Abstract
Purpose
To review the epidemiology, risk factors, microbiologic spectrum, and treatment of microbial keratitis during a five-year period at an urban public hospital with comparison to an adjacent private university practice.
Methods
Retrospective chart review in the 5-year interval 2009 through 2014 Primary outcome measures included patient age at presentation, best-corrected visual acuity (BCVA), risk factors, culture and sensitivities, treatment, and complication occurrence.
Results
528 eyes with microbial keratitis were identified, 318 in the public cohort and 210 in the private cohort. Contact lens wear was the most common risk factor in the public cohort while ocular surface disease was the most common risk factor in the private cohort. Gram-positive organisms represented 47.3%, gram-negative organisms 32.1%, fungal organisms 13.6%, and Acanthamoeba 6.4% of corneal isolates. Gentamicin resistance was 4.4% and tobramycin resistance was 2.9%. The inpatient treatment rate of the public cohort was 40% compared to 4% in the private cohort. In the public cohort, average BCVA at resolution was 20/82 [logMAR 0.61]. For the private cohort, average BCVA at resolution was 20/73 [logMAR 0.56]. The perforation rate was 8% in the public cohort compared to 4% in the private cohort. 6% of cases underwent urgent penetrating keratoplasty in the public cohort versus 2% in the private cohort.
Conclusions
Microbial keratitis remains a clinical challenge in the urban public hospital setting. The risk profile of patients presenting in the public hospital setting may be different from patients presenting in a private care setting. Public hospital patients may present later in the course of their infection and thus have a higher rate of complications regardless of effective antimicrobial therapy.
Keywords: microbial keratitis, corneal ulcer, infectious keratitis
Introduction
Microbial keratitis is a sight-threatening infection of the cornea characterized by an epithelial defect and underlying stromal infiltrate. The clinical outcome depends upon prompt effective treatment for the underlying pathogen.1 Recent reports of increasing antibiotic resistance among ocular pathogens is cause for grave concern given the shift among ophthalmologists from culture-driven treatment with multiple, fortified, compounded antibiotic agents to empiric treatment with widely available commercial preparations.2–5
We recently described the results of microbial keratitis therapy at an urban public hospital.6 Outcomes during the study period were compared to outcomes at the same hospital a decade prior.7 Fears of increasing antimicrobial resistance were not realized over the two study intervals. Changes in treatment from routine culture and inpatient treatment with fortified antibiotics to greater outpatient treatment with fluoroquinolone monotherapy resulted in improved visual outcomes. This study expands upon the prior study to examine the similarities and differences between patients treated at the urban public hospital and the adjacent university faculty practice.
Materials and Methods
This study was approved by the University of Texas Southwestern (UTSW) Medical Center Institutional Review Board and conforms to the tenets of the Declaration of Helsinki. The UTSW ophthalmology physicians billing records between September, 2009 and August, 2014 were queried for ICD-9 codes for corneal ulcers (370.00, 370.01, 370.02, 370.03, 370.04, 370.05, 370.06, 370.0). The medical records of patients treated at the urban public hospital system, Parkland Health and Hospital Systems, were identified and categorized as public patients for the purposes of this study. The medical records of patients treated at the Aston Clinic, Clements University Hospital, St. Paul University Hospital, and Zale Lipshy University hospital, private university facilities on the same medical campus, were identified and categorized as private patients. The patients’ clinical history, past medical history, and ophthalmic medical notes were then reviewed for inclusion in the study. Inclusion criteria were patients who presented during the study period and underwent a comprehensive ophthalmologic examination and were diagnosed and treated for microbial keratitis. Exclusion criteria were suspicion or confirmation of viral or noninfectious keratitis. Data transformations and statistical analyses using Pearson’s correlation coefficient, Student’s T test, and Z test for independent populations were performed in SAS Enterprise Guide 6.1 (Cary, NC).
Best-corrected visual acuity (BCVA) was determined as previously described.6 BCVA, spectacle, contact lens, or pinhole, was recorded at both presentation and resolution of the keratitis. Average BCVA was determined by first converting the visual acuity to log of the minimum angle of resolution (logMAR) then taking the average of the logMAR values. Counting fingers vision was converted to snellen equivalent by assuming fingers are the size of a 200 letter. Hand motions vision was considered 10 times worse than count fingers.8 The patient’s keratitis was considered resolved following urgent penetrating keratoplasty, enucleation, or evisceration, and the vision was not used for calculating average BCVA. Light perception or worse vision was recorded but excluded from average BCVA calculations.
