Abstract
Parenteral nutrition (PN) is essential to the practice of neonatology. While PN is life-sustaining, it associated with a host of complications including parenteral nutrition-associated liver disease (PNALD) and central line-associated bloodstream infections (CLASBIs), which carry a high morbidity and mortality and pose a burden to the healthcare system. Evidence has emerged that the dose and composition of intravenous lipid products can alter the incidence of PNALD and CLASBIs. However, other patient- and PN-related factors, such as prematurity, birth weight, and gastrointestinal anatomy and function, must not be ignored. In order improve neonatal care, future research is still needed to optimize the content of PN and decrease the incidence PNALD and CLASBIs.
Keywords: parenteral nutrition, neonates, lipids, parenteral nutrition-associated liver disease, infections
Introduction
Parental nutrition (PN) is an essential part of the medical management of critically ill neonates. The primary goal of PN is to maintain hydration and electrolyte balance, and to promote growth and neurodevelopment without adverse complications. For a myriad of reasons, up to 70% of neonates in the neonatal intensive care unit (NICU) are prescribed PN at some point, and approximately 16,000 children receive PN in the home setting in the United States (US) (Box 1).1,2 Without PN, children who are unable to consume sufficient enteral nutrition would succumb to malnutrition, dehydration, and electrolyte derangements.
Box 1. Neonatal populations at high risk for complications secondary to parenteral nutrition (PN).
| Populations at High-Risk for PN Associated Complications |
|---|
|
Although outcomes in poorly resourced countries do not mirror what has been witnessed in well-resourced countries, PN has undoubtedly contributed to the improved survival of infants with gastrointestinal disorders and premature neonates worldwide.3–6 The survival rate of neonates born in the United States with gastroschisis is approximately 90% to 97%.7,8 By comparison, mortality rates in poorly resourced countries vary between 40% and 100% depending on surgical and medical treatments including the availability of PN.3,5 Despite benefits, PN does not come without complications. Whereas some of these complications are transient with recovery, others are associated with an increased risk of morbidity and mortality—specifically parenteral nutrition-associated liver disease (PNALD) and central line-associated bloodstream infections (CLASBIs) (Fig. 1).2 The purpose of this review is to summarize some of the commonly encountered complications associated with PN in the neonatal population.
Figure 1.
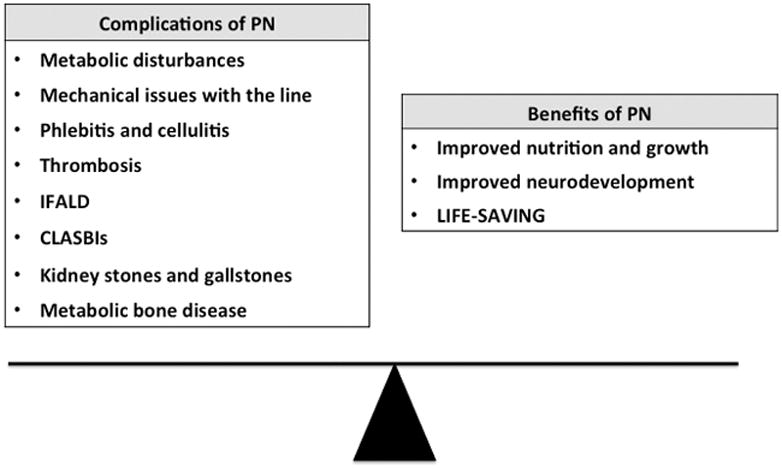
Common complications and benefits associated with parenteral nutrition (PN). PNALD, parenteral nutrition-associated liver disease (PNALD). CLASBIs, central line-associated bloodstream infections.
Metabolic Complications
Lipid Intolerance
Lipid emulsions provide long-chain polyunsaturated fatty acids (LC-PUFAs), which are important for a multitude of reasons; they maintain the integrity of the cell membrane and serve as precursors to eicosanoids and prostanoids (Fig. 2).9,10 The United States Food and Drug Administration (FDA)-approved and most frequently prescribed intravenous lipid product in the United States, Intralipid® 20% (Frensenius Kabi, Uppsala, Sweden), is derived entirely from soybean oil (SO), which mainly contains omega-6 fatty acids. SO contains the essential LC-PUFAs, linoleic and α-linolenic acid, but lacks the downstream products, arachidonic acid (ARA), eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA), which are important for cerebral and retinal development as well as the immune system (see Fig. 2, Table 1). Owing to increased demand, immature metabolic machinery, and limited stores, it is not surprising that premature neonates and children dependent on parenteral nutrition for a prolonged period are prone to developing omega-6 and omega-3 PUFA deficiencies, which are linked to morbidities such as chronic lung disease and neurodevelopmental impairment.9,11
Figure 2.
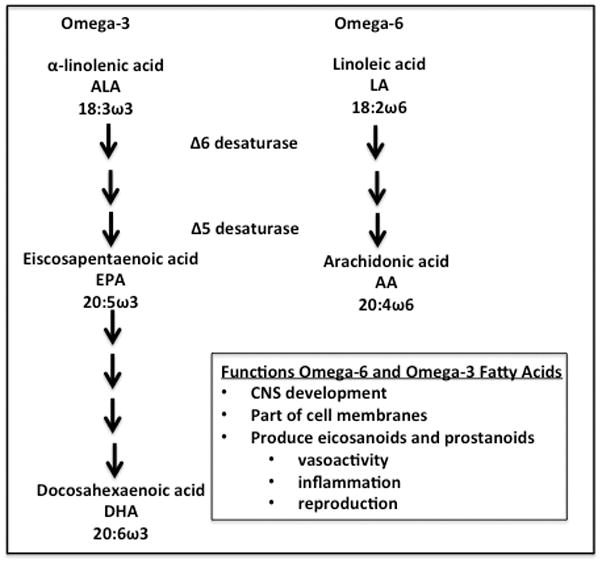
Omega-3 and omega-6 fatty acids and their functions.
Table 1.
