Abstract
Objectives
To develop a model to predict hypertension risk among children with incident elevated blood pressure (BP); to test the external validity of the model.
Methods
A retrospective cohort study was conducted in 3 organizations: Kaiser Permanente Colorado was the model derivation site; HealthPartners of Minnesota and Kaiser Permanente Northern California served as external validation sites. During study years 2006 through 2012, all children aged 3 through 17 years with incident elevated BP in an outpatient setting were identified. The predictor variables were demographic and clinical characteristics collected during routine care. Cox proportional hazards regression was used to predict subsequent hypertension, and diagnostic statistics were used to assess model performance.
Results
Among 5598 subjects at the derivation site with incident elevated BP, 160 (2.9%) developed hypertension during the study period. Eight characteristics were used to predict hypertension risk: age; sex; race; BP preceding incident elevated BP; body mass index percentile; systolic BP percentile; diastolic BP percentile; and clinical setting of the incident elevated BP. At the derivation site, the model discriminated well between those at higher versus lower risk of hypertension (c-statistic 0.77). At external validation sites, the observed risk of hypertension was higher than the predicted risk, and the model showed poor discrimination (c-statistic ranged from 0.64 to 0.67).
Conclusions
Among children with incident elevated BP, a risk model demonstrated good internal validity with respect to predicting subsequent hypertension. However, the risk model did not perform well at two external validation sites, which may limit transportability to other settings.
Keywords: hypertension, elevated blood pressure, predictive risk model, screening, electronic health records
INTRODUCTION
Hypertension in childhood is relatively common, affecting 2% to 5% of the population,1–3 and is associated with hypertension during adulthood.4–6 Although a strategy of universal blood pressure (BP) screening during childhood has been recommended for decades,7 in 2013 the U.S. Preventive Services Task Force (USPSTF) found insufficient evidence to support universal BP screening in children,8 a conclusion that sparked a great deal of debate.9, 10 However, the American Academy of Pediatrics recommends universal BP measurement beginning at 3 years of age,11 a position that has not changed since publication of the USPSTF findings.
Even in the context of a universal BP screening strategy, identifying children with hypertension can be challenging.12–14 Hypertension in children is defined as a BP ≥ 95th percentile for age, sex, and height percentile on 3 separate occasions.7, 15 When a BP is elevated in the hypertensive range in an asymptomatic individual, a repeat measurement within 1 to 2 weeks is recommended. However, appropriate follow up of abnormal BPs does not occur consistently,16 and hypertension in childhood often goes unrecognized.12–14
Developing a means to better differentiate between children at higher versus lower risk of hypertension has been proposed as a strategy to improve hypertension recognition.17, 18 Predictive risk models can be used to risk-stratify individuals based on clinical and demographic characteristics, in order to predict the likelihood of future disease states.19 Among children with an incident elevated BP, a predictive risk model could potentially identify those at higher and lower risk of developing hypertension. In settings with electronic health records (EHRs), predictive risk models can be integrated into clinical decision support tools for use during routine care, as long as the risk models are based upon data readily available within the EHR.20–22
Our study objective was to develop a risk model to predict the likelihood of hypertension among children with an incident elevated BP in the hypertensive range. Specifically, we sought to derive a predictive risk model for hypertension using clinical and demographic characteristics available within an EHR at a large health care system. Because our goal was to develop a pragmatic tool that could be used in a variety of clinical settings, we also assessed the external validity of the predictive risk model,23 by examining the performance of the model in two other health care systems.
METHODS
Study Setting
This investigation was conducted as part of a retrospective observational study of pediatric hypertension, described in detail elsewhere.24 Three large integrated health care delivery systems participated: HealthPartners of Minnesota, Kaiser Permanente Colorado (KPCO), and Kaiser Permanente Northern California (KPNC). Primary care to children is provided at 18 medical offices at HealthPartners, 28 offices at KPCO, and > 30 offices at KPNC. The human subjects review board at HealthPartners approved the study, and KPCO and KPNC ceded research oversight to HealthPartners. Written consent was not required.
The 3 study sites used the Epic EHR (Madison, WI), which captures demographic data, health plan enrollment information, diagnosis codes, and vital signs. BP values were captured in specific EHR fields, and multiple BP measurements could be recorded at each visit. The EHR did not display BP percentiles.
Study Cohort
Inclusion Criteria
We used the following steps to develop a cohort of children aged 3 through 17 years with an incident elevated BP in the hypertensive range. At HealthPartners and KPCO, all subjects with a BP measured during 2006 through 2012 were eligible for inclusion. Because KPNC was implementing their EHR on a staggered basis during 2006–2007, the cohort at KPNC was restricted to 3 KPNC sub-regions with early EHR implementation, and restricted to years 2008 through 2012. At KPNC, a 50% random sample was selected for study, in order to create cohorts of roughly similar size at all 3 study sites. For study inclusion, subjects were required to have continuous health plan enrollment for at least 6 months before, and at least 12 months after, the date of their incident elevated BP. Subjects were also required to have at least 2 additional BPs measured on subsequent occasions after their incident elevated BP.
