Abstract
Severe mycoplasma pneumonia is a rare entity with only 0.5–2% of cases having a fulminant course. We present a 74-year-old woman with hypertension, diabetes mellitus and remote history of marginal zone B-cell lymphoma admitted with abdominal pain and diarrhea of 1–2 days associated with body-aches, dyspnea, dry cough and weight loss for 2–3 weeks. On physical exam, she was febrile, tachypneic, tachycardic and hypoxic on room air. Chest examination revealed diffuse crackles and end-expiratory wheezes. Laboratory tests showed anemia, acute-on-chronic kidney injury and hyaline casts and epithelial cells in the urine analysis. Chest roentgenogram and computed tomograhphy scan showed pulmonary infiltrates. Intravenous ceftriaxone and azithromycin with bronchodilators were initiated. Her clinical course was complicated by hypoxic respiratory failure, hemoptysis, and worsening of infiltrates, requiring intubation and mechanical ventilation. Bronchoscopic bronchoalveolar lavage was consistent with diffuse alveolar hemorrhage (DAH). The patient's serum was positive for IgM antibody to Mycoplasma pneumoniae [1134 U/mL] and Anti-I-specific IgM-cold-agglutining [1:40]. A diagnosis of severe mycoplasma infection with DAH was made. The patient was treated with an additional course of doxycycline, pulse dose steroids and plasmapharesis with good clinical response. Surgical lung biopsy showed focal acute lung injury. Bone marrow biopsy and fat pad biopsy were normal. She was liberated from mechanical ventilation and discharged. She returned within 24 hours of discharge with cardiac arrest and new onset right-bundle-branch-block. We hypothesize our patient had severe mycoplasma pneumonia with DAH and multisystem complications of the same including a possible venous thrombo-embolic episode leading to her demise.
Keywords: Mycoplasma pneumonia, Diffuse alveolar hemorrhage, Multisystem involvement, Complications of mycoplasma
Abbreviations
- BAL
Bronchoalveolar lavage
- CAP
Community Acquired Pneumonia
- CARDS
Community-acquired Respiratory Distress Syndrome
- DAH
Diffuse Alveolar Hemorrhage
- EBV
Epstein Barr Virus
- MP
Mycoplasma pneumoniae
1. Introduction
While an estimated 2 million cases of mycoplasma infections happen every year in USA, the true incidence of this health issue remains unknown [1]. It has been reported as a cause of community acquired pneumonia in 5–35% cases in worldwide literature, with more than 50% patients requiring only outpatient treatment [2]. Severe mycoplasma with mycoplasma pneumonia (MP)-associated diffuse alveolar hemorrhage (DAH) is rare [3], [4]. Multi-system involvement of MP is well known, but not a clinical identity which is easily recognizable [5].
We present as interesting case of MP associated DAH and multisystem involvement of MP.
2. Case report
A 74-year-old woman with controlled hypertension, diabetes mellitus type 2, and remote history of marginal zone B-cell lymphoma, was admitted in the summer of 2016 with abdominal pain, watery diarrhea of 1–2 days duration associated with body-aches, progressive dyspnea, and dry cough of 2–3 weeks duration. She reported unintentional weight loss of 20 pounds during this time. She denied recent travel, sick contact, or hospitalization. She was a light smoker of 3–5 cigarettes/day. On physical exam, she was an elderly woman in mild distress, febrile (38.9 °C), tachypneic (RR 22 breath/min), tachycardic (HR 110 beats/min), hypoxic with oxygen saturation of 92% on ambient air. Auscultation of chest revealed diffuse crackles and end-expiratory wheezes. Rest of the physical exam was within normal limits. Laboratory tests showed anemia, acute-on-chronic kidney injury and hyaline casts and epithelial cells in the urine analysis (Table 1). Electrocardiogram indicated sinus arrhythmia with new first-degree atrioventricular block. Chest roentgenogram and computed tomograhphy scan revealed pulmonary infiltrates (Fig. 1). Antibiotics for community-acquired pneumonia (CAP) (ceftriaxone and azithromycin) and bronchodilators were initiated. Her clinical course was complicated by hypoxic respiratory failure, hemoptysis, and worsening of infiltrates, requiring intubation and mechanical ventilation. Bronchoscopic bronchoalveolar lavage (BAL) was consistent with diffuse alveolar hemorrhage (DAH) (Fig. 2).
