Abstract
BACKGROUND
Colorectal cancer screening rates for African American patients remain sub-optimal. Patient decision aids designed with an entertainment-education approach have been shown to improve saliency and foster informed decision making. The purpose of this investigation was to assess whether an entertainment-education decision aid tailored for African American patients improved patients’ decision making, attitudes, intentions, or colorectal cancer screening behavior.
METHODS
Eighty-nine participants were randomized to view a patient decision aid video containing culturally-tailored information about colorectal cancer screening options and theory-based support in decision making, presented in an entertainment-education format, or an attention-control video about hypertension that contained similarly-detailed information. Participants met with their clinician and then completed follow-up questionnaires assessing their knowledge, decisional conflict, self-advocacy, attitudes, perceived social norms, and intentions. At three months, completion of screening was assessed by chart review.
RESULTS
Viewing the culturally-tailored decision aid significantly increased African American patients’ knowledge of colorectal cancer screening recommendations and options. It also significantly reduced their decisional conflict and improved their self-advocacy. No significant differences were observed in participants’ attitudes, norms, or intentions. At three months, 23% of all patients had completed a colonoscopy.
CONCLUSIONS
Designing targeted, engaging patient decision aids for groups that receive sub-optimal screening holds promise for improving patient decision making and self-advocacy, and additional research is warranted to investigate their effectiveness in clinical practices with sub-optimal screening rates, and on downstream behaviors such as repeat testing.
Keywords: Colorectal cancer, screening, decision aids, African American, choice behaviors
INTRODUCTION
Despite the decrease in total colorectal cancer incidence and mortality over the past decade, colorectal cancer remains the third most common cancer diagnosis and the second leading cause of cancer death in the United States.1 While a 90% 5-year survival rate exists when colorectal cancer is detected early, only 40% of colorectal cancers are diagnosed early, most likely due to low screening rates.1 Multiple colorectal cancer screening methods are recommended and considered equally effective for early detection.2–7
Underserved groups and individuals with lower educational attainment are less likely to be screened. The American Cancer Society reports that 56% of African Americans 50 years or older met colorectal cancer screening guidelines, compared to 62% of Caucasians.3, 8 Several high-quality educational programs exist, but behavioral barriers may be contributing to the screening gap, such as negative attitudes, social pressures, and difficulties applying the medical information at a personal level to make well-informed decisions.9, 10
Evidence-based decision support tools, such as patient decision aids, may be an effective way to diminish these barriers. A recent systematic review showed that patients exposed to a colorectal cancer screening decision aid had greater knowledge, were more likely to be interested in screening, and were more likely to complete screening.11,12 Additionally, approaches such as entertainment education have been used to create culturally-tailored decision aids that improve saliency of the medical information by providing engaging and relevant stories that model informed decision making (e.g., soap-opera-like scenes of a group of friends discussing how they talked with their doctors about a health care decision).13–15 The purpose of this investigation was to assess whether an entertainment education decision aid tailored for African American patients improved decision making, attitudes, intentions, or colorectal cancer screening behavior.
MATERIALS AND METHODS
Study design
This randomized controlled trial of a tailored African American colorectal cancer screening decision aid video versus an attention control video was registered on ClinicalTrials.gov (NCT01492049). The MD Anderson Institutional Review Board and Kelsey Research Education Committee provided ethical review and approval (August 7, 2012) for this study. Informed consent was obtained from all participants.
Materials: Intervention and Control Videos
Development of the decision aid intervention included content review by an expert panel, paper prototyping, video production, and pilot testing using cognitive interviews. In addition to the International Patient Decision Aid Standards (IPDAS) Collaboration guidelines, three complementary models provided the conceptual framework that guided the design of the study, the intervention, and evaluation (Figure 1). The Ottawa Decision Support Framework focuses on addressing modifiable factors to improve patient decision making, such as increasing knowledge, decreasing decisional conflict, and fostering self-advocacy skills.16, 17 The Integrated Model of Behavior is based on the theory of reasoned action, planned behavior, health belief model, and social cognitive theory.18–20 It posits that a behavior (e.g., completing colorectal cancer screening) is most likely to occur if the individual has positive attitudes about colorectal cancer screening, positive perceived social norms regarding screening, and a sense of self-efficacy for completing the task. These factors lead to stronger intentions to engage in screening. The Edutainment Decision Aid Model, was used to improve saliency for African Americans and to ensure the decision aid was accessible across literacy levels.13 This approach intersperses educational and decision support content, including tailored soap-opera-like scenes of individuals modeling decision making behaviors.