Exclusion Criteria
Subjects were excluded if they turned 18 years old or died within 12 months following the date of their incident elevated BP. International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes from encounter diagnoses were used to exclude additional subjects. Because the goal was to develop a cohort with incident elevated BP, we excluded children with a diagnosis of hypertension (ICD-9-CM codes 401.xx through 405.xx) on or prior to the date of their incident elevated BP. We also excluded subjects with certain comorbid conditions known to cause hypertension, including acute or chronic renal disease (189.0, 283.11, 580.xx through 587.xx, 589.xx, 590.0x, 593.71, 593.72, 593.73, 593.81, V42.0, V56.x, 866.xx), aortic coarctation (747.1x), and pregnancy (630.xx through 677.xx, V22.xx through V24.xx, and V27.xx through V39.xx).
Identifying Incident Elevated BP
The BPs used for this study were measured by medical assistants or registered nurses during routine clinical care, using protocols based on published guidelines for proper BP measurement technique.7 Within each health system, staff members received formal training in BP measurement based on national guidelines7 at the time of their hire; however, additional details regarding the BP training, such as whether a specific training manual was used, are not available. At HealthPartners and KPCO, BP was measured predominantly using aneroid sphygmomanometers; at KPNC, Welch Allyn oscillometric devices (Skaneateles Falls, NY) were used.
Because a height percentile is needed to categorize BPs in childhood,7 we required a height measurement within 90 days before or after the respective BP measurement. Because national guidelines recommend BP measurement at every health care encounter,7 we examined BP measurements from all primary care, medical specialty, and surgical specialty outpatient visits. Measurements from emergency department and inpatient settings were excluded.
After developing the study cohort, we identified all individuals with an incident elevated BP in the hypertensive range, defined as a systolic BP and/or diastolic BP ≥ 95th percentile for age, sex, and height percentile.7 National guidelines recommend repeating an elevated BP at the time of the visit7; in routine practice, providers are likely to make clinical decisions about BP follow-up on the basis of the repeat measurement. Therefore, when multiple BPs were measured on the same day, the last recorded BP was used in analyses. Based upon national guidelines,7 hypertensive stage was categorized into 3 mutually exclusive categories: stage 1 systolic elevation (systolic 95th percentile to the 99th percentile plus 5 mm Hg); stage 2 systolic elevation (systolic > 99th percentile plus 5 mm Hg); or diastolic-only elevation (a diastolic ≥ 95th percentile with a systolic < 95th percentile).
Identifying Hypertension
Among subjects with an incident elevated BP, we determined which children subsequently developed hypertension. Hypertension was defined as: 1) three consecutive elevated BPs in the hypertensive range on separate occasions; or 2) an ICD-9-CM diagnosis code for hypertension after the date of incident elevated BP plus the initiation of an anti-hypertensive medication; or 3) an ICD-9-CM diagnosis code for hypertension, with the diagnosis confirmed by a manual review of the subject’s EHR. Manual review of the EHR, performed by physicians at each site, was done to exclude subjects in whom hypertension had been miscoded or “ruled out” rather than confirmed.
Analytic Methods
Derivation and Internal Validation of Predictive Risk Model
The predictive risk model was developed using the study cohort at KPCO. We used Cox proportional hazards regression to predict hypertension among children with an incident elevated BP. Time for each individual was calculated as the number of days after an incident elevated BP until a subject either: 1) met the study definition of hypertension; or 2) was censored event-free. Censoring events were: the end of the study observation period, disenrollment in the health plan, turning 18 years old ≥ 12 months after the incident elevated BP, or death ≥ 12 months after the incident elevated BP.
The covariates included in the regression model were: systolic and diastolic BP percentiles of the incident elevated BP, prior pre-hypertensive BP (systolic or diastolic BP ≥ 90th percentile but < 95th percentile), body mass index (BMI) percentile, asthma, diabetes, age, sex, race, census tract median income, and census tract education. All covariates had < 10% missing values, and missing covariate values were imputed using simple imputation: the cohort mean value was used for continuous variables, and the modal value was used for categorical variables. Continuous variables were tested as linear variables, clinically meaningful categorical variables, and restricted cubic splines, and we used the data form that resulted in the best fit within regression models. The use of restricted cubic splines allows for a non-monotonic relationship between a given covariate and hypertension risk. All covariates associated with the outcome of hypertension at p < 0.25 were considered for the fully adjusted multivariate model. Risk scores for each individual were then determined based upon the final multivariate regression model.
After a final predictive risk model was derived, we evaluated the model’s internal validity25 at KPCO. The bootstrap corrected c-statistic was used as a measure of model discrimination, defined as the degree to which those with hypertension were identified as higher risk by the model.25 We also assessed several measures of model calibration, defined as the degree to which the expected outcome from the model (hypertension or no hypertension) matched the observed outcome for every subject.25 Calibration was measured through a calibration slope and the Hosmer-Lemeshow test.26 Calibration was also examined using plots of expected and observed cumulative incidence of hypertension over time by predicted risk quintiles.
