Abstract
Background
Merkel cell carcinoma (MCC) is an aggressive skin cancer with a recurrence rate of >40%. Of the 2000 MCC cases/year in the USA, most are caused by the Merkel cell polyomavirus (MCPyV). Antibodies to MCPyV-oncoprotein (T-antigens) have been correlated with MCC tumor burden. We prospectively validated the clinical utility of MCPyV oncoprotein antibody titers for MCC prognostication and surveillance.
Methods
MCPyV-oncoprotein antibody detection was optimized in a clinical laboratory. A cohort of 219 patients with newly-diagnosed MCC were followed prospectively (median follow-up 1.9 years). Among seropositive patients, antibody titer and disease status were serially tracked.
Results
Antibodies to MCPyV-oncoproteins were rare among healthy individuals (1%) but present in most MCC patients (114 of 219, 52%, p<0.01). Seropositivity at diagnosis independently predicted decreased recurrence risk (HR=0.58; p=0.04) in multivariate analyses adjusted for age, sex, stage, and immunosuppression. Following initial treatment, seropositive patients whose disease did not recur had rapidly falling titers that became negative by a median of 8.4 months. Among seropositive patients who underwent serial evaluation (71 patients; 282 timepoints), an increasing oncoprotein titer had a positive predictive value of 66% for clinically evident recurrence while a decreasing titer had a negative predictive value of 97%.
Conclusions
Determination of oncoprotein antibody titer assists in the clinical management of newly diagnosed MCC patients by stratifying them into a higher risk seronegative cohort in whom radiologic imaging may play a more prominent role, and into a lower-risk seropositive cohort whose disease status can be tracked in part via oncoprotein antibody titer.
Introduction
Merkel cell carcinoma (MCC) is a neuroendocrine skin cancer with an incidence of 0.6 per 100,000,1 corresponding to approximately 2,000 new cases annually in the United States based on 2015 census data.2 Age, sun exposure, and male sex are risk factors for MCC,3 and immunosuppression portends poorer outcome.4, 5 MCC has a recurrence rate of >40%.6 This high recurrence rate indicates a need for data-driven surveillance approaches.
In 2008, a causative polyomavirus (Merkel cell polyomavirus/MCPyV) was identified in 80% of MCCs7 (Fig 1A). MCPyV is common worldwide, with 60% of adults demonstrating serologic evidence of prior infection.8-11 Infection often occurs in childhood and is typically self-limited.11-13 However, among patients who develop MCC, MCPyV integrates into the human genome and undergoes tumor-specific truncating mutations and thus can no longer replicate (Fig 1B).7, 14 Instead, viral oncoproteins (T-antigens) are persistently expressed in MCC tumors and help to promote cell cycle progression and tumorigenesis through multiple mechanisms,15 including inhibition of the tumor-suppressor pRb,16 stabilization of the oncoprotein c-Myc,17 and evasion of innate immunity.18, 19 These oncoproteins are detectable by immunohistochemistry in 70-100% of MCCs.16, 17
Figure 1. Rationale for a viral serologic assay for Merkel cell carcinoma (MCC) recurrence.
Panel A: Clinical and microscopic characteristics of a Merkel cell polyomavirus (MCPyV) positive MCC arising on sun-exposed skin. Tumor sections containing stroma (pink on H/E staining) demonstrate MCC-specific expression of cytokeratin-20 (CK20) in a perinuclear dot-like pattern and express viral large T-Ag oncoprotein (CM2B4 antibody31) (scale bar = 50 μm). Panel B: Schematic of the Merkel cell polyomavirus (MCPyV) genome7 and oncoproteins that are persistently expressed in human MCCs. The small and large T-Ag oncoproteins share an amino-terminal domain (common T-Ag) that is recognized by antibodies produced by the majority of patients with MCPyV-positive tumors22. The ‘X’ symbols indicate the region in which truncating mutations clonally occur in individual tumors. Panel C: Schematic of MCC development and relative MCPyV-oncoprotein antibody titers. Panel D: Distribution of antibody titers among control subjects and MCC patients. 1% of healthy blood donors (n=100) are seropositive as compared with 52% of MCC patients (n=219) at the time of diagnosis.
90% of persons with MCC produce antibodies to the MCPyV capsid proteins.8 High titers of anti-capsid antibodies at presentation have been reported to be a favorable prognostic factor. 20, 21 However, these antibodies (which mark previous exposure) are also detectable in >60% of healthy adults.8, 10 Furthermore, titers of antibodies to the MCPyV capsid protein do not vary with MCC tumor burden21, 22 and thus could not serve as a biomarker for recurrence. Given limitations of anti-capsid antibodies, we instead focused on antibodies against MCPyV-oncoprotein. These antibodies are rarely detectable in healthy individuals, but are prevalent among MCC patients.21, 22 In a discovery case series of 20 patients, we observed that titers increased with rising MCC burden and fell after tumor excision.22 Similarly, others have shown that patients with blood draws at the time of recurrence are more likely to have detectable antibodies than those with draws at the time of remission, although longitudinal patient-specific data was not presented.21
In this study, using a large, prospective validation cohort of 219 newly diagnosed patients followed over a 5-year period, we tested the clinical utility of MCPyV-oncoprotein antibodies in MCC management. To maximize clinical applicability, the assay was first established in a hospital-based laboratory. We tested two clinical roles for oncoprotein antibody quantitation: initial MCC prognostication and as a marker for disease recurrence following definitive therapy (Fig 1C). Our results suggest that MCPyV-oncoprotein antibody titer is a biomarker that can assist in optimizing MCC management.