Figure 1.
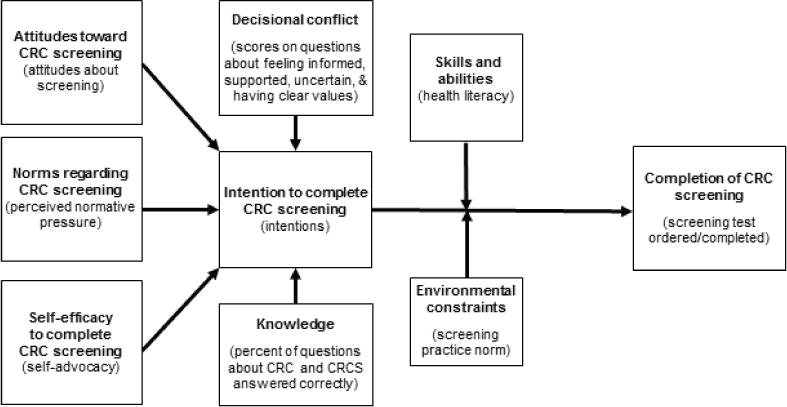
Conceptual framework of colorectal cancer (CRC) screening decisions (and selected study outcomes)12–19
The educational components of the decision aid video described the anatomy of the digestive system and colon, how colorectal cancer forms, who is at high risk of developing it, and morbidity/mortality rates. They also discussed how colorectal cancer can potentially be prevented if polyps are detected and removed. Three screening options (colonoscopy, fecal occult blood test, and sigmoidoscopy) were compared with respect to how each test works, how it is performed, preparations required by the patient, accuracy, recommended frequency, and other pros and cons. The decision support components included statements encouraging patients to talk to their provider about colorectal cancer screening, to ask questions, and to share their concerns and preferences. Scenes depicting an African American family making a decision about colorectal cancer screening modeled decision-making skills and behaviors.
The hypertension video was selected as an attention control because it provided similar educational content (e.g. anatomy, treatment options, prevention, and risk/benefit information), but it lacked the decision support and tailored education entertainment components. Table 1 compares the key design elements between the intervention and control videos.
Table 1.
Comparison of the intervention (decision aid) and attention-control (education) videos.
| Design Elements | Intervention Video | Control Video |
|---|---|---|
| Medical Information | Text, graphics, and narration describing colorectal cancer, screening options and risks/benefits | Text, graphics, and narration describing hypertension treatment options and risks/benefits |
| Decision Support | Theory-based dramatized scenes modeling decision-making skills | None |
| User targeting | African Americans aged 49–75 years | None |
| Total Length | 30 minutes | 11 minutes |
Study participants and procedures
Patients were eligible if they were 49–75 years old (i.e., appropriate for considering screening by their next birthday), were African American, had a scheduled office visit, were due for colorectal cancer screening, and were able to speak and write English. Patients were ineligible if they had a history of polyps or colorectal cancer; or were up-to-date on screening (i.e., fecal occult blood test within the last year, flexible sigmoidoscopy with the last five years, or colonoscopy within the last 10 years).
Participants were recruited from November 2012 to June 2013 from internal medicine and family medicine outpatient clinics at three tertiary care centers that serve a racially, ethnically, and economically diverse patient population in the greater Houston area. Research assistants reviewed electronic medical records to identify potential participants. Clinic staff called potential participants and screened for eligibility using a standardized script. Research assistants then confirmed eligibility, reviewed study procedures, enrolled willing volunteers, and scheduled a study visit one hour prior to their next clinic visit. Interviewers and participants were blinded until baseline questionnaires were completed. Participants were randomized using computer-generated permuted blocks in a two-to-one ratio, intervention to control. After viewing their randomly-assigned video, participants completed post-intervention questionnaires. All participants completed follow-up telephone interviews 1–3 weeks later (variance due to time needed to reach some participants) that assessed decision-making, attitudes, and intentions regarding screening. Screening completion was confirmed by medical chart review at three months. Participants were provided with a $50 gift card at the baseline study visit.
