Heart failure with preserved ejection fraction (HFpEF) is not one single disease but a syndrome comprised of multiple pathophysiologic mechanisms.1, 2 The assortment of underlying causes of HFpEF and its consequential phenotypic diversity is what makes this syndrome so unique and, unfortunately, so difficult to treat. Despite the heterogeneity of HFpEF, a common thread exists. Regardless of the predominate subtype of HFpEF, the left atrium (LA) plays a central role in the underlying disease process and the symptoms that results from it.
Multiple studies have shown the diagnostic and prognostic importance of LA size and pressure in HFpEF.3–6 Beyond these parameters, echocardiographic speckle tracking strain has made it possible to non-invasively measure the functional components of the LA including reservoir, conduit, and contractile (booster) strain. LA reservoir strain, in particular, has strong prognostic value in HFpEF, and outperforms left ventricular (LV) and right ventricular longitudinal strain in this regard.7 The mechanistic insight gained from evaluating LA mechanics in patients with HFpEF has created a paradigm shift in our thinking of this chamber. It appears that the LA is not simply being a passive marker of disease severity—it is a critical, active component of the HFpEF syndrome. Indeed, in the setting of HFpEF, reduced LA strain (indicative of intrinsic LA mechanical dysfunction) is a major driver of both elevated pulmonary vascular resistance and decreased peak oxygen consumption (VO2) on cardiopulmonary exercise testing, which leads to exercise intolerance and adverse outcomes (Figure 1).7
Figure 1. The Central Role of the Left Atrium in the Pathogenesis of Heart Failure with Preserved Ejection Fraction.
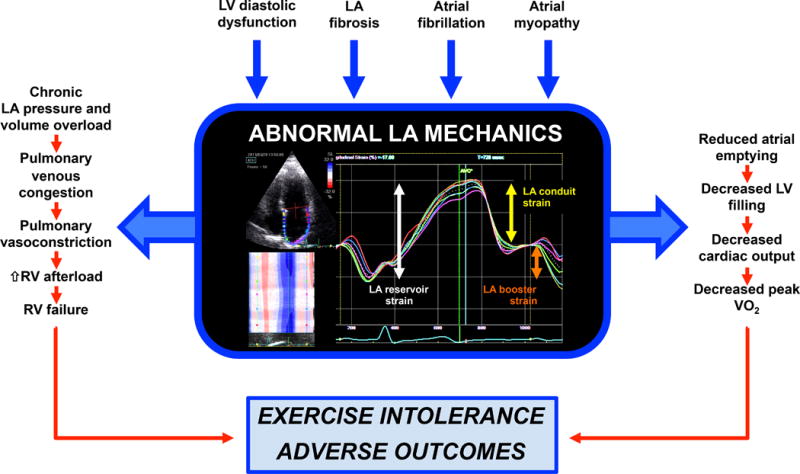
LV diastolic dysfunction, LA fibrosis, atrial fibrillation, and primary LA myopathy can all contribute to abnormal LA mechanics in HFpEF, which can manifest as abnormalities in LA reservoir function (which occurs during LV systole and reflects the ability of the LA to fill); LA conduit function (which occurs during early LV diastole and reflects the contribution of the LA to early LV filling [coincides with early mitral inflow (E wave)]); and LA booster function (which occurs during late diastole and reflects LA contractile function [coincides with late mitral inflow (A wave)]). Abnormal LA mechanics result in: (1) pulmonary venous congestion, pulmonary vasoconstriction, and RV failure; and (2) decreased LA emptying, reduced LV filling, and reduced cardiac output during exertion. These abnormalities combine to ultimately result in exercise intolerance and adverse outcomes in HFpEF patients.
LA = left atrium; LV = left ventricle; RV = right ventricle
In this issue of the Journal, von Roeder et al. designed a remarkably detailed study by combining echocardiography, cardiovascular magnetic resonance (CMR) imaging, cardiopulmonary exercise testing, and invasive hemodynamics with conductance catheters to further characterize the role of LA function in HFpEF patients.8 The authors included 22 patients with HFpEF and 12 controls. HFpEF was defined by signs and symptoms of heart failure, LV ejection fraction > 50%, and objective evidence of increased LV filling pressures (based on echocardiographic criteria and/or elevated N-terminal pro-B-type natriuretic peptide levels). LA reservoir and conduit strain were lower in HFpEF compared to controls, and in the HFpEF patients, LA conduit strain was associated with peak VO2 even after adjusting for LV stiffness (beta) and relaxation (tau). Of the LV properties on invasive conductance catheter analysis, LA conduit strain correlated best with the volume of early LV filling and less so with intrinsic LV stiffness or relaxation, which is not surprising given the role of LA conduit strain in driving LV filling during early diastole.
