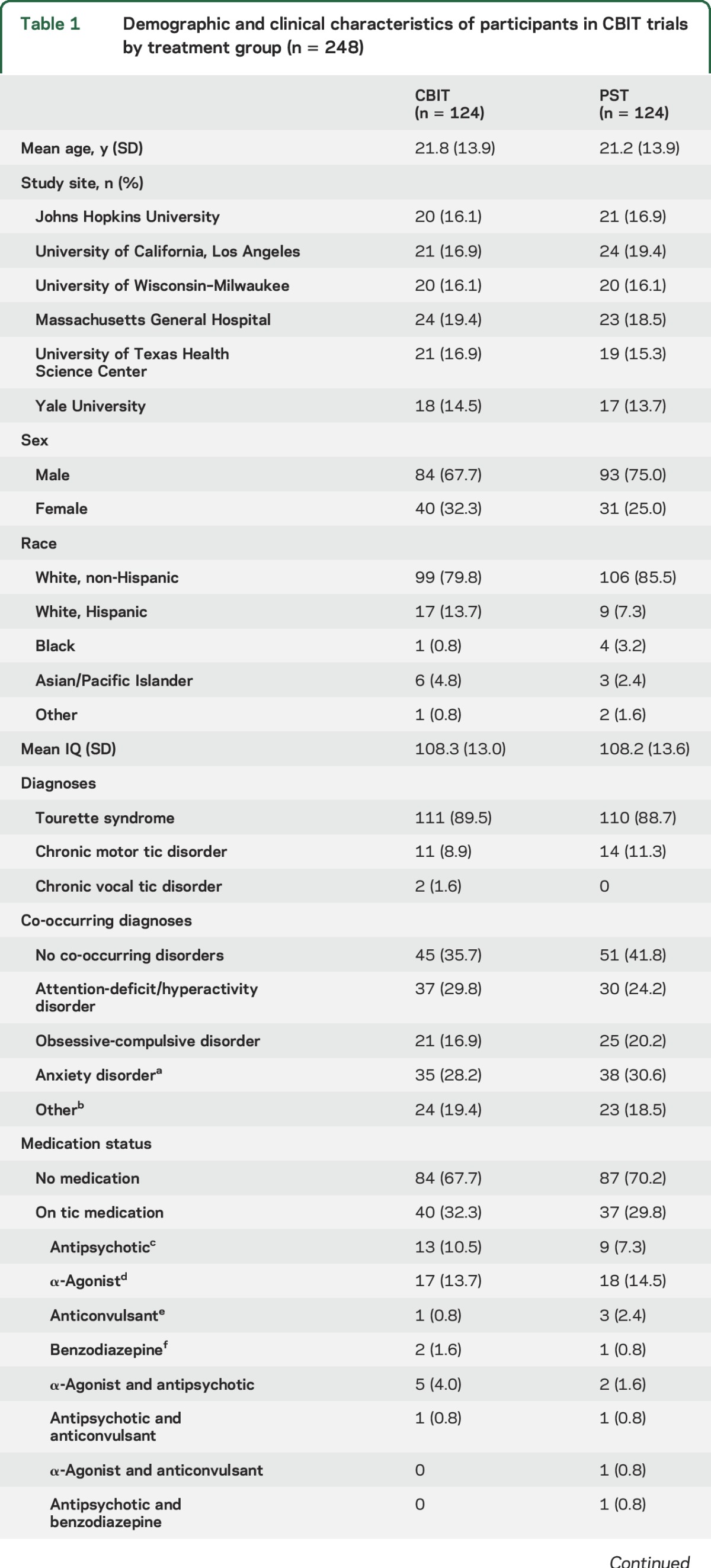
The combined sample included 177 male and 71 female participants (n = 248), mean (SD) age 21.5 (±13.9) years, range 9–69 years. There were no significant group differences on any demographic or clinical variables (table 1). Additional characteristics of the child and adult participants have been reported elsewhere.9,10,23,27
Table 1.
Demographic and clinical characteristics of participants in CBIT trials by treatment group (n = 248)
Moderation.
The presence of tic medication significantly moderated response to CBIT, favoring those on no tic medication (F1,222 = 7.07, p = 0.01) (figure). The 4-level tic medication variable (no tic medication, antipsychotics only, α2 agonists only, or both) also significantly moderated treatment response (F3,210 = 3.26, p = 0.023) (table 2). Table 3 shows least squares means (LSM) and SD for participants receiving and not receiving medication by the treatment condition. Using LSM, the treatment comparison at week 10 in the CBIT condition for participants receiving vs not receiving medication was statistically significant (t = 2.09, p = 0.04). For participants on α2 agonists, the comparison of week 10 LSM revealed no difference between CBIT and PST (t = 0.82, p = 0.41). In contrast, participants in the PST condition who were receiving α2 agonists showed significantly greater tic reduction that participants not on tic medication (t = −2.37, p = 0.019).
Figure. Moderating effects of tic medication on Yale Global Tic Severity Scale (YGTSS) Total Tic score.