Measures
Baseline questionnaires assessed patient characteristics, including health literacy using a single item literacy screener (“How often do you have someone else help you read hospital materials?”).21 Responses were categorized into high health literacy (“none of the time” and “a little of the time”) or low health literacy (“some of the time”, “most of the time”, and “all of the time”). Knowledge was assessed using a 15-item questionnaire developed for this study, and responses were summed (wrong/unsure=0 and correct=1), with higher scores indicating greater knowledge.
Attitudes towards, and perceived social normative pressures about, colorectal cancer screening were assessed using a modified Integrative Model Scale, which assesses each construct with three items.19 Participants indicated their level of agreement from 1 = strongly disagree to 5 = strongly agree. Negative attitudes were reverse scored. Responses were summed to obtain a maximum score of 15 for each construct, with higher scores indicating more positive attitudes and perceived social norms regarding screening. Intentions to be screened was assessed using three items indicating their level of agreement from 1 = strongly disagree to 5 = strongly agree. Responses were summed for a total possible score of 15, with higher scores indicating greater intentions to get screened.
Post-intervention questionnaires assessed participants’ knowledge, decision-making, and screening behaviors. The low-literacy 10-item Decisional Conflict Scale and four subscale (Informed, Value Clarity, Support, and Uncertainty) scores were summed (yes = 0, unsure = 2, and no = 4) and scaled to a maximum of 100 points, with lower scores indicating less conflict.22 The 12-item Patient Self-Advocacy Scale was scored (yes = 1, unsure = 2, no = 3), summed, and divided by 12 for an average score, with lower scores indicating greater self-advocacy.23 Chart review at three months after the study visit confirmed colorectal cancer screening test orders and completion.
Data Analysis
Data analyses included confirmation of equal randomization at baseline, review of the distribution of variables, univariable analyses (ANOVA for continuous variables, chi-square tests for categorical variables), and multivariable modeling (ANCOVA for continuous variables, co-variables retained if significant at α = 0.2). For binary outcomes, crude and adjusted logistic regression models tested the effect of the intervention. All statistical analysis was performed using SPSS software (IBM SPSS Statistics, version 23).
The target sample size of 88 participants was selected to detect an effect size of 0.6 on the Decisional Conflict Scale (DCS). Decisional conflict is primary measure of decision quality and an intermediate measure in the process of screening uptake. The purpose of patient decision aids is to help patients make a well-informed, values-congruent decision among two or more medically-relevant options, and this decision aid was designed to prepare patients for a consultation with their doctor, at which many other factors may have impacted the screening decision (e.g., contraindications, lack of time, etc.). The Decisional Conflict Scale is a widely-used measure of patients’ perceptions of whether their decision-making process is informed, based on personal values (i.e., the relative importance they place on the likelihood of risks and benefits), supported, certain, and effective. Analyses assessed the effect of viewing the intervention video compared to the control video on decisional conflict, knowledge, self-advocacy, attitudes, perceived normative pressure, intentions, and screening behavior. Additional analyses confirmed that there was no interaction between health literacy and intervention status with respect to post-intervention knowledge scores using a linear model with an interaction term.
RESULTS
Participants
Fifty-nine patients were randomized to the intervention arm and 30 were randomized to the control arm (Figure 2). One patient from each arm was lost to follow-up and one patient randomized to the control arm was misassigned, received the intervention, and was subsequently dropped from the analysis. Chart reviews at 3 months were completed for all participants. The study was completed as planned and no unintended harms were observed.
Figure 2.