Of note, only 36% of the HFpEF patients enrolled in the study were taking diuretics—and none were previously hospitalized for heart failure—both of which suggest a relatively early HFpEF phenotype, thereby decreasing the generalizability of the results. However, it is remarkable that even in the early stages of the HFpEF syndrome, LA conduit strain is already significantly diminished. It is also worth noting that patients in atrial fibrillation (AF) at the time of the study were excluded, and there were no significant differences in baseline characteristics, CMR measures, or invasive hemodynamics between the 5 patients with paroxysmal AF (but in sinus rhythm at the time of the study) and the remaining 17 patients. Taken together, these findings suggest that LA mechanical failure is an early driver of the HFpEF syndrome, and therefore central to its pathogenesis. It is well known that there is a high prevalence of LV diastolic dysfunction in the community; however, why only some but not all of these individuals develop HFpEF has been a major unanswered question in cardiovascular epidemiology. The findings of the study by von Roeder et al. suggest that it may be the vulnerability of the LA to LV diastolic dysfunction in some individuals that explains the difference between asymptomatic LV diastolic dysfunction and the development of symptomatic HFpEF.
One of the most unique aspects of the study by von Roeder et al. is the use of CMR-derived feature tracking rather than echocardiographic speckle tracking strain to measure the three phases of LA strain and strain rate. While the LA endocardial border is generally better visualized with CMR compared to echo, the low temporal resolution (25–30 frames per cardiac cycle) may limit the accuracy of strain measures. In addition, the feasibility of this technique is uncertain as the authors excluded an unknown number of segments due to poor quality tracking. Little data exists correlating feature tracking with the more commonly used speckle tracking strain of the LA.9 These differences are important, and should be considered when comparing the results of the present study to prior studies of the LA in HFpEF that used speckle-tracking echocardiography. Nevertheless, any noise in the data introduced by CMR feature-tracking measurement of LA strain would have decreased the strength of the identified correlations. Given the high correlation between LA conduit strain and peak VO2, the findings by von Roeder et al. show that calculation of LA strain by feature-tracking (which can be applied retrospectively to any CMR with cine images) could be a very useful technique for future evaluation of the LA in heart failure syndromes.
Regardless of the method used to measure strain, the results of the present study are intriguing and, once again, position the LA as a key player in the pathophysiology of HFpEF. Thoughtful data analysis reveals a strong association between LA conduit strain and peak VO2 independent of LA pressure, which the authors hypothesize is due to reduced early LV filling volume during exercise given the strong correlation between this measure and LA conduit strain. Although a larger sample size might have shown more of an association between peak VO2 and LV stiffness, the findings of this paper re-emphasize the concept of an underlying LA myopathy in HFpEF—which is possibly primary in some patients—and the idea that it can be targeted for therapeutic intervention.
LA fibrosis is likely a major component of the remodeling process that occurs in HFpEF as it is in patients with AF and severe mitral regurgitation.10, 11 Many of the clinical risk factors associated with HFpEF—obesity, hypertension, diabetes—may directly contribute to LA fibrosis through inflammation and oxidative stress.12, 13 The effects of the LA myopathy in HFpEF become hemodynamically apparent when the LA is stressed with a fluid challenge or exercise as evidenced by large v waves (despite the lack of significant dynamic mitral regurgitation), which are the result of a non-compliant LA (Figure 2).
Figure 2. Hemodynamic Evidence of Left Atrial Non-Compliance Brought Out by Fluid Challenge in a Patient with Heart Failure with Preserved Ejection Fraction.
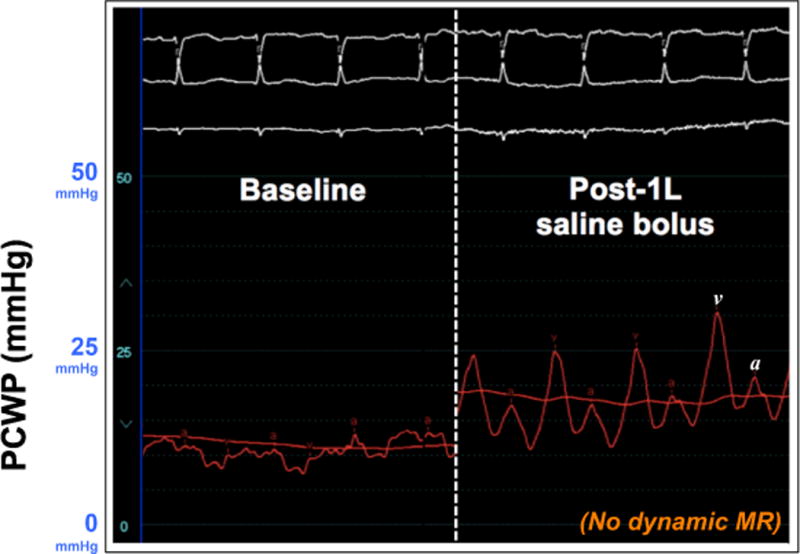
The hemodynamic pressure tracings shown here are from a patient with early HFpEF and reduced left atrial conduit strain. Left panel: Baseline PCWP was upper normal (13 mmHg at end-expiration). Right panel: After 1-liter normal saline bolus over 10 minutes, the PCWP waveform changed dramatically with prominent v waves up to 30 mmHg at end-expiration. In the absence of significant mitral regurgitation (which was confirmed in this case by simultaneous echocardiography), the prominent v waves reflect the inability of the left atrium to accommodate the increased load.