Composition of two different intravenous lipid emulsions, soybean oil (Intralipid®) and fish oil (Omegaven®).
| Intralipid® | Omegaven® | SMOF® | |
|---|---|---|---|
|
| |||
| Vitamin E (mg/L) | 38 | 250 | 47.6 |
| Phytosterols (mg/L) | 343 | 0 | 48 |
|
| |||
| Oil Source | |||
|
| |||
| Soybean oil % | 100 | 0 | 30 |
| Fish oil % | 0 | 100 | 15 |
| Coconut oil % | 0 | 0 | 30 |
| Olive oil % | 0 | 0 | 25 |
|
| |||
| Fat Composition | |||
|
| |||
| Linoleic acid (g/100cc) | 5 | 0.1–0.7 | 2.85 |
| Arachidonic acid (g/100cc) | 0 | 0.1–0.4 | 0.05 |
| α-Linolenic acid (g/100cc) | 0.9 | <0.2 | 0.275 |
| Eicosapentaenoic acid (g/100cc) | 0 | 1.28–2.82 | 0.25 |
| Docosahexaenoic acid (g/100cc) | 0 | 1.44–3.09 | 0.05 |
While parenteral lipids serve as an important source of calories, improve metabolic efficiency, and prevent essential fatty acid deficiencies, they can exacerbate or cause hypertriglyceridemia and/or hyperglycemia. Particularly in the face of sepsis, lipids may impair insulin sensitivity and increase gluconeogenesis, and result in lipid and glucose intolerance.12 The long-term risks of serum hypertriglyceridemia, unlike those for hyperglycemia or hypoglycemia, are unknown.13–15 The temporary withholding or long-term restriction of lipids also remains controversial.9,16,17 At the same time, the benefit of providing early and high-dose parenteral lipids shortly after birth is unclear. In 2 meta-analyses, this strategy alone did not appear to decrease common neonatal morbidities.18,19 However, the prescription of 2 to 3 g/kg/d of parenteral lipids in combination with approximately 2.5 to 3.5 g/kg/d of amino acids at birth may facilitate growth by improving nitrogen balance and anabolism.20
Glucose Intolerance
Parenteral glucose infusions, particularly when prescribed at high glucose infusion rates, can result in abnormal serum glucose concentrations. After birth, the incidence of hyperglycemia in the very low birth weight (VLBW) population is inversely proportional to gestational age and birth weight, and can approach an incidence rate of 75%.13–15 Hyperglycemia and hypoglycemia are associated with increased neonatal morbidity and mortality.13–15 In the NIRTURE (Neonatal Insulin Replacement Therapy in Europe) study, a multisite, double-blind randomized controlled trial, insulin therapy in the first week of life in VLBW neonates was reported to improve glycemic control and decrease weight loss.21 However, subjects who received insulin were more likely to become hypoglycemic, die at 28 days of life, and develop ventricular hemorrhage or periventricular lesions in comparison with controls.21 Interestingly, in a study of neonates with a mean gestational age of 24 weeks, a 60% reduction in the parenteral glucose infusion rate from approximately 8.4 to 3.4 mg/kg/min reduced serum glucose concentrations by 30% without an increased incidence of serum hypoglycemia or change in hepatic gluconeogenesis.22 In order to combat hyperglycemia, the early introduction of parenteral amino acids has been shown to decrease the risk of hyperglycemia by stimulating endogenous insulin production.23–27
Amino Acid-Related Complications
In a double-blind randomized controlled study by Blanco and colleagues,25 extremely low birth weight (ELBW, birth weight < 1 kg) neonates were assigned to 1 of 2 interventions: early and high-dose amino acids in the form of Aminosyn PF® (Abbott Laboratories, Chicago, IL) or a control dose. The early and high-dose group was prescribed a target goal of approximately 4 g/kg/d by day of life 3, whereas the control group’s amino acids were advanced to approximately 3 g/kg/d in the first couple of days of life. In comparison with the control group, the early and high-dose group exhibited decreased growth during follow-up. Whereas neurodevelopmental scores at 18 months postmenstrual age were decreased in the interventional group when compared with controls, at 24 months the cognitive scores were similar between the 2 arms. Of notable concern was the negative correlation discovered between various plasma amino acid concentrations and growth and Mental Developmental Index (MDI).28,29 It remains unclear as to whether this finding is secondary to the target dose of 4 g/kg/d, with 3 g/kg/d or less in the first few days of life being more appropriate, specific outliers, and/or the specific amino acid formulation. Nevertheless, other studies have not replicated these results and have actually demonstrated the converse. Once a protein and energy deficit occurs, it is difficult to make up. Appropriate provisions of protein and energy promote lean body mass and linear growth—which may be just as important (if not more) as body weight.24,30,31
As expected, concentrations of serum blood urea nitrogen (BUN) and, in some subjects, serum ammonia, increased in the early and high-dose amino acid group in comparison with the control group in the aforementioned study by Blanco and colleagues.20,25,28,29,31 As with hypertriglyceridemia, it remains debatable as to what constitutes a clinically significant state of uremia. What is an appropriate aminogram for VLBW infants, and when parenteral protein should be decreased considering the implications of a protein deficit, also remain questionable.20,25,27 Higher BUN concentrations are to be expected when clinicians prescribe amino acids at a higher dose immediately after birth, and in most cases reflect amino acid oxidation and protein turnover, not toxicity.
Summary
Although the early introduction of parenteral amino acids and lipids after birth may be associated with transient metabolic complications, numerous studies have demonstrated that PN and continued refinements in PN care have resulted in improved growth and long-term neurodevelopment that have far-researching benefits beyond the NICU.4,6,24,26,32 Although significant gains have been made, one should remember that neonates, both preterm and term, who depend on PN in the NICU, still have a high rate of growth failure that persists even after hospital discharge.33,34
Parenteral nutrition-associated liver disease
Although PN is life-sustaining, it is associated with PNALD, which carries high morbidity and mortality in the pediatric population.2 PNALD, a heterogeneous liver injury consisting of cholestasis, steatosis, fibrosis, and even cirrhosis, is characteristically defined as the development of persistent, direct hyperbilirubinemia when other causes of liver disease are excluded in patients who have received prolonged courses of PN. As serum direct bilirubin rises, mortality and the need for a small bowel or combined small bowel-liver transplant increases.1,35 While liver biopsy is considered the gold standard for PNALD diagnosis, this type of invasive surveillance carries risks related to bleeding and anesthesia. As a result, clinicians routinely rely upon laboratory evaluations, specifically serum direct bilirubin concentrations and liver function tests, to monitor PNALD. However, it is well recognized that histological injury begins soon after PN initiation and does not correlate with bilirubin concentrations. In fact, bilirubins can be normal in the presence of severe histological damage.36
PNALD risk factors can broadly be characterized as either patient-related or PN-related (Box 2). PNALD patient-related risk factors mainly center around the percentage of calories a patient tolerates enterally versus parenterally, which in turn depends on the patient’s gastrointestinal function and anatomy, such as length of small-bowel remnant, gastrocolonic continuity, and presence of an ileocecal valve. By contemporary analyses, approximately 25% of neonates with gastrointestinal disorders will develop PNALD.37 The incidence and likelihood of developing PNALD vary by specific gastrointestinal diagnosis and report.1,37,38 Moreover, PNALD incidence increases significantly when a congenital gastrointestinal diagnosis is complicated by any stage of necrotizing enterocolitis.1,37–41 For children who develop short bowel syndrome (SBS), 67% will develop PNALD and 17% will progress to end-stage liver failure.42 In addition, prematurity, birth weight, infections, and perhaps an individual’s genetic makeup appear to be important drivers for PNALD.1,41 The odds ratio for developing cholestasis among neonates with a birth weight of less than 750 g is 13.1, whereas for a neonate with a birth weight between 1 and 1.5 kg this decreases to 2.8.1 In one study, despite receiving fewer PN days, small for gestational age neonates, in a comparison with appropriate for gestational age controls, had an odds ratio of 3.3 for developing cholestasis.41
Box 2. Common risk factors for parenteral nutrition-associated liver disease (PNALD). CLASBI, central line associated bloodstream infection. SGA, small for gestational age. IUGR, intrauterine growth restriction.