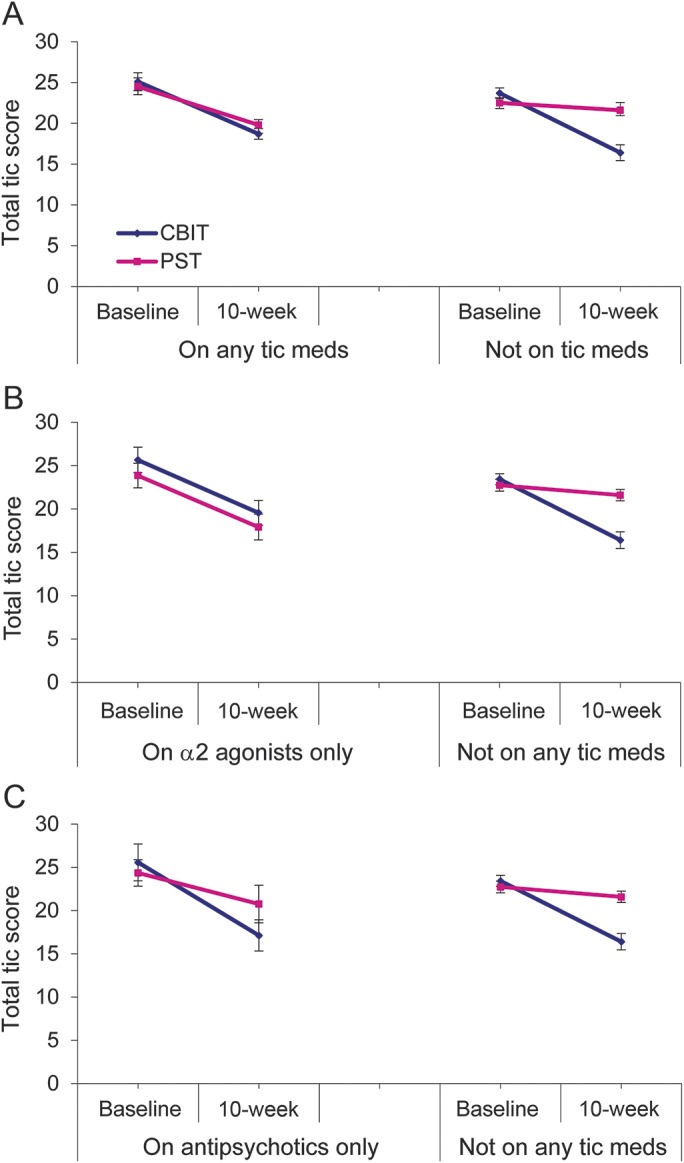
(A) Change from baseline in participants on any tic-suppressing medication (n = 77) to those not on tic medication. (B, C) Change in YGTSS in participants receiving α2 agonists only (n = 37) and antipsychotic medication only (n = 25). Error bars represent standard