PCWP = pulmonary capillary wedge pressure; MR = mitral regurgitation
LA strain appears to be a sensitive measure of these intrinsic LA myopathic changes. In a small study comparing HFpEF patients to hypertensive controls, LA reservoir strain failed to appropriately augment during passive leg raise in patients with HFpEF.14 As shown by von Roeder et al. these alterations in LA mechanics (LA conduit function in particular) might occur early in HFpEF before significant volume overload develops. To further test this hypothesis, a large, population-based cohort study involving older adults (age 60–100 years) attending the Multi-Ethnic Study of Atherosclerosis (MESA) Year-15 exam (n~3,500+ at 6 sites across the United States) is currently underway to better understand the prevalence, pathogenesis, and phenomics of early HFpEF. Cardiac mechanics, including LA speckle-tracking strain with and without passive leg raise, is a critical component of the MESA examination and should provide insight into early HFpEF at the population level in older adults.
In HFpEF patients, elevated LA pressure is a major contributor to dyspnea and exercise intolerance; thus, it is not surprising that several devices that indirectly or directly decompress the LA are being utilized and tested in patients with HFpEF. The CardioMEMS device is a wireless, implanted pulmonary artery pressure sensor and monitor that transmits hemodynamic data daily using a wireless radiofrequency transmitter. The single-blinded, multicenter The CardioMEMS Heart Sensor Allows Monitoring of Pressure to Improve Outcomes in New York Heart Association Class III Heart Failure Patients (CHAMPION) trial showed that tight control of pulmonary artery diastolic pressure (a surrogate for LA pressure) resulted in significantly lower hospitalization rates in heart failure patients, including those with LV ejection fraction > 40%.15
An alternate strategy for decompressing the LA is via the creation of an interatrial shunt. The Reduce Elevated Left Atrial Pressure in Heart Failure (REDUCE LAP-HF I) Trial is a prospective, randomized, placebo-controlled clinical trial to evaluate a transcatheter interatrial shunt (Corvia Medical IASD System II) in patients with symptomatic heart failure and LVEF > 40%.16 The device is a 1-piece self-expanding metal cage with a double-disc design that allows right to left shunting through a hole (barrel) in its center. Previous non-randomized, open-label trials have shown that this device is safe and is associated improved exercise capacity and symptoms, but these findings need confirmation in randomized studies. The primary effectiveness outcome in the ongoing randomized, controlled REDUCE LAP-HF I trial—which has completed enrollment—is the change in supine exercise pulmonary capillary wedge pressure at 1 month after device implantation.
Another novel device designed to directly decompress the LA is a micropump-based form of circulatory support in which blood from the LA is pumped to the aorta. A simulation study modeling both LA and LV mechanical circulatory support evaluated the effect of these devices on the hemodynamics of 4 distinct HFpEF phenotypes (hypertrophic cardiomyopathy; infiltrative cardiomyopathy; non-hypertrophic, non-hypertensive; and “garden variety” [associated with common comorbidities].17 For all phenotypes, the LA assist device increased cardiac output and decreased pulmonary and LA pressures. In addition, the LA assist device was less likely than the LV assist device to induce suction because of the presence of an enlarged LA but normal or small LV in the various HFpEF phenotypes.
The study by von Roeder et al. adds to the growing literature on the role of the LA in HFpEF, and provides further rationale for the aforementioned devices, which all demonstrate the potential power of focusing on the LA as a therapeutic target in HFpEF. Nevertheless, several questions remain. How and when does LA myopathy develop? Can LA dysfunction identify HFpEF patients early, before they develop more overt forms of HFpEF? How does LA remodeling evolve over time, and when is it best to consider LA device therapy? Is there a HFpEF subtype that is a primary LA myopathy? Further studies evaluating the mechanics, hemodynamics, and tissue characteristics of the LA both at rest and under stress (e.g., exercise, volume loading) are needed to answer these questions.