| Common Risk Factors for PNALD | |
|---|---|
|
| |
| Patient-related | PN-related |
|
| |
|
|
Intravenous Fatty Acid Emulsions and PNALD
Recent studies have demonstrated that the dose and composition of intravenous fatty acid emulsions may play an important role in the development and progression of PNALD.16,17,39,40,43,44 SO has been traditionally prescribed at an approximate dose of 0.5 to 4 g/kg/d. The American Academy of Pediatrics recommends a maximum dose of 3 g/kg/d of intravenous lipids. An intravenous lipid emulsion composed entirely of fish oil (FO), commercially available as Omegaven® 10% (Fresenius Kabi, Hamburg, Germany), is composed mainly of the omega-3 fatty acids EPA and DHA (Table 1). FO is not currently FDA-approved but is available outside of the United States, and is prescribed at 1 g/kg/d. Studies have provided evidence that when 1 g/kg/d of exclusive FO is substituted for SO, direct hyperbilirubinemia is more likely to resolve and the incidences of death and transplant may be reduced.44–46 European studies have also demonstrated that mixed fatty acid emulsions, such as SMOF® (Fresenius Kabi, Hamburg, Germany), which contain soybean, fish, olive, and coconut oils, are associated with improved liver function, decreased markers of inflammation and oxidative injury, and increased antioxidant activity (see Table 1).47–50 Whereas FO and products containing FO appear to biochemically reverse PNALD, their effect on histology, which is equally (if not more) important, remains unknown.36
There is also evidence that the incidence and progression of PNALD can be modified by decreasing the SO dose alone.16,40,51 When compared with a historical cohort who received the standard SO dose, surgical neonates with cholestasis who received 1 g/kg of SO twice a week had an increased incidence of cholestasis resolution (42% vs 10%). However, 8 of 13 neonates developed a triene:tetraene ratio of greater than 0.05 but less than 0.2, and without physical manifestations of an essential fatty acid deficiency.16 Traditionally, an essential fatty acid deficiency has been defined as a triene:tetraene ratio of greater than 0.2.
In a retrospective study of 214 neonates by Sanchez and colleagues51 and a randomized controlled pilot study of 28 subjects by Rollins and colleagues40, lipid sparing appeared to prevent and slow down the onset of cholestasis. By contrast, in a much smaller retrospective review by Nehra and colleagues,52 neonates with gastrointestinal disorders who received 1 g/kg/d of SO had a similar incidence of cholestasis when compared with neonates who received 2 to 3 g/kg/d of SO. Of note, there was a trend toward an increased rate of change in direct bilirubin in the 2 to 3 g/kg/d group compared with the 1 g/kg/d group, which did not reach statistical significance (P=0.05).
All published studies investigating the efficacy and safety of SO sparing (1 vs 2–3 g/kg/d ) for cholestasis prevention or treatment, with the exception of one small, randomized controlled pilot trial by Rollins and colleagues,40 are single-center retrospective or uncontrolled prospective investigations.16,51–53 As a result, methodological issues in study design and confounding variables, specifically advances in neonatal and nutritional care, have led to conflicting results.16,51–53
Likewise, all FO studies have been performed at one institution and most have relied upon historical controls.44–46 In a small randomized, controlled study of 19 neonates of less than 3 months of age, there was no difference in the incidence of cholestasis and maximum serum direct bilirubin concentrations between the FO and SO groups. Interestingly, one subject in each group crossed over to the other study arm because of a serum direct hyperbilirubinemia. The study was stopped early because of the unexpected low incidence of PNALD and concerns for futility.39
One concern with lipid minimization is the decreased energy intake from fat and the risk for a deficiency of essential fatty acids. Postnatal growth restriction and necrotizing enterocolitis in the premature population are inversely related to gestational age and directly linked to poor neurodevelopment.34 Neonates with congenital gastrointestinal disorders are also at risk for long-term suboptimal growth and neurodevelopment.54 To compensate for decreased calories many clinicians increase glucose infusion rates, which may promote cholestasis and steatosis. Although most lipid-sparing studies with either FO or SO have not demonstrated an increase in growth failure, these studies are not designed to examine such an outcome. Studies on long-term growth and development are clearly lacking.16,39,40,45,46,51 Advantages to mixed lipid emulsion such as SMOF® are that they contain omega-3 fatty acids, can be dosed at 2 to 3 g/kg/d, and may better facilitate growth (see Table 1).
With regard to a deficiency of essential fatty acids, it does not seem likely that neonates receiving 1 g/kg/d and upward of FO or SO will develop a biochemical deficiency of essential fatty acids as measured by a serum triene:tetraene ratio.10,16,45,46 However, deficiencies of specific fatty acids with lipid sparing and even toxicities with FO and SO may be possible, and may have unknown short-term and long-term consequences.11
Neonates, such as VLBWs and neonates with congenital gastrointestinal disorders who develop SBS, may possibly reap the most benefit from the potential hepatoprotective properties of lipid sparing or alternative lipid emulsions. However, one must also remember that these same infants are also at very high risk for nutritional deficiencies and cognitive delays.16,34,41 As a result, it remains unclear if the possible benefit of lipid sparing (PNALD prevention and treatment) outweighs the possible risks (poor growth and neurodevelopment) in these high-risk groups. While some risk factors that predict clear and definitive intestinal failure and advanced PNALD are present at birth, many risk factors do not unfold until later in the patient’s hospital course, making it difficult for clinicians to determine when to initiate FO or SO dose reduction (see Box 2)1,2,37,41,55 Complicating this dilemma even further, the required duration for these therapies is unknown.45
In summary, direct comparisons of FO versus SO and 1 g/kg/d versus 3 g/kg/d of SO are complicated by the following: (1) published lipid-sparing studies have targeted different populations; (2) primary outcomes are different (cholestasis prevention vs treatment); (3) investigations have used different doses and durations; and (4) issues with trial design and sample size.16,39,40,44–46,51–53,56 Considering the mounting evidence that FO may prevent or delay the need for transplant, it may be unethical to conduct a randomized controlled trial comparing FO with SO at 1 g/kg/d in children with advanced PNALD who are at high risk for liver failure or who have liver failure.1,35,44–46 Considering the importance of PNALD prevention, it would behoove the research community to initiate well-powered, randomized, multisite, long-term studies, with or without a factorial design, to determine whether lipid sparing (FO vs SO, each dosed at 1 g/kg/d and/or 1 g/kg/d vs 3 g/kg/d of SO) safely prevents cholestasis.