Methods
MCC patients
Patients with pathologist-verified MCC were prospectively enrolled in an IRB-approved natural history cohort study and provided written informed consent. 465 patients provided consent and had blood drawn, of whom 219 were newly diagnosed (<= 90 days) and included in further analyses. Blood was collected in red-top tubes and shipped overnight at ambient temperature to our Specimen-Processing Facility between 6/2008-10/2013. Sera were stored at -80°C. Grossly hemolyzed samples were excluded. Clinical follow-up was obtained through 2/18/2014.
Population controls
Sera from Seattle-based blood-donors (Supplemental Table 1) were tested.
MCPyV oncoprotein antibody detection
Serology assays were performed at the University of Washington Clinical Immunology Laboratory (www.merkelcell.org/sero). Glutathione-S-transferase(GST)-tagged MCPyV small T-antigen protein was produced recombinantly in Rosetta E. coli, purified, and linked to a Luminex® bead.8, 22 GST was employed as negative control and run concurrently. Sera were applied at 1:100, 1:1000, and 1:10,000 dilutions. Blocking was performed with superblock (Millipore CBS-K at 0.025%), and antibodies were detected with a biotinylated anti-human IgG secondary antibody (1:1000 dilution, Kierkegaard-Perry) and streptavidin-phycoerythrin-detection. Every plate included 24 dilutions of a standard pool (derived from 14 strongly-positive patients). Titers for individual sera were calculated using weighted non-linear regression. The threshold for a negative titer was set as <75 because 99% of normal control subjects without MCC had oncoprotein antibody titers below 75. The positive threshold was set at ≥150 because assay results above this level were highly reproducible. Based on these performance characteristics, titers <75 were defined as seronegative, ≥150 as seropositive, and titers between 75 and 150 as “borderline”. Patients who had initial titers ≥75 were considered to be antibody-producers.
Classification of surveillance draw values
Serial blood draws were considered “rising” if the titer value was ≥150 and increased ≥20% from the prior draw. Draws were considered “falling” if the titer decreased by at least 20% from the prior draw or was <75. All other draws were considered “stable”. The 20% threshold for a change in titer was pre-determined based on run-to-run variability across samples of varying titers (Supplemental Fig 1A). Draws occurred at 3-6 month intervals, based on NCCN guidelines23 that suggest disease assessment be performed at this interval. Supplemental Table 2 details the timing of draws and numbers of patients ‘at-risk’ at various timepoints.
For patients who recurred, all draws up to and including the time of first recurrence were included in the serial draw analysis. Only the first recurrence of MCC was considered for each patient; draws that occurred >45 days after the first recurrence were excluded. A blood draw value was paired with recurrence status if the recurrence was clinically or radiologically detected within a 45 day window before or after the draw.
Statistical analysis
Comparisons between the proportion of MCC patients and healthy persons seropositive for MCPyV were performed using the Fisher's-Exact test. Demographic factors between seropositive and seronegative MCC patients were compared using logistic regression. Recurrence-free survival (RFS) was defined as the interval from the date of initial diagnostic biopsy to the date of first disease recurrence, last follow-up, or death. The risk of recurrence for clinical prognostic factors (age, sex, stage, and immune suppression) was estimated using a Cox proportional hazards model. A multivariate Cox model was used to compare recurrence-free survival between antibody positive and negative patients.
To evaluate whether changes in oncoprotein titer could be used to detect first recurrences of MCC, sensitivity, specificity, positive and negative predictive values were determined using the generalized estimating equations approach. A linear model with an autocorrelation structure (AR(1)) was employed to account for multiple observations within a person. Comparisons between fractions of patients with recurrence within 45 days of falling, rising and stable titers were performed with Fisher's exact test.
Analyses were performed using SAS version 9.4 or STATA version 11.0, and employed a statistical significance threshold of 5%.