errors. CBIT = Comprehensive Behavioral Intervention for Tics; PST = psychoeducation and supportive therapy.
Table 2.
Baseline characteristics examined as potential moderators of treatment response (n = 248)
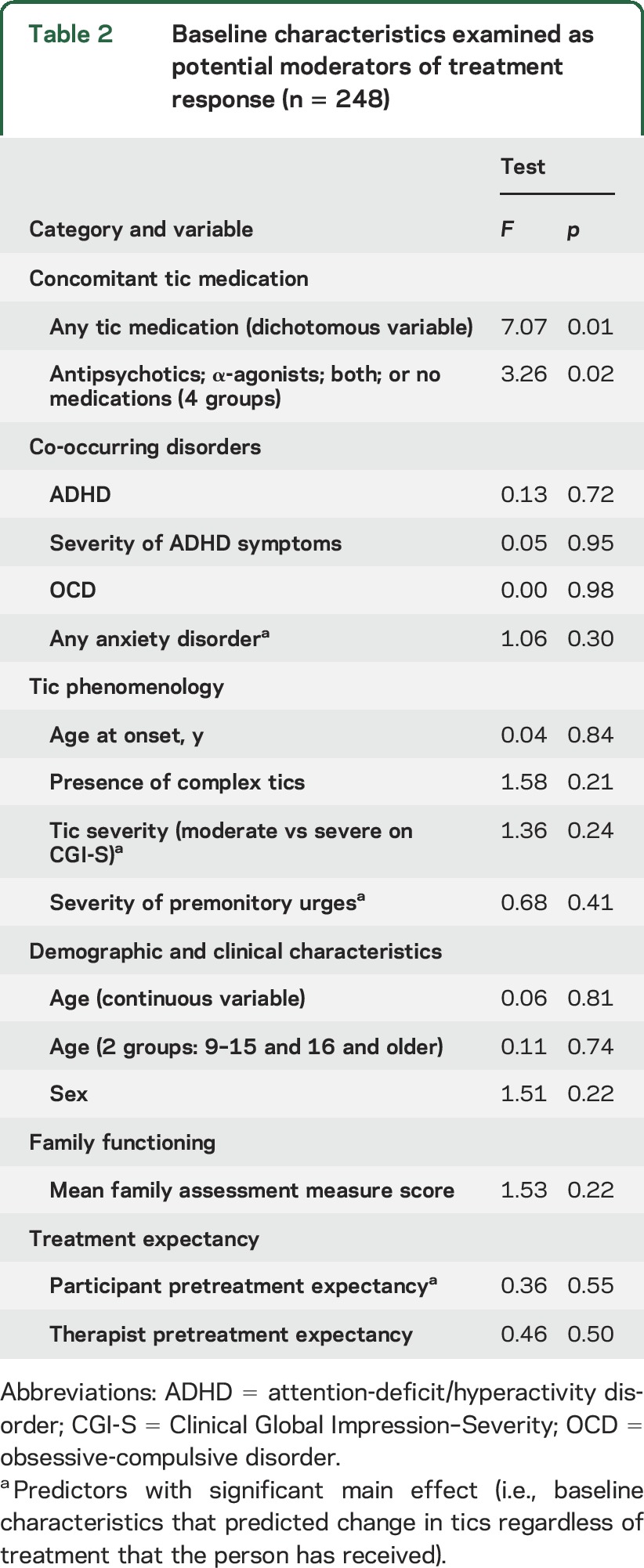
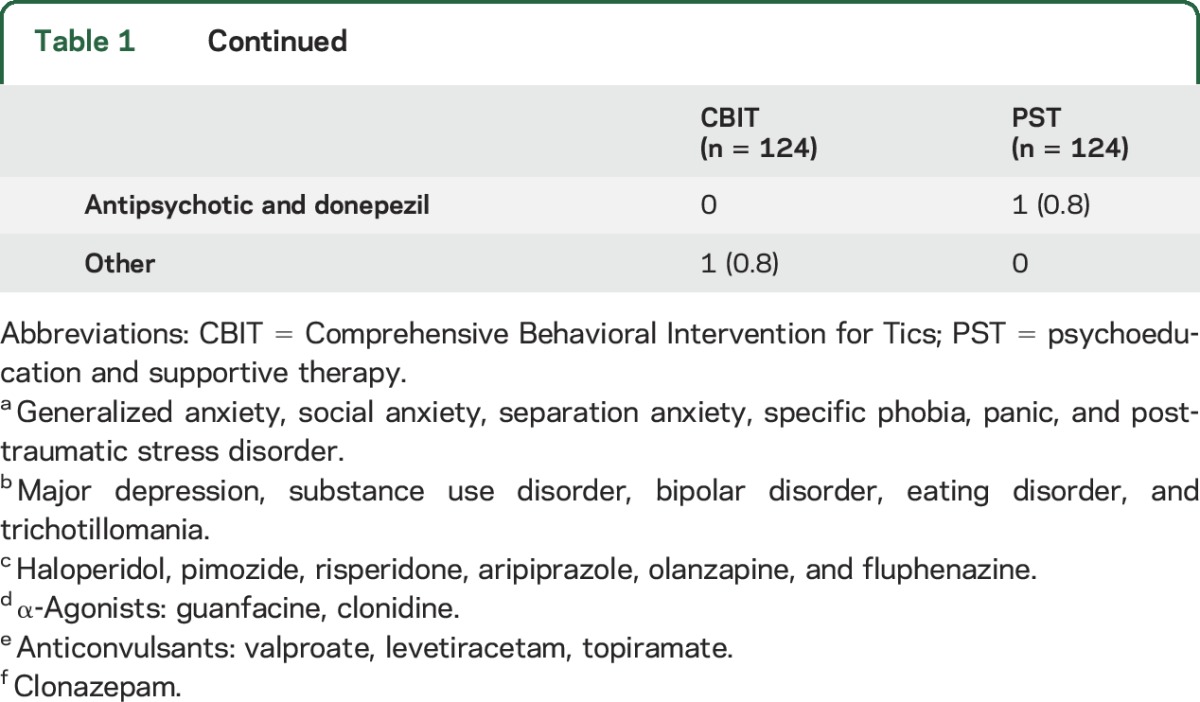
Table 3.