Table 1.
Laboratory results.
| Test | Result | Test | Result |
|---|---|---|---|
| Hemoglobin (12–16 g/dL) | 9.1 | Serum sodium (135–145 meq/L) | 137 |
| Hematocrit (42–51%) | 28.9 | Lactic acid (0.5–1.6 mM/L) | 0.6 |
| Platelet count (150–400 K/μL) | 311 | Blood urea nitrogen (6–20 mg/dL) | 21 |
| White blood cell count (4.8–10.8 k/uL) | 5.5 | Creatinine (0.5–1.5 mg/dL) | 2.2 |
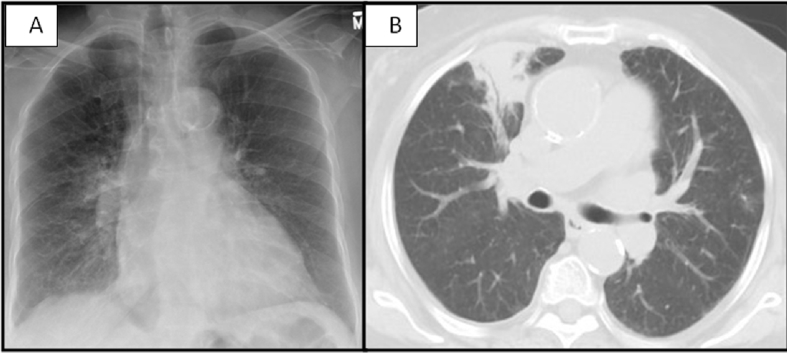
Fig. 1.
A: Chest radiograph showing right perihilar infiltrate with bronchial cuffing. B: Chest computed tomography with hyperinflated lungs, centrilobular emphysema, scattered blebs and bullae, consolidation with air bronchograms in the right upper and lower lobes, and small right pleural effusion (One Column Fitting Image).
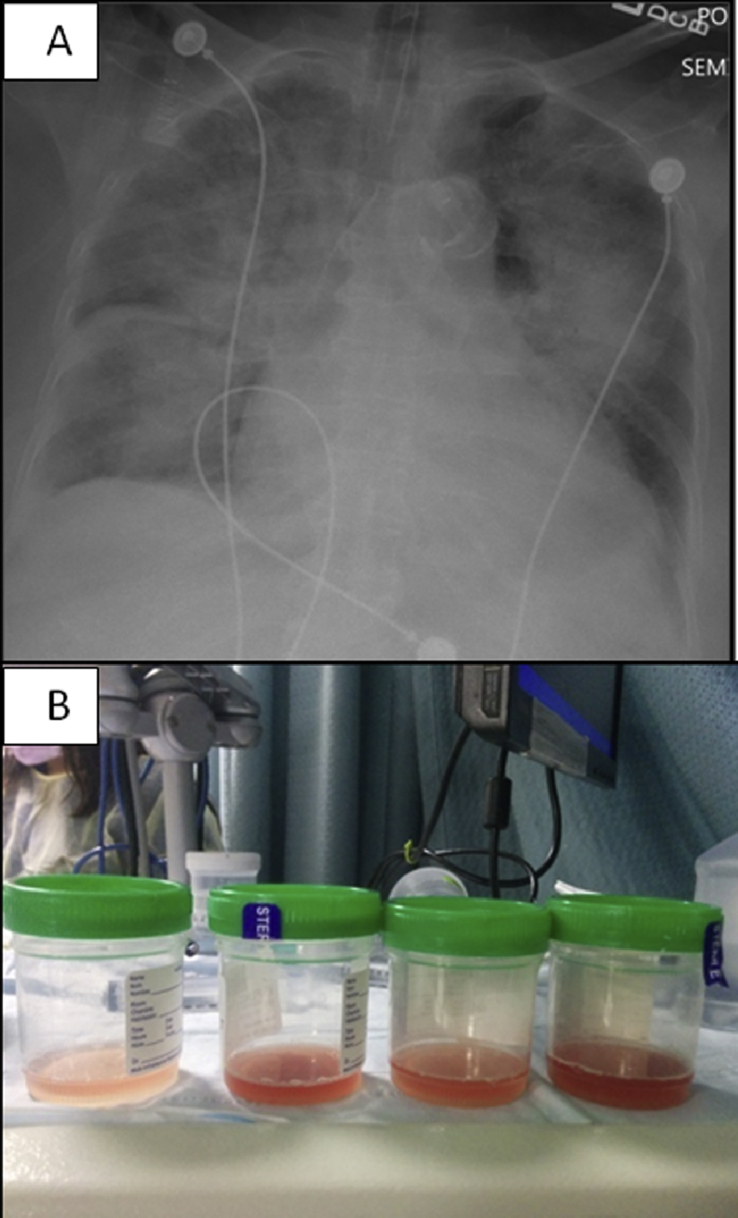
Fig. 2.
