Abstract
The anterior region of the left superior temporal gyrus/superior temporal sulcus (aSTG/STS) has been implicated in two very different cognitive functions: sentence processing and social-emotional processing. However, the vast majority of the sentence stimuli in previous reports have been of a social or social-emotional nature suggesting that sentence processing may be confounded with semantic content. To evaluate this possibility we had subjects read word lists that differed in phrase/constituent size (single words, 3-word phrases, 6-word sentences) and semantic content (social-emotional, social, and inanimate objects) while scanned in a 7 T environment. This allowed us to investigate if the aSTG/STS responded to increasing constituent structure (with increased activity as a function of constituent size) with or without regard to a specific domain of concepts, i.e., social and/or social-emotional content. Activity in the left aSTG/STS was found to increase with constituent size. This region was also modulated by content, however, such that social-emotional concepts were preferred over social and object stimuli. Reading also induced content type effects in domain-specific semantic regions. Those preferring social-emotional content included aSTG/STS, inferior frontal gyrus, posterior STS, lateral fusiform, ventromedial prefrontal cortex, and amygdala, regions included in the “social brain”, while those preferring object content included parahippocampal gyrus, retrosplenial cortex, and caudate, regions involved in object processing. These results suggest that semantic content affects higher-level linguistic processing and should be taken into account in future studies.
Keywords: Affective, Emotion, fMRI, Language, Social brain
1. Introduction
There is considerable evidence that the more anterior regions of the temporal lobes (ATL) play a critical role in processing conceptual information related to people, especially concerning their emotional state and situations. For example, activation of the ATL, bilaterally, has commonly been observed in neuroimaging studies of social and emotional processing (Olson et al., 2007, 2013; Simmons and Martin 2009; Wong and Gallate 2012). In addition, the atrophy of anterior temporal cortex prominently affects emotional behavior (Snowden et al., 2001; Chan et al., 2009). At a finer-grained level of analysis, functional neuroimaging studies have linked a preference for processing social and social-emotional over non-social concepts to a specific portion of the ATL centered on the most anterior aspect of the superior temporal gyrus and sulcus (aSTG/STS) (Zahn et al., 2007; Simmons et al., 2010). Consistent with these findings, the aSTG/STS has been shown to be part of a broader ‘social brain’ network based on neuropsychological and neuroimaging functional connectivity studies that have revealed covariation of neural activity between the aSTG/STS and medial prefrontal cortex (mPFC), lateral fusiform gyrus, precuneus, posterior STS, as well as the amygdala (Burnett and Blakemore 2009; Simmons et al., 2010; Gotts et al., 2012; Simmons and Martin 2012; Pascual et al., 2013).
In contrast, a separate neuroimaging literature strongly implicates the left aSTG/STS in sentence processing (e.g., Mazoyer et al., 1993; Vandenberghe et al., 2002; Humphries et al., 2006; Rogalsky and Hickok 2009; Fedorenko et al., 2010; Brennan et al., 2012). These studies suggest the aSTG/STS plays a major role in sentence-level processing (as opposed to more basic lexical/word-level processes), perhaps by aiding in the composition of sentence meaning through combinatorial semantic processing (Vandenberghe et al., 2002) and/or building syntactic structure (Humphries et al., 2006; Brennan et al., 2012). Pallier et al. (2011) extended these findings by parametrically modulating phrase or “constituent” size (thereby increasing constituent structure and conceptual complexity). They observed increasing activity with increasing constituent size for real word but not pseudoword constituents in the left anterior temporal lobe, centered on the most anterior portion of the STG/STS. Taken together, these findings suggest that semantic content is necessary for creating constituent structure representations, and that, at least in this brain region, constituent structure computations and higher-level (phrasal or sentential) conceptual representations are intertwined.
A closer look at the stimuli and location of activation in the neuroimaging sentence studies reveals, however, that the conceptual representation and sentence literatures, though seemingly disparate, may have more in common than previously appreciated. The social > non-social findings and sentence > word list findings appear to center on the same region of the aSTG/STS, and the vast majority of stimuli used in the sentence studies describe social situations (i.e., involving humans – including human interactions that are emotional in nature) (typically over 90% as detailed in the Appendices to these reports). For example: “That girl in the park was playing the violin”, “Her nervous boyfriend took the ring out of his pocket” (Rogalsky and Hickok, 2009); “Hardly any survivors were found after the earthquake”, “The baby is staying with his grandmother” (Vandenberghe et al., 2002); “The man on vacation lost a bag and a wallet” (Humphries et al., 2006), and so on. This strong bias towards social, as well as social-emotional content raises the possibility that the aSTG/STS activations in previous sentence processing studies may have been confounded by the semantic content of the stimuli used in those experiments. The goal of our study was to directly address this possibility by evaluating the relationship between content and sentence processing in the ATL by modulating both the type of semantic content and the amount of constituent structure. Specifically, we sought to determine whether the ATL contained distinct regions involved in sentence processing – regardless of semantic content – or whether sentence processing in the ATL was constrained by meaning. To accomplish this, we scanned subjects with a 7 T scanner while they read rapid, serially presented lists of six words that varied in semantic content type (people in emotional situations, people in non-emotional situations, inanimate objects, and jabberwocky) and constituent size (single words, 3-word phrases, and 6-word sentences).
2. Materials and methods
2.1. Subjects
Twenty-two right-handed native English-speaking subjects (mean age 26, 10 females) participated in the experiment. Two subjects were excluded from further analysis due to excessive head motion (leaving 20 subjects, 9 females, mean age 26). All subjects were screened and did not report any neurological or psychiatric problems. Written informed consent was obtained from each subject in accordance with a National Institutes of Health Institutional Review Board approved protocol. Subjects were paid for their participation.