Least squares means (SD) Yale Global Tic Severity Scale Total Tic scores by treatment group stratified by tic medication, presence of ADHD, OCD, and anxiety disorders, and by moderate vs severe tics
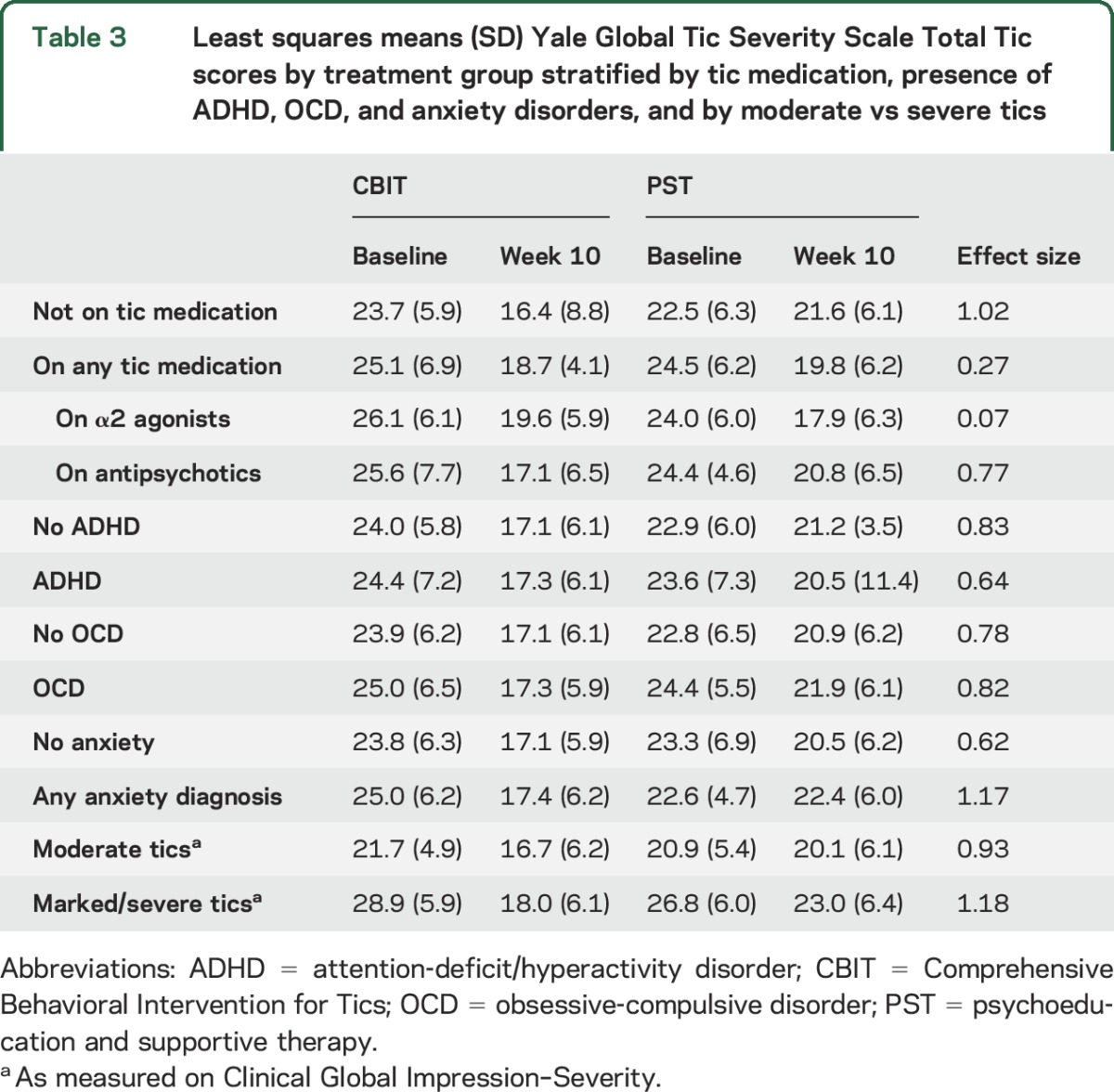
The presence of ADHD did not moderate treatment effect in participants <18 years old (n = 152) (F1,132 = 0.01, p = 0.94) or in the combined child and adult samples (n = 248, F1,222 = 0.13, p = 0.72). Moderating effects of ADHD symptom severity tested using ADHD-RS z scores were not significant in children and adults separately or in the combined sample (F1,219 = 0.79, p = 0.38). The presence of OCD or any anxiety disorder exclusive of OCD did not moderate treatment response (F1,222 = 0.01, p = 0.1 and F1,222 = 1.06, p = 0.30, respectively).
There were no significant treatment interactions with age at onset, presence of complex tics, tic severity on the CGI-S, the severity of premonitory urges, sex, BFAM, participant, or therapist expectancy (table 2). Baseline age (centered variable), as a continuous variable or when dichotomized into younger and older age groups, did not moderate outcome.