Results
Prevalence of MCPyV-oncoprotein seropositivity
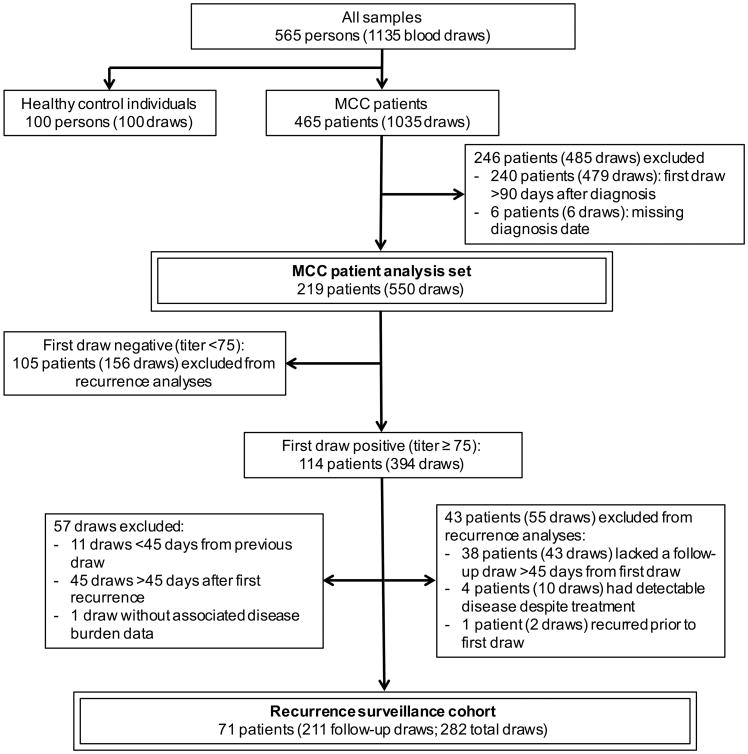
A total of 465 patients with Merkel cell carcinoma were enrolled in our prospective cohort natural history study and had at least one associated blood draw (n=465 patients, 1035 blood-draws). Of the 465 patients, 219 had a blood draw ≤90 days after diagnosis and were included in further analyses (Fig 2). Analysis was limited to newly-diagnosed patients in order to reduce late-entry enrollment bias and because titers fall quickly after therapy.
Figure 2. Patient inclusion schematic for demographic and outcomes analyses.
219 patients were evaluable for demographic and survival analyses and 71 patients were both seropositive and had serial draws and thus could be included in recurrence analyses. An additional 43 patients were seropositive at diagnosis but excluded from survival analyses due to lack of a follow-up draw (38 patients), inability to receive definitive therapy (4 patients), or recurrence prior to first draw (1 patient).
Among 219 newly diagnosed patients with MCC, 114 (52%) were MCPyV-oncoprotein seropositive at time of diagnosis. This was markedly increased as compared to the population prevalence of MCPyV oncoprotein seropositivity of 1%, as determined from screening 100 healthy blood bank donors (p<0.01, Fig 1D). Furthermore, titers were higher among patients with MCC: no control subject had an antibody titer ≥ 150, compared to 45% of MCC patients at diagnosis (p<0.01).
Characteristics of MCC patients who produced oncoprotein antibodies
Demographics of seropositive and seronegative MCC patients are compared in Table 1. Immune suppressed persons were less likely to produce detectable antibodies (p≤0.01). Serostatus was associated with the location of the primary lesion (p≤0.01). There were high rates of oncoprotein antibody seropositivity among patients with sun-protected MCCs (buttocks; 88% seropositive) and among patients with occult primary lesions (73%). Stage at diagnosis was significantly associated with seropositivity to MCPyV-oncoprotein. Specifically, patients diagnosed with stage II or III MCC were more likely to be seropositive than patients with stage I MCC.
Table 1. Patient characteristics and MCPyV oncoprotein antibody status among 219 MCC patients.
A total of 219 newly diagnosed patients with MCC were followed prospectively, of whom 114 (52%) were MCPyV-oncoprotein antibody positive at diagnosis. Patients with a sun-protected or occult primary, higher stage at diagnosis, or younger age at diagnosis were significantly more likely to be seropositive, whereas immunosuppressed persons were significantly less likely to be seropositive. One patient lacked staging information.
| MCPyV Oncoprotein Antibody Status at MCC Diagnosis | |||
|---|---|---|---|
|
|
|||
| Seronegative (n=105) | Seropositive (n=114) | ||
| Characteristics | No. (%) | No. (%) | P-value |
|
| |||
| Sex | 0.98 | ||
|
| |||
| Female | 44 (47.3) | 49 (52.7) | |
| Male | 61 (48.4) | 65 (51.6) | |
|
| |||
| Age at diagnosis | 0.01 | ||
|
| |||
| ≤65 | 35 (38.4) | 56 (61.5) | |
| >65 | 70 (54.7) | 58 (45.3) | |
|
| |||
| Immune suppressed | 0.004 | ||
|
| |||
| No | 86 (43.9) | 110 (56.1) | |
| Yes | 19 (82.6) | 4 (17.4) | |
|
| |||
| Primary site | 0.03 | ||
|
| |||
| Head & neck | 25 (50.7) | 34 (49.3) | |
| Buttock | 1 (12.5) | 7 (87.5) | |
| Upper Limb | 45 (62.5) | 27 (37.5) | |
| Lower Limb | 14 (46.7) | 16 (53.3) | |
| Trunk | 10 (76.9) | 3 (23.1) | |
| Occult Primary | 10 (27.0) | 27 (73.0) | |
|
| |||
| Stage at MCC diagnosis | 0.001 | ||
|
| |||
| I | 60 (65.2) | 32 (34.8) | |
| II | 7 (25.9) | 20 (74.1) | |
| III | 35 (38.5) | 56 (61.5) | |
| IV | 3 (37.5) | 5 (62.5) | |
MCPyV antibody seropositivity at diagnosis and recurrence-free survival
Of the 219 MCC patients included in the analysis of oncoprotein antibodies, 67 had an observed recurrence, with 52 (78% of recurring patients) recurring within 12 months of diagnosis. There were 51 deaths (35 attributable to MCC and 16 due to other causes). The median follow-up for patients still alive at last contact was 681 days (1.9 years). The analysis of recurrence-free survival, prognostic factors and MCPyV antibody serostatus is presented in Table 2. Higher stage and male sex were associated with increased risk of recurrence (p<0.01 and p=0.02 respectively). Age and immune suppression were not significantly associated with the risk of recurrence (p=0.19 and 0.21, respectively). Importantly, MCPyV antibody seropositive status was independently associated with a 42% decreased risk of recurrence (hazard ratio = 0.58, 95% confidence interval 0.36-0.97) in the multivariate model adjusting for known prognostic factors (Table 2).