Despite these unanswered questions, one fact is clear: it is time for us to focus on the LA, which has thus far been overshadowed by the LV in the field of HFpEF. Ever since the first comprehensive description of HFpEF18 we have argued about the best LV ejection fraction cut-off for HFpEF; debated the best term for HFpEF (“diastolic heart failure”, “preserved systolic function”, “normal ejection fraction”, or “preserved ejection fraction”); and quibbled about whether or not LV diastolic dysfunction should be part of the definition of HFpEF, all the while focusing on the left ventricle. Maybe it is time to think outside of the proverbial box. In-depth studies of the LA in HFpEF such as those by von Roeder et al. and others7, 19—along with the availability of novel therapeutic options—have placed the long overdue spotlight on the LA in HFpEF.
Acknowledgments
Disclosures: Dr. Freed has received grant support from Bayer/International Society for Heart and Lung Transplantation. Dr. Shah has received grant support from Actelion, AstraZeneca, Corvia, and Novartis; and consulting fees from Actelion, AstraZeneca, Bayer, Ironwood, Novartis, Pfizer, and Sanofi. Dr. Shah is supported by grants from the American Heart Association (16SFRN28780016, and 15CVGPSD27260148) and National Institutes of Health (R01 HL107557 and R01 HL127028).
References
- 1.Shah SJ, Katz DH, Deo RC. Phenotypic spectrum of heart failure with preserved ejection fraction. Heart Fail Clin. 2014;10:407–18. doi: 10.1016/j.hfc.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shah SJ, Kitzman DW, Borlaug BA, van Heerebeek L, Zile MR, Kass DA, Paulus WJ. Phenotype-Specific Treatment of Heart Failure With Preserved Ejection Fraction: A Multiorgan Roadmap. Circulation. 2016;134:73–90. doi: 10.1161/CIRCULATIONAHA.116.021884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takemoto Y, Barnes ME, Seward JB, Lester SJ, Appleton CA, Gersh BJ, Bailey KR, Tsang TS. Usefulness of left atrial volume in predicting first congestive heart failure in patients > or = 65 years of age with well-preserved left ventricular systolic function. Am J Cardiol. 2005;96:832–6. doi: 10.1016/j.amjcard.2005.05.031. [DOI] [PubMed] [Google Scholar]
- 4.Russo C, Jin Z, Homma S, Rundek T, Elkind MS, Sacco RL, Di Tullio MR. Left atrial minimum volume and reservoir function as correlates of left ventricular diastolic function: impact of left ventricular systolic function. Heart. 2012;98:813–20. doi: 10.1136/heartjnl-2011-301388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gottdiener JS, Kitzman DW, Aurigemma GP, Arnold AM, Manolio TA. Left atrial volume, geometry, and function in systolic and diastolic heart failure of persons > or =65 years of age (the cardiovascular health study) Am J Cardiol. 2006;97:83–9. doi: 10.1016/j.amjcard.2005.07.126. [DOI] [PubMed] [Google Scholar]
- 6.Kurt M, Wang J, Torre-Amione G, Nagueh SF. Left atrial function in diastolic heart failure. Circ Cardiovasc Imaging. 2009;2:10–5. doi: 10.1161/CIRCIMAGING.108.813071. [DOI] [PubMed] [Google Scholar]
- 7.Freed BH, Daruwalla V, Cheng JY, Aguilar FG, Beussink L, Choi A, Klein DA, Dixon D, Baldridge A, Rasmussen-Torvik LJ, Maganti K, Shah SJ. Prognostic Utility and Clinical Significance of Cardiac Mechanics in Heart Failure With Preserved Ejection Fraction: Importance of Left Atrial Strain. Circ Cardiovasc Imaging. 2016;9 doi: 10.1161/CIRCIMAGING.115.003754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.von Roeder M, Rommel K-P, Kowallick J, Blazek S, Besler C, Fengler K, Lotz J, Hasenfuss G, Lucke C, Gutberlet M, Schuler G, Schuster A, Lurz P. The Influence of Left Atrial Function on Exercise Capacity and Left Ventricular Function in Patients with Heart Failure and Preserved Ejection Fraction. Circ Cardiovasc Imaging. 2017;XX:XX–XX. doi: 10.1161/CIRCIMAGING.116.005467. [DOI] [PubMed] [Google Scholar]