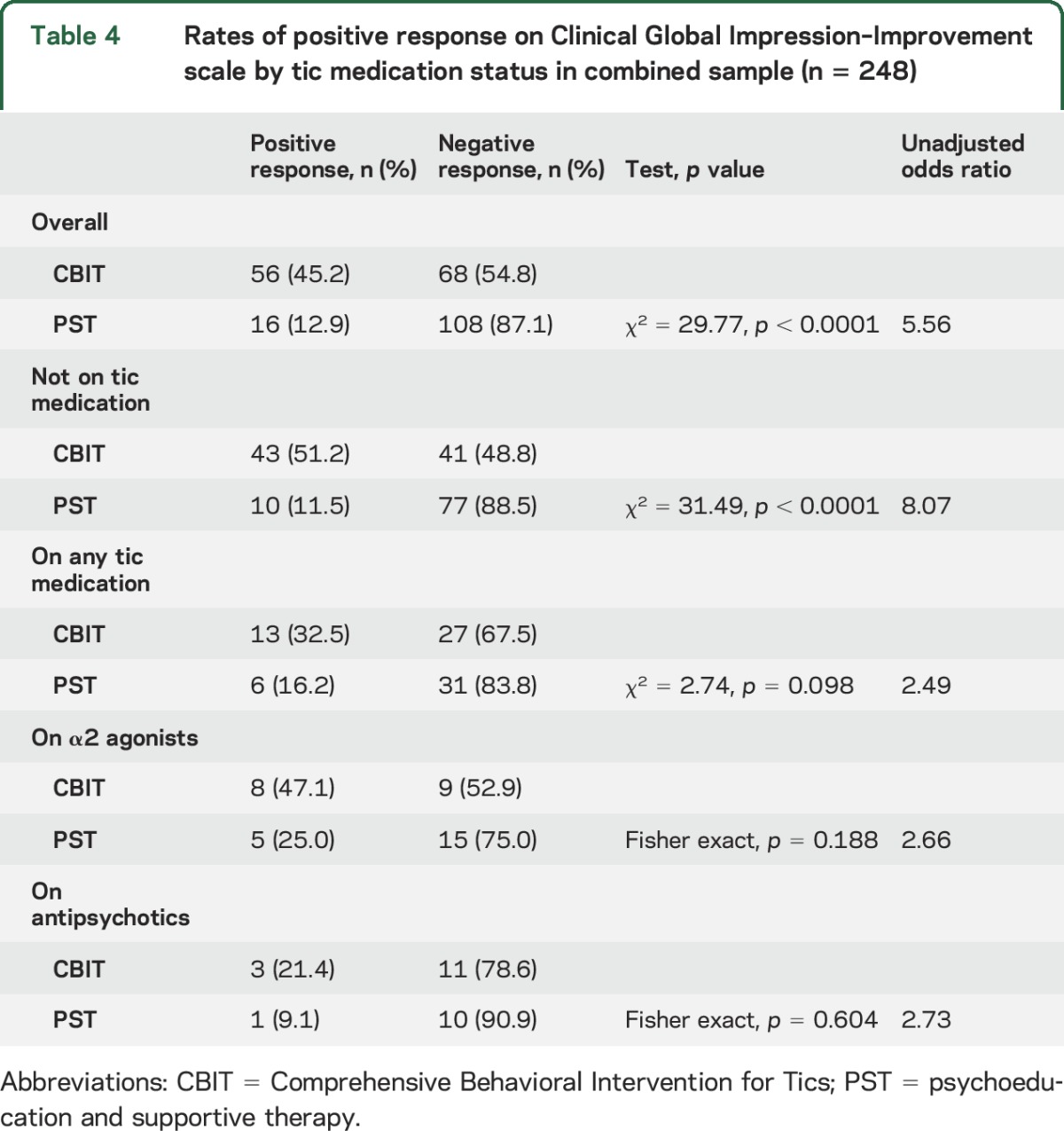
The effect size for CBIT vs PST was 1.02 for participants not on tic medication compared to 0.27 for those on tic medication. To evaluate further the moderating effects of tic medication, we compared participants on no tic medication, antipsychotics only, α2 agonists only, or both (table 3 and figure). Participants on α2 agonists (CBIT n = 17; PST n = 20) showed similar improvement in both groups (t = 0.82, p = 0.41; effect size = 0.07). By contrast, for participants on antipsychotic medication (CBIT n = 14; PST n = 11), the treatment difference was only slightly lower than the treatment difference for participants not on tic medication (t = 1.39, p = 0.16; effect size = 0.77). Table 4 shows the overall rate of positive response on the CGI-I and by tic medication status. For participants on no tic medication, the rate of positive response in CBIT was significantly greater than for PST. For participants on a tic medication, the response rate for CBIT was twice that for PST, but this difference was not significant.
Table 4.
Rates of positive response on Clinical Global Impression–Improvement scale by tic medication status in combined sample (n = 248)
Predictors.
There were no significant main effects on age at onset, presence of complex tics, sex, or family functioning. Greater overall severity at baseline as measured on the CGI-S predicted a greater reduction of tics regardless of treatment condition (F1,222 = 8.22, p = 0.005). Greater severity of premonitory urges at baseline predicted lesser tic reduction in both treatments (F1,225 = 8.13, p = 0.005). Positive expectancy by participants was a significant predictor of treatment benefit (F1,210 = 6.72, p = 0.01). Anxiety disorders (exclusive of OCD) predicted lower tic reduction at week 10 regardless of treatment condition (F1,222 = 4.17, p = 0.042). Otherwise co-occurring psychiatric disorders did not predict treatment outcome.
DISCUSSION
Data from the child and adult CBIT vs PST trials (n = 248) were combined to examine moderators and predictors of treatment response. Compared to participants on tic medication, the treatment effect of CBIT was significantly larger in participants on no tic medication. This differential response was evident in the YGTSS Total Tic score and the rate of positive response on the CGI-I scored by a blinded evaluator. The presence of a lifetime diagnosis of ADHD, OCD, or other anxiety disorders did not moderate treatment response to CBIT, nor did age, sex, current severity of ADHD symptoms, tic severity, or severity of premonitory urges.
The moderating effect of tic-suppressing medication warrants closer inspection. First, the use of tic-suppressing medication may indicate greater tic severity. At baseline, there was no difference in YGTSS Total Tic scores in participants on tic medication compared to those not taking a tic medication. However, because the premedication tic severity for those on tic medication is unknown, the role of tic severity in these analyses is unclear.