Table 2. Relationship of MCC recurrence risk to selected clinical characteristics.
Hazard ratios are shown for MCC recurrence risk. On multivariate Cox regression, MCPyV oncoprotein antibody seropositivity at diagnosis was associated with significantly improved recurrence-free survival. Stage and sex were also significant. N=219 patients with newly diagnosed MCC.
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
|
|
||||||
| Hazard Ratio | 95% CI | P-Value (global) | Hazard Ratio | 95% CI | P-value (global) | |
| Stage | < 0.01 | <0.01 | ||||
|
| ||||||
| I | 1.00 | Reference | 1.00 | Reference | ||
| II | 2.49 | 1.18-5.25 | 3.13 | 1.42-6.90 | ||
| III | 2.48 | 1.43-4.30 | 3.00 | 1.68-5.33 | ||
| IV | 6.89 | 2.87-16.52 | 8.46 | 3.34-21.43 | ||
|
| ||||||
| Sex | <0.01 | 0.02 | ||||
|
| ||||||
| Female | 1.00 | Reference | 1.00 | Reference | ||
| Male | 2.11 | 1.29-3.47 | 1.79 | 1.08-2.95 | ||
|
| ||||||
| Age | 0.18 | 0.19 | ||||
|
| ||||||
| ≤65 years | 1.00 | Reference | 1.00 | Reference | ||
| >65 years | 1.38 | 0.86-2.20 | 1.40 | 0.85-2.32 | ||
|
| ||||||
| Immune suppression | 0.18 | 0.21 | ||||
|
| ||||||
| No | 1.00 | Reference | 1.00 | Reference | ||
| Yes | 1.54 | 0.81-2.93 | 1.56 | 0.78-3.12 | ||
|
| ||||||
| Oncoprotein antibody | 0.1 | 0.04 | ||||
|
| ||||||
| Seronegative | 1.00 | Reference | 1.00 | Reference | ||
| Seropositive | 0.68 | 0.44-1.08 | 0.58 | 0.36-0.97 | ||
MCPyV-oncoprotein serology quantitative assay performance
We hypothesized that MCPyV oncoprotein antibodies might be useful not only for initial prognostication, but as an ongoing biomarker in seropositive patients. In order for this to be possible, the assay needs to be both readily clinically available and reproducible. We established the assay in a CLIA certified clinical laboratory and measured its performance and quantitative reproducibility. After assay optimization at a hospital clinical immunology laboratory, the coefficient of variation for MCPyV-oncoprotein serology ranged from 17% to 27% (Supplemental Fig 1A) and the assay was highly linear (Supplemental Fig 1C). Potentially confounding factors were assessed. Storage at ambient temperature for up to 14 days had no effect on titer (Supplemental Fig 1B), suggesting delay in shipping of sera does not meaningfully affect results. We compared red-top to gold-top “serum separator” tubes and observed no effect of tube type (Supplemental Fig 1D). Finally, to determine whether various serum conditions affected titer, we mixed sera of defined titer with varying amounts of sera containing high levels of rheumatoid factor, polyclonal immunoglobulins or sera that was lipemic, icteric, or hemolyzed. No reproducible interference was observed. This suggests that the viral protein antibody titer is both reproducible and quantitative, allowing for serial measurement over time.
Changes in viral oncoprotein antibody titer as a biomarker of MCC recurrence
Based on prior observations that MCC patients who did not recur typically had a rapid and sustained fall in MCPyV-oncoprotein titer, we hypothesized that among MCPyV antibody-positive patients, serial analyses of titer over time would be an informative marker for recurrence. To allow for a consistent analysis population, we focused on first recurrences among patients who were seropositive at diagnosis and had been rendered free of detectable disease. In total, 71 oncoprotein seropositive patients with MCC met criteria (Fig 2) for serial observation. Among these patients, a total of 282 blood draws were performed: 71 initial “time of diagnosis” draws, and 211 subsequent “surveillance” draws. Seventeen patients recurred during the study period of which 16 patients had serology testing within 45 days of the recurrence. The median time to recurrence was 273 days (range 81-1146).