Corneal cultures were performed as previously described.7 Briefly, calcium alginate swabs were used to directly inoculate specimens onto chocolate agar, blood agar, thioglycollate broth, and Sabouraud’s dextrose agar. The plated samples were then submitted to the appropriate institution’s clinical laboratory, either Parkland or UTSW for gram stain and culture. Confocal microscopy was performed at UTSW and interpreted by a cornea faculty member when Acanthamoeba was suspected regardless of whether the patient was treated at Parkland or UTSW.
Results
Demographics and Risk Factors
Five hundred twenty-eight eyes of 526 patients were identified that met the inclusion and exclusion criteria. Three hundred eighteen eyes were treated at the public facilities. Two hundred ten eyes were treated at the private facilities. The average age of the two patient cohorts were significantly different, 42.9 years in the public cohort and 51.3 years in the private cohort (t = −4.65, p < 0.01). Of the public hospital cohort, 130 eyes (41%) had a history of contact lens wear, 88 eyes (28%) had a pre-existing history of ocular surface disease, 55 eyes (17%) had preceding ocular trauma, and 13 eyes (4%) were under topical steroid therapy prior to the development of their corneal infections. Of the private patient cohort, 90 eyes (43%) had a history of contact lens wear, 95 eyes (45%) had pre-existing ocular surface disease, 14 eyes (7%) had ocular trauma, and 21 eyes (10%) were receiving topical steroid drops (Figure 1). There were statistically significant differences in pre-existing ocular surface disease, ocular trauma, and topical steroid use but not contact lens wear (Table 1).
Figure 1. Risk factors for microbial keratitis.
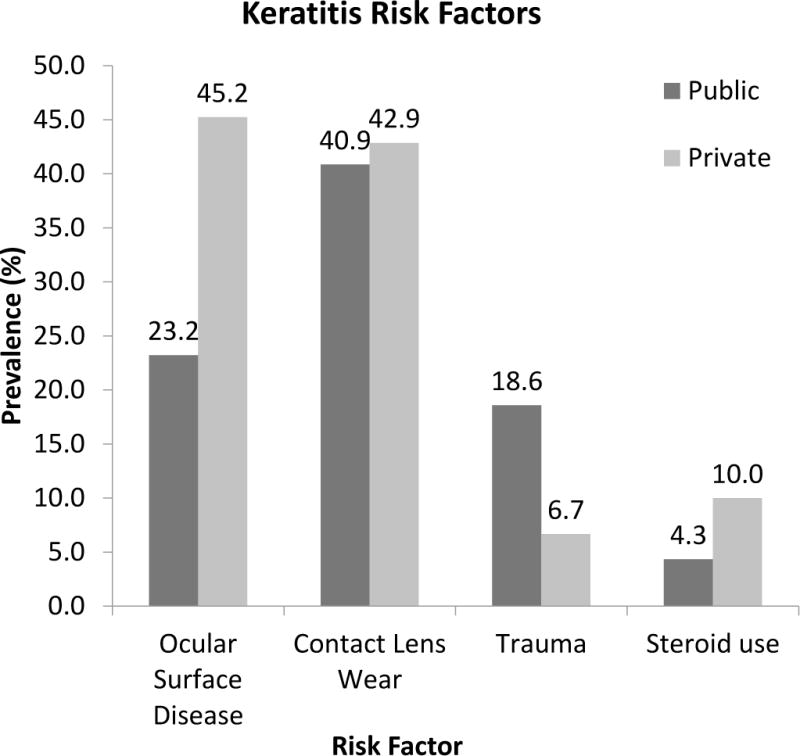
2009 – 2014 comparison of public (n = 318) and private (n = 210) patient cohorts.
Table 1. Risk factors for microbial keratitis.
Comparison of risk factors for microbial keratitis among public and private patient cohorts using a Z test to compare proportional differences between two independent populations.
| % Public (n = 318) |
% Private (n = 210) |
Z-score | p-value | |
|---|---|---|---|---|
| Contact lens wear | 40.9 | 42.9 | 0.3 | >.05 |
| Ocular surface disease | 23.2 | 45.2 | 5.5 | <.001 |
| Trauma | 18.6 | 6.7 | −4.2 | <.001 |
| Steroid use | 4.3 | 10.0 | 2.6 | <.001 |
Note: some patients had multiple risk factors.