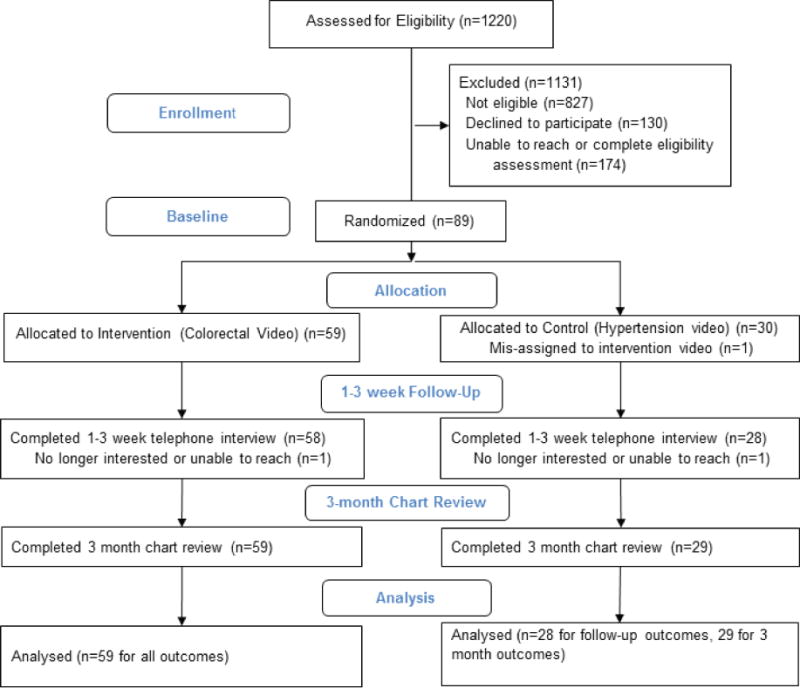
Consolidated Standards of Reporting Trials diagram.
Intervention and control patients did not differ significantly on baseline characteristics (Table 2). Patients were primarily less than 60 years of age, female, married, privately-insured, and health literate. About 30% had a high school education or less. No differences were observed between recruitment sites.
Table 2.
Participant Demographic Characteristics.
| Characteristic | Intervention (n = 59) n (%) |
Control (n = 29) n (%) |
P-value |
|---|---|---|---|
| Age in years (mean, min-max, SD) | 57.7 (49–73, 7.4) | 57.4 (49–71, 5.9) | 0.861 |
| Gender | 0.552 | ||
| Male | 20 (34) | 8 (28) | |
| Female | 39 (66) | 21 (72) | |
| Highest level of education | 0.962 | ||
| High school or less | 18 (31) | 9 (31) | |
| College or more | 41 (69) | 20 (69) | |
| Marital status | 0.322 | ||
| Married/long term relationship | 31 (53) | 12 (41) | |
| Single/divorced/widowed/other | 28 (47) | 17 (58) | |
| Type of health insurance | 0.902 | ||
| Private only | 44 (75) | 22 (76) | |
| Medicare/Medicare and/or other | 15 (25) | 7 (24) | |
| Health literacy3 | 0.192 | ||
| High | 48 (81) | 20 (69) | |
| Low | 11 (19) | 9 (31) |
Abbreviations: SD = Standard Deviation.
One-way ANOVA between-groups significance test.
Pearson’s chi-square significance test. Exact test p-values are reported if cell counts were <5.
Single Item Literacy Screening question.
Knowledge, decisional conflict, self-advocacy, and attitudes and intentions towards screening
Table 3 presents participants’ scores on the self-reported outcomes at baseline (knowledge, attitudes, norms, intentions) and follow-up (all scales), assessed using two-sided ANCOVA models adjusted for covariates. Both groups had comparable baseline knowledge scores (p = 0.64), and participants who viewed the decision aid had significantly greater pre-post increases in knowledge scores (2.3 versus 0.4, p < 0.01). Participants who viewed the decision aid reported significantly lower (improved) decisional conflict total and subscale scores than the control group (p < 0.01), as well as significantly lower (improved) self-advocacy scores (p = 0.01). There were no significant differences between intervention and control groups in mean pre-post change scores for attitudes, perceived normative pressures, or intentions to get screened.
Table 3.
Participants’ attitudes and beliefs about screening.
| Outcome | Intervention (n = 58) | Control (n = 28) | P-value adjusted1 | ||
|---|---|---|---|---|---|
|
| |||||
| Baseline | Follow-up | Baseline | Follow-up | ||
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | ||
| KnowledgeA | 8.9 (3.1) | 11.6 (2.4) | 9.2 (2.9) | 9.6 (2.5) | <0.012 |
| Decisional conflictA | — | 11.0 (16.7) | — | 39.6 (27.7) | <0.013 |
| Informed subscaleA | — | 15.8 (27.8) | — | 58.0 (38.8) | <0.014 |
| Values clarity subscaleA | — | 16.7 (28.1) | — | 38.9 (40.0) | <0.015 |
| Support subscaleA | — | 5.2 (15.0) | — | 18.5 (24.2) | <0.016 |
| Uncertainty subscaleA | — | 6.0 (17.1) | — | 44.4 (44.6) | <0.017 |
| Patient self-advocacyA,B | — | 1.6 (0.3) | — | 1.8 (0.3) | 0.018 |
| Attitudes about screeningA,B | 9.7 (2.0) | 9.4 (2.2) | 8.9 (1.7) | 8.6 (2.3) | 0.499 |
| Perceived normative pressureA,B | 10.5 (2.2) | 10.6 (1.9) | 11.6 (2.0) | 10.6 (2.2) | 0.4910 |
| Intention to get screenedA,B | 12.5 (2.1) | 13.0 (1.5) | 12.1 (2.6) | 12.8 (1.6) | 0.6911 |
Abbreviations: SD = Standard Deviation
Based on the Ottawa Decision Support Framework.