Second, of the 248 participants, only 77 (31%) were on a tic-suppressing medication (primarily antipsychotics or α2 agonists). As shown in table 3, the difference in YGTSS change scores between CBIT and PST was 1.7 points for participants on any tic medication compared to a difference of 6.4 points for participants not on tic medication (effect sizes 0.27 and 1.02, respectively). Although the numbers are small, there were differences by drug class. The CBIT-PST YGTSS change-score difference for participants on antipsychotic medications was 4.9 points (effect size 0.77), which was similar to the improvement in participants on no tic medication. By contrast, for participants on α2 agonists, the difference in YGTSS change scores between treatments was 0.4 points (effect size 0.07). This attenuated effect size was largely due to the 6.1-point improvement in the YGTSS in the PST group on α2 agonists compared to a 6.5-point reduction in CBIT. When examined categorically on the CGI-I, participants on no tic medication showed a higher positive response rate than those on tic medication with no apparent difference by drug class.
Although participants not on tic medication showed greater magnitude of benefit and likelihood of positive response, participants on tic medication also showed improvement with CBIT. Thus, within the CBIT group, there was a 7.3-point reduction in the YGTSS Total Tic score in participants not on tic-suppressing medication. This is remarkably similar to the 6.5-point reduction for CBIT in participants receiving α2 agonists for tics and the 8.5-point reduction in participants on antipsychotics for tics. The 6.1-point decrease in tic severity in the PST group on α2 agonists for tics reduced the treatment difference between CBIT and PST for all participants on tic medication and may explain the significant interaction. The smaller CBIT-PST treatment difference in participants on α2 agonists need not imply that patients on α2 agonists should not be offered CBIT.
The predictor analysis identified 4 variables that affected outcome regardless of treatment assignment: co-occurring anxiety disorders, severity of premonitory urges, participant positive expectancy, and higher overall severity as measured on the CGI-S. Participants with a co-occurring anxiety disorder showed less tic reduction after 10 weeks. Although CBIT includes brief relaxation training, incorporation of more active anxiety and stress management strategies in CBIT may be useful. The severity of premonitory urges was also associated with lower tic reduction. Despite the deliberate teaching on early detection of the urge and initiation of voluntary competing response in CBIT, premonitory urges did not improve with CBIT.9,10 The current analysis suggests that the severity of premonitory urges impeded improvement in both study treatments. Greater levels of premonitory urges may be associated with more treatment-resistant forms of CTDs.28
Positive expectancy for change among study participants and parents was associated with greater tic reduction over time. This nonspecific effect suggests that some participants and their parents were optimistic that therapeutic attention to the problem of chronic tics could be beneficial.29 When overall severity was dichotomized on CGI-S of moderate vs marked or greater, participants with greater overall severity also showed greater tic reduction over time regardless of treatment condition.
Positive expectations for treatment depend, at least in part, on agreement between the patient and the clinician on the goals of treatment as well as the specific components of the therapy.30 Thus, open discussion about the goal (tic reduction) and techniques of CBIT (e.g., awareness training and competing response training) between the therapist and the patient is warranted.
These results should be considered in light of limitations. First, the CBIT studies were designed to test main effects. By definition, moderator and predictor analyses are exploratory. Second, although the effect size and likelihood of positive response were lower for participants on tic medication, the subgroups by medication class were small, making it difficult to interpret the findings.
CBIT is helpful for reducing tics in children and adults regardless of coexisting tic medication treatment, although the difference between CBIT and PST was smaller for participants on tic-suppressing medications. PST was designed to include psychoeducation and support that are part of good clinical practice and it should be provided to patients in conjunction with medication management for tics. The presence of common co-occurring conditions in children and adults with CTDs did not moderate treatment effects, suggesting that CBIT is effective for reducing tic severity in patients with stable co-occurring conditions.
GLOSSARY
- ADHD
attention-deficit/hyperactivity disorder
- ADHD-RS
Attention Deficit Hyperactivity Disorder Rating Scale
- BFAM
Brief Family Assessment Measure III
- CBIT
Comprehensive Behavioral Intervention for Tics
- CGI-I
Clinical Global Impression–Improvement
- CGI-S
Clinical Global Impression–Severity
- CTD
chronic tic disorder
- LSM
least squares means
- OCD
obsessive-compulsive disorder
- PST
psychoeducation and supportive therapy
- TS
Tourette syndrome
- YGTSS
Yale Global Tic Severity Scale
AUTHOR CONTRIBUTIONS
Dr. Sukhodolsky had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Drs. Sukhodolsky, Woods, Piacentini, Wilhelm, and Peterson, L. Katsovich, and Drs. Dziura, Walkup, and Scahill. Acquisition of data: Drs. Sukhodolsky, Woods, Piacentini, Wilhelm, Peterson, Walkup, and Scahill. Analysis and interpretation of data: Drs. Sukhodolsky, Woods, Piacentini, Wilhelm, and Peterson, L. Katsovich, and Drs. Dziura, Walkup, and Scahill. Drafting of the manuscript: Drs. Sukhodolsky, Woods, Piacentini, Wilhelm, Dziura, Walkup, and Scahill. Critical revision of the manuscript for important intellectual content: Drs. Sukhodolsky, Woods, Piacentini, Wilhelm, Dziura, Walkup, and Scahill. Statistical analysis: Dr. Sukhodolsky, L. Katsovich, and Drs. Dziura and Scahill. Obtained funding: Drs. Piacentini, Woods, Wilhelm, Peterson, Walkup, and Scahill. Administrative, technical, or material support: Drs. Sukhodolsky, Woods, Piacentini, Wilhelm, and Peterson, L. Katsovich, and Drs. Walkup and Scahill.