Lipids, Phytosterols, and PNALD
Understanding the reasons why FO, SO dose reduction, and mixed lipid emulsions may modify the incidence and progression of PNALD could provide important clues to the etiology of this disease. Such clues may uncover targets for future preventive strategies and therapeutics for PNALD. All 3 lipid strategies reduce the liver’s exposure to phytosterols, which are known to interfere with bile acid transport, resulting in biliary sludge: the hallmark of pediatric PNALD (Fig. 3).43,57–60 Phytosterols are found in vegetable foods and include campesterol, stimgasterol, and sitosterol. SO is made up of 43% cholesterol and 57% phytosterols. FO, by comparison, is made up of cholesterol only.61 There is linear correlation between PN duration and increasing concentrations of serum phytosterols.61 SO dose reduction and FO have been associated with decreased serum phytosterols.62
Figure 3.
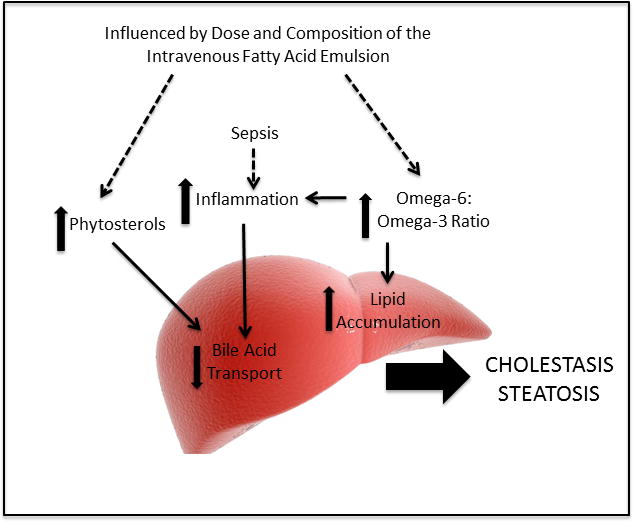
Proposed etiology of parenteral nutrition-associated liver disease.
Approximately 5% to 10% of orally ingested phytosterols are absorbed by the intestine while 90% to 95% are excreted in the feces by the enterocyte apical ABCG5/G8 transporter. However, intravenous fatty acids bypass this transporter and rely on the canicular ABCG5/G8 transporter in the hepatocyte to secrete phytosterols into the bile. Sitosterol inhibits cholesterol 7α-hydroxlase, the rate-limiting step that converts cholesterol into bile acids, which plays an important role in lipid metabolism.63 Moreover, stigmasterol antagonizes bile acid nuclear receptors, liver X receptor (LXR) and farnesoid X receptor (FXR). FXR protects the liver from hepatoxic bile acids by reducing bile acid import via (1) suppression of the bile acid cotransporter (NTCP, SLC10A1), (2) reduction of bile acid synthesis by suppressing CYP7A1, and (3) enhancing bile acid efflux resulting from upregulation of the bile salt export pump (BSEP) ABC11 and organic solute transporter (OST) (see Fig. 3). In animal models, FXR knockouts develop liver injury, whereas treatment with FXR agonists protect against the development of cholestasis.64 In cell lines, stigmasterol antagonizes BSEP.65 Moreover, mice infused with PN and FO exhibited less liver injury compared to those infused with PN and SO. When stigmasterol was added to FO, these animals developed cholestasis.43 Lastly, BSEP is developmentally regulated and is not expressed or completely functional in the neonatal period, placing preterm neonates at high risk for PNALD.66
Lipids, Inflammation, Oxidant Injury and PNALD
Bile acid transport is not only regulated not only by phytosterols but also inflammation (see Fig. 3). Critically ill neonates are at high risk for systemic inflammation, specifically bloodstream infections. The high mortality associated with long-term PN stems not only from liver failure but also CLASBIs, which can result in multiorgan failure and death.2 Ninety percent of patients with intestinal resections develop cholestasis after sepsis, and serum direct bilirubins increase considerably after sepsis.55 Lipopolysaccharides and cytokines alter the expression of specific bile acid transporters (BSEP and NTCP), decreasing bile flow.67,68 When liver macrophages were stimulated with either stigmasterol, lipopolysaccride, or placebo, cells subjected to stigmasterol or lipopolysaccride showed increased transcription of interleukin-6 (IL-6) and tumor necrosis factor α (TNF-α).43
In a large meta-analysis, lipid products that contained FO or higher concentrations of omega-3 fatty acids were associated with a 25% reduction in infections (relative risk 0.75, 95% confidence interval 0.56–1.00). This finding may be the result of the immunomodulatory properties of omega-3 fatty acids and antioxidants in these preparations or chance.19 SO is composed mainly of omega-6 fatty acids, which produce a cascade of proinflammatory bioactive compounds, and contains a small concentration of the antioxidant vitamin E. FO contains mainly anti-inflammatory omega-3 fatty acids and a higher concentration of vitamin E (see Table 1). Lipid emulsions that provide a more appropriate omega-6:omega-3 fatty acid ratio and contain EPA and DHA have been shown to decrease inflammatory mediators such as TNF-α, nuclear factor κβ, and IL-6, and increase proresolving lipid mediators, such as resolvins and protectins.57,58 In neonates and animals, FO and mixed emulsions increase the concentrations of vitamin E and A and reduce lipid peroxidation, as measured by F2-isoprostane and total antioxidant potential.47,49,50,57,58,69
High omega-6:omega-3 fatty acid ratios also affect lipid metabolism by regulating peroxisome proliferator-activated receptor (PPAR) and sterol regulatory element binding proteins (SREBPs).57,70,71 PPAR regulates fatty acid storage, whereas SREBPs are involved in cholesterol synthesis and fatty acid uptake. As fat accumulates in hepatic cells, phagocytic dysfunction and impaired endotoxin clearance occurs, resulting in hepatic injury. In fact, when rodents were provided with a high-carbohydrate, high-fat diet, supplementation with EPA resulted in improved insulin sensitivity, increased lipolysis, and decreased TNF-α and PPAR compared with animals who received a similar diet without EPA.72
LXR, which is regulated by phytosterols and inflammatory products, reduces production of apolipoprotein E, resulting in impaired clearance of chylomicrons and low-density lipoproteins.73 In animal models, FXR agonists have been shown to be heptoprotective by decreasing apolipoprotein CIII, which inhibits hepatic uptake of lipid-rich particles.74 As a result, a lipid emulsion with a high omega-6:omega-3 fatty acid ratio may alter lipid trafficking, thereby promoting steatosis, yet another histological feature of PNALD (see Fig. 3).