Based on the Integrative Model of Behavior.
Two-sided Analysis of Covariance (ANCOVA) significance test.
Interpretations, score ranges, and model adjustments:
Adjusted for health literacy, education, and marital status.
Adjusted for baseline knowledge score, health literacy, and education.
Adjusted for baseline knowledge score, health literacy, and education.
Adjusted for health literacy and education.
Adjusted for age and health insurance.
Adjusted for health literacy and education.
Adjusted for marital status.
Adjusted for baseline attitude and marital status.
Adjusted for baseline norms, health literacy, and age.
Adjusted for baseline intentions score, health literacy, education, health insurance, and age.
Screening behavior
Overall, 22% of all participants reported ordering screening test by 1 – 3 weeks after the clinical visit, and chart review at three months indicated that 47% of all participants had ordered and 23% had completed a colorectal cancer screening test (all colonoscopies). There were no significant differences between the intervention and control group for either outcome (Table 4). Sub-analyses about the patient-clinician consultation indicated that participants who viewed the decision aid may have had higher intentions to discuss screening with their clinician (p = 0.06) and may be able to more frequently discuss their screening preferences with their doctor (76% versus 38%, p = 0.07).
Table 4.
Study participants’ screening behaviors.
| Intervention (n = 59) n (%) |
Control (n = 29) n (%) |
Total (n = 88) n (%) |
P-valueB | |
|---|---|---|---|---|
| Ordered screening testA | 26 (45) | 15 (52) | 41 (47) | 0.50 |
| Completed screening at 3 monthsA | 12 (21) | 8 (28) | 20 (23) | 0.45 |
Based on the Integrative Model of Behavior.
Effect of randomization to intervention vs control group.
DISCUSSION
The entertainment-education decision aid about colorectal cancer screening significantly improved African American patients’ knowledge, reduced their decisional conflict, and increased their sense of self-advocacy. No differences were observed between intervention and control regarding patient-reported attitudes, perceived normative social pressure, or intentions to discuss screening with their physician. Chart review confirmed that 47% of both groups had ordered and 23% had completed a colonoscopy within three months.
The entertainment-education decision aid used in this study was effective in improving patient’s knowledge by 20% compared to control. This increase is similar to the effects seen across decision aid studies and by studies evaluating decisions aids for colorectal cancer screening.24 A majority of decision aid studies have been successful in significantly improving knowledge, either from baseline to post-intervention or compared to a control group.24
An increase in screening knowledge is postulated to lead to lower decisional conflict,25 which was observed in our study. Notable improvements were observed in patients’ decisional conflict levels across all four constructs – feeling informed, being more clear about how they valued the risk/benefit trade-offs, feeling supported in their decision, and feeling more certain about the decision. Participants who viewed the decision aid had a mean decisional conflict score of 11, while those who viewed the control video had a mean score of 40. Scores below 25 are associated with implementing decisions; scores over 37.5 are associated with delaying decisions or feeling unsure about implementation.22 Additional studies indicate that for every unit increase in decisional conflict, patients are 59 times more likely to change their mind, 23 times more likely to delay their decision, 5 times more likely to express decisional regret, and 19 times more likely to blame their doctor for negative clinical outcomes, independent of the patient’s age or knowledge scores.22 Other studies of colorectal cancer screening decision aids have measured the effect of a decision aid on decisional conflict; at least two other studies also achieved lower decisional conflict scores in the intervention groups compared to control conditions.25, 26 Future studies may wish to assess the effect of viewing a colorectal cancer screening decision aid on post-screening outcomes such as regret, blame, and adherence to subsequent screening recommendations.