External Validation of Predictive Risk Model
After the predictive risk model was derived and evaluated at KPCO, the external validity of the model was tested at HealthPartners and KPNC. External validation was performed by calculating the predicted risk of hypertension for subjects at HealthPartners and KPNC, using the model parameters derived at KPCO. Performing external validation in this manner assumes that the baseline risk (i.e. hazard) of hypertension is the same across the derivation and validation cohorts.27, 28 Model discrimination and calibration were assessed at the external validation sites.27, 28 However, because the baseline risk differed across the study cohorts, we also recalibrated the predictive risk model using the baseline hazard at each site.27, 28 Data management was performed using SAS 9.2 (Cary, NC), and statistical analyses were performed using R (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Derivation and Internal Validation of Predictive Risk Model
Within a cohort of 153110 children at KPCO, we identified 6710 subjects (4.4%) with an incident elevated BP in the hypertensive range. Of these, 1112 of 6710 subjects (16.6%) were excluded because they did not have at least 2 additional BPs measured on separate occasions after their incident elevated BP. Compared to the 1112 subjects excluded from the final analytic cohort, the 5598 included had a longer time of health plan enrollment following the incident elevated BP (mean 42 months enrollment for included vs. 29 months for excluded, p<0.001) and were younger (mean 10.3 years old for included vs. 10.9 years for excluded, p<0.001). The baseline sociodemographic and clinical characteristics of subjects included in the analytic cohort at KPCO are presented in Table 1 (second column).
Table 1.
Baseline sociodemographic and clinical characteristics of subjects with an incident elevated BPa at derivation and validation study sites.
| Characteristic | Model derivation site,b n=5598 | Model validation site A,b n=1377 | Model validation site B,b n=5135 |
|---|---|---|---|
| Age in years, mean (SD) | 10.3 (3.92) | 10.3 (4.32) | 9.5 (4.63) |
| Sex, n (%) | |||
| Male | 3053 (54.5) | 719 (52.2) | 2572 (50.1) |
| Female | 2545 (45.5) | 658 (47.8) | 2563 (49.9) |
| Race/ethnicity, n (%) | |||
| Asian/Pacific Islander | 186 (3.3) | 85 (6.2) | 973 (18.9) |
| Black | 247 ( 4.4) | 257 (18.7) | 385 (7.5) |
| Hispanic | 1164 (20.8) | 56 (4.1) | 1599 (31.1) |
| Other racial/ethnic group | 198 (3.5) | 107 (7.8) | 75 (1.5) |
| White | 3233 (57.8) | 855 (62.1) | 1821 (35.5) |
| Missing | 570 (10.2) | 17 (1.2) | 282 (5.5) |
| Household income of census tract in dollars, median (IQR) | 61741 (47323, 76192) | 55341 (41106, 68036) | 61389 (46250, 76760) |
| Body mass index percentile, n (%) | |||
| < 85th | 3244 (57.9) | 742 (53.9) | 2559 (49.8) |
| 85th to < 95th | 956 (17.1) | 246 (17.9) | 972 (18.9) |
| ≥ 95th | 1378 (24.6) | 375 (27.2) | 1564 (30.5) |
| Missing | 20 (0.4) | 14 (1.0) | 40 (0.8) |
| Category of incident BP elevation,c n (%) | |||
| Stage 1 systolic elevation | 3024 (54.0) | 980 (71.2) | 3623 (70.6) |
| Stage 2 systolic elevation | 262 (4.7) | 69 (5.0) | 348 (6.8) |
| Diastolic elevated, systolic not elevated | 2312 (41.3) | 328 (23.8) | 1164 (22.7) |
| BP immediately preceding incident elevated BP, n (%) | |||
| Normotensive | 3916 (70.0) | 727 (52.8) | 2078 (40.5) |
| Pre-hypertensive | 593 (10.6) | 179 (13.0) | 734 (14.3) |
| Missing | 1089 (19.5) | 471 (34.2) | 2323 (45.2) |
| Clinical setting of incident elevated blood pressure, n (%) | |||
| Primary care, well-child care | 1235 (22.1) | 624 (45.3) | 2500 (48.7)d |
| Primary care, non-well-child care | 2896 (51.7) | 697 (50.6) | 2115 (41.2)d |
| Medical and surgical specialty | 1467 (26.2) | 56 (4.1) | 341 (6.6)d |
SD indicates standard deviation; IQR, inter-quartile range; BP, blood pressure
Incident elevated BP defined as systolic and/or diastolic BP ≥ 95th percentile for age, height, and sex.
In pair-wise comparisons between study sites (site A vs. derivation site; site B vs. derivation site), all characteristics listed in this table were significantly different between sites (p < 0.01), except for the comparisons of age and sex between site A and the derivation site, which were not significantly different.
Represents 3 mutually exclusive categories: stage 1 systolic elevation (systolic 95th percentile to the 99th percentile plus 5 mm Hg); stage 2 systolic elevation (systolic > 99th percentile plus 5 mm Hg); or diastolic-only elevation (a diastolic ≥ 95th percentile with a systolic < 95th percentile)
Clinical setting of incident elevated BP was missing for n=179 (3.5%) of subjects from model validation site B.
Among 5598 subjects with an incident elevated BP, 160 (2.9%) developed hypertension during the study observation period. As presented in Table 2, eight characteristics were used to predict hypertension risk: age; sex; race; BP preceding incident elevated BP; BMI percentile; systolic BP percentile; diastolic BP percentile; and clinical setting of the incident elevated BP. The model fit was best when BMI, systolic BP, and diastolic BP percentiles were treated as restricted cubic splines. Asthma, diabetes, census tract median income, and census tract education were not associated with hypertension and were excluded from the final model.