Cost of PN Complications
Considering that evidence-based and cost-effective medicine is essential to neonatal practice, clinicians must remember that PN can come with a significant price. For example, it is unknown whether the potential nutritional benefit of a short course of PN for a low birth weight neonate (birth weight < 2 kg) outweighs the daily cost of PN, which is approximately US $500. In one study, a NICU feeding protocol decreased PN duration by approximately 5 days, in turn leading to a significant reduction in cost: approximately $385,000 in hospital savings in 1 year.75
PN is life-saving, but complications such as PNALD and CLASBIs cannot be ignored. Once end-stage liver disease develops, the only remaining life-saving measure is combined liver-intestinal transplant.2 Indications for transplant, either intestine or combined liver-intestine, include not only advanced PNALD, but also a history of repeated CLABSIs, which can result in catheter removals and replacements and eventual loss of vascular access. Five-year post-transplant survival is 50% to 70%, and the cost of transplant is approximately $900,000 in the first year post-transplant and $375,000 each year thereafter.2,45 After transplant, patients are still not free of complications—which include graft rejection and deadly infections and malignancies secondary to immunosuppression. With respect to CLABSIs, it is estimated that more than 2 million nosocomial infections occur each year in the United States. Almost half of all deaths in the NICU after 2 weeks of life are attributed to nosocomial infections, many related to catheters, and increase the average NICU stay by a mean of 24 days.76,77
Summary
PN has revolutionized neonatology. However, potential long-term complications such as PNALD and CLABSIs are life-threatening, pose an economic burden to society, and profoundly affect the quality of lives of patients and their families. Research and collaboration is warranted to: (1) optimize PN delivery and composition to more closely mimic placental nutrient supply and that of a healthy, breastfed term infant, (2) identify biomarkers that reflect PN intolerance that are clinically relevant and predict the early onset of adverse complications such as PNALD and CLASBIs, and (3) reduce the incidence of PNALD and CLASBIs by improving current practices and developing new preventive strategies and therapeutic modalities. Together, basic scientists and translational and clinical researchers, along with clinicians and families, can improve PN care to enhance the health of all children.
Key Points.
Although parenteral nutrition (PN) is life-saving, it is associated with myriad of complications, some of which are transient and others life-threatening, including parenteral nutrition-associated liver disease (PNALD) and central line-associated bloodstream infections (CLASBIs).
Although fish oil-based lipid emulsions can biochemically reverse PNALD, clinicians should bear in mind that the development and progression of PNALD is multifactorial.
It remains to be determined whether dose reduction of lipid emulsions can prevent PNALD without sacrificing growth and neurodevelopment.
The phytosterol, long chain polyunsaturated fatty acid, and antioxidant content of intravenous lipid products seems to play an important role in the pathogenesis of PNALD.
Research is needed with regard to optimizing the PN content for the neonatal population so as to safely promote growth and neurodevelopment.
Contributor Information
Kara L Calkins, Assistant Clinical Professor, University of California, Los Angeles, Department of Pediatrics, Division of Neonatology and Developmental Biology, Mattel Children’s Hospital, University of California, Los Angeles.
Robert S. Venick, Associate Clinical Professor, University of California, Los Angeles, Department of Pediatrics, Division of Gastroenterology, Mattel Children’s Hospital, University of California, Los Angeles
Sherin U. Devaskar, Distinguished Professor, University of California, Los Angeles, Department of Pediatrics, Division of Neonatology and Developmental Biology, Mattel Children’s Hospital, University of California, Los Angeles
References
- 1.Christensen RD, Henry E, Wiedmeier SE, et al. Identifying patients, on the first day of life, at high-risk of developing parenteral nutrition-associated liver disease. J Perinatol. 2007;27(5):284–290. doi: 10.1038/sj.jp.7211686. [DOI] [PubMed] [Google Scholar]
- 2.Squires RH, Duggan C, Teitelbaum DH, et al. Natural history of pediatric intestinal failure: initial report from the pediatric intestinal failure consortium. J Pediatr. 2012;161(4):723–728. doi: 10.1016/j.jpeds.2012.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arnold M. Is the incidence of gastroschisis rising in South Africa in accordance with international trends? A retrospective analysis at Pretoria Academic and Kalafong Hospitals, 1981–2001. S Afr J Surg. 2004;42(3):86–88. [PubMed] [Google Scholar]
- 4.Poindexter BB, Langer JC, Dusick AM, et al. Early provision of parenteral amino acids in extremely low birth weight infants: relation to growth and neurodevelopmental outcome. J Pediatr. 2006;148(3):300–305. doi: 10.1016/j.jpeds.2005.10.038. [DOI] [PubMed] [Google Scholar]
- 5.Sekabira J, Hadley GP. Gastroschisis: a third world perspective. Pediatr Surg Int. 2009;25(4):327–329. doi: 10.1007/s00383-009-2348-4. [DOI] [PubMed] [Google Scholar]
- 6.Stephens BE, Walden RV, Gargus RA, et al. First-week protein and energy intakes are associated with 18-month developmental outcomes in extremely low birth weight infants. Pediatrics. 2009;123(5):1337–1343. doi: 10.1542/peds.2008-0211. [DOI] [PubMed] [Google Scholar]
- 7.Bradnock TJ, Marven S, Owen A, et al. Gastroschisis: one year outcomes from national cohort study. BMJ. 2011;343:d6749. doi: 10.1136/bmj.d6749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mills JA, Lin Y, Macnab YC, et al. Perinatal predictors of outcome in gastroschisis. J Perinatol. 2010;30(12):809–813. doi: 10.1038/jp.2010.43. [DOI] [PubMed] [Google Scholar]