STUDY FUNDING
This work was supported by grant R01MH070802 (Dr. Piacentini) with subcontracts to Drs. Woods, Scahill, Wilhelm, Peterson, and Walkup, and by grants R01MH069877 (Dr. Wilhelm), R01MH069874 (Dr. Scahill), and R01MH069875 (Dr. Petersen) from the National Institute of Mental Health with subcontracts to Drs. Piacentini and Woods. Dr. Sukhodolsky was supported by a career award from NIMH (K01MH079130). Drs. Scahill and Dziura received support from the Yale University Clinical and Translational Sciences Award grant UL1 RR024139 from the National Center for Research Resources, NIH. This study was also supported by Tourette Syndrome Association funding to Dr. Scahill. The funding organization had no role in the design or conduct of the study; in the collection, management, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript.
DISCLOSURE
D. Sukhodolsky received research support and speaker's honoraria from the Tourette Association of America and receives royalties from Guilford Press. D. Woods receives speaker's honoraria from the Tourette Association of America and royalties from Guilford Press and Oxford University Press. J. Piacentini received research support from Pfizer and the Tourette Association of America, speaker's honoraria from the International Obsessive Compulsive Disorder Foundation, the Trichotillomania Learning Center Foundation for Body-Focused Repetitive Behaviors, and the Tourette Association of America, and royalties from Guilford Press and Oxford University Press. S. Wilhelm received research support in the form of free medication and matching placebo for National Institute of Mental Health–funded studies from Forest Laboratories, presenters for the Massachusetts General Hospital Psychiatry Academy in educational programs supported through independent medical education grants from pharmaceutical companies, and salary support from Novartis. She receives royalties from Elsevier Publications, Springer Publications, Guilford Publications, New Harbinger Publications, and Oxford University Press, and speaking honoraria from the International Obsessive Compulsive Disorder Foundation and the Tourette Association of America. She received payment from the Association for Behavioral and Cognitive Therapies for her role as Associate Editor for Behavior Therapy as well as from John Wiley & Sons, Inc., for her role as Associate Editor for Depression & Anxiety. A. Peterson has received research support and speaker's honoraria from the Tourette Association of America and receives royalties from Oxford University Press. L. Katsovich and J. Dziura report no disclosures relevant to the manuscript. J. Walkup received research support in the form of free drug and placebo for National Institute of Mental Health–funded studies from Eli Lilly, Pfizer, and Abbott, and received payment for a one-time consultation with Shire. He received speaker honoraria from the Tourette Syndrome Center for Disease Control and Prevention outreach educational programs, American Academy of Child and Adolescent Psychiatry, and American Psychiatric Association. Dr. Walkup receives royalties from Guilford Press and Oxford University Press. Dr. Walkup receives grant funding from the Hartwell Foundation and the Tourette Association of America, and is an unpaid advisor to Anxiety Disorders Association of America, Consumer Reports, and Trichotillomania Learning Center. L. Scahill served as a consultant to Neuren, Bracket, MedAdvante, Roche, Coronado, and Supernus, received speaker's honoraria from the Tourette Association of America, and receives royalties from Guilford Press and Oxford University Press. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Scahill L, Dalsgaard S, Bradbury K. The prevalence of Tourette syndrome and its relationship to clinical features. In: Martino D, Leckman JF, eds. Tourette Syndrome. New York: Oxford University Press; 2013:121–136. [Google Scholar]
- 2.Bloch MH, Leckman JF. Clinical course of Tourette syndrome. J Psychosom Res 2009;67:497–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sukhodolsky DG, Scahill L, Zhang H, et al. Disruptive behavior in children with Tourette's syndrome: association with ADHD comorbidity, tic severity, and functional impairment. J Am Acad Child Adolesc Psychiatry 2003;42:98–105. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Prevalence of diagnosed Tourette syndrome in persons aged 6-17 years: United States, 2007. MMWR Morb Mortal Wkly Rep 2009;58:581–585. [PubMed] [Google Scholar]
- 5.Olfson M, Crystal S, Gerhard T, Huang C, Walkup JT, Scahill L. Patterns and correlates of tic disorder diagnoses in privately and publicly insured youth. J Am Acad Child Adolesc Psychiatry 2011;50:119–131. [DOI] [PubMed] [Google Scholar]
- 6.Murphy TK, Lewin AB, Storch EA, Stock S. Practice parameter for the assessment and treatment of children and adolescents with tic disorders. J Am Acad Child Adolesc Psychiatry 2013;52:1341–1359. [DOI] [PubMed] [Google Scholar]