Table 2.
Among subjects with an incident elevated BPa at the model derivation site, multivariate analyses of factors associated with meeting the study definition of hypertension.
| Characteristic | Adjustedb,c hazard ratios (95% CI) | P value |
|---|---|---|
| Age in years | 1.10 (1.05, 1.16) | <0.001 |
| Sex | ||
| Male | 1.40 (1.01, 1.95) | 0.044 |
| Female | 1.00 (Ref) | |
| Race | ||
| Non-white | 1.00 (0.72, 1.39) | 0.99 |
| White | 1.00 (Ref) | |
| Normotensive BP immediately preceding incident elevated BP | ||
| Yes | 1.00 (Ref) | 0.045 |
| No | 1.39 (1.01, 1.93) | |
| BMI percentiled | NA | NA |
| Systolic BP percentiled | NA | NA |
| Diastolic BP percentiled | NA | NA |
| Clinical setting of incident elevated BP | ||
| Primary care, well-child care | 1.00 (Ref) | |
| Primary care, non-well-child care | 1.01 (0.66, 1.55) | 0.949 |
| Medical and surgical specialty | 1.32 (0.84, 2.10) | 0.231 |
CI indicates confidence interval; BP, blood pressure; BMI, body mass index; NA, not applicable; Ref, referent.
Incident elevated BP defined as systolic and/or diastolic BP ≥ 95th percentile for age, height, and sex.
Multivariate Cox proportional hazards regression analyses, adjusted for age, sex, race, BP preceding incident elevated BP, BMI percentile, systolic BP percentile, diastolic BP percentile, and clinical setting of incident BP measurement.
Asthma, diabetes, census tract median income, and census tract education were not associated with hypertension and were excluded from the final model.
BMI percentile, systolic BP percentile, and diastolic BP percentile were modeled using splines; this method does not produce a summary hazard ratio and confidence interval for each unit increase.
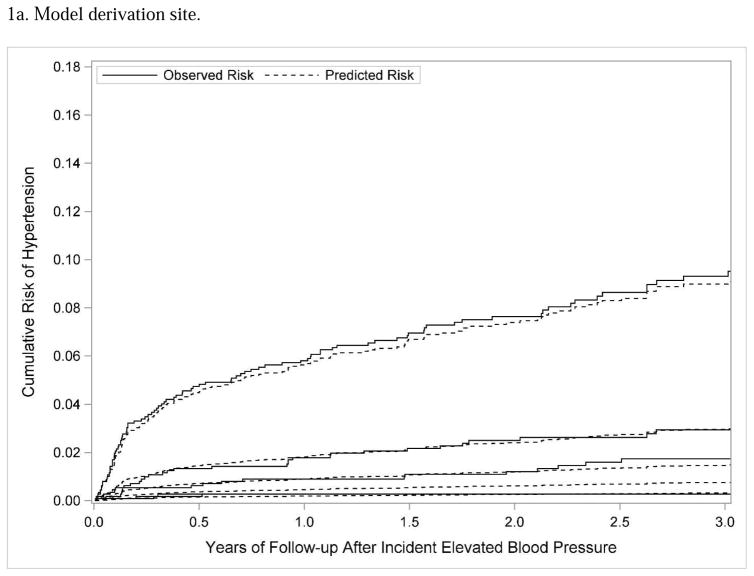
Measures of discrimination, calibration, and classification were used to assess the internal validity25 of the predictive risk model at KPCO. The uncorrected c-statistic for the model was 0.81, and the bootstrap corrected c-statistic was 0.77, indicating that the model effectively discriminated between high-risk and low-risk subjects.27 In Figure 1a, subjects were divided into 5 quintiles according to predicted hypertension risk, and the predicted and observed hypertension risk was plotted over time. This figure visually demonstrates effective discrimination (the curves for higher risk quintiles showed separation over time from lower risk quintiles) and effective calibration (the predicted risk of hypertension closely tracked the observed risk over time for each quintile of risk).27 The Hosmer-Lemeshow goodness-of-fit statistic (p=0.24) was also consistent with effective calibration. Finally, a threshold or cut-off value of risk was used to assess classification measures such as sensitivity and specificity. As shown in Table 3, the observed risk of hypertension at the derivation site (KPCO) was well below 1% for the lowest two quintiles of risk. If the risk scores were dichotomized as lower risk (quintiles 1 and 2) versus higher risk (quintiles 3, 4, and 5), the model would have a sensitivity of 95.0%, a specificity of 41.0%, and a positive predictive value of 4.5% with respect to predicting hypertension.