We again observed that most MCC patients who were rendered free of disease had a rapid and sustained fall in MCPyV-oncoprotein titer. Among patients who were initially seropositive, the median interval between MCC diagnosis and when antibodies were no longer detectable was 8.4 months. Only 4/30 (13%) evaluable recurrence-free patients still had detectable MCPyV-oncoprotein antibodies at two years.
In our prospective cohort, we tested whether a falling titer was clinically reassuring. There were 176 falling-titer samples from 62 patients. Among these 176 samples, there were only 4 false negative blood draws occurring within 45 days of recurrence (2.2%, Fig 3A and Fig 3B). We statistically determined the specificity and negative predictive value using a generalized estimating equations (GEE) approach (which allows for accounting for multiple observations within the same individual; see statistical methods section). With this, the overall specificity of a falling titer was 89% (95% CI: 82-96%), and negative predictive value was 97% (95% CI: 94%-100%) (Supplemental Table 3).
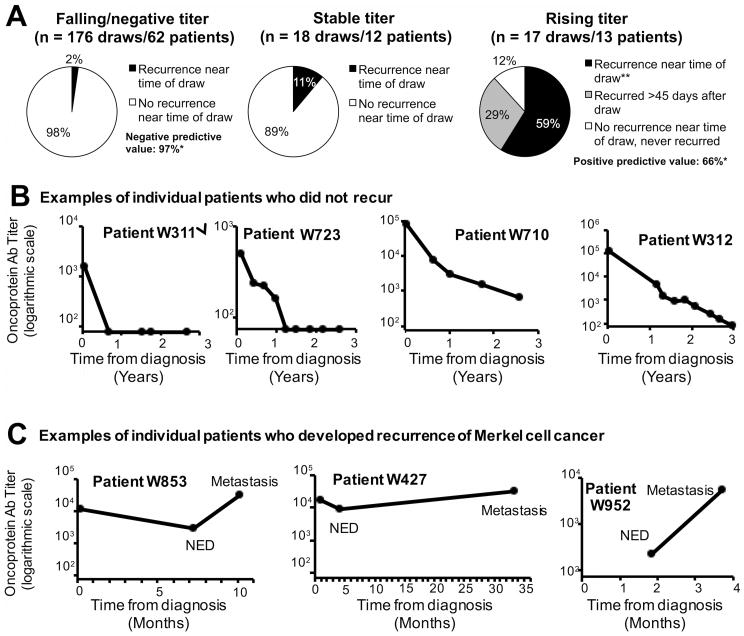
Figure 3. Prospective validation of serial MCPyV antibody levels as a marker for recurrence among patients who are antibody positive at diagnosis.
A total of 71 patients were antibody positive at diagnosis, definitively treated, and had serial follow-up draws (n=282 draws: 71 initial and 211 follow-up) during the study period. These patients were followed prospectively to determine whether MCPyV antibody trend between draws could be used clinically as a tumor marker/biomarker for recurrence. A) Association of titer trend with recurrence. A total of 176 draws were falling by >20% as compared to previous draw. In 98% of cases, patients were without evidence of disease by clinical assessment (exam and/or scans). *After statistically accounting for multiple draws in an individual subject, the negative predictive value (likelihood that a falling titer represented no progression) was 97%. Conversely, there were 17 draws from 13 patients associated with a rising titer. In 59% of cases, recurrence could be detected at the time of the positive/rising titer while an additional 29% developed overt metastasis after the study period. The positive predictive value for detectable recurrence at the time of rising titer was 66%. **p<0.05 for proportion of patients in recurrence, comparing patients with rising titer(59%) with patients with falling/negative titer (2%). The number of patients in the three groups (stable, failing, or rising titers) is greater than 71 because some patients were evaluable in more than one category (such as a patient who initially had a falling titer and then later had a rising titer). B) Examples of individual patients who did not recur. 4 of the 54 patients who did not recur during the study period are shown. Approximately half of patients who did not recur became seronegative during the study period, at a median of 9 months. Note the logarithmic Y-axis (titer) and the X (time) axis in years. C) Examples of individual patients who recurred. 3 of the 17 patients who developed recurrence during the study period are shown. Note that the X (time) axis is now depicted in months.
Interestingly, among the “false-negative” patients with falling titers but clinical progression, three of four were in patients who developed small metastases immediately after removal of large-volume primary and/or bulky nodal disease. These three patients in fact had overall decreases in tumor burden that were accurately reflected by the serologic test. Thus, in high risk patients an initial post-treatment scan should be considered.
There was a small population of patients who had a readily detectable stable (<20% change) MCPyV-oncoprotein titer (12 patients, 18 of 211 follow-up draws). In only 2 of these cases (11%) was recurrence/progression detected within 45 days of the draw. This was not statistically different from that of a falling titer (p = 0.10) (Fig 3A).