Microbiology and Antibiotic Susceptibility
Corneal culture and confocal microscopy identified a total of 328 isolates between the 2 patient populations (Table 2). Of the total organisms isolated, 47.3% were gram positive, 32.1% were gram negative, 13.6% were fungal, and 6.4% were amebic. Eyes from public hospital patients had fewer gram-positive and more gram negative and fungal infections compared to the private patient population (Figure 2). Two of 6 confocal examinations of the public cohort were positive for acanthamoeba. Eighteen of 27 confocal examinations of the private cohort were positive for acanthamoeba. Two corneal cultures isolated acanthamoeba in the private cohort, only one of which revealed cysts on confocal microscopy. There were no cultures performed for acanthamoeba in the public cohort. Coagulase-negative Staphylococcus was the most common gram-positive isolates from both the public and private patient populations. Pseudomonas aeruginosa were the most common gram-negative isolates from both the public and private patient populations. While Fusarium species was the most common fungal isolate from the public patient population, Candida species was the most common fungal isolate from the private patient population. Gram-positive organisms were correlated with both ocular surface disease and topical steroid use (PCC = 0.21, p < 0.01 and PCC = 0.18, p < 0.01 respectively). Both Acanthamoeba and Pseudomonas infections were correlated with prior contact lens wear (PCC = 0.14, p < 0.01 and PCC = 0.24, p < 0.01 respectively). No particular microbe or group of microbes was associated with trauma in this study.
Table 2. Microbiologic spectrum of microbial keratitis: 2009–2014.
Corneal cultures of microbial keratitis at Parkland Health and Hospital Systems and University of Texas Southwestern Medical Center, 5-year period, 2009–2014. (MRSA = methicillin-resistant Staphylococcus aureus, MSSA = methicillin-sensitive Staphylococcus aureus)
| No. | % of Class | % of Total | |
|---|---|---|---|
|
Gram-Positive Organisms
| |||
| Coagulase-negative staphylococcus | 63 | 40.4% | 19.1% |
| Non speciated | 50 | 32.1% | 15.2% |
| Staphylococcus epidermidis | 12 | 7.7% | 3.6% |
| Staphylococcus warneri | 1 | 0.6% | 0.3% |
| Alpha hemolytic streptococcus | 34 | 21.8% | 10.3% |
| Non-speciated | 10 | 6.4% | 3.0% |
| Streptococcus pneumoniae | 17 | 10.9% | 5.2% |
| Staphylococcus aureus | 25 | 16.0% | 7.6% |
| MSSA | 21 | 13.5% | 6.4% |
| MRSA | 4 | 2.6% | 1.2% |
| Bacillus species not cereus | 8 | 5.1% | 2.4% |
| Corynebacterium | 4 | 2.6% | 1.2% |
| Other gram-positve | 22 | 14.1% | 6.7% |
|
| |||
| Total gram positive | 156 | 100% | 47.3% |
|
| |||
|
Gram-Negative Organisms
| |||
| Pseudomonas aeruginosa | 45 | 42.5% | 13.6% |
| Moraxella catarrhalis | 11 | 10.4% | 3.3% |
| Serratia marcescens | 10 | 9.4% | 3.0% |
| Klebsiella pneumoniae | 5 | 4.7% | 1.5% |
| Moraxella lacunata | 5 | 4.7% | 1.5% |
| Proteus mirabilis | 3 | 2.8% | 0.9% |
| Haemophilus influenzae | 2 | 1.9% | 0.6% |
| Other gram-negative | 25 | 23.6% | 7.6% |
|
| |||
| Total gram negative | 85 | 100.0% | 32.1% |
|
| |||
|
Fungal Organisms
| |||
| Bipolaris species | 11 | 24.4% | 3.3% |
| Candida species | 11 | 24.4% | 3.3% |
| Fusariam species | 9 | 28.1% | 4.1% |
| Aspergillus species | 4 | 8.9% | 1.2% |
| Other fungal | 9 | 20.0% | 2.7% |
|
| |||
| Total fungal | 32 | 100.0% | 14.5% |
|
| |||
|
Parasitic Organisms
| |||
| Acanthamoeba | 21 | 6.4% | |
|
| |||
| Total Organisms | 328 | ||
Figure 2. Microbiology of keratitis.