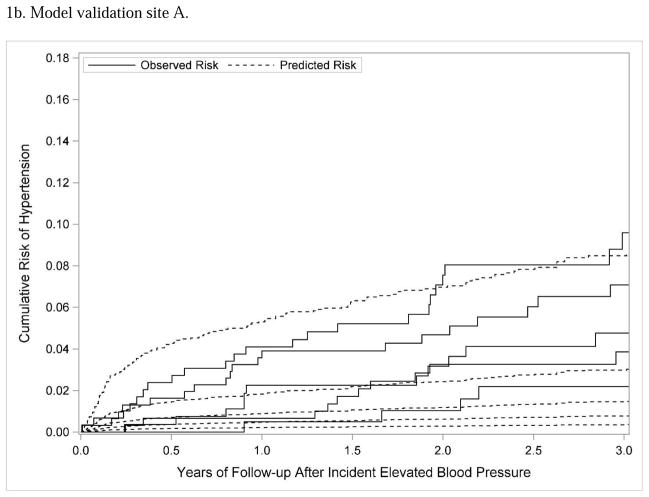
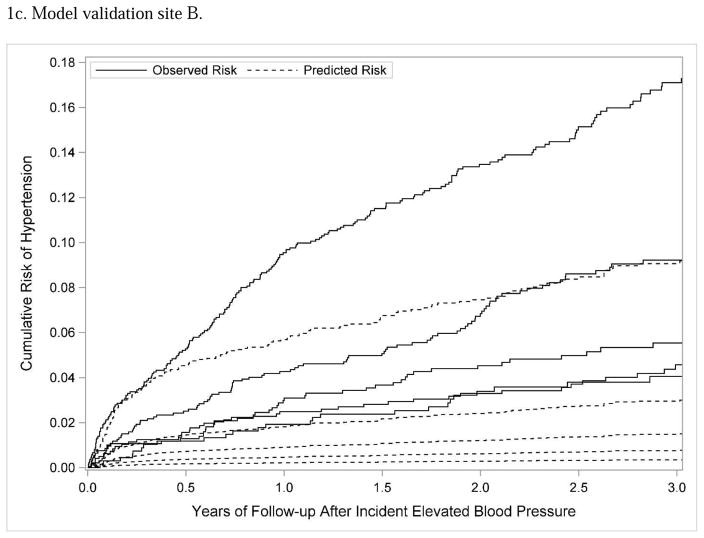
Figure 1.
Subjects were divided into 5 quintiles according to predicted hypertension risk; the figure shows Lumley plots of the predicted risk of hypertension (dotted lines) compared to the observed risk of hypertension (solid lines) across the 5 different quintiles of risk at each site.
Table 3.
The observed cumulative risk of hypertension at 3 years, categorized by quintiles of the prediction model risk scoresa developed at the derivation site and applied to two external validation sites.
| n | 3-year cumulative risk of hypertension | |
|---|---|---|
| Risk score categories, model derivation site (total n=5598) | ||
| 1 (lowest) | 1119 | 0.3% |
| 2 | 1120 | 0.3% |
| 3 | 1120 | 1.7% |
| 4 | 1120 | 2.9% |
| 5 (highest) | 1119 | 9.3% |
| Risk score categories, model validation site A (total n=1377) | ||
| 1 (lowest) | 202 | 2.2% |
| 2 | 268 | 4.8% |
| 3 | 305 | 3.9% |
| 4 | 308 | 7.1% |
| 5 (highest) | 294 | 9.6% |
| Risk score categories, model validation site B (total n=5135) | ||
| 1 (lowest) | 675 | 4.1% |
| 2 | 962 | 4.6% |
| 3 | 937 | 5.5% |
| 4 | 1197 | 9.2% |
| 5 (highest) | 1364 | 17.1% |
The predictive model risk scores were categorized by quintiles at the model derivation site; the same score thresholds for each quintile were then applied to the two eternal validation sites; because risk scores were higher at the external validation sites, more individuals appeared in the higher risk quintiles at external validation sites.
External Validation of Predictive Risk Model
At validation site A, in a cohort of 90023 children, 2351 (2.6%) had an incident elevated BP and met study eligibility criteria; 974 of 2351 subjects (41.4%) were excluded because they did not have at least 2 additional BPs, and the remaining 1377 subjects were included in model validation. At validation site B, among 200000 children, 10515 (5.3%) had an incident elevated BP and were study-eligible; 5380 of 10515 subjects (51.2%) were excluded because they did not have at least 2 additional BPs during the study observation period, and the remaining 5135 subjects were included in model validation. At both sites, those included in the final cohort had a longer time of health plan enrollment than those excluded (site A, 43 months enrollment among included vs. 30 months among excluded, p<0.001; site B, 36 months vs. 29 months, p<0.001). The baseline sociodemographic and clinical characteristics of the external validation cohorts are presented in Table 1 (third and fourth columns).
The predictive risk model derived at KPCO was used to predict hypertension risk separately at the two external validation sites. The bootstrap corrected c-statistics for validation sites A and B were 0.64 and 0.67, respectively, indicating relatively poor discrimination between those at higher versus lower risk of hypertension. At each external validation site, the model was updated; updating in this context means that the parameter estimates were recalculated for each covariate. The bootstrap corrected c-statistic for the updated model for sites A and B was 0.61 and 0.69, respectively, showing little improvement with model discrimination when the model was updated. Model calibration was also relatively poor; as shown in Figures 1b and 1c, the observed risk of hypertension was substantially higher than the predicted risk across different quintiles of risk. Dichotomizing the subjects as lower risk (quintiles 1 and 2) versus higher risk (quintiles 3, 4, and 5), the model had lower sensitivity and specificity at the external validation sites (site A: sensitivity 78.9%, specificity 34.9%; site B: sensitivity 84.5%, specificity 33.4%) than at the model derivation site.