2.2. Experimental stimuli and design
All stimuli were strings of 6 English words. These strings varied in terms of their semantic content (4 levels of Content Type: Social-Emotional, Social, Object, Jabberwocky) and the size of the largest grammatical constituent that they could form (3 levels of Constituent Size: 1-word, 3-word, 6-word) in a fully-crossed factorial design with 12 conditions in total. We generated these stimuli by first creating 160 6-word sentences for each type of meaningful Content Type (Social-Emotional, Social, Object). The content types for sentences were created as follows. The Social-Emotional and Social sentences contained words referring to people either by a proper name (‘John’) or the name of an occupation/title (‘architect’). Social-Emotional sentences additionally contained at least one word taken from an emotional norming study (Warriner et al., 2013) which had either strongly positive or negative valence ratings (> 7 or o 3 on a 9-point scale) and medium to high arousal ratings (> 4 on a 9-point scale where 9 referred to excited and 1 to calm). These sentences described people in emotional situations (e.g. in which they felt happy, sad, angry, fearful, surprised, disgusted, etc.). Object sentences contained no people-related words but instead featured nouns denoting inanimate entities like tools, buildings, office supplies, clothing, books, nature, etc. All sentences were right-branching and past tense. Repetition of open class words was kept to a minimum across all stimuli (12.6%). For each of these content types, 40 of the 160 sentences we constructed were randomly selected to be used in the 6-word Constituent Size condition and 40 were randomly selected for the 1-word condition. For the 1-word condition, these 40 were scrambled and regrouped so that the 6 words created unrelated word strings (similar to other approaches, e.g., Humphries et al., 2006; Pallier et al., 2011). The 3-word condition was created by extracting as many non-overlapping 3-word constituents as possible from the remaining 80 sentences (about 90–100 3-word constituents). We then randomly paired 3-word constituents together to create 40 6-word stimuli with two 3-word phrases that did not create a sentence. The Jabberwocky conditions were created by randomly selecting 40 trials of each constituent size type from the pool of real-word trials and replacing all open class words with pseudowords (largely taken from those generated by the English Lexicon Project database; Balota et al., 2007) with the same number of letters and same morphological ending (or a morphological ending more typical of that word class). Suffixes that were mainly person-related (e.g., “-neer” and “-ist” for professions) and personal pronouns were replaced with neutral counterparts to remove all social content from Jabberwocky sentences. In this manner, 40 trials were created for the Jabberwocky 6-word condition, 40 trials for the Jabberwocky 3-word condition, and 40 trials for the Jabberwocky 1 -word condition. All stimuli are included in the Supplementary materials, Section 3.
These factors, Content Type and Constituent Size, are orthogonal in the sense that the Content Type manipulation modulates the type of conceptual information (i.e., social-emotional, social, object) but not the amount of coherent semantic information or syntax, and the Constituent Size manipulation modulates the amount of coherent semantic information and syntax but does not modulate type of conceptual information. Thus, as designed, they are suitable for addressing our questions. Additionally, the open class words were balanced across conditions ([Social-Emotional, Social, Object] × [1, 3, 6]) for word length and for the log(HAL frequency) from norms of the English Lexicon Project (Balota et al., 2007). A 2-way ANOVA revealed no difference in length across Content Type (Social-Emotional mean = 6.42, Social mean = 6.32, Object mean = 6.26, F(1370) = 0.77, p = 0.45), Constituent Size (6-word mean = 6.25, 3-word mean = 6.40, 1-word mean = 6.36, F (1370) = 0.8, p = 0.45), and no interaction (F(1370) = 1.65, p = 0.15). There also was no difference for log frequency across Content Type (Social-Emotional mean = 9.00, Social mean = 9.15, Object mean = 9.04, F(1370) = 0.68, p = 0.5), Constituent Size (6-word mean = 9.06, 3-word mean = 8.95, 1-word mean = 9.19, F(1370) = 1.56, p = 0.21), and no interaction (F(1370) = 0.48, p = 0.75). Additionally, available concreteness ratings of the open class words (from a norming study by Brysbaert et al., 2013) were matched between Social and Object conditions (2-sided t-test: t(892) = 0.70, p = 0.49) when proper names were considered fully concrete.
Trials were presented using Presentation software (Neurobehavioral Systems, Inc) in a fast event-related design with words appearing sequentially for 300 ms each (Fig. 1) on the screen (total of 1800 ms for 6 words). A fixation was then presented after the words with variable ISI (minimum 2200 ms) with optimal jitter and randomization calculated using Optseq2. Words were presented in black letters against a gray background in Arial size 48 font. Stimuli were presented in 10 runs, each about 5 min 30 s, with short breaks between them. The beginning of the run started with 12 s of fixation to allow the scanner to reach a steady state. About twice a minute, a trial which requested that the subject press a left or right button would appear (“please press the left/ right button now”), and responses were recorded. This allowed us to assess whether subjects were maintaining attention throughout the experiment. The order and timing of conditions remained constant across subjects, but the list of stimuli were customized for each subject by randomly drawing from the stimulus set to avoid order effects. Subjects were given instructions to read the stimuli that appear on the screen and press the button when the trial instructed them to do so.
Fig. 1.
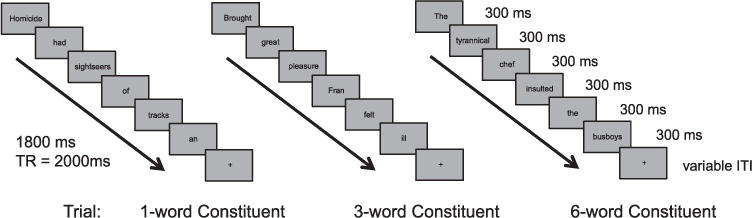
Example trials and timing for 1-word, 3-word, and 6-word constituent conditions.
Physiological data were acquired while subjects performed the experiment. We monitored cardiac and respiratory signals with the Biopac setup (Biopac Systems, Inc.; signals sampled at 500 Hz). Custom Matlab scripts were used to measure heart rate and possible changes across conditions. We computed the average heart rate from the cardiac recordings (sampled at 500 Hz) for each condition by extracting the peak value of a spectrum calculated by performing a 2048-point FFT on the 4 s after each stimulus (since 4 s was the minimum time of a trial). As we selected the peak based on if it was a local maximum > 0 Hz, the DC (direct current) component was not extracted. A repeated-measures ANOVA was used to test for a significant difference between heart rates of the Social-Emotional, Social, and Object conditions.
2.3. MRI acquisition
Data were collected on a 7 T Siemens MRI scanner with a 32-channel head coil located at the NIH Clinical Center NMR Research Facility. The session began with 2 high-resolution anatomical scans (MPRAGE: 192 axial slices, 1.0 mm thick, field of view (FOV) = 25.6cm, acquisition matrix = 256 × 256; the Proton Density scan used the same parameters) which were followed by a gradient-echo echo-planar image (EPI) scan with a 1.6 mm isotropic voxel (repetition time = 2000 ms, echo time = 27 ms, flip angle = 70°, FOV = 20cm, acquisition matrix = 125 × 125, 43 oblique slices, 1.6 mm thick, 0.16 mm inter-slice gap, 168 volumes collected with a GRAPPA acceleration factor of 3). Before both MPRAGE and EPI acquisitions, we performed 3D shimming to mitigate any magnetic field inhomogeneities. The oblique slices did not cover the whole brain on account of the increased spatial resolution, but they did cover all of temporal and occipital cortices as well as inferior frontal gyrus (IFG) such that all perisylvian language areas were included (see Supplemental materials, Section 1, Supplementary Fig. 1).