Previously, we found improvements in self-advocacy for an entertainment-education decision aid administered in a setting where patients would be expected to have low health literacy.15 In the current study, with a broader range of literacy levels, greater self-advocacy was also observed for patients receiving the entertainment-education decision aid. These findings are encouraging as the Edutainment Decision Aid Model includes modeling of desired behaviors and has the potential to impact perceptions of self-advocacy in decision making. Other studies have examined intervention effects on self-efficacy/self-advocacy, with mixed results. Two randomized trials achieved significantly higher self-efficacy each in intervention27 and control28 groups. One uncontrolled trial achieved significant pre-post increase in self-efficacy. More research is needed to examine how decision aids can be designed to increase screening self-efficacy.
Viewing the decision aid did not appear to affect behavioral determinants such as attitudes, perceived normative pressure, or intentions at the time of the initial clinical consultation (constructs from the modified Integrative Model18, 19); however, sub-group analyses suggested non-significant trends towards higher intentions to discuss screening preferences with their clinician and higher patient-reported rates of discussion in the clinical consultation. Consistent with our findings, the majority of colorectal cancer screening decision aid studies found no significant effect of decision aids on screening intentions,25, 28–32 whereas attitudes towards screening were either the same28 or more negative26, 33 in decision aid viewers than in controls. This could be attributed to patients having a clearer picture of the risks associated with screening after viewing a decision aid.
Limitations
The Integrated Model of Behavior includes several external, environmental, and contextual factors that may account for limitations observed in this study, such as clinical practice variations, interpersonal communication, and the potential for successful behaviors to improve attitudes over time.18, 19 This study was not able to assess the interaction between patient and clinician during the consultation, which may have included competing priorities or contraindications to screening at this visit. There was minimal variation regarding which screening test was ordered, suggesting an underlying practice pattern.
Further, the null effect observed on attitudes and perceived normative social pressure about colorectal cancer screening may have been due to a ceiling effect for the measures from the modified Integrated Model Scale (e.g., patient reports of screening intentions tend to be high) or the short follow-up period. Additional measurement development studies may be needed for this instrument.
Finally, this study was not designed to identify components of the intervention that led to improvements in decisional outcomes, nor identify subgroups of patients for whom the intervention was most impactful in making informed decisions. The decision aid was compared to an attention-control video for the purpose of assessing its impact as a patient decision aid; however, future studies may wish to assess the value of the entertainment education approach by comparing the entertainment education decision aid to a standard colorectal cancer screening decision aid video.
Conclusion
Viewing an education-entertainment tailored patient decision aid about colorectal cancer screening improved African American patients’ knowledge and self-advocacy about colorectal cancer screening. Notably, it greatly reduced their decisional conflict across all four constructs – feeling well-informed, more clear about how they valued the risk/benefit trade-offs, more supported in their decision, and more certain about the decision – and shifted them from delaying decisions to implementing decisions. Designing tailored patient decision aids holds promise for improving patient decision making and self-advocacy, and additional research is warranted to investigate their effect in clinical practices that have sub-optimal screening rates and for downstream behaviors such as repeat testing.
Acknowledgments
We acknowledge the assistance of research staff at the Kelsey-Seybold Clinic with data collection. We are grateful for the guidance on the content of the intervention from Drs. Navkiran Shokar, Sally Vernon, and Sarah Hawley. We thank Dr. Gary Deyter for his assistance in editing.
Financial Support
The project was supported by grants from the National Cancer Institute (R21CA132669, R25TCA057730). Dr. Ashley Housten was supported by the National Cancer Institute of the National Institutes of Health under Award Number R25CA057730 (PI: Shine Chang, PhD) and by the Cancer Center Support Grant CA016672 (PI: Ronald DePinho, MD). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or National Institutes of Health. This work was also supported in part by a grant from The University of Texas MD Anderson Cancer Center Duncan Family Institute for Cancer Prevention and Risk Assessment. It was also supported for Dr. Suzanne K. Linder by Award Number R24HS022134 from the Agency for Healthcare Research and Quality and Award Number RP140020 from the Cancer Prevention Research Institute of Texas.
Footnotes
Potential Competing Interests
The authors declare no conflicts of interest associated with this manuscript.
Specific Author Contributions
Study Planning: SKL, MLJ, GSR, RJV
Study Conduct: SKL, VBL, RJV
Data Collection, Analyses, and Interpretation: GK, LML, AJH, ASH, RJV
Drafting and Editing the Manuscript: All authors
All authors have reviewed and approved the final submitted draft.