- 9.Nandivada P, Carlson SJ, Cowan E, et al. Role of parenteral lipid emulsions in the preterm infant. Early Hum Dev. 2013;89(Suppl 2):S45–49. doi: 10.1016/j.earlhumdev.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 10.Le HD, Meisel JA, de Meijer VE, et al. The essentiality of arachidonic acid and docosahexaenoic acid. Prostaglandins Leukot Essent Fatty Acids. 2009;81(2–3):165. doi: 10.1016/j.plefa.2009.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robinson DT, Carlson SE, Murthy K, et al. Docosahexaenoic and arachidonic acid levels in extremely low birth weight infants with prolonged exposure to intravenous lipids. J Pediatr. 2013;162(1):56–61. doi: 10.1016/j.jpeds.2012.06.045. [DOI] [PubMed] [Google Scholar]
- 12.Boden G. Effects of free fatty acids on gluconeogenesis and glycogenolysis. Life Sci. 2003;72(9):977–988. doi: 10.1016/s0024-3205(02)02350-0. [DOI] [PubMed] [Google Scholar]
- 13.Hey E. Hyperglycaemia and the very preterm baby. Semin Fetal Neonatal Med. 2005;10(4):377–387. doi: 10.1016/j.siny.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 14.Beardsall K, Vanhaesebrouck S, Ogilvy-Stuart AL, et al. Prevalence and determinants of hyperglycemia in very low birth weight infants: cohort analyses of the NIRTURE study. J Pediatr. 2010;157(5):715–719. e711–713. doi: 10.1016/j.jpeds.2010.04.032. [DOI] [PubMed] [Google Scholar]
- 15.Hays SP, Smith EO, Sunehag AL. Hyperglycemia is a risk factor for early death and morbidity in extremely low birth-weight infants. Pediatrics. 2006;118(5):1811–1818. doi: 10.1542/peds.2006-0628. [DOI] [PubMed] [Google Scholar]
- 16.Cober MP, Killu G, Brattain A, et al. Intravenous fat emulsions reduction for patients with parenteral nutrition-associated liver disease. J Pediatr. 2012;160(3):421–427. doi: 10.1016/j.jpeds.2011.08.047. [DOI] [PubMed] [Google Scholar]
- 17.Venick RS, Calkins K. The impact of intravenous fish oil emulsions on pediatric intestinal failure-associated liver disease. Curr Opin Organ Transplant. 2011;16(3):306–311. doi: 10.1097/MOT.0b013e32834670eb. [DOI] [PubMed] [Google Scholar]
- 18.Simmer K, Rao SC. Early introduction of lipids to parenterally-fed preterm infants. Cochrane Database Syst Rev. 2005;(2):CD005256. doi: 10.1002/14651858.CD005256. [DOI] [PubMed] [Google Scholar]
- 19.Vlaardingerbroek H, Veldhorst MA, Spronk S, et al. Parenteral lipid administration to very-low-birth-weight infants--early introduction of lipids and use of new lipid emulsions: a systematic review and meta-analysis. Am J Clin Nutr. 2012;96(2):255–268. doi: 10.3945/ajcn.112.040717. [DOI] [PubMed] [Google Scholar]
- 20.Vlaardingerbroek H, Vermeulen MJ, Rook D, et al. Safety and efficacy of early parenteral lipid and high-dose amino acid administration to very low birth weight infants. J Pediatr. 2013;163(3):638–644. e631–635. doi: 10.1016/j.jpeds.2013.03.059. [DOI] [PubMed] [Google Scholar]
- 21.Beardsall K, Vanhaesebrouck S, Ogilvy-Stuart AL, et al. Early insulin therapy in very-low-birth-weight infants. N Engl J Med. 2008;359(18):1873–1884. doi: 10.1056/NEJMoa0803725. [DOI] [PubMed] [Google Scholar]
- 22.Chacko SK, Ordonez J, Sauer PJ, et al. Gluconeogenesis is not regulated by either glucose or insulin in extremely low birth weight infants receiving total parenteral nutrition. J Pediatr. 2011;158(6):891–896. doi: 10.1016/j.jpeds.2010.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mahaveer A, Grime C, Morgan C. Increasing early protein intake is associated with a reduction in insulin-treated hyperglycemia in very preterm infants. Nutr Clin Pract. 2012;27(3):399–405. doi: 10.1177/0884533612438730. [DOI] [PubMed] [Google Scholar]
- 24.Thureen PJ, Melara D, Fennessey PV, et al. Effect of low versus high intravenous amino acid intake on very low birth weight infants in the early neonatal period. Pediatr Res. 2003;53(1):24–32. doi: 10.1203/00006450-200301000-00008. [DOI] [PubMed] [Google Scholar]
- 25.Blanco CL, Falck A, Green BK, et al. Metabolic responses to early and high protein supplementation in a randomized trial evaluating the prevention of hyperkalemia in extremely low birth weight infants. J Pediatr. 2008;153(4):535–540. doi: 10.1016/j.jpeds.2008.04.059. [DOI] [PubMed] [Google Scholar]
- 26.Dinerstein A, Nieto RM, Solana CL, et al. Early and aggressive nutritional strategy (parenteral and enteral) decreases postnatal growth failure in very low birth weight infants. J Perinatol. 2006;26:436–442. doi: 10.1038/sj.jp.7211539. [DOI] [PubMed] [Google Scholar]
- 27.Hay WW, Jr, Thureen PJ. Early postnatal administration of intravenous amino acids to preterm, extremely low birth weight infants. J Pediatr. 2006;148(3):291–294. doi: 10.1016/j.jpeds.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 28.Blanco CL, Gong AK, Green BK, et al. Early changes in plasma amino acid concentrations during aggressive nutritional therapy in extremely low birth weight infants. J Pediatr. 2011;158(4):543–548. e541. doi: 10.1016/j.jpeds.2010.09.082. [DOI] [PubMed] [Google Scholar]
- 29.Blanco CL, Gong AK, Schoolfield J, et al. Impact of early and high amino acid supplementation on ELBW infants at 2 years. J Pediatr Gastroenterol Nutr. 2012;54(5):601–607. doi: 10.1097/MPG.0b013e31824887a0. [DOI] [PubMed] [Google Scholar]
- 30.Gravari E, Radmacher PG, Adamkin DH, et al. Amino acid profiles and serial blood urea nitrogen levels in infants less than 1250 g receiving early parenteral nutrition. J Neonatal Perinatal Med. 2012;5(2):149–153. [Google Scholar]
- 31.Poindexter BB, Ehrenkranz RA, Stoll BJ, et al. Effect of parenteral glutamine supplementation on plasma amino acid concentrations in extremely low-birth-weight infants. Am J Clin Nutr. 2003;77(3):737–743. doi: 10.1093/ajcn/77.3.737. [DOI] [PubMed] [Google Scholar]
- 32.Denne SC, Poindexter BB. Evidence supporting early nutritional support with parenteral amino acid infusion. Semin Perinatol. 2007;31(2):56–60. doi: 10.1053/j.semperi.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 33.Dusick AM, Poindexter BB, Ehrenkranz RA, et al. Growth failure in the preterm infant: can we catch up? Semin Perinatol. 2003;27(4):302–310. doi: 10.1016/s0146-0005(03)00044-2. [DOI] [PubMed] [Google Scholar]