2.4. MRI data analysis
MRI data were processed using the AFNI software package (Cox, 1996) for general linear modeling and performing a mixed-effects ANOVA. Preprocessing of each subject’s EPI data included several steps: de-spiking, slice-time correction, removal of first 6 volumes (before the scanner reached equilibrium magnetization), de-obliquing, registration of all volumes to the first volume collected as it was closest to the anatomical collection, spatial smoothing with a 3 mm full-width half-maximum Gaussian filter, and normalization by the mean signal in a given run for all volumes. The MPRAGE was normalized by the proton density image to achieve uniformity of the anatomical scan, and the resulting anatomical scan was also de-obliqued and aligned to the first EPI volume. Both EPI and anatomical scans were transformed to the Talairach and Tournoux (1988) standard space with their respective voxel resolutions conserved.
Twelve regressors were created for the experimental conditions as well as 6 regressors of no interest for the motion estimates (estimated from the output of the volume registration) and third-order polynomial regressors for baseline shifts. These regressors were convolved with a set of piecewise linear splines or ‘tent’ basis functions as they fit a wider variety of possible hemodynamic responses. We used 9 tent functions placed every 2 s to estimate the response for the 16 s following the start of the trial. Beta weights were then calculated from the regression model for each condition at each voxel for each subject. Those beta weights corresponding to 4–8 s of the hemodynamic response were averaged (to estimate the peak response) and used in subsequent analysis. For group analysis, a repeated measures mixed-effects ANOVA on Constituent Size and Content Type was performed on these averaged beta weights at each voxel. The Jabberwocky condition was not included in the ANOVA as its Constituent Size effect is not consistent (Pallier et al., 2011), but it was examined in post-hoc tests.
We assessed if the aSTG/STS is involved in real word sentence processing in two ways, as both have previously been used in the literature to test for sentence effects (e.g., Humphries et al., 2006; Pallier et al., 2011). First, we examined the main effect of Constituent Size and tested for increasing activity with increasing constituent size as explained below. Second, we directly contrasted sentences and single word conditions using the 6-word v. 1-word statistical map. In both cases, a whole-brain map was created and was corrected for multiple comparisons at a False Discovery Rate (FDR) of q = 0.05 (Genovese et al., 2002). The map for the main effect of Constituent Size was thresholded at 10 voxels to avoid weak, spurious activation. For all statistical maps in this report, clusters were defined as a contiguous set of voxels sharing at least one corner. A cluster was termed aSTG/STS if the peak activation was anterior to the limen insula (Insausti et al., 1998; Simmons et al., 2010; y = 3 in the left hemisphere, y = 5 in the right). Whether it was aSTG or aSTS was determined based on visual inspection in all three orientations. Any clusters posterior to the limen insula were named mid- and posterior- to differentiate them as separate from the anterior clusters.
We examined the direction of the Constituent Size effect (1-word, 3-word, 6-word) within each real word Constituent Size cluster by using the approach of best-fit lines taken by Pallier et al. (2011). For each subject, we extracted the average beta weights across a cluster separately for the 1-word, 3-word, and 6-word conditions. We then fit the beta weights of these conditions with a best-fit line for each subject. Finally, to test if the grand-average best-fit line slope was positive, we used two-sided t-tests. In addition to testing the real word Constituent Size effect, we used the same approach to test for a Jabberwocky Constituent Size effect. Pallier et al. found that most best-fit lines were not linear but logarithmic, and we confirmed that logarithmic functions best fit our data as well. Plots in the figures are on a log-linear scale, so these logarithmic responses appear linear on this scale.
We were also interested if any aSTG/STS Constituent Size cluster exhibited a Content Type bias. Within these clusters, we extracted beta weights from all voxels for Social-Emotional, Social, and Object conditions, averaged beta weights across the voxels and across subjects for each condition, and tested for Content Type differences with paired two-sided t-tests.
We also investigated whether reading produces real word Content Type effects in other brain regions by examining the main effect of Content Type, corrected for multiple comparisons at a False Discovery Rate (FDR) of q = 0.05 and thresholded at 10 voxels. Differences driving any main effects of Content Type were tested with paired two-sided t-tests. Post-hoc tests were done on beta weights that had been averaged across voxels within a cluster and across subjects for each condition.
3. Results
3.1. Behavioral and physiological results
Accuracy scores on the occasional trials requesting a button press were near ceiling (mean 96%) demonstrating that subjects maintained attention while reading. Given the differences in rated arousal between Social-Emotional and the other conditions (see Section 2.2), we wondered if this would translate to differences in heart rate between the conditions. A repeated-measures ANOVA did not find a significant difference between heart rates (Social-Emotional mean = 1.24 Hz (beats/second), Social mean = 1.26 Hz, Object mean = 1.25 Hz, Repeated Measures ANOVA: F(2,38) = 1.10, p40.1). Thus the Social-Emotional condition does not seem to engage the autonomic nervous system more than the other conditions.
3.2. Main effect of Constituent Size
To address whether aSTG/STS is involved in sentence processing, we examined the main effect of Constituent Size, which revealed four clusters (Table 1). Within the area of primary interest, the left anterior temporal lobe, one cluster peaked in aSTG/STS (Fig. 2). This cluster exhibited a significantly positive slope for the real word best-fit line (p < 0.001) but not the Jabberwocky best-fit line (p > 0.1), and these slopes were significantly different (p < 0.001) suggesting that real word sentence processing is preferred here. We were also interested in the sensitivity of this sentence region to semantic content to see if meaning constrains sentence processing here. This region also demonstrated a Content Type bias for Social-Emotional stimuli (p < 0.001 for both Social-Emotional 4 Social and Social-Emotional > Object), suggesting that sentence processing of social-emotional content is preferred. Clusters also appeared in left IFG triangularis, in right aSTG/STS, and spanning left aSTG/IFG or-bitalis, and they all demonstrated significantly positive slopes for real words (p < 0.001) but not Jabberwocky (p > 0.1) and differences between these slopes (p < 0.05). They also demonstrated Content Type biases towards Social-Emotional stimuli (p < 0.001 for Social-Emotional > Object). See Table 2 for all individual statistics of slopes as well as Content Type biases. As expected based on these positive slopes, the contrast of 6-word > 1-word also revealed clusters in left aSTG/STS (peak coordinates: −48, 15, −13) and left IFG triangularis (−54, 23, 12), replicating findings of previous studies which have used a sentence > single words contrast rather than the Constituent Size to investigate sentence processing (e.g., Humphries et al., 2006).
Table 1.