Comparison of Derivation and External Validation Cohorts
We performed post-hoc analyses to better understand potential reasons for the poor performance of the predictive risk model at external validation sites. The case mix between the derivation and external validation cohorts differed, and case mix differences are known to affect predictive risk models.29 For example, the proportion of subjects with obesity, stage 1 systolic elevation, stage 2 systolic elevation, and a prior pre-hypertensive measurement was significantly higher in the external validation cohorts compared to the derivation cohort (p<0.001 comparing each characteristic between derivation and validation sites). Case mix differences could be due to true differences in disease incidence across study sites, or due to differences in care practices resulting in different study populations. For example, the proportion of subjects excluded because they lacked at least 2 additional qualifying BPs during the study observation period was significantly lower at the derivation site (16.6%) compared to sites A (41.4%) and B (51.2%, [overall chi-squared p <0.001]).
DISCUSSION
Within a cohort of children with incident elevated BP at a large integrated health care system, we developed a risk model predicting the likelihood of subsequent hypertension. We then examined the external validity of the risk model in two other health care systems. The performance of the model at the derivation site was good, as the model appropriately discriminated high-risk from low-risk individuals, and observed outcomes (hypertension or no hypertension) followed predicted outcomes in the population over time. However, the model did not perform as well at the external validation sites, with poor discrimination and calibration, even when accounting for differences in baseline risk of hypertension between the derivation and external validation sites.
While predictive risk models have been developed in diverse settings for a variety of health conditions,19, 28 we are unaware of other published models predicting the likelihood of hypertension among children with an incident elevated BP. In settings with EHRs, a predictive risk model for hypertension could be integrated into clinical decision support tools to improve hypertension recognition.20–22 However, the relatively poor external validity of our predictive risk model indicates that it is not currently suitable for widespread dissemination, because it may not perform well in different health care environments.23, 30 Many examples exist of predictive risk models with good internal validity, which were never validated in patients and settings external to the development environment;28 these risk models may have been implemented in new settings with disappointing results. In the current study, we had the unique opportunity to perform external validation immediately after developing the risk model, and doing so highlighted the limitations of the risk model that had been developed.
Despite their similarities as large integrated health care delivery organizations, the three study sites differed in their BP screening practices, such as regarding whether BP was routinely measured at specialty visits or non-well-child visits in primary care, and these differences may have contributed to poor external validation of the risk model. The use of different BP measurement devices across sites also could have negatively affected external validity, as aneroid and oscillometric devices are known to yield different BP measurements within the same subject.31 Experience measuring BPs in children could vary depending on the clinical setting (e.g. primary care versus specialty care), which could have affected BP accuracy. These differences are systematic, in that they result from the care systems in place that determine who has their BP measured, by whom, using which BP devices, and could lead to enough differences in hypertension detection across sites to explain the poor external validation of the risk model.
In addition, relevant patient characteristics (also referred to as case mix29) differed across study sites. Subjects at validation sites were more likely to be obese, and more likely to have stage 2 systolic BP elevation at their incident elevated BP measurement, than subjects at the derivation site. Presence of obesity and stage 2 systolic BP elevation would likely confer higher risk of subsequent hypertension,32, 33 and the risk of hypertension was demonstrably higher at validation sites compared to the derivation site. Although these baseline covariates (systolic BP percentile, prior BP, and BMI percentile) were included in our regression model predicting hypertension risk, case mix differences across sites could nonetheless have contributed to poor external validation,29 because the relative weights of various covariates were based upon a population at lower risk of eventually developing hypertension.30 What explains the difference in hypertension risk across sites? Study inclusion and exclusion criteria could have contributed to this difference: subjects at validations sites with at least 2 BPs following their incident elevated BP may have differed from those with less than 2 follow up BPs in important but unmeasured ways.
This investigation is subject to a number of limitations. The BPs were measured during routine care; although staff at study sites were trained in BP measurement using standard protocols, it was not possible to independently verify the accuracy of BP measurements taken at hundreds of clinics across thousands of patient visits. BPs were examined from all primary care, medical specialty, and surgical specialty outpatient visits, and the accuracy of BP measurements could have varied by visit type and child age. For example, circumstances during non-well-child care encounters, such as a child experiencing an acute illness, fever, or pain, could have affected the accuracy of measured BPs.
In addition, patient problem lists were not examined, because it was not possible to determine whether the diagnoses listed were active versus resolved; this approach could have affected our ability to exclude subjects with known (i.e., secondary) causes of hypertension. Although subjects had a mean length of follow up of 3.5 years after their incident elevated BP, some subjects may have developed hypertension after the end of the study observation period. Additional risk factors for hypertension, such as a family history of hypertension,34 were not routinely captured within the EHR, and therefore could not be used in the predictive risk model. Because of missing race/ethnicity data, and differences in how race/ethnicity data was recorded across sites, we were forced to use a suboptimal measure of race/ethnicity, which could have affected the internal and external validity of the final model. Finally, this study was conducted in 3 large health care organizations; results may not generalize to other clinical settings, which may have different protocols and equipment for measuring BPs in children.
In conclusion, among children with incident elevated BP, a predictive risk model developed at one site performed well at predicting subsequent hypertension. However, the risk model did not perform as well at two external sites, which may limit its current transportability to other settings.
What’s New.
Among children with incident elevated blood pressure, a predictive risk model developed at one site performed well at predicting subsequent hypertension. However, the risk model did not perform well at two external validation sites, which may limit its current transportability.
Acknowledgments
This study was supported by the National Heart, Lung, and Blood Institute at the National Institutes of Health (1R01HL093345-01A1; PI: Patrick J. O’Connor). The study sponsor was not involved in study design, collection of data, analyses, or manuscript preparation. The findings and conclusions reported herein are those of the authors, and do not necessarily reflect the opinions of the study sponsor. Portions of this work were presented at the Pediatric Academic Societies’ Annual Meeting, May 5, 2014, Vancouver, British Columbia, Canada. We would like to acknowledge Nicole K. Trower, Heather M. Tavel, and Malini Chandra, who helped develop the datasets used for this study, as well as Nancy E. Sherwood, who provided scientific input regarding preliminary findings from this work.
Abbreviations
- BP
blood pressure
- BMI
body mass index
- ICD-9-CM
International Classification of Diseases, 9th Revision, Clinical Modification
- EHR
electronic health record
- mm Hg
millimeters of mercury
- USPSTF
U.S. Preventive Services Task Force
Footnotes
Disclosures and conflicts of interest: Joan Lo has received research funding from Sanofi unrelated to this work. All authors declare that they have no conflicts of interest relevant to this article to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sinaiko AR, Gomez-Marin O, Prineas RJ. Prevalence of “significant” hypertension in junior high school-aged children: the Children and Adolescent Blood Pressure Program. J Pediatr. 1989;114:664–669. doi: 10.1016/s0022-3476(89)80718-8. [DOI] [PubMed] [Google Scholar]
- 2.Sorof JM, Lai D, Turner J, Poffenbarger T, Portman RJ. Overweight, ethnicity, and the prevalence of hypertension in school-aged children. Pediatrics. 2004;113:475–482. doi: 10.1542/peds.113.3.475. [DOI] [PubMed] [Google Scholar]
- 3.McNiece KL, Poffenbarger TS, Turner JL, Franco KD, Sorof JM, Portman RJ. Prevalence of hypertension and pre-hypertension among adolescents. J Pediatr. 2007;150:640–644. 644. doi: 10.1016/j.jpeds.2007.01.052. [DOI] [PubMed] [Google Scholar]
- 4.Chen X, Wang Y. Tracking of blood pressure from childhood to adulthood: a systematic review and meta-regression analysis. Circulation. 2008;117:3171–3180. doi: 10.1161/CIRCULATIONAHA.107.730366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lauer RM, Clarke WR. Childhood risk factors for high adult blood pressure: the Muscatine Study. Pediatrics. 1989;84:633–641. [PubMed] [Google Scholar]
- 6.Kelly RK, Thomson R, Smith KJ, Dwyer T, Venn A, Magnussen CG. Factors affecting tracking of blood pressure from childhood to adulthood: the Childhood Determinants of Adult Health Study. J Pediatr. 2015 doi: 10.1016/j.jpeds.2015.07.055. [DOI] [PubMed] [Google Scholar]
- 7.National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114:555–576. [PubMed] [Google Scholar]
- 8.Thompson M, Dana T, Bougatsos C, Blazina I, Norris SL. Screening for hypertension in children and adolescents to prevent cardiovascular disease. Pediatrics. 2013;131:490–525. doi: 10.1542/peds.2012-3523. [DOI] [PubMed] [Google Scholar]
- 9.Brady TM, Redwine KM, Flynn JT American Society of Pediatric N. Screening blood pressure measurement in children: are we saving lives? Pediatr Nephrol. 2014;29:947–950. doi: 10.1007/s00467-013-2715-1. [DOI] [PubMed] [Google Scholar]
- 10.Samuels JA, Bell C, Flynn JT. Screening children for high blood pressure: where the US Preventive Services Task Force went wrong. J Clin Hypertens(Greenwich) 2013;15:526–527. doi: 10.1111/jch.12147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.American Academy of Pediatrics. Bright Futures Recommendations for Preventive Pediatric Health Care. American Academy of Pediatrics; 2015. [Google Scholar]
- 12.Hansen ML, Gunn PW, Kaelber DC. Underdiagnosis of hypertension in children and adolescents. JAMA. 2007;298:874–879. doi: 10.1001/jama.298.8.874. [DOI] [PubMed] [Google Scholar]
- 13.Brady TM, Solomon BS, Neu AM, Siberry GK, Parekh RS. Patient-, provider-, and clinic-level predictors of unrecognized elevated blood pressure in children. Pediatrics. 2010;125:e1286–1293. doi: 10.1542/peds.2009-0555. [DOI] [PubMed] [Google Scholar]