References
- 1.American Cancer Society. Colorectal Cancer Facts & Figures. American Cancer Society; Atlanta: 2016. pp. 2014–2016. [Google Scholar]
- 2.Burt RW, Barthel JS, Dunn KB, et al. NCCN clinical practice guidelines in oncology. Colorectal cancer screening. J Natl Compr Canc Netw. 2010;8:8–61. doi: 10.6004/jnccn.2010.0003. [DOI] [PubMed] [Google Scholar]
- 3.Dolan NC, Ferreira MR, Fitzgibbon ML, et al. Colorectal cancer screening among African-American and white male veterans. Am J Prev Med. 2005;28:479–482. doi: 10.1016/j.amepre.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 4.McFarland EG, Levin B, Lieberman DA, et al. Revised colorectal screening guidelines: joint effort of the American Cancer Society, U.S. Multisociety Task Force on Colorectal Cancer, and American College of Radiology. Radiology. 2008;248:717–720. doi: 10.1148/radiol.2483080842. [DOI] [PubMed] [Google Scholar]
- 5.Qaseem A, Denberg TD, Hopkins RH, Jr, et al. Screening for colorectal cancer: a guidance statement from the American College of Physicians. Ann Intern Med. 2012;156:378–386. doi: 10.7326/0003-4819-156-5-201203060-00010. [DOI] [PubMed] [Google Scholar]
- 6.Smith RA, Manassaram-Baptiste D, Brooks D, et al. Cancer screening in the United States, 2014: a review of current American Cancer Society guidelines and current issues in cancer screening. CA Cancer J Clin. 2014;64:30–51. doi: 10.3322/caac.21212. [DOI] [PubMed] [Google Scholar]
- 7.U. S. Preventive Services Task Force. Screening for colorectal cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;149:627–637. doi: 10.7326/0003-4819-149-9-200811040-00243. [DOI] [PubMed] [Google Scholar]
- 8.DeSantis C, Naishadham D, Jemal A. Cancer statistics for African Americans, 2013. CA Cancer J Clin. 2013;63:151–166. doi: 10.3322/caac.21173. [DOI] [PubMed] [Google Scholar]
- 9.Davis TC, Dolan NC, Ferreira MR, et al. The role of inadequate health literacy skills in colorectal cancer screening. Cancer Invest. 2001;19:193–200. doi: 10.1081/cnv-100000154. [DOI] [PubMed] [Google Scholar]
- 10.Miller DP, Jr, Brownlee CD, McCoy TP, et al. The effect of health literacy on knowledge and receipt of colorectal cancer screening: a survey study. BMC Fam Pract. 2007;8:16. doi: 10.1186/1471-2296-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Volk RJ, Linder SK, Lopez-Olivo MA, et al. Patient decision aids for colorectal cancer screening: a systematic review and meta-analysis. Am J Prev Med. 2016;51(5):779–791. doi: 10.1016/j.amepre.2016.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stacey D, Legare F, Col NF, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2014(1):CD001431. doi: 10.1002/14651858.CD001431.pub4. [DOI] [PubMed] [Google Scholar]
- 13.Jibaja-Weiss ML, Volk RJ. Utilizing computerized entertainment education in the development of decision aids for lower literate and naive computer users. J Health Commun. 2007;12:681–697. doi: 10.1080/10810730701624356. [DOI] [PubMed] [Google Scholar]
- 14.McCaffery KJ, Holmes-Rovner M, Smith SK, et al. Addressing health literacy in patient decision aids. BMC Med Inform Decis Mak. 2013;13(2):S10. doi: 10.1186/1472-6947-13-S2-S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Volk RJ, Jibaja-Weiss ML, Hawley ST, et al. Entertainment education for prostate cancer screening: a randomized trial among primary care patients with low health literacy. Patient Educ Couns. 2008;73:482–89. doi: 10.1016/j.pec.2008.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Connor AM, Jacobsen MJ, Stacey D. An evidence-based approach to managing women’s decisional conflict. J Obstet Gynecol Neonatal Nurs. 2002;31:570–581. doi: 10.1111/j.1552-6909.2002.tb00083.x. [DOI] [PubMed] [Google Scholar]
- 17.Volk R, Llewellyn-Thomas H, Stacey D, et al. The international patient decision aids standards (IPDAS) collaboration’s quality dimensions: theoretical rationales, current evidence, and emerging issues. BMC Med Inform and Dec Making. 2013;13(2) doi: 10.1186/1472-6947-13-S2-S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fishbein M, Hennessy M, Kamb M, et al. Using intervention theory to model factors influencing behavior change. Project RESPECT. Eval Health Prof. 2001;24:363–384. doi: 10.1177/01632780122034966. [DOI] [PubMed] [Google Scholar]