Regions showing a main effect of Constituent Size, FDR corrected, with a 10 voxel threshold. Cluster extent, peak Talairach coordinates, and peak F-statistic are listed for each cluster.
| Region | Number of voxels | x | y | z | Peak F-stat |
|---|---|---|---|---|---|
| L aSTG/STS | 35 | −45 | 15 | −15 | 22.5 |
| L IFG triangularis | 28 | −53 | 27 | 14 | 23.1 |
| R aSTG/STS | 25 | 43 | 12 | −18 | 22.5 |
| L aSTG/IFG | 21 | −48 | 20 | −10 | 25.3 |
Fig. 2.
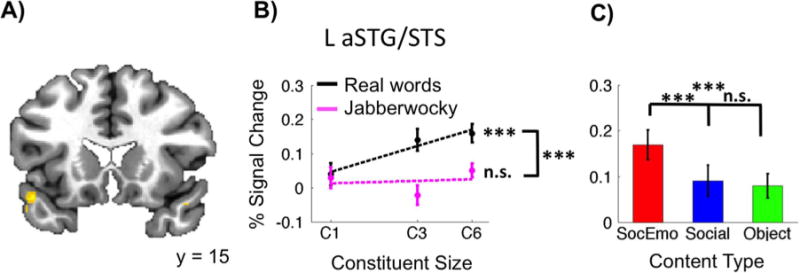
Constituent Size modulated anterior temporal lobe activity. A) Group maps plotted on the TT_N27 anatomy revealed a cluster within left aSTG/STS. B) This cluster demonstrated increasing Constituent Size effects for real words but not jabberwocky. A significant difference between the real word and jabberwocky slopes existed. C) This region also demonstrated a Content Type bias towards Social-Emotional stimuli. The cluster survived multiple comparisons correction (FDR, q = 0.05). The line plot is on a log-linear scale, with best-fit lines fitted to the beta values. Both graphs show percent changes for each condition from the mean signal, which was calculated across all runs, and error bars on graphs indicate ± 1 SEM. Two-sided t-tests were used for all post-hoc tests (***: p < 0.001;**: p < 0.01;*: p < 0.05; t: p < 0.1; n.s.: p > 0.1).
Table 2.
Individual statistics for Constituent Size clusters. All tests were 2-sided t-tests with 19° of freedom.
| Region | Real Word Slope > 0 | Jabber Slope > 0 | Real > Jabber Slope | Soc-Emo > Social | Soc-Emo > Object | Social > Object |
|---|---|---|---|---|---|---|
| L aSTG/STS | t = 6.83 | t = 0.47 | t = 4.086 | t = 4.86 | t = 4.10 | t = 0.52 |
| p < 0.001 | p > 0.1 | p < 0.001 | p < 0.001 | p < 0.001 | p > 0.1 | |
| L IFG triangularis | t = 6.52 | t = −0.40 | t = 3.10 | t = 3.10 | t = 3.50 | t = 1.05 |
| p < 0.001 | p > 0.1 | p = 0.006 | p = 0.006 | p = 0.002 | p > 0.1 | |
| R aSTG/STS | t = 6.37 | t = −0.06 | t = 3.01 | t = 1.40 | t = 4.43 | t = 1.30 |
| p < 0.001 | p > 0.1 | p = 0.007 | p > 0.1 | p < 0.001 | p > 0.1 | |
| L aSTG/IFG | t = 5.94 | t = 0.10 | t = 2.46 | t = 2.14 | t = 2.38 | t = 0.85 |
| p < 0.001 | p > 0.1 | p = 0.024 | p = 0.046 | p = 0.028 | p > 0.1 |
3.3. Main effect of Content Type
Since reading demonstrated a Social-Emotional bias within sentence processing regions, we examined if Content Type preferences were more widespread while reading. A number of regions did display these preferences (Table 3). They grouped into those that preferred Social/Social-Emotional over Object conditions (Fig. 3A: peaking in left aSTS, left aSTG, left IFG orbitalis, left IFG triangularis, left mid-STS (mSTS), left posterior STS (pSTS), left lateral fusiform, right aSTS, right mSTS, and right anterior parahippocampal gyrus (PHG), p < 0.05 for 17/20 comparisons of Social-Emotional > Object, and Social > Object) and those that preferred Object over Social/Social-Emotional conditions (Fig. 3B: left PHG-1, left PHG-2, left retrosplenial cortex (RSC), left Caudate-1, and left Caudate-2, p < 0.05 for 9/10 comparisons of Object > Social, and Object > Social-Emotional). See Table 4 for all individual statistics. As can be seen in the graphs of Fig. 3, all clusters demonstrated a graded effect across content types with Social-Emotional and Object at the extremes. If we look at the difference between these extremes across the brain (Social-Emotional v. Object), an additional set of Social-Emotional preferring brain regions (FDR corrected at q = 0.05, 5 voxel extent threshold) emerges which are part of the broader ‘social brain’ network including right ventromedial PFC (R vmPFC, 2, 41, −8) and left amygdala (−19, −7, −15) (see Supplementary materials, Section 2, Table S1 for full table of results).
Table 3.
Regions showing a main effect of content type, FDR corrected with a 10 voxel threshold. Cluster extent, peak Talairach coordinates, and peak F-statistic are listed for each cluster.
| Region | Number of voxels | x | y | z | Peak F-stat | ||
|---|---|---|---|---|---|---|---|
| Regions with Social-Emotional bias | |||||||
| L IFG orbitalis | 504 | −51 | 25 | 3 | 34.6 | ||
| L aSTS | 319 | −48 | 17 | −21 | 24.9 | ||
| L pSTS | 286 | −45 | −39 | 1 | 21.9 | ||
| L mSTS | 227 | −53 | −12 | −10 | 23.7 | ||
| L aSTG | 43 | −48 | 12 | −8 | 16.7 | ||
| L lateral fusiform | 25 | −38 | −41 | −15 | 13.7 | ||
| R aPHC | 24 | 26 | −13 | −18 | 14.3 | ||
| R aSTS | 15 | 45 | 17 | −28 | 15.6 | ||
| R mSTS | 11 | 51 | −15 | −10 | 11 | ||
| L IFG triangularis | 10 | −38 | 22 | 11 | 14.9 | ||
| Regions with Object bias | |||||||
| L RSC | 63 | −16 | −60 | 22 | 13.8 | ||
| L Caudate-1 | 22 | −18 | −9 | 22 | 26.4 | ||
| L PHG-1 | 12 | −29 | −25 | −20 | 12.3 | ||
| L PHG-2 | 11 | −29 | −37 | −12 | 12.9 | ||
| L Caudate-2 | 11 | −16 | −21 | 17 | 13.4 | ||
Fig. 3.