- 14.Bijlsma MW, Blufpand HN, Kaspers GJ, Bokenkamp A. Why pediatricians fail to diagnose hypertension: a multicenter survey. J Pediatr. 2014;164:173–177. e177. doi: 10.1016/j.jpeds.2013.08.066. [DOI] [PubMed] [Google Scholar]
- 15.Kavey RW, Simons-Morton DG, de Jesus JM National Heart Lung and Blood Institute. Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: summary report. Pediatrics. 2011;128(Suppl 5):S213–S256. doi: 10.1542/peds.2009-2107C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Daley MF, Sinaiko AR, Reifler LM, et al. Patterns of care and persistence after incident elevated blood pressure. Pediatrics. 2013;132:e349–355. doi: 10.1542/peds.2012-2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chiolero A, Bovet P, Paradis G. Screening for elevated blood pressure in children and adolescents: a critical appraisal. JAMA Pediatr. 2013;167:266–273. doi: 10.1001/jamapediatrics.2013.438. [DOI] [PubMed] [Google Scholar]
- 18.Bloetzer C, Bovet P, Chiolero A. Performance of targeted screening for the identification of hypertension in children. J Hypertens. 2015;33(Suppl 1):e34. doi: 10.1097/HJH.0000000000000560. [DOI] [PubMed] [Google Scholar]
- 19.Moons KG, Altman DG, Vergouwe Y, Royston P. Prognosis and prognostic research: application and impact of prognostic models in clinical practice. BMJ. 2009;338:b606. doi: 10.1136/bmj.b606. [DOI] [PubMed] [Google Scholar]
- 20.Kharbanda EO, Nordin JD, Sinaiko AR, et al. TeenBP: development and piloting of an EHR-linked clinical decision support system to improve recognition of hypertension in adolescents. EGEMS (Wash DC) 2015;3:1142. doi: 10.13063/2327-9214.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suresh S. Big data and predictive analytics: applications in the care of children. Pediatr Clin North Am. 2016;63:357–366. doi: 10.1016/j.pcl.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 22.Brady TM, Neu AM, Miller ER, 3rd, Appel LJ, Siberry GK, Solomon BS. Real-time electronic medical record alerts increase high blood pressure recognition in children. Clin Pediatr (Phila) 2015;54:667–675. doi: 10.1177/0009922814559379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bleeker SE, Moll HA, Steyerberg EW, et al. External validation is necessary in prediction research: a clinical example. J Clin Epidemiol. 2003;56:826–832. doi: 10.1016/s0895-4356(03)00207-5. [DOI] [PubMed] [Google Scholar]
- 24.Lo JC, Sinaiko A, Chandra M, et al. Prehypertension and hypertension in community-based pediatric practice. Pediatrics. 2013;131:e415–424. doi: 10.1542/peds.2012-1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moons KG, Kengne AP, Woodward M, et al. Risk prediction models: I. Development, internal validation, and assessing the incremental value of a new (bio)marker. Heart. 2012;98:683–690. doi: 10.1136/heartjnl-2011-301246. [DOI] [PubMed] [Google Scholar]
- 26.Hosmer DW, Lemeshow S, Sturdivant RX. Applied Logistic Regression. 3. Hoboken, NJ: John Wiley & Sons, Inc; 2013. Assessing the Fit of the Model; pp. 153–226. [Google Scholar]
- 27.Royston P, Altman DG. External validation of a Cox prognostic model: principles and methods. BMC Med Res Methodol. 2013;13:33. doi: 10.1186/1471-2288-13-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Collins GS, de Groot JA, Dutton S, et al. External validation of multivariable prediction models: a systematic review of methodological conduct and reporting. BMC Med Res Methodol. 2014;14:40. doi: 10.1186/1471-2288-14-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Steyerberg E. Clinical Prediction Models: A Practical Approach to Development, Validation, and Updating. Springer; New York: 2008. Patterns of External Validity; pp. 333–360. [Google Scholar]
- 30.Toll DB, Janssen KJ, Vergouwe Y, Moons KG. Validation, updating and impact of clinical prediction rules: a review. J Clin Epidemiol. 2008;61:1085–1094. doi: 10.1016/j.jclinepi.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 31.Eliasdottir SB, Steinthorsdottir SD, Indridason OS, Palsson R, Edvardsson VO. Comparison of aneroid and oscillometric blood pressure measurements in children. J Clin Hypertens (Greenwich) 2013;15:776–783. doi: 10.1111/jch.12196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koebnick C, Black MH, Wu J, et al. High blood pressure in overweight and obese youth: implications for screening. J Clin Hypertens (Greenwich) 2013;15:793–805. doi: 10.1111/jch.12199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bucher BS, Ferrarini A, Weber N, Bullo M, Bianchetti MG, Simonetti GD. Primary hypertension in childhood. Curr Hypertens Rep. 2013;15:444–452. doi: 10.1007/s11906-013-0378-8. [DOI] [PubMed] [Google Scholar]
- 34.Bloetzer C, Paccaud F, Burnier M, Bovet P, Chiolero A. Performance of parental history for the targeted screening of hypertension in children. J Hypertens. 2015;33:1167–1173. doi: 10.1097/HJH.0000000000000560. [DOI] [PubMed] [Google Scholar]