- 7.Weisman H, Qureshi IA, Leckman JF, Scahill L, Bloch MH. Systematic review: pharmacological treatment of tic disorders: efficacy of antipsychotic and alpha-2 adrenergic agonist agents. Neurosci Biobehav Rev 2013;37:1162–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Woods DW, Piacentini JC, Chang SW, et al. Managing Tourette Syndrome: A Behavioral Intervention. New York: Oxford University Press; 2008. [Google Scholar]
- 9.Piacentini J, Woods DW, Scahill L, et al. Behavior therapy for children with Tourette disorder: a randomized controlled trial. JAMA 2010;303:1929–1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilhelm S, Peterson AL, Piacentini J, et al. Randomized trial of behavior therapy for adults with Tourette's disorder. Arch Gen Psychiatry 2012;69:795–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frank E, Cassano GB, Rucci P, et al. Predictors and moderators of time to remission of major depression with interpersonal psychotherapy and SSRI pharmacotherapy. Psychol Med 2011;41:151–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guy W. Clinical Global Impression Scales (CGI). ECDEU Assessment Manual for Psychopharmacology (Publication 76-338). Washington, DC: Department of Health, Education, and Welfare; 1976. [Google Scholar]
- 13.Leckman JF, Riddle MA, Hardin MT, et al. The Yale Global Tic Severity Scale: initial testing of a clinician-rated scale of tic severity. J Am Acad Child Adolesc Psychiatry 1989;28:566–573. [DOI] [PubMed] [Google Scholar]
- 14.Silverman WK, Albano AM. Anxiety Disorders Interview Schedule for DSM-IV, Child Version. New York: Oxford University Press; 1996. [Google Scholar]
- 15.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders, Clinician Version (SCID-CV). Washington, DC: American Psychiatric Press; 1996. [Google Scholar]
- 16.DuPaul GJ, Power TJ, Anastopoulos AD, Reid R. ADHD Rating Scale IV. New York: Guilford; 1998. [Google Scholar]
- 17.Wechsler D. Wechsler Abbreviated Scales of Intelligence. San Antonio, TX: The Psychological Corporation; 1999. [Google Scholar]
- 18.Holdnack HA. Wechsler Test of Adult Reading. San Antonio, TX: The Psychological Corporation; 2001. [Google Scholar]
- 19.Hinshaw SP. Moderators and mediators of treatment response for children with attention-deficit/hyperactivity disorder: the multimodal treatment study of children with attention-deficit/hyperactivity disorder. Arch Gen Psychiatry 1999;56:1088–1096. [DOI] [PubMed] [Google Scholar]
- 20.Farmer C, Lecavalier L, Yu S, et al. Predictors and moderators of parent training efficacy in a sample of children with autism spectrum disorders and serious behavioral problems. J Autism Dev Disord 2012;42:1037–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Compton SN, Peris TS, Almirall D, et al. Predictors and moderators of treatment response in childhood anxiety disorders: results from the CAMS trial. J Consult Clin Psychol 2014;82:212–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kraemer HC, Wilson GT, Fairburn CG, Agras WS. Mediators and moderators of treatment effects in randomized clinical trials. Arch Gen Psychiatry 2002;59:877–883. [DOI] [PubMed] [Google Scholar]
- 23.McGuire JF, Nyirabahizi E, Kircanski K, et al. A cluster analysis of tic symptoms in children and adults with Tourette syndrome: clinical correlates and treatment outcome. Psychiatry Res 2013;210:1198–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Woods DW, Piacentini J, Himle MB, Chang S. Premonitory Urge for Tics Scale (PUTS): initial psychometric results and examination of the premonitory urge phenomenon in youths with tic disorders. J Dev Behav Pediatr 2005;26:397–403. [DOI] [PubMed] [Google Scholar]
- 25.Skinner H, Steinhauer P, Santa-Barbara J. Family Assessment Measure III (FAM-III). North Tonawanda, NY: Multi-Health Systems; 1995. [Google Scholar]
- 26.Brown H, Prescott R. Applied Mixed Models in Medicine. 2nd ed. Chichester, VT: John Wiley & Sons; 2006. [Google Scholar]
- 27.Specht MW, Woods DW, Piacentini J, et al. Clinical characteristics of children and adolescents with a primary tic disorder. J Dev Phys Disabil 2011;23:15–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Banaschewski T, Woerner W, Rothenberger A. Premonitory sensory phenomena and suppressibility of tics in Tourette syndrome: developmental aspects in children and adolescents. Dev Med Child Neurol 2003;45:700–703. [DOI] [PubMed] [Google Scholar]
- 29.Rutherford BR, Sneed JR, Tandler JM, Rindskopf D, Peterson BS, Roose SP. Deconstructing pediatric depression trials: an analysis of the effects of expectancy and therapeutic contact. J Am Acad Child Adolesc Psychiatry 2011;50:782–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cohen M, Beard C, Björgvinsson T. Examining patient characteristics as predictors of patient beliefs about treatment credibility and expectancies for treatment outcome. J Psychother Integr 2015;25:90–99. [Google Scholar]