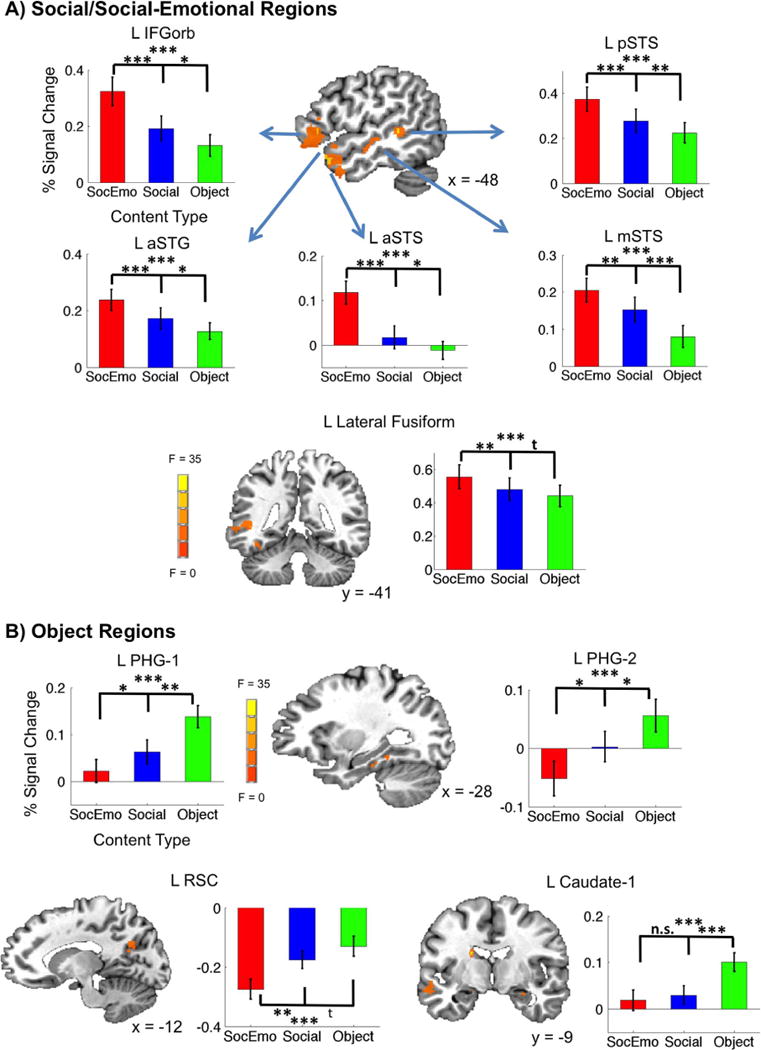
Domain-specific social/social-emotional and object regions. A) Group average maps plotted on the TT_N27 anatomy reveal left frontal and temporal clusters (L IFG orbitalis, L aSTG, L aSTS, L mSTS, L pSTS, and L lateral fusiform) and plots show these areas most strongly prefer Social-Emotional stimuli followed by Social and Object stimuli. B) Left parahippocampal gyrus (L PHG-1 and PHG-2), L retrosplenial cortex (L RSC), and left caudate clusters display main effects and the accompanying plots show these areas most strongly prefer Object stimuli followed by Social and Social-Emotional stimuli. All clusters survived multiple comparisons correction (FDR, q = 0.05). Graphs show percent changes for each condition from the mean signal, which was calculated across all runs, and error bars on graphs indicate ± 1 SEM. Post-hoc two-sided t-tests were performed between Content Type conditions (***: p < 0.001;**: p < 0.01;*: p < 0.05; t: p < 0.1; n.s.: p > 0.1).
Table 4.
Individual statistics for content type clusters. All tests were 2-sided t-tests with 19° of freedom.
| Regions with Social-Emotional bias
| |||
|---|---|---|---|
| Region | Soc-Emo > Social | Soc-Emo > Object | Social > Object |
| L IFG orbitalis | t = 5.38 | t = 6.56 | t = 2.74 |
| p < 0.001 | p < 0.001 | p = 0.013 | |
| L aSTS | t = 5.38 | t = 6.56 | t = 2.74 |
| p < 0.001 | p < 0.001 | p = 0.013 | |
| L pSTS | t = 6.82 | t = 8.54 | t = 3.43 |
| p < 0.001 | p < 0.001 | p = 0.002 | |
| L mSTS | t = 3.06 | t = 8.64 | t = 3.96 |
| p = 0.006 | p < 0.001 | p < 0.001 | |
| L aSTG | t = 4.15 | t = 6.13 | t = 2.30 |
| p < 0.001 | p < 0.001 | p = 0.033 | |
| L lateral fusiform | t = 3.37 | t = 6.16 | t = 1.98 |
| p = 0.003 | p < 0.001 | p = 0.062 | |
| R aPHC | t = 1.96 | t = 5.85 | t = 4.07 |
| p = 0.065 | p < 0.001 | p < 0.001 | |
| R aSTS | t = 5.41 | t = 5.40 | t = −0.39 |
| p < 0.001 | p < 0.001 | p > 0.1 | |
| R mSTS | t = 2.99 | t = 4.44 | t = 2.18 |
| p = 0.008 | p < 0.001 | p = 0.042 | |
| L IFG triangularis | t = 4.25 | t = 4.21 | t = 1.29 |
| p < 0.001 | p < 0.001 | p > 0.1 | |
| Regions with Object bias
| |||
|---|---|---|---|
| Region | Social > Soc-Emo | Object > Soc-Emo | Object > Social |
| L RSC | t = 3.49 | t = 5.23 | t = 2.03 |
| p = 0.003 | p < 0.001 | p = 0.057 | |
| L Caudate-1 | t = 1.29 | t = 6.06 | t = 4.80 |
| p > 0.1 | p < 0.001 | p < 0.001 | |
| L PHG-1 | t = 2.28 | t = 5.69 | t = 3.36 |
| p = 0.028 | p < 0.001 | p = 0.003 | |
| L PHG-2 | t = 2.66 | t = 4.37 | t = 2.84 |
| p = 0.015 | p < 0.001 | p = 0.011 | |
| L Caudate-2 | t = 1. 7 2 | t = 5.45 | t = 3.48 |
| p > 0.1 | p < 0.001 | p = 0.003 | |
4. Discussion
Consistent with previous studies, we found that activity in the left aSTG/STS was strongly modulated by Constituent Size, thereby providing further evidence that this region is involved in sentence processing (e.g., Brennan et al., 2012; Fedorenko et al., 2010; Humphries et al., 2006; Pallier et al., 2011; Rogalsky and Hickok 2009; Vandenberghe et al., 2002; although see Wilson et al., 2013; Mesulam et al., 2015 for a different view). In addition, and consistent with the findings of Pallier et al. (2011), we failed to find a Constituent Size effect for jabberwocky in this region, thus suggesting that conceptual representations are a fundamental component of sentence processing in the aSTG/STS.