- 9.Hoit BD. Reply: Feature tracking cardiac magnetic resonance imaging in the assessment of left atrial function. J Am Coll Cardiol. 2014;63:2435–6. doi: 10.1016/j.jacc.2014.02.544. [DOI] [PubMed] [Google Scholar]
- 10.Kuppahally SS, Akoum N, Burgon NS, Badger TJ, Kholmovski EG, Vijayakumar S, Rao SN, Blauer J, Fish EN, Dibella EV, Macleod RS, McGann C, Litwin SE, Marrouche NF. Left atrial strain and strain rate in patients with paroxysmal and persistent atrial fibrillation: relationship to left atrial structural remodeling detected by delayed-enhancement MRI. Circ Cardiovasc Imaging. 2010;3:231–9. doi: 10.1161/CIRCIMAGING.109.865683. [DOI] [PubMed] [Google Scholar]
- 11.Cameli M, Lisi M, Righini FM, Massoni A, Natali BM, Focardi M, Tacchini D, Geyer A, Curci V, Di Tommaso C, Lisi G, Maccherini M, Chiavarelli M, Massetti M, Tanganelli P, Mondillo S. Usefulness of atrial deformation analysis to predict left atrial fibrosis and endocardial thickness in patients undergoing mitral valve operations for severe mitral regurgitation secondary to mitral valve prolapse. Am J Cardiol. 2013;111:595–601. doi: 10.1016/j.amjcard.2012.10.049. [DOI] [PubMed] [Google Scholar]
- 12.Kitzman DW, Shah SJ. The HFpEF Obesity Phenotype: The Elephant in the Room. J Am Coll Cardiol. 2016;68:200–3. doi: 10.1016/j.jacc.2016.05.019. [DOI] [PubMed] [Google Scholar]
- 13.Goldberger JJ, Arora R, Green D, Greenland P, Lee DC, Lloyd-Jones DM, Markl M, Ng J, Shah SJ. Evaluating the Atrial Myopathy Underlying Atrial Fibrillation: Identifying the Arrhythmogenic and Thrombogenic Substrate. Circulation. 2015;132:278–91. doi: 10.1161/CIRCULATIONAHA.115.016795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Obokata M, Negishi K, Kurosawa K, Arima H, Tateno R, Ui G, Tange S, Arai M, Kurabayashi M. Incremental diagnostic value of la strain with leg lifts in heart failure with preserved ejection fraction. JACC Cardiovasc Imaging. 2013;6:749–58. doi: 10.1016/j.jcmg.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 15.Adamson PB, Abraham WT, Bourge RC, Costanzo MR, Hasan A, Yadav C, Henderson J, Cowart P, Stevenson LW. Wireless pulmonary artery pressure monitoring guides management to reduce decompensation in heart failure with preserved ejection fraction. Circ Heart Fail. 2014;7:935–44. doi: 10.1161/CIRCHEARTFAILURE.113.001229. [DOI] [PubMed] [Google Scholar]
- 16.Feldman T, Komtebedde J, Burkhoff D, Massaro J, Maurer MS, Leon MB, Kaye D, Silvestry FE, Cleland JG, Kitzman D, Kubo SH, Van Veldhuisen DJ, Kleber F, Trochu JN, Auricchio A, Gustafsson F, Hasenfubeta G, Ponikowski P, Filippatos G, Mauri L, Shah SJ. Transcatheter Interatrial Shunt Device for the Treatment of Heart Failure: Rationale and Design of the Randomized Trial to REDUCE Elevated Left Atrial Pressure in Heart Failure (REDUCE LAP-HF I) Circ Heart Fail. 2016;9:e003025. doi: 10.1161/CIRCHEARTFAILURE.116.003025. [DOI] [PubMed] [Google Scholar]
- 17.Burkhoff D, Maurer MS, Joseph SM, Rogers JG, Birati EY, Rame JE, Shah SJ. Left atrial decompression pump for severe heart failure with preserved ejection fraction: theoretical and clinical considerations. JACC Heart Fail. 2015;3:275–82. doi: 10.1016/j.jchf.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 18.Dougherty AH, Naccarelli GV, Gray EL, Hicks CH, Goldstein RA. Congestive heart failure with normal systolic function. Am J Cardiol. 1984;54:778–82. doi: 10.1016/s0002-9149(84)80207-6. [DOI] [PubMed] [Google Scholar]
- 19.Melenovsky V, Hwang SJ, Redfield MM, Zakeri R, Lin G, Borlaug BA. Left atrial remodeling and function in advanced heart failure with preserved or reduced ejection fraction. Circ Heart Fail. 2015;8:295–303. doi: 10.1161/CIRCHEARTFAILURE.114.001667. [DOI] [PubMed] [Google Scholar]