In comparison to a falling or stable titer, which was clinically reassuring, a majority of patients with a rising titer were found to have recurrence/progression within 45 days of the rising titer. There were 17 blood draws from 13 patients that were classified as rising (titer value was ≥150 and increased ≥20% from the prior draw). In 59% of cases (10 draws/10 patients), recurrence/progression was detected within 45 days of the blood draw (p<0.01 as compared to falling titer; Fig 3A and Fig 3C). Of the 7 remaining “false positive” draws (from 3 patients), 5 (29%) came from a single patient (W-763) that had an FDG-avid node concerning for recurrence (but inaccessible to biopsy during study period) who then developed additional sites of metastatic disease that was biopsy proven to represent MCC recurrence after the study closed. This patient is shown in grey shading in Fig 3A. If this patient is counted as false positive, the sensitivity is 63% (95% confidence interval: 39-87%) and positive predictive value is 66% (95% CI: 33-98%). If instead this patient is considered to be a true positive, positive predictive value improves to 83%.
Among the 10 patients with a rising titer and contemporaneous recurrence, 3 had locally recurrent disease and 7 had distant metastatic disease. Importantly, all 7 patients with distant metastatic disease had new metastatic disease on scans but were clinically asymptomatic and had no palpable disease on physical exam.
Discussion
Merkel cell carcinoma (MCC) is an aggressive cutaneous malignancy with a recurrence rate of >40%. Here we report in a large prospective validation cohort a clinically available virus directed assay that can identify two populations of patients at diagnosis: a MCPyV-oncoprotein seronegative group at higher risk of recurrence who may benefit from closer imaging surveillance and a MCPyV oncoprotein seropositive group for whom serial MCPyV antibody titer assessment may assist in ongoing surveillance.
Recurrence-free survival was decreased in oncoprotein antibody-negative patients in a stage-independent fashion. Immune suppression4, 5, 24 has consistently been reported as an adverse prognostic factor for MCC, and the absence of identifiable MCPyV in tumor tissue is sometimes reported to be an adverse prognostic factor (our multivariate models did account for known immunosuppression).25, 26 The worsened outcome observed among MCPyV-oncoprotein antibody-negative patients is similar to that reported for poor outcomes among patients with low or absent titers of antibodies recognizing MCPyV capsid.21, 20 Capsid antibodies were not tested directly in this study. However, anti-oncoprotein antibodies have several advantages: they are more specific to MCC (as these antibodies are only rarely detected in healthy persons) and most notably anti-oncoprotein antibodies vary with disease burden (as compared to capsid antibodies which typically remain constant). Increased clinical and radiographic surveillance for MCPyV-oncoprotein antibody-negative patients may be warranted, given their higher-risk MCC, and the inability to use serology to monitor for recurrence.
Among patients who make MCPyV-oncoprotein antibodies, we found a rising antibody titer frequently indicated MCC recurrence (PPV 66%) whereas a falling titer was highly reassuring (NPV 97%). This negative predictive value provides significant clinical utility and may help to best direct the use of other testing such as scans. Of note, there were only four false negatives, three of which occurred in the immediate post-excision setting where there had been an overall decrease in burden of disease during that interval. This suggests that with the first (∼3 month time point) falling titer, a scan should be considered. After this, scans for “antibody makers” could mostly be reserved for a clinical symptom change or a rising MCPyV-oncoprotein antibody titer. Although this study focused on detection of first recurrences, available longitudinal data suggest this test may also be useful for detecting later MCC recurrences if the titer decreases markedly following treatment of first recurrence.
This study had several limitations. Patients were treated with several modalities, including surgery, radiotherapy, and a combination thereof, meaning therapy was not uniform, but did reflect ‘real world’ variation. Blood samples were collected at diverse centers across the US and shipped with a delay in processing of up to 3 days. Of note, our data indicate that this delay should not affect assay results based on tests of serum antibody stability at room temperature (Supplemental Fig 1B). Finally, as a necessity of study design, and in order to maximize enrollment and minimize bias from delayed entry, we report only on first recurrences.
There is controversy as to whether early detection of asymptomatic distant metastatic cancer improves outcomes. Such early detection is only beneficial if available therapies are more effective for low-burden disease. Indeed, early detection of metastatic MCC may not have been particularly beneficial when therapy was limited to cytotoxic chemotherapy (typically only palliative in nature). However, the viral etiology of most MCC tumors and the strong association of MCC with immune suppression suggest great potential for effective immunotherapy (with the associated possibility for long-term disease control). Indeed, several promising immunotherapy trials targeting MCC are being conducted, and a recently reported trial indicated a greater than 50% response rate to immune checkpoint blockade in advanced MCC with median durability of response already greater than that of cytotoxic chemotherapy in MCC.27-29 In melanoma, response rates to immune checkpoint inhibitors have been observed to be higher, with longer progression free survival in patients with lower burden of disease at time of treatment.30 Therefore, early detection of distant metastasis in MCC offers an opportunity to change clinical management in ways that are likely to lead to improved patient outcomes, particularly in the emerging era of immune therapy for MCC.