- 19.Frosch DL, Legare F, Fishbein M, et al. Adjuncts or adversaries to shared decision-making? Applying the Integrative Model of behavior to the role and design of decision support interventions in healthcare interactions. Implement Sci. 2009;4:73. doi: 10.1186/1748-5908-4-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zikmund-Fisher BJ, Windschitl PD, Exe N, et al. ‘I’ll do what they did”: social norm information and cancer treatment decisions. Patient Educ Couns. 2011;85:225–229. doi: 10.1016/j.pec.2011.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morris NS, MacLean CD, Chew LD, et al. The Single Item Literacy Screener: evaluation of a brief instrument to identify limited reading ability. BMC Fam Pract. 2006;7:21. doi: 10.1186/1471-2296-7-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O’Connor AM. User Manual - Decisional conflict scale(s) Ottawa Health Research Institute; Ottawa: 2010. [Google Scholar]
- 23.Brashers DE, Haas SM, Neidig JL. The patient self-advocacy scale: measuring patient involvement in health care decision-making interactions. Health Commun. 1999;11:97–121. doi: 10.1207/s15327027hc1102_1. [DOI] [PubMed] [Google Scholar]
- 24.Volk RJ, Linder SK, Lopez-Olivo MA, et al. Patient Decision Aids for Colorectal Cancer Screening: A Systematic Review and Meta-Analysis. Am J Prev Med. doi: 10.1016/j.amepre.2016.06.022. [accepted 2016];[In Press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dolan JG, Frisina S. Randomized controlled trial of a patient decision aid for colorectal cancer screening. Med Decis Making. 2002;22:125–139. doi: 10.1177/0272989X0202200210. [DOI] [PubMed] [Google Scholar]
- 26.Smith SK, Trevena L, Simpson JM, et al. A decision aid to support informed choices about bowel cancer screening among adults with low education: randomised controlled trial. BMJ. 2010;341:c5370. doi: 10.1136/bmj.c5370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jerant A, Kravitz RL, Rooney M, et al. Effects of a tailored interactive multimedia computer program on determinants of colorectal cancer screening: a randomized controlled pilot study in physician offices. Patient Educ Couns. 2007;66:67–74. doi: 10.1016/j.pec.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 28.Frosch DL, Legare F, Mangione CM. Using decision aids in community-based primary care: a theory-driven evaluation with ethnically diverse patients. Patient Educ Couns. 2008;73:490–496. doi: 10.1016/j.pec.2008.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Griffith JM, Fichter M, Fowler FJ, et al. Should a colon cancer screening decision aid include the option of no testing? A comparative trial of two decision aids. BMC Med Inform Decis Mak. 2008;8:10. doi: 10.1186/1472-6947-8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Griffith JM, Lewis CL, Brenner AR, et al. The effect of offering different numbers of colorectal cancer screening test options in a decision aid: a pilot randomized trial. BMC Med Inform Decis Mak. 2008;8:4. doi: 10.1186/1472-6947-8-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trevena LJ, Irwig L, Barratt A. Randomized trial of a self-administered decision aid for colorectal cancer screening. J Med Screen. 2008;15:76–82. doi: 10.1258/jms.2008.007110. [DOI] [PubMed] [Google Scholar]
- 32.Wolf AM, Schorling JB. Does informed consent alter elderly patients’ preferences for colorectal cancer screening? Results of a randomized trial. J Gen Intern Med. 2000;15:24–30. doi: 10.1046/j.1525-1497.2000.01079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Steckelberg A, Hulfenhaus C, Haastert B, et al. Effect of evidence based risk information on “informed choice” in colorectal cancer screening: randomised controlled trial. BMJ. 2011;342:1–7. doi: 10.1136/bmj.d3193. [DOI] [PMC free article] [PubMed] [Google Scholar]