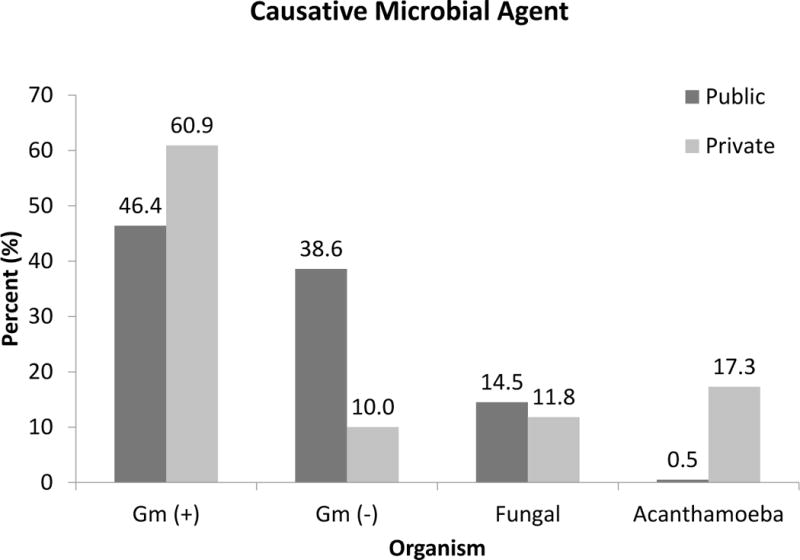
2009 – 2014 comparison of microbiologic isolates from public (n = 220) and private (n = 108) cohorts.
Three cases of methicillin-resistant Staphylococcus aureus were isolated from the public patient population and 1 case was isolated from the private patient population. Five of 114 isolates (4.4%) tested for gentamicin resistance were found to be resistant. Two of 68 isolates (2.9%) tested for tobramycin resistance were found to be resistant. One isolate of pseudomonas was found to have gentamicin resistance but not tobramycin resistance. The only pathogens tested and found to harbor resistance to fluoroquinolones were 1 isolate of E. coli and 1 isolate of Nocardia. No pathogens were found to exhibit vancomycin resistance.
Treatment
At the public facilities, corneal cultures were performed on 232 eyes (73%) and of those, 153 eyes (66%) had culture positive results compared to the private facilities where corneal cultures were performed on 118 eyes (54%) and of those, 86 eyes (75%) had culture positive results. One hundred twenty six patients (40%) at the public hospital received inpatient treatment of their corneal infections compared to 9 patients (4%) at the private facilities. At the public facility, 137 eyes (43%) received fluoroquinolone monotherapy as the initial therapy for their keratitis and 153 eyes received a combination of fortified vancomycin and gentamicin as initial therapy (48%). Nineteen eyes were begun on initial antifungal agents on presentation (6%). One eye was initially treated with polyhexamethylene biguanide (PHMB). At the private facility, 110 eyes (52%) were started on fluoroquinolone monotherapy, 66 eyes (31%) were started on fortified antibiotics, 10 eyes (5%) on anti-fungals, and 15 eyes (7%) on anti-amoebic therapy (Figure 3).
Figure 3. Treatment of microbial keratitis.
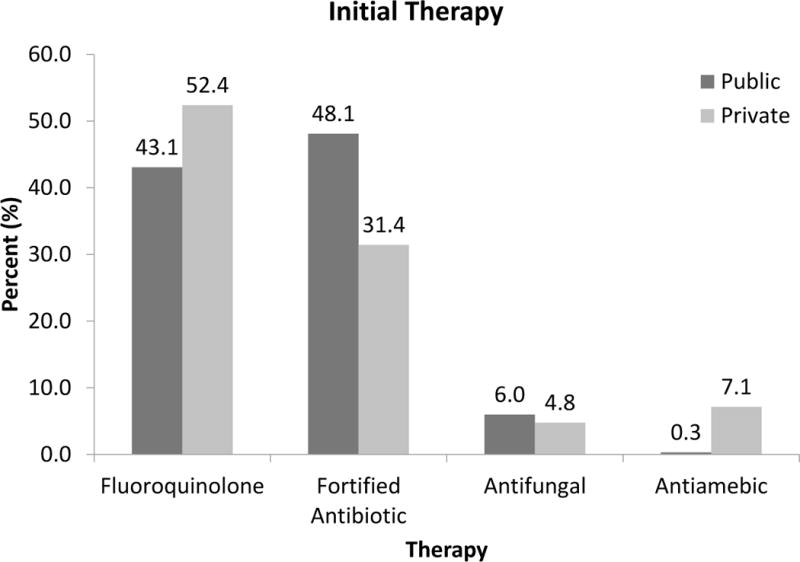
2009 – 2014 comparison of public (n = 318) and private (n = 210) patient cohorts.