Since the end of follow-up of this cohort in February 2014, we have found this assay to be useful in several aspects of clinical care in the Seattle Merkel cell carcinoma program. Our patients are now routinely tested for MCPyV-oncoprotein antibodies at diagnosis, and if antibodies are detected, testing is typically carried out every 3 months while at significant risk of recurrence (about 3-4 years). A rising titer prompts clinical and radiographic evaluation. Oncoprotein-negative patients are often followed with imaging during their first 2-3 years. Providers from other centers can readily access this assay as a clinical “send-out” test.
In summary, over 50% of MCC patients make MCPyV-oncoprotein antibodies and those who do not are at higher risk for recurrence and may benefit from closer follow-up with imaging. Viral oncoprotein antibodies have clinical utility for the early detection of occult recurrent or distant metastatic disease. Prompt recognition and treatment of metastatic disease may be associated with better outcomes by allowing for immunotherapy to begin at a time of lower disease burden.
Supplementary Material
Acknowledgments
We thank Leonor Busuego, the FHCRC Specimen Processing Facility, Dafina Ibrani, Betsy Williams, Vanna Maggs, Audrey Ruhland, Krista Stafstrom for technical and logistical assistance, and Stephanie Lee for critical advice.
Funding sources: NIH R01-CA162522 and K24-CA139052 to PN, NCATS Grant TL1 TR000422 to AM, and the MCC patient gift fund at UW.
Footnotes
Conflicts of interest: None
References
- 1.Albores-Saavedra J, Batich K, Chable-Montero F, Sagy N, Schwartz AM, Henson DE. Merkel cell carcinoma demographics, morphology, and survival based on 3870 cases: a population based study. J Cutan Pathol. 2010;37:20–27. doi: 10.1111/j.1600-0560.2009.01370.x. [DOI] [PubMed] [Google Scholar]
- 2.United States Census Population Clock. [accessed August 17, 2015]; Available from URL: http://www.census.gov/popclock/
- 3.Heath M, Jaimes N, Lemos B, et al. Clinical characteristics of Merkel cell carcinoma at diagnosis in 195 patients: the AEIOU features. J Am Acad Dermatol. 2008;58:375–381. doi: 10.1016/j.jaad.2007.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brewer JD, Shanafelt TD, Otley CC, et al. Chronic lymphocytic leukemia is associated with decreased survival of patients with malignant melanoma and Merkel cell carcinoma in a SEER population-based study. J Clin Oncol. 2012;30:843–849. doi: 10.1200/JCO.2011.34.9605. [DOI] [PubMed] [Google Scholar]
- 5.Paulson KG, Iyer JG, Blom A, et al. Systemic immune suppression predicts diminished Merkel cell carcinoma-specific survival independent of stage. J Invest Dermatol. 2013;133:642–646. doi: 10.1038/jid.2012.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fields RC, Busam KJ, Chou JF, et al. Five hundred patients with Merkel cell carcinoma evaluated at a single institution. Ann Surg. 2011;254:465–473. doi: 10.1097/SLA.0b013e31822c5fc1. discussion 473-465. [DOI] [PubMed] [Google Scholar]
- 7.Feng H, Shuda M, Chang Y, Moore PS. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science. 2008;319:1096–1100. doi: 10.1126/science.1152586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carter JJ, Paulson KG, Wipf GC, et al. Association of Merkel cell polyomavirus-specific antibodies with Merkel cell carcinoma. J Natl Cancer Inst. 2009;101:1510–1522. doi: 10.1093/jnci/djp332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pastrana DV, Tolstov YL, Becker JC, Moore PS, Chang Y, Buck CB. Quantitation of human seroresponsiveness to Merkel cell polyomavirus. PLoS Pathog. 2009;5:e1000578. doi: 10.1371/journal.ppat.1000578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Touze A, Gaitan J, Arnold F, et al. Generation of Merkel cell polyomavirus (MCV)-like particles and their application to detection of MCV antibodies. J Clin Microbiol. 2010;48:1767–1770. doi: 10.1128/JCM.01691-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martel-Jantin C, Filippone C, Tortevoye P, et al. Molecular epidemiology of merkel cell polyomavirus: evidence for geographically related variant genotypes. J Clin Microbiol. 2014;52:1687–1690. doi: 10.1128/JCM.02348-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martel-Jantin C, Pedergnana V, Nicol JT, et al. Merkel cell polyomavirus infection occurs during early childhood and is transmitted between siblings. J Clin Virol. 2013;58:288–291. doi: 10.1016/j.jcv.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 13.Chen T, Hedman L, Mattila PS, et al. Serological evidence of Merkel cell polyomavirus primary infections in childhood. J Clin Virol. 2011;50:125–129. doi: 10.1016/j.jcv.