- 34.Cole CR, Hansen NI, Higgins RD, et al. Very low birth weight preterm infants with surgical short bowel syndrome: incidence, morbidity and mortality, and growth outcomes at 18 to 22 months. Pediatrics. 2008;122(3):e573–e582. doi: 10.1542/peds.2007-3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Willis TC, Carter BA, Rogers SP, et al. High rates of mortality and morbidity occur in infants with parenteral nutrition–associated cholestasis. JPEN J Parenter Enteral Nutr. 2010;34(1):32–37. doi: 10.1177/0148607109332772. [DOI] [PubMed] [Google Scholar]
- 36.Fitzgibbons SC, Jones BA, Hull MA, et al. Relationship between biopsy-proven parenteralnutrition-associated liver fibrosis and biochemical cholestasis in children with short bowel syndrome. J Pediatr Surg Jan. 2010;45(1):95–99. doi: 10.1016/j.jpedsurg.2009.10.020. discussion 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Javid PJ, Malone FR, Dick AA, et al. A contemporary analysis of parenteral nutrition–associated liver disease in surgical infants. J Pediatr Surg. 2011;46(10):1913–1917. doi: 10.1016/j.jpedsurg.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 38.Baird R, Eeson G, Safavi A, et al. Institutional practice and outcome variation in the management of congenital diaphragmatic hernia and gastroschisis in Canada: a report from the Canadian Pediatric Surgery Network. J Pediatr Surg. 2011;46(5):801–807. doi: 10.1016/j.jpedsurg.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 39.Nehra D, Fallon EM, Potemkin AK, et al. A Comparison of 2 Intravenous Lipid Emulsions: Interim Analysis of a Randomized Controlled Trial. JPEN J Parenter Enteral Nutr. 2013:1–9. doi: 10.1177/0148607113492549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rollins MD, Ward RM, Jackson WD, et al. Effect of decreased parenteral soybean lipid emulsion on hepatic function in infants at risk for parenteral nutrition-associated liver disease: a pilot study. J Pediatr Surg. 2013;48(6):1348–1356. doi: 10.1016/j.jpedsurg.2013.03.040. [DOI] [PubMed] [Google Scholar]
- 41.Robinson DT, Ehrenkranz RA. Parenteral nutrition-associated cholestasis in small for gestational age infants. J Pediatr. 2008;152(1):59–62. doi: 10.1016/j.jpeds.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 42.Grant D, Abu-Elmagd K, Reyes J, et al. 2003 report of the intestine transplant registry: a new era has dawned. Ann Surg. 2005;241(4):607–613. doi: 10.1097/01.sla.0000157265.85388.a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.El Kasmi KC, Anderson AL, Devereaux MW, et al. Phytosterols promote liver injury and kupffer cell activation in parenteral nutrition-associated liver disease. Sci Transl Med. 2013;5(206):206ra137. doi: 10.1126/scitranslmed.3006898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Premkumar MH, Carter BA, Hawthorne KM, et al. High rates of resolution of cholestasis in parenteral nutrition-associated liver disease with fish oil-based lipid emulsion monotherapy. J Pediatr. 2013;162(4):793–798. doi: 10.1016/j.jpeds.2012.10.019. [DOI] [PubMed] [Google Scholar]
- 45.Calkins KL, Dunn JCY, Shew SB, et al. Pediatric intestinal failure–associated liver disease is reversed with 6 months of intravenous fish oil. JPEN J Parenter Enteral Nutr. 2013 Jul 26;2013:1–11. doi: 10.1177/0148607113495416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gura KM, Lee S, Valim C, et al. Safety and efficacy of a fish-oil-based fat emulsion in the treatment of parenteral nutrition-associated liver disease. Pediatrics. 2008;121(3):e678–686. doi: 10.1542/peds.2007-2248. [DOI] [PubMed] [Google Scholar]
- 47.Goulet O, Antebi H, Wolf C, et al. A new intravenous fat emulsion containing soybean oil, medium-chain triglycerides, olive oil, and fish oil: a single-center, double-blind randomized study on efficacy and safety in pediatric patients receiving home parenteral nutrition. JPEN J Parenter Enteral Nutr. 2010;34(5):485–495. doi: 10.1177/0148607110363614. [DOI] [PubMed] [Google Scholar]
- 48.Koletzko B, Goulet O. Fish oil containing intravenous lipid emulsions in parenteral nutrition-associated cholestatic liver disease. Curr Opin Clin Nutr Metab Care. 2010;13(3):321. doi: 10.1097/MCO.0b013e3283385407. [DOI] [PubMed] [Google Scholar]
- 49.Skouroliakou M, Konstantinou D, Koutri K, et al. A double-blind, randomized clinical trial of the effect of ω-3 fatty acids on the oxidative stress of preterm neonates fed through parenteral nutrition. Eur J Clin Nutr. 2010;64(9):940–947. doi: 10.1038/ejcn.2010.98. [DOI] [PubMed] [Google Scholar]
- 50.Tomsits E, Pataki M, Tölgyesi A, et al. Safety and efficacy of a lipid emulsion containing a mixture of soybean oil, medium-chain triglycerides, olive oil, and fish oil: a randomised, double-blind clinical trial in premature infants requiring parenteral nutrition. J Pediatr Gastroenterol Nutr. 2010;51(4):514–521. doi: 10.1097/MPG.0b013e3181de210c. [DOI] [PubMed] [Google Scholar]
- 51.Sanchez SE, Braun LP, Mercer LD, et al. The effect of lipid restriction on the prevention of parenteral nutrition-associated cholestasis in surgical infants. J Pediatr Surg. 2013;48(3):573–578. doi: 10.1016/j.jpedsurg.2012.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nehra D, Fallon EM, Carlson SJ, et al. Provision of a soy-based intravenous lipid emulsion at 1 g/kg/d does not prevent cholestasis in neonates. JPEN J Parenter Enteral Nutr. 2013;37(4):498–505. doi: 10.1177/0148607112453072. [DOI] [PubMed] [Google Scholar]
- 53.Rangel SJ, Calkins CM, Cowles RA, et al. Parenteral nutrition-associated cholestasis: an American Pediatric Surgical Association Outcomes and Clinical Trials Committee systematic review. J Pediatr Surg. 2012;47(1):225–240. doi: 10.1016/j.jpedsurg.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 54.South A, Marshall D, Bose C, et al. Growth and neurodevelopment at 16 to 24 months of age for infants born with gastroschisis. J Perinatol. 2008;28(10):702–706. doi: 10.1038/jp.2008.71. [DOI] [PubMed] [Google Scholar]