Outcomes
In the public patient cohort, BCVA was logMAR 1.43 (20/542) at presentation compared to logMAR 0.80 (20/126) in the private patient cohort (t = 6.68, p<0.01) with 10.7% of eyes with light perception vision or worse compared to 7.6%. In the public patient cohort, BCVA was logMAR 0.61 (20/82) at resolution compared to logMAR 0.56 (20/73) in the private patient cohort (t = 0.62, p<0.01) with 8.2% of eyes with light perception or worse compared to 3.8%. (Figure 4) Twenty-four eyes at the public institution (7.5%) developed corneal perforations compared to 8 eyes at the private institution (4%). At the public institution, 19 eyes (6.0%) underwent urgent keratoplasty and 11 eyes (3.5%) underwent urgent enucleation or evisceration compared to the private institution where 5 eyes (2.3%) underwent urgent keratoplasty and 9 eyes (4.2%) underwent urgent enucleation or evisceration (Figure 5).
Figure 4. Visual outcomes of microbial keratitis.
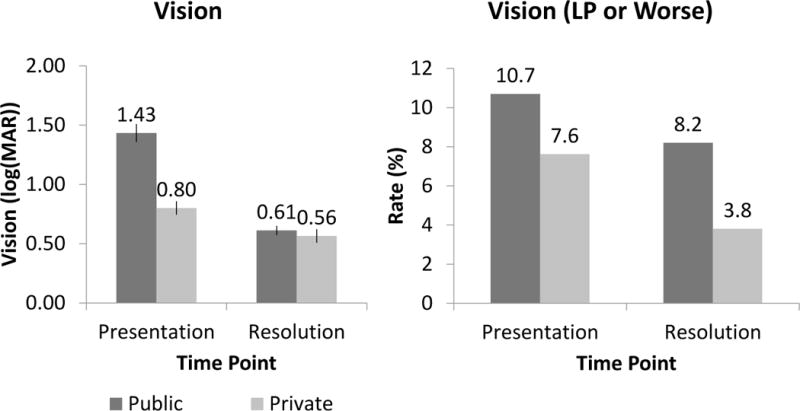
2009 – 2014 comparison of public (n = 318) and private (n = 210) patient cohorts in (a) log(MAR) and (b) portion with light perception or worse vision.
Figure 5. Complications of microbial keratitis.
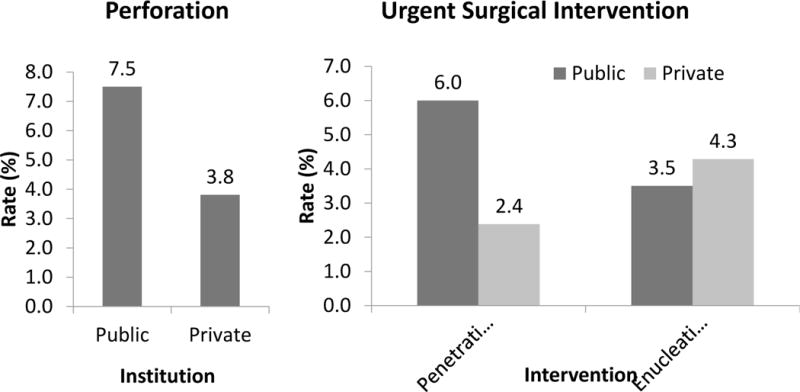
2009 – 2014 comparison of public (n = 318) and private (n = 210) patient cohorts for (a) perforation rate, and (b) urgent surgical rate.