Importantly however, the content of these representations mattered. Specifically, activity in the region of aSTG/STS defined by the Constituent Size effect was biased towards material describing social-emotional situations over content devoid of social meaning (Herve et al., 2013; Simmons et al., 2010; Zahn et al., 2007).
This bias towards social-emotional content was not limited to the left aSTG/STS but rather was seen in multiple brain regions, including the orbital region of inferior frontal gyrus (IFG orbitalis), ventromedial prefrontal cortex (vmPFC), and the amygdala. Each of these regions have been linked to different aspects of social processing (Adolphs 2009; Frith and Frith, 2012). For example, these areas are active when reading emotional, relative to non-emotional words (Kensinger and Corkin 2004; Burnett et al., 2009), and damage to vmPFC and amygdala results in social-emotional processing impairments (Barrash et al., 2000; Adolphs et al., 2002). Two other areas associated with social processing, the left lateral region of the fusiform gyrus and the posterior portion of the STS also showed a social bias. Both of these posterior brain regions have been strongly associated with perceiving and knowing about the form and motion of biological entities, respectively, whether represented by pictures or by highly abstract stimuli (e.g., moving geometric forms interpreted as conveying social interactions and written passages about social situations: Castelli et al., 2000; Martin and Weisberg 2003; Speer et al., 2009; Deen and McCarthy 2010; Saygin et al., 2010).
In contrast, other brain regions, including left parahippocampal cortex, retrosplenial cortex, and caudate (e.g., Kraut et al., 2002; Bar et al., 2008), preferred inanimate object over social and social-emotional content. Taken together, these results show that word reading tasks can be used to map broad sets of content-sensitive of brain areas.
Our results also impact the debate regarding the domain-general/ specific nature of representations in the ATL. The initial proposal by Patterson and colleagues argued that the ATL contained a domain-general hub that was necessary for representing all semantic concepts (e.g., Patterson et al., 2007). However, our findings, as well as numerous previous reports, suggest that the most anterior portion of the ATL, typically centered on the aSTG/STS, plays a central role in a domain-specific neural system for representing social/emotional concepts (e.g., Zahn et al., 2007, 2009; Ross and Olson, 2010; Simmons et al., 2010; for review see Olson et al., 2013; Simmons and Martin, 2009). Indeed, both resting state functional connectivity studies of the human brain (Simmons et al., 2010; Pascual et al., 2013) and anatomical tracing studies in the monkey (Saleem et al., 2008) show that the aSTG/STS is part of a network of regions that respond to social and emotional stimuli, including medial prefrontal cortex, the amygdala and anterior aspect of the hippocampus. These data provide a direct challenge for models that posit single, domain-general hub for all semantic concepts (e.g., Lambon Ralph, 2014). To support that idea one would have to argue that either the aSTG/STS provides only a valence bias, and thus does not represent concepts, per se (Rice et al., 2015) or that the ATL hub represents all concepts except those concerned with social processes.
While others have speculated that the sentences > word lists activation in ATL is related to syntactic structure building (e.g., Brennan et al., 2012), the lack of a Jabberwocky Constituent Size effect (also absent in Pallier et al., 2011) suggests that syntax alone is not the main function. Instead, sentences may cause more activation because more complex social/emotional concepts are preferred over simpler ones. Within STG, Honey and colleagues (Honey et al., 2012) find that regions further from primary auditory cortex process information over longer temporal windows suggesting that more anterior and posterior regions of STG may prefer longer, more complex stimuli. They propose that higher-order areas may be involved in processing longer, more complex stimuli generally. We find that in the case of aSTG/STS, longer, more complex social/emotional stimuli are preferred.
The strong effect of social-emotional content on activity in aSTG/ STS raises the possibility that this finding reflected the emotional/ arousing nature of the stimuli, rather than their social content, per se. Although our data can not rule out this possibility, our findings clearly indicate that the emotional and or arousing nature of these sentences can not account for all of our findings given that other brain regions, such as parahippocampal cortex, responded minimally to these stimuli while showing a strong bias for inanimate object content. Studies that directly control for emotional valance and arousal of both social and non-social stimuli (e.g., Yang et al ., 2012) will be needed to address the differential roles that emotional valance, arousal, and social content may play in modulating activity in aSTG/STS.
We were motivated to perform this study at higher field strength to see if sentence and social-emotional processing co-localized in the ATL at a fine resolution. There are benefits but also limitations in using a 7 T scanner however. The increased field strength increases signal amplitude, allowing for smaller voxels than typically used at 3 T or 1.5 T. Smaller voxels minimize partial volume effects, which also improve gray matter signal fidelity. Additionally, the BOLD component from extravascular small vessels (likely located closer to gray matter) increases more than the extravascular large vessels when moving to higher fields, so spatial specificity is thought to increase with increasing field strength (Krüger et al., 2001). But artifacts can also be magnified by the increased field. MRI susceptibility effects increase at 7 T, so signal dropout is greater in regions near air-tissue boundaries such as medial temporal regions than in lower field strengths. Pre-acquisition 3D shimming is one approach to mitigate field in-homogeneities, and it was performed in this study. Additionally, physiological artifacts are magnified at higher field strengths. While the ratio of physiological noise to thermal noise more than doubles for larger voxels (3 × 3 × 3 mm3), it only increases by ~50% for smaller voxels (1 × 1 × 3 mm3) (Triantafyllou et al., 2005). Thus choosing a smaller voxel in our case helped to minimize the relative contribution of physiological noise. According to Hutton et al. (2011), modeling and removing these physiological variables increases tSNR at 7 T, but this increase is minimal at smaller voxel sizes in the range we used. Also using smaller voxels generally reduces brain coverage since more time is needed to collect more samples, but we carefully positioned our coverage over relevant peri-sylvian language regions including the full temporal lobes.
In conclusion, our study provides evidence that the aSTG/STS and left IFG are biased towards processing sentences over shorter constituents. But importantly, these regions prefer processing social-emotional concepts over non-social concepts. The preference for social-emotional concepts also holds in other areas important for social-emotional processing, including IFG orbitalis, pSTS, lateral fusiform, vmPFC, and amygdala, while inanimate object concepts were dominant in regions associated with object processing, i.e., PHG, RSC, and caudate. These results highlight the importance of taking semantic content into account when studying reading and other aspects of linguistic processing.