- 55.Sondheimer JM, Asturias E, Cadnapaphornchai M. Infection and cholestasis in neonates with intestinal resection and long-term parenteral nutrition. J Pediatr Gastroenterol Nutr. 1998;27(2):131–137. doi: 10.1097/00005176-199808000-00001. [DOI] [PubMed] [Google Scholar]
- 56.Cowan E, Nandivada P, Puder M. Fish oil-based lipid emulsion in the treatment of parenteral nutrition-associated liver disease. Curr Opin Pediatr. 2013;25(2):193–200. doi: 10.1097/MOP.0b013e32835e02ac. [DOI] [PubMed] [Google Scholar]
- 57.Kalish BT, Le HD, Gura KM, et al. A metabolomic analysis of two intravenous lipid emulsions in a murine model. PLoS One. 2013;8(4):e59653. doi: 10.1371/journal.pone.0059653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kalish BT, Le HD, Fitzgerald JM, et al. Intravenous fish oil lipid emulsion promotes a shift toward anti-inflammatory proresolving lipid mediators. American journal of physiology. Am J Physiol Gastrointest Liver Physiol. 2013;305(11):G818–828. doi: 10.1152/ajpgi.00106.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kurvinen A, Nissinen MJ, Gylling H, et al. Effects of long-term parenteral nutrition on serum lipids, plant sterols, cholesterol metabolism, and liver histology in pediatric intestinal failure. J Pediatr Gastroenterol Nutr. 2011;53(4):440–446. doi: 10.1097/MPG.0b013e3182212130. [DOI] [PubMed] [Google Scholar]
- 60.Bindl L, Lütjohann D, Buderus S, et al. High plasma levels of phytosterols in patients on parenteral nutrition: a marker of liver dysfunction. J Pediatr Gastroenterol Nutr. 2000;31(3):313–316. doi: 10.1097/00005176-200009000-00022. [DOI] [PubMed] [Google Scholar]
- 61.Forchielli ML, Bersani G, Tala S, et al. The spectrum of plant and animal sterols in different oil-derived intravenous emulsions. Lipids. 2010;45(1):63–71. doi: 10.1007/s11745-009-3371-x. [DOI] [PubMed] [Google Scholar]
- 62.Btaiche IF, Khalidi N. Parenteral nutrition-associated liver complications in children. Pharmacotherapy. 2002;22(2):188–211. doi: 10.1592/phco.22.3.188.33553. [DOI] [PubMed] [Google Scholar]
- 63.Boberg KM, Stabursvik A, Bjorkhem I, et al. Dehydroxylation of a 7 beta-hydroxy-C27 plant sterol in rat liver. Biochim Biophys Acta. 1989;1004(3):321–326. doi: 10.1016/0005-2760(89)90079-9. [DOI] [PubMed] [Google Scholar]
- 64.Liu Y, Binz J, Numerick MJ, et al. Hepatoprotection by the farnesoid X receptor agonist GW4064 in rat models of intra- and extrahepatic cholestasis. J Clin Invest Dec. 2003;112(11):1678–1687. doi: 10.1172/JCI18945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Carter BA, Taylor OA, Prendergast DR, et al. Stigmasterol, a soy lipid–derived phytosterol, is an antagonist of the bile acid nuclear receptor FXR. Pediatr Res. 2007;62(3):301–306. doi: 10.1203/PDR.0b013e3181256492. [DOI] [PubMed] [Google Scholar]
- 66.Tomer G, Ananthanarayanan M, Weymann A, et al. Differential developmental regulation of rat liver canalicular membrane transporters Bsep and Mrp2. Pediatr Res. 2003;53(2):288–294. doi: 10.1203/01.PDR.0000047509.54253.01. [DOI] [PubMed] [Google Scholar]
- 67.Moseley RH, Wang W, Takeda H, et al. Effect of endotoxin on bile acid transport in rat liver: a potential model for sepsis-associated cholestasis. Am J Physiol. 1996;271(1 Pt 1):G137–146. doi: 10.1152/ajpgi.1996.271.1.G137. [DOI] [PubMed] [Google Scholar]
- 68.Ghose R, Zimmerman TL, Thevananther S, et al. Endotoxin leads to rapid subcellular re-localization of hepatic RXRalpha: A novel mechanism for reduced hepatic gene expression in inflammation. Nucl Recept. 2004;2(1):4. doi: 10.1186/1478-1336-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Deshpande G, Simmer K, Deshmukh M, et al. Randomized trial of fish oil (SMOFlipid) and olive oil lipid (clinoleic) in very preterm neonates. J Pediatr Gastroenterol Nutr. 2013 Sep 16; doi: 10.1097/mpg.0000000000000174. [DOI] [PubMed] [Google Scholar]
- 70.Perez-Echarri N, Perez-Matute P, Marcos-Gomez B, et al. Down-regulation in muscle and liver lipogenic genes: EPA ethyl ester treatment in lean and overweight (high-fat-fed) rats. J Nutr Biochem. 2009;20(9):705–714. doi: 10.1016/j.jnutbio.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 71.Takeuchi H, Kojima K, Sekine S, et al. Effect of dietary n-6/n-3 ratio on liver n-6/n-3 ratio and peroxisomal beta-oxidation activity in rats. J Oleo Sci. 2008;57(12):649–657. doi: 10.5650/jos.57.649. [DOI] [PubMed] [Google Scholar]
- 72.Perez-Matute P, Perez-Echarri N, Martinez JA, et al. Eicosapentaenoic acid actions on adiposity and insulin resistance in control and high-fat-fed rats: role of apoptosis, adiponectin and tumour necrosis factor-alpha. Br J Nutr. 2007;97(2):389–398. doi: 10.1017/S0007114507207627. [DOI] [PubMed] [Google Scholar]
- 73.Mak PA, Laffitte BA, Desrumaux C, et al. Regulated expression of the apolipoprotein E/C-I/C-IV/C-II gene cluster in murine and human macrophages. A critical role for nuclear liver X receptors alpha and beta. J Biol Chem. 2002;277(35):31900–31908. doi: 10.1074/jbc.M202993200. [DOI] [PubMed] [Google Scholar]
- 74.Claudel T, Inoue Y, Barbier O, et al. Farnesoid X receptor agonists suppress hepatic apolipoprotein CIII expression. Gastroenterology. 2003;125(2):544–555. doi: 10.1016/s0016-5085(03)00896-5. [DOI] [PubMed] [Google Scholar]
- 75.Butler TJ, Szekely LJ, Grow JL. A standardized nutrition approach for very low birth weight neonates improves outcomes, reduces cost and is not associated with increased rates of necrotizing enterocolitis, sepsis or mortality. J Perinatol. 2013;33(11):851–857. doi: 10.1038/jp.2013.66. [DOI] [PubMed] [Google Scholar]
- 76.Mahieu LM, De Muynck AO, Ieven MM, et al. Risk factors for central vascular catheter-associated bloodstream infections among patients in a neonatal intensive care unit. J Hosp Infect. 2001;48(2):108–116. doi: 10.1053/jhin.2001.0984. [DOI] [PubMed] [Google Scholar]
- 77.Polin RA, Saiman L. Nosocomial infections in the neonatal intensive care unit. Neoreviews. 2003;4(3):e81–e89. [Google Scholar]