Discussion
Demographics and Risk Factors
The patient populations presenting at the public and private institutions were significantly different despite their close proximity. Parkland Health and Hospital Systems is the public hospital system for Dallas County residents and serves as a safety net for underinsured patients. University of Texas Southwestern Medical Center is a tertiary referral center and has the only medical school affiliated ophthalmology department in North Texas. The patients at the public cohort were younger and more than twice as likely to have sustained trauma in association with the corneal infections. The patients in the private cohort were almost twice as likely to have ocular surface disease and more than twice as likely to be using topical corticosteroids in association with their corneal infections. Both cohorts of patients had contact lens wear rates higher than the study in the prior decade consistent with rates reported by others.7, 9 We attribute these findings to patients at the private university facilities more likely to be established patients and thus have known ophthalmic disease compared to the patients at the public facilities who often presented through the emergency department with acute symptoms. While contact lens wear is reported to be the single largest risk factor for microbial keratitis overall, the patients at the academic facilities are often undergoing complex treatment and are more likely to have corneal disease requiring topical steroid therapy such as recent corneal transplantation.10, 11
Microbiology and Antibiotic Susceptibility
The microbiologic spectrum of microbial keratitis of the two patient cohorts was expected because of the differing risk profiles. The prior study of public hospital patients found pseudomonas infection to be associated with contact lens wear and gram-positive bacteria to be associated with ocular surface disease and topical steroid use.6 Results from the total combined populations confirm these associations and also confirms a suspected correlation between contact lens wear and acanthamoeba. Our private university setting is equipped with a confocal microscope establishing its local role as an acanthamoeba referral center.
Isolates from microbial keratitis from both populations continue to exhibit high susceptibility to common topical preparations. One recent study suggests antibiotic resistance may be greater in microbes isolated from elderly patients compared to younger patients.4 While there was a significant difference in the average age of our 2 patient populations, the difference was less than 10 years and we found no difference in resistance patterns between the 2 populations. One limitation of our study is that antibiotic susceptibility is not routinely tested on all staphylococcus isolates at any of our institutions. Large studies have not shown a relation between antibiotic resistance and clinical outcomes and we are still challenged to develop a satisfactory model for determining in vivo efficacy. Furthermore, ophthalmic antibiotic preparations are applied topically at concentrations orders of magnitude greater than typical minimum inhibitory concentrations with resulting high corneal concentrations.12–14
Treatment
Despite the shift towards outpatient treatment previously reported at our public hospital, patients remain much more likely to be admitted for inpatient care, have culture of their eye infection, and be placed on multiple broad spectrum antibiotics compared to patients at adjacent private facilities.6 Microbial keratitis in our public patient cohort also appears to present at a later more advanced stages compared to the private patient cohort. Though ophthalmologists have been moving away from routine culture and fortified antibiotic therapy to fluoroquinolone monotherapy and empiric treatment as a group, many still prefer more intensive therapy and culture when the infection appears more serious.15
Outcomes
Although our public patient cohort presented for evaluation and treatment with significantly worse vision compared to our private patient cohort, many patients in the public cohort regained visual acuity similar to the private cohort at resolution of their infections. As discussed previously, both cohorts, public and private, demonstrated significantly better outcomes than the same public population previously reported 10 years earlier.7 We attribute this to the high efficacy of modern fourth-generation fluoroquinolone therapy, which remain effective as broad-spectrum therapy.4 While the corneal perforation and need for urgent surgery have decreased in the public patient population, these complications remain higher than our private patient cohort.
Conclusions
Microbial keratitis remains a challenging infection to treat in the urban public hospital patient population despite the availability of highly effective ophthalmic antibiotics. The risk profile of patients presenting in the public hospital setting appears to be different from those of patients presenting in the private setting, which in part reflects the expected microbiology of infectious keratitis. Public patients present late compared to their counterparts with greater access to care. Despite widespread clinical concern, increasing numbers of resistant organisms has not been observed at either of our institutions. Importantly, visual outcomes continue to improve in both populations despite an increasing trend to monotherapy outpatient treatment.
Acknowledgments
Source of Funding:
Supported in part by EY020799 and an unrestricted grant from Research to Prevent Blindness, Inc, New York, NY
Footnotes
Conflicts of Interest:
No conflicts of interests.