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 14.Shuda M, Feng H, Kwun HJ, et al. T antigen mutations are a human tumor-specific signature for Merkel cell polyomavirus. Proc Natl Acad Sci U S A. 2008;105:16272–16277. doi: 10.1073/pnas.0806526105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Church CD, Nghiem P. How does the Merkel polyomavirus lead to a lethal cancer? Many answers, many questions, and a new mouse model. J Invest Dermatol. 2015;135:1221–1224. doi: 10.1038/jid.2015.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Houben R, Shuda M, Weinkam R, et al. Merkel cell polyomavirus-infected Merkel cell carcinoma cells require expression of viral T antigens. J Virol. 2010;84:7064–7072. doi: 10.1128/JVI.02400-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kwun HJ, Shuda M, Feng H, Camacho CJ, Moore PS, Chang Y. Merkel cell polyomavirus small T antigen controls viral replication and oncoprotein expression by targeting the cellular ubiquitin ligase SCFFbw7. Cell Host Microbe. 2013;14:125–135. doi: 10.1016/j.chom.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shahzad N, Shuda M, Gheit T, et al. The T antigen locus of Merkel cell polyomavirus downregulates human Toll-like receptor 9 expression. J Virol. 2013;87:13009–13019. doi: 10.1128/JVI.01786-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Griffiths DA, Abdul-Sada H, Knight LM, et al. Merkel cell polyomavirus small T antigen targets the NEMO adaptor protein to disrupt inflammatory signaling. J Virol. 2013;87:13853–13867. doi: 10.1128/JVI.02159-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Touze A, Le Bidre E, Laude H, et al. High levels of antibodies against merkel cell polyomavirus identify a subset of patients with merkel cell carcinoma with better clinical outcome. J Clin Oncol. 2011;29:1612–1619. doi: 10.1200/JCO.2010.31.1704. [DOI] [PubMed] [Google Scholar]
- 21.Samimi M, Molet L, Fleury M, et al. Prognostic value of antibodies to Merkel cell polyomavirus T-Antigens and VP1 protein in Merkel cell carcinoma patients. Br J Dermatol. 2015 doi: 10.1111/bjd.14313. [DOI] [PubMed] [Google Scholar]
- 22.Paulson KG, Carter JJ, Johnson LG, et al. Antibodies to merkel cell polyomavirus T antigen oncoproteins reflect tumor burden in merkel cell carcinoma patients. Cancer Res. 2010;70:8388–8397. doi: 10.1158/0008-5472.CAN-10-2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bichakjian CK, Olencki T, Alam M, et al. Merkel cell carcinoma, version 1.2014. J Natl Compr Canc Netw. 2014;12:410–424. doi: 10.6004/jnccn.2014.0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnson ME, Zhu F, Li T, et al. Absolute lymphocyte count: a potential prognostic factor for Merkel cell carcinoma. J Am Acad Dermatol. 2014;70:1028–1035. doi: 10.1016/j.jaad.2014.01.890. [DOI] [PubMed] [Google Scholar]
- 25.Sihto H, Kukko H, Koljonen V, Sankila R, Bohling T, Joensuu H. Clinical factors associated with Merkel cell polyomavirus infection in Merkel cell carcinoma. J Natl Cancer Inst. 2009;101:938–945. doi: 10.1093/jnci/djp139. [DOI] [PubMed] [Google Scholar]
- 26.Bhatia K, Goedert JJ, Modali R, Preiss L, Ayers LW. Immunological detection of viral large T antigen identifies a subset of Merkel cell carcinoma tumors with higher viral abundance and better clinical outcome. Int J Cancer. 2010;127:1493–1496. doi: 10.1002/ijc.25136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iyer JGBA, Doumani R, Lewis C, Anderson A, Ma C, Parvatheneni U, Bhatia S, Nghiem P. Response rate and durability of chemotherapy for metastatic Merkel cell carcinoma among 62 patients. J Clin Oncol. 2014;32:5s. doi: 10.1002/cam4.815. (suppl; abstr 9091). 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nghiem PT, Bhatia S, Lipson EJ, et al. PD-1 Blockade with Pembrolizumab in Advanced Merkel-Cell Carcinoma. N Engl J Med. 2016 doi: 10.1056/NEJMoa1603702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mantripragada K, Birnbaum A. Response to Anti-PD-1 Therapy in Metastatic Merkel Cell Carcinoma Metastatic to the Heart and Pancreas. Cureus. 2015;7:e403. doi: 10.7759/cureus.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nishino M, Giobbie-Hurder A, Ramaiya NH, Hodi FS. Response assessment in metastatic melanoma treated with ipilimumab and bevacizumab: CT tumor size and density as markers for response and outcome. J Immunother Cancer. 2014;2:40. doi: 10.1186/s40425-014-0040-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shuda M, Arora R, Kwun HJ, et al. Human Merkel cell polyomavirus infection I. MCV T antigen expression in Merkel cell carcinoma, lymphoid tissues and lymphoid tumors. Int J Cancer. 2009;125:1243–1249. doi: 10.1002/ijc.24510. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.