A: Chest radiograph showing bilateral diffuse infiltrates. B: Progressively hemorrhagic BAL fluid (One Column Fitting Image).
The course was complicated by atrial fibrillation, intermittent bronchospasm, and acute kidney injury. The patient was positive for IgM antibody to Mycoplasma pneumoniae [1134 U/mL; normal: <770 U/mL] and Anti-I-specific IgM-cold-agglutining [1:40; normal: none detected]. Hence a diagnosis of severe mycoplasma pneumonia associated with DAH and acute hypoxic respiratory failure was made. She received a course of doxycycline after receiving a course of azithromycin. Due to severe respiratory compromise and persistent hemoptysis, she received pulse steroid (1 g methylprednisolone for 3 days) followed by tapering doses of steroid with minimal response. She received nine cycles of plasmapheresis with clinical-radiological improvement. Surgical lung biopsy showed focal acute lung injury and airway-centered combination of mild chronic inflammation, focal peribronchiolar metaplasia and fibrosis with absence of eosinophils, vasculitis, granulomas, micro abscesses, food particles or lymphoproliferative disorder. Bone marrow biopsy and fat pad biopsy to evaluate for malignancy were normal.
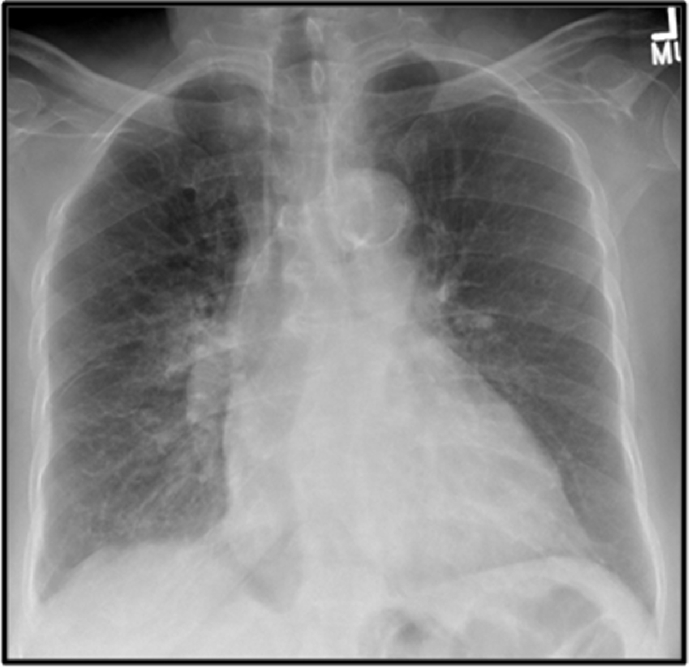
The clinical-radiological-pathological findings and serology were consistent with severe MP. She was liberated from mechanical ventilation and discharged to a rehabilitation center. Her chest roengenogram on discharge is as shown (Fig. 3). She returned within 24 hours of discharge with cardiac arrest and new onset right-bundle-branch-block. There was no hemoptysis; laboratory parameters were unchanged. Resuscitation was unsuccessful. We believe our patient had severe MP pneumonia along with pulmonary, cardiac, renal, hematological complications and a possible venous thrombo-embolic episode leading to her demise. We hypothesize that our patient had a venous thrombo-embolic episode since when she returned to the emergency room, she was hypoxic with a saturation of 86%, her electrocardiogram showed a new onset right bundle branch block and her proBNP level was 18185 pg/mL (0–450 pg/mL).