Presented in part at the Association for Research in Vision and Ophthalmology Annual Meeting, May 01-05, 2016, Seattle, Washington
References
- 1.Jones DB. Early Diagnosis and Therapy of Bacterial Corneal Ulcers. International Ophthalmology Clinics. 1973;13(4):1–30. doi: 10.1097/00004397-197312000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Hsu HY, Nacke R, Song JC, Yoo SH, Alfonso EC, Israel HA. Community Opinions in the Management of Corneal Ulcers and Ophthalmic Antibiotics: A Survey of 4 States. Eye & Contact Lens: Science & Clinical Practice. 2010;36(4):195–200. doi: 10.1097/icl.0b013e3181e3ef45. [DOI] [PubMed] [Google Scholar]
- 3.Adebayo A, Parikh JG, Mccormick SA, et al. Shifting trends in in vitro antibiotic susceptibilities for common bacterial conjunctival isolates in the last decade at the New York Eye and Ear Infirmary. Graefes Arch Clin Exp Ophthalmol Graefe’s Archive for Clinical and Experimental Ophthalmology. 2010;249(1):111–119. doi: 10.1007/s00417-010-1426-6. [DOI] [PubMed] [Google Scholar]
- 4.Asbell PA, Sanfilippo CM, Pillar CM, Decory HH, Sahm DF, Morris TW. Antibiotic Resistance Among Ocular Pathogens in the United States. JAMA Ophthalmol JAMA Ophthalmology. 2015;133(12):1445. doi: 10.1001/jamaophthalmol.2015.3888. [DOI] [PubMed] [Google Scholar]
- 5.Ni N, Nam E, Hammersmith K, et al. Seasonal Geographic Antimicrobial Resistance Patterns in Microbial Keratitis. Cornea. 2015;1 doi: 10.1097/ico.0000000000000352. [DOI] [PubMed] [Google Scholar]
- 6.Truong DT, Bui MT, Memon P, et al. Microbial Keratitis at an Urban Public Hospital: A 10-Year Update. J Clin Exp Ophthalmol Journal of Clinical & Experimental Ophthalmology. 2015;06(06) doi: 10.4172/2155-9570.1000498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pachigolla G, Blomquist P, Cavanagh HD. Microbial Keratitis Pathogens and Antibiotic Susceptibilities: A 5-Year Review of Cases at an Urban County Hospital in North Texas. Eye & Contact Lens: Science & Clinical Practice. 2007;33(1):45–49. doi: 10.1097/01.icl.0000234002.88643.d0. [DOI] [PubMed] [Google Scholar]
- 8.Holladay J. Proper method for calculating average visual acuity. Journal of Refractive Surgery. 1997;13(4):388–391. doi: 10.3928/1081-597X-19970701-16. [DOI] [PubMed] [Google Scholar]
- 9.Sand D, She R, Shulman IA, Chen DS, Schur M, Hsu HY. Microbial Keratitis in Los Angeles. Ophthalmology. 2015;122(5):918–924. doi: 10.1016/j.ophtha.2014.11.027. [DOI] [PubMed] [Google Scholar]
- 10.Cope JR, Collier SA, Rao MM, et al. Contact Lens Wearer Demographics and Risk Behaviors for Contact Lens-Related Eye Infections – United States, 2014. MMWR Morbidity and Mortality Weekly Report MMWR Morb Mortal Wkly Rep. 2015;64(32):865–870. doi: 10.15585/mmwr.mm6432a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dart J. Contact lenses and other risk factors in microbial keratitis. The Lancet. 1991;338(8768):650–653. doi: 10.1016/0140-6736(91)91231-i. [DOI] [PubMed] [Google Scholar]
- 12.Coster DJ, Badenoch PR. Host, microbial, and pharmacological factors affecting the outcome of suppurative keratitis. British Journal of Ophthalmology. 1987;71(2):96–101. doi: 10.1136/bjo.71.2.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim RY, Cooper KL, Kelly LD. Predictive factors for response to medical therapy in bacterial ulcerative keratitis. Graefe’s Arch Clin Exp Ophthalmol Graefe’s Archive for Clinical and Experimental Ophthalmology. 1996;234(12):731–738. doi: 10.1007/bf00189353. [DOI] [PubMed] [Google Scholar]
- 14.Segreti J, Jones RN, Bertino JS. Challenges in Assessing Microbial Susceptibility and Predicting Clinical Response to Newer-Generation Fluoroquinolones. Journal of Ocular Pharmacology and Therapeutics. 2012;28(1):3–11. doi: 10.1089/jop.2011.0072. [DOI] [PubMed] [Google Scholar]
- 15.Mcleod SD, Debacker CM, Viana MA. Differential Care of Corneal Ulcers in the Community Based on Apparent Severity. Ophthalmology. 1996;103(3):479–484. doi: 10.1016/s0161-6420(96)30668-4. [DOI] [PubMed] [Google Scholar]