Supplementary Material
Acknowledgments
We thank Sophie Wohltjen for help with data analysis and Steve Gotts for helpful discussions. This study was supported by the Intramural Research Program of the National Institute of Mental Health, National Institutes of Health, and it was conducted under NIH Clinical Study Protocol 93-M-0170 (ZIAMH002588); NCT00001360.
Appendix A. Supplementary material
Supplementary data associated with this article can be found in the online version at http://dx.doi.org/10.1016/j.neuropsychologia.2016.06.019.
References
- Adolphs R. The social brain: neural basis of social knowledge. Annu Rev Psychol. 2009;60:693–716. doi: 10.1146/annurev.psych.60.110707.163514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolphs R, Baron-Cohen S, Tranel D. Impaired recognition of social emotions following amygdala damage. J Cogn Neurosci. 2002;14:1264–1274. doi: 10.1162/089892902760807258. [DOI] [PubMed] [Google Scholar]
- Balota DA, Yap MJ, Cortese MJ, Hutchison KA, Kessler B, Loftis B, Neely JH, Nelson R, Simpson GB, Treiman R. The english lexicon project. Behav Res Methods. 2007;39:445–459. doi: 10.3758/bf03193014. [DOI] [PubMed] [Google Scholar]
- Bar M, Aminoff E, Schacter DL. Scenes unseen: the parahippocampal cortex intrinsically subserves contextual associations, not scenes or places per se. J Neurosci. 2008;28:8539–8544. doi: 10.1523/JNEUROSCI.0987-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrash J, Tranel D, Anderson SW. Acquired personality disturbances associated with bilateral damage to the ventromedial prefrontal region. Dev Neuropsychol. 2000;18:355–381. doi: 10.1207/S1532694205Barrash. [DOI] [PubMed] [Google Scholar]
- Brennan J, Nir Y, Hasson U, Malach R, Heeger DJ, Pylkkänen L. Syntactic structure building in the anterior temporal lobe during natural story listening. Brain Lang. 2012;120:163–173. doi: 10.1016/j.bandl.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brysbaert M, Warriner AB, Kuperman V. Concreteness ratings for 40 thousand generally known English word lemmas. Behav Res Methods. 2013:1–8. doi: 10.3758/s13428-013-0403-5. [DOI] [PubMed] [Google Scholar]
- Burnett S, Blakemore SJ. Functional connectivity during a social emotion task in adolescents and in adults. Eur J Neurosci. 2009;29:1294–1301. doi: 10.1111/j.1460-9568.2009.06674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett S, Bird G, Moll J, Frith C, Blakemore SJ. Development during adolescence of the neural processing of social emotion. J Cogn Neurosci. 2009;21:1736–1750. doi: 10.1162/jocn.2009.21121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelli F, Happé F, Frith U, Frith C. Movement and mind: a functional imaging study of perception and interpretation of complex intentional movement patterns. NeuroImage. 2000;12:314–325. doi: 10.1006/nimg.2000.0612. [DOI] [PubMed] [Google Scholar]
- Chan D, Anderson V, Pijnenburg Y, Whitwell J, Barnes J, Scahill R, Stevens JM, Barkhof F, Scheltens P, Rossor MN, Fox NC. The clinical profile of right temporal lobe atrophy. Brain. 2009;132:1287–1298. doi: 10.1093/brain/awp037. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res Int J. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Deen B, McCarthy G. Reading about the actions of others: biological motion imagery and action congruency influence brain activity. Neuropsychologia. 2010;48:1607–1615. doi: 10.1016/j.neuropsychologia.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorenko E, Hsieh PJ, Nieto-Castañón A, Whitfield-Gabrieli S, Kanwisher N. New method for fMRI investigations of language: defining ROIs functionally in individual subjects. J Neurophysiol. 2010;104:1177–1194. doi: 10.1152/jn.00032.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith CD, Frith U. Mechanisms of social cognition. Annu Rev Psychol. 2012;63:287–313. doi: 10.1146/annurev-psych-120710-100449. [DOI] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. NeuroImage. 2002;15:870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Gotts SJ, Simmons WK, Milbury LA, Wallace GL, Cox RW, Martin A. Fractionation of social brain circuits in autism spectrum disorders. Brain. 2012;135:2711–2725. doi: 10.1093/brain/aws160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herve PY, Razafimandimby A, Jobard G, Tzourio-Mazoyer N. A shared neural substrate for mentalizing and the affective component of sentence comprehension. PLoS ONE. 2013;8:1–8. doi: 10.1371/journal.pone.0054400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honey CJ, Thesen T, Donner TH, Silbert LJ, Carlson CE, Devinsky O, Doyle WK, Rubin N, Heeger DJ, Hasson U. Slow cortical dynamics and the accumulation of information over long timescales. Neuron. 2012;76:423–434. doi: 10.1016/j.neuron.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries C, Binder JR, Medler DA, Liebenthal E. Syntactic and semantic modulation of neural activity during auditory sentence comprehension. J Cogn Neurosci. 2006;18:665–679. doi: 10.1162/jocn.2006.18.4.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton C, Josephs O, Stadler J, Featherstone E, Reid A, Speck O, Bernarding J, Weiskopf N. The impact of physiological noise correction on fMRI at 7 T. NeuroImage. 2011;57:101–112. doi: 10.1016/j.neuroimage.2011.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insausti R, Juottonen K, Soininen H, Insausti AM, Partanen K, Vainio P, Laakso MP, Pitkänen A. MR volumetric analysis of the human entorhinal, perirhinal, and temporopolar cortices. Am J Neuroradiol. 1998;19:659–671. [PMC free article] [PubMed] [Google Scholar]
- Kensinger EA, Corkin S. Two routes to emotional memory: distinct neural processes for valence and arousal. Proc Natl Acad Sci USA. 2004;101:3310–3315. doi: 10.1073/pnas.0306408101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krüger G, Kastrup A, Glover GH. Neuroimaging at 1.5 T and 3.0 T: comparison of oxygenation-sensitive magnetic resonance imaging. Magn Reson Med. 2001;45:595–604. doi: 10.1002/mrm.1081. [DOI] [PubMed] [Google Scholar]
- Kraut MA, Kremen S, Moo LR, Segal JB, Calhoun V, Hart J. Object activation in semantic memory from visual multimodal feature input. J Cogn Neurosci. 2002;14:37–47. doi: 10.1162/089892902317205302. [DOI] [PubMed] [Google Scholar]
- Lambon Ralph MA. Neurocognitive insights on conceptual knowledge and its breakdown. Philos Trans R Soc Lond B Biol Sci. 2014;369:20120392. doi: 10.1098/rstb.2012.0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A, Weisberg J. Neural foundations for understanding social and mechanical concepts. Cogn Neuropsychol. 2003;20:575–587. doi: 10.1080/02643290342000005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazoyer BM, Tzourio N, Frak V, Syrota A, Murayama N, Levrier O, Salamon G, Dehaene S, Cohen L, Mehler J. The cortical representation of speech. J Cogn Neurosci. 1993;5:467–479. doi: 10.1162/jocn.1993.5.4.467. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Thompson CK, Weintraub S, Rogalski EJ. The Wernicke conundrum and the anatomy of language comprehension in primary progressive aphasia. Brain. 2015;54:awv154. doi: 10.1093/brain/awv154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson IR, Plotzker A, Ezzyat Y. The enigmatic temporal pole: a review of findings on social and emotional processing. Brain. 2007;130:1718–1731. doi: 10.1093/brain/awm052. [DOI] [PubMed] [Google Scholar]
- Olson IR, McCoy D, Klobusicky E, Ross LA. Social cognition and the anterior temporal lobes: a review and theoretical framework. Soc Cogn Affect Neurosci. 2013;8:123–133. doi: 10.1093/scan/nss119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallier C, Devauchelle AD, Dehaene S. Cortical representation of the constituent structure of sentences. Proc Natl Acad Sci USA. 2011:201018711. doi: 10.1073/pnas.1018711108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual B, Masdeu JC, Hollenbeck M, Makris N, Insausti R, Ding SL, Dickerson BC. Large-scale brain networks of the human left temporal pole: a functional connectivity MRI study. Cereb Cortex. 2013;60:bht260. doi: 10.1093/cercor/bht260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson K, Nestor PJ, Rogers TT. Where do you know what you know? The representation of semantic knowledge in the human brain. Nat Rev Neurosci. 2007;8:976–987. doi: 10.1038/nrn2277. [DOI] [PubMed] [Google Scholar]
- Rice GE, Hoffman P, Lambon Ralph MA. Graded specialization within and between the anterior temporal lobes. Ann NY Acad Sci. 2015;1359:84–97. doi: 10.1111/nyas.12951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogalsky C, Hickok G. Selective attention to semantic and syntactic features modulates sentence processing networks in anterior temporal cortex. Cereb Cortex. 2009;19:786–796. doi: 10.1093/cercor/bhn126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross LA, Olson IR. Social cognition and the anterior temporal lobes. NeuroImage. 2010;49:3452–3462. doi: 10.1016/j.neuroimage.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleem KS, Kondo H, Price JL. Complementary circuits connecting the orbital and medial prefrontal networks with the temporal, insular, and opercular cortex in the macaque monkey. J Comp Neurol. 2008;506:659–693. doi: 10.1002/cne.21577. [DOI] [PubMed] [Google Scholar]
- Saygin AP, McCullough S, Alac M, Emmorey K. Modulation of BOLD response in motion-sensitive lateral temporal cortex by real and fictive motion sentences. J Cogn Neurosci. 2010;22:2480–2490. doi: 10.1162/jocn.2009.21388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons WK, Martin A. The anterior temporal lobes and the functional architecture of semantic memory. J Int Neuropsychol Soc. 2009;15:645–649. doi: 10.1017/S1355617709990348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons WK, Martin A. Spontaneous resting-state BOLD fluctuations reveal persistent domain-specific neural networks. Soc Cogn Affect Neurosci. 2012;7:467–475. doi: 10.1093/scan/nsr018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons WK, Reddish M, Bellgowan PSF, Martin A. The selectivity and functional connectivity of the anterior temporal lobes. Cereb Cortex. 2010;20:813–825. doi: 10.1093/cercor/bhp149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowden JS, Bathgate D, Varma A, Blackshaw A, Gibbons ZC, Neary D. Distinct behavioural profiles in frontotemporal dementia and semantic dementia. J Neurol Neurosurg Psychiatry. 2001;70:323–332. doi: 10.1136/jnnp.70.3.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speer NK, Reynolds JR, Swallow KM, Zacks JM. Reading stories activates neural representations of visual and motor experiences. Psychol Sci. 2009;20:989–999. doi: 10.1111/j.1467-9280.2009.02397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain: 3-D Proportional System: An Approach to Cerebral Imaging. 1st. Thieme., Stuttgart; New York: 1988. [Google Scholar]
- Triantafyllou C, Hoge RD, Krueger G, Wiggins CJ, Potthast A, Wiggins GC, Wald LL. Comparison of physiological noise at 1.5 T, 3 T and 7 T and optimization of fMRI acquisition parameters. NeuroImage. 2005;26:243–250. doi: 10.1016/j.neuroimage.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Vandenberghe R, Nobre AC, Price CJ. The response of left temporal cortex to sentences. J Cogn Neurosci. 2002;14:550–560. doi: 10.1162/08989290260045800. [DOI] [PubMed] [Google Scholar]
- Warriner AB, Kuperman V, Brysbaert M. Norms of valence, arousal, and dominance for 13,915 English lemmas. Behav Res Methods. 2013;45:1191–1207. doi: 10.3758/s13428-012-0314-x. [DOI] [PubMed] [Google Scholar]
- Wilson SM, DeMarco AT, Henry ML, Gesierich B, Babiak M, Mandelli ML, Miller BL, Gorno-Tempini ML. What role does the anterior temporal lobe play in sentence-level processing? Neural correlates of syntactic processing in semantic variant primary progressive aphasia. J Cogn Neurosci. 2013;26:970–985. doi: 10.1162/jocn_a_00550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong C, Gallate J. The function of the anterior temporal lobe: a review of the empirical evidence. Brain Res. 2012;1449:94–116. doi: 10.1016/j.brainres.2012.02.017. [DOI] [PubMed] [Google Scholar]
- Yang J, Bellgowan PSF, Martin A. Threat, domain-specificity and the human amygdala. Neuropsychologia. 2012;50:2566–2572. doi: 10.1016/j.neuropsychologia.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahn R, Moll J, Krueger F, Huey ED, Garrido G, Grafman J. Social concepts are represented in the superior anterior temporal cortex. Proc Natl Acad Sci USA. 2007;104:6430–6435. doi: 10.1073/pnas.0607061104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahn R, Moll J, Iyengar V, Huey ED, Tierney M, Krueger F, Grafman J. Social conceptual impairments in frontotemporal lobar degeneration with right anterior temporal hypometabolism. Brain. 2009;132:604–616. doi: 10.1093/brain/awn343. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