Fig. 3.
Chest radiograph showing resolution of previously visualized infiltrates (One Column Fitting Image).
3. Discussion
Mycoplasma pneumoniae (MP) infections are a worldwide cause of pneumonia affecting all age groups with variable severity; Severe mycoplasma infections are rare, with only 0.5–2% cases have a fulminant course [6].
Manifestations of MP may be multi-systemic (Table 2) [5]. Organs can be affected by one of three mechanisms: (1) direct, with bacterium present at the site of inflammation and activation of local inflammatory cytokines; (2) indirect, due to the effect of an altered immune response; or (3) vascular occlusion or impairment leading to organ dysfunction [5].
Table 2.
Manifestations of Mycoplasma pneumoniae infections.
| Organ involved (Incidence) | Manifestations |
|---|---|
| Pulmonary | Asthma exacerbation Tracheobronchitis Pneumonia: lobar and multilobar infiltrates Diffuse Alveolar Hemorrhage |
| Gastrointestinal (25%) | Nausea, vomiting, abdominal pain Diarrhea Anorexia |
| Cardiovascular | Myocarditis, pericarditis Cardiac arrhythmias Thrombotic events |
| Neurologic | Meningitis, encephalitis, optic neuritis Guillain-Barre syndrome |
| Renal | Acute tubular necrosis, glomerulonephritis, interstitial nephritis Renal artery thrombosis |
| Musculoskeletal/Skin | Erythema nodosum, cutaneous leukocytoclastic vasculitis Erythema multiforme, Stevens-Johnson syndrome MP-associated mucositis Myopathy, arthritis and rhabdomyolysis |
| Thrombotic (due to antiphospholipid antibodies) | Pulmonary embolism Splenic artery and left atrium and right ventricle thrombosis Aortic thrombosis |
| Others | Vasculitis (positive antineutrophil cytoplasmic antibodies) Cytopenias, cold agglutinins - induced autoimmune hemolytic anemia Kawasaki disease |
The pathogenesis of pulmonary MP includes local cytotoxic injury and poorly understood exaggerated immunological response. Autoimmune phenomena can be prominent and attributed to antigenic mimicry and direct cell activation. Cold agglutinins to the I-antigen of human red blood cells are a common finding [7]. Adhesion molecules P30 and P1 are found in an attachment organelle of the pathogen for cytoadherence [2]. A potential exotoxin, an ADP-ribosyltransferase in the pathogen's genome called community-acquired respiratory distress syndrome (CARDS) toxin, causes vacuolation and ciliostasis in cultured host cells [2]. Mycoplasma-infected type II pneumocytes show higher levels of interleukin (IL)- 8, tumor necrosis factor-αand IL-1-β mRNA production, resulting in increased cytokine levels and recruitment of lymphocytes and inflammatory cells [8], [9]. Cytokines can result in diffuse alveolar epithelial membrane injury leading to DAH; this has been demonstrated in stem cell transplant patients [10]. Mycoplasma-induced IgM cold agglutinins cross-react with I antigem containing erythrocyte glycoproteins. In the bronchial epithelium, the I antigen is contained in long-chain sialo-oligosaccharides that can serve as receptors for MP [2]. Disruption and cytotoxicity of lung tissue leading to DAH can be produced by the CARDS toxin, hydrogen peroxide, and superoxide, causing injury to epithelial cells, and damaged red blood cells due to anti-I cross-reactivity may extravasate into the alveolar space [2], [6], [8], [9], [10].
Causative factors for DAH are dependent on the patient's immune status and can be classified as either non-infective or infective. In immunocompromised patients, infective agents of DAH include cytomegalovirus, adenovirus, invasive aspergillosis, Mycoplasma, Legionella, and Strongyloides [11]. In immunocompetent patients, influenza A (H1N1), dengue, leptospirosis, malaria, and Staphylococcus aureus infection have been associated with DAH [11]. Severe mycoplasma with MP-associated DAH is rare, with only two cases identified in the English literature [3], [4].
Diagnosis is based on findings of hemoptysis, anemia, diffