18F-AV-1451 is a tau PET radioligand, characterized by high affinity for paired helical filaments (PHF) of hyperphosphorylated tau in neurofibrillary tangles (NFTs). Postmortem tissue studies have shown AV-1451 binding selectively to tau (3R + 4R isoforms) but not β-amyloid pathology.1–3 Human studies evaluating AV-1451 tracer retention in aging and Alzheimer disease (AD) have indicated that in vivo binding patterns parallel existing neuropathologic staging of tau.4,5
Despite the applicability of this ligand for imaging tau in aging and dementia, in certain conditions tracer binding might complicate image interpretation. Pathologic studies have found AV-1451 also showing retention unrelated to tau; this off-target binding may reflect iron deposition, neuromelanin, or vascular factors.1,3 Off-target binding can complicate quantification in research studies and clinical trials; for example, choroid plexus signal may affect accurate measurement of hippocampal signal.5 In this report we expand on this by demonstrating tracer uptake in meningiomas, vascular malformations, and remote infarcts, suggesting that in studies using AV-1451 and other tau tracers to study aging and disease, researchers must carefully examine PET images for focal accumulations of tracer and MRI for incidental findings that may produce spurious elevations of signal that do not reflect the presence of age or AD-related pathology.
Methods.
In ongoing studies in our laboratory, we discovered 5 cases (2 normal controls, 3 patients: 1 AD, 1 mild cognitive impairment [MCI], and 1 Parkinson disease [PD]) with surprising, atypical increases in AV-1451 uptake in regions of incidental neuroimaging findings. The institutional review boards of all participating institutions approved the study and informed consent was obtained from all participants. In each participant, T1 magnetization-prepared rapid gradient echo and fluid-attenuated inversion recovery (FLAIR) MRI were available, as were 80–100 minutes AV-1451 PET standardized uptake value ratio (SUVR) images. We also generated a global cortical amyloid value from 11C-Pittsburgh compound B distribution value ratio images for amyloid positivity classification.5 PET images used a cerebellar gray matter reference region. AV-1451 and FLAIR images were linearly aligned to T1 images and resliced. Target regions of interest (ROIs; 9 × 9 × 9 mm cubes) were drawn on T1 images, centered on the observed imaging findings, as were ROIs in the hemisphere contralateral to the findings. Images and histograms demonstrating AV-1451 signal in target and contralateral ROIs are displayed in figure 1 and figure e-1 at Neurology.org.
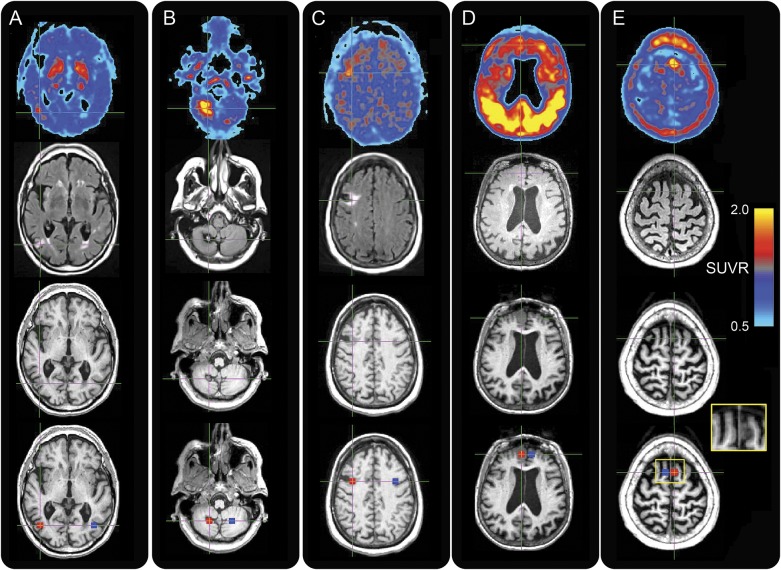
Figure 1. Elevated AV-1451 tracer uptake seen in incidental neuroimaging findings.
(A–E) Axial images for participants A (1st column), B (2nd column), C (3rd column), D (4th column), and E (5th column). Images (left hemisphere on right) are 18F-AV-1451 standardized uptake value ratio (SUVR) (1st row), fluid-attenuated inversion recovery (2nd row), T1 (3rd row), and target and contralateral regions of interest (red and blue masks in 4th row) overlaid on T1. Participant D exhibits strong tracer retention in the cortex, consistent with the participant's diagnosis of Alzheimer disease. Participant E’s inset shows close-up of meningioma on T1.
Patients.
Participant A (table e-1) is an 80-year-old amyloid-negative cognitively normal man whose T1 images reveal right temporal hypointensity surrounded by hyperintensity on FLAIR images, consistent with chronic infarction. Participant B is an 80-year-old borderline amyloid-positive cognitively normal woman whose MRI sequences reveal the characteristic appearance of a right cerebellar cavernous malformation. Participant C is a 67-year-old borderline amyloid-positive woman with PD whose imaging is also consistent with a remote infarct, located in the right frontal lobe. Participant D is a 77-year-old amyloid-positive woman with AD with a right medial frontal meningioma. Participant E is an 80-year-old amyloid-positive woman with amnestic MCI with a left superior frontal meningioma. We found elevated AV-1451 signal associated with the chronic infarcts, cavernous malformation, and meningiomas. For all participants, AV-1451 signal is markedly increased in the location of the target ROI covering the incidental imaging finding. ROI SUVR values are shown as histograms with x-axes representing voxel SUVRs, and with means listed in table e-1. SUVRs associated with each finding (histograms labeled “target”) are correspondingly elevated, with higher mean SUVR values compared to the contralateral ROI (histograms labeled “contralateral”).
Discussion.
The elevated signal surrounding the infarcts in participants A and C is consistent with existing neuropathologic data in human ischemic tissue. For example, tau-2 immunoreactivity is observed in glial cells in the setting of ischemia,6 and Alz-50 reactive neurons (indicative of misfolded tau) are associated with ischemic infarction; while these could represent PHF tau, these inclusions do not have the immunohistochemical features of NFTs or tau accumulating in AD.7 However, evidence suggests that elevated AV-1451 uptake in the other types of lesions are unlikely to be related to tau binding.1,3 AV-1451 uptake in meningiomas may represent binding to calcified structures, uptake in cavernous malformations and infarcts may represent binding to iron deposits or hemorrhagic by-products, and uptake in infarcts might also indicate binding to gliotic tissue.1,3 Specifically, elevated AV-1451 signal has been reported in other tissues that are calcified (such as choroid plexus) or involve iron deposition (e.g., colocalization of AV-1451 with Prussian blue staining).3 Gliosis-related binding increases could also be consistent with binding to degenerating white matter in tau-negative frontotemporal lobar degeneration cases.3 There are common features shared by all of these observations and our findings. Vascular permeability differences in lesions may also contribute to increased tracer retention. These eventualities are not necessarily exclusive, and histopathologic confirmation will be necessary to understand the in vivo tracer binding patterns.
Supplementary Material
Footnotes
Supplemental data at Neurology.org
Author contributions: S.N.L.: data analysis, data interpretation, study concept and design, drafting the manuscript. N.A.: data analysis, data interpretation, drafting the manuscript. J.R.W.: data analysis, data interpretation, drafting the manuscript. R.L.J.: data analysis, data interpretation, drafting the manuscript. G.D.R.: data analysis, data interpretation, drafting the manuscript, critical revision of the manuscript for important intellectual content. W.J.J.: acquisition of data, study concept and design, data analysis, data interpretation, drafting the manuscript, critical revision of the manuscript for important intellectual content, study supervision. S.N.L. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis and the conduct of the research.
Study funding: This research was supported by NIH grants R01-AG034570 (W.J.J.), R01-AG045611 (G.D.R., R.L.J.), P50-AG23501 (Dr. Bruce L. Miller, G.D.R.), F32-AG050389 (S.N.L.), and the Tau Consortium (WJ.J., G.D.R.). No funding sources had any role in the collection, analysis, or interpretation of data. The authors declare no competing financial interests.
Disclosure: S. Lockhart, N. Ayakta, J. Winer, and R. La Joie report no disclosures relevant to the manuscript. G. Rabinovici receives research support from Avid Radiopharmaceuticals, GE Healthcare, and Piramal, and has received consulting fees or speaking honoraria from Roche, Eisai, Lundbeck, Piramal, and Putnam. W. Jagust has served as a consultant to BioClinica, Genentech, and Novartis Pharmaceuticals. Avid Radiopharmaceuticals enabled use of the 18F-AV-1451 tracer but did not provide direct funding and was not involved in data analysis or interpretation. Go to Neurology.org for full disclosures.
References
- 1.Marquie M, Normandin MD, Vanderburg CR, et al. Validating novel tau positron emission tomography tracer [F-18]-AV-1451 (T807) on postmortem brain tissue. Ann Neurol 2015;78:787–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xia CF, Arteaga J, Chen G, et al. [18F]T807, a novel tau positron emission tomography imaging agent for Alzheimer's disease. Alzheimers Dement 2013;9:666–676. [DOI] [PubMed] [Google Scholar]
- 3.Lowe VJ, Curran G, Fang P, et al. An autoradiographic evaluation of AV-1451 Tau PET in dementia. Acta Neuropathol Commun 2016;4:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnson KA, Schultz A, Betensky RA, et al. Tau positron emission tomographic imaging in aging and early Alzheimer disease. Ann Neurol 2016;79:110–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schöll M, Lockhart SN, Schonhaut DR, et al. PET imaging of tau deposition in the aging human brain. Neuron 2016;89:971–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Uchihara T, Tsuchiya K, Nakamura A, Ikeda K. Appearance of tau-2 immunoreactivity in glial cells in human brain with cerebral infarction. Neurosci Lett 2000;286:99–102. [DOI] [PubMed] [Google Scholar]
- 7.Uchihara T, Tsuchiya K, Kondo H, Hayama T, Ikeda K. Widespread appearance of Alz-50 immunoreactive neurons in the human brain with cerebral infarction. Stroke 1995;26:2145–2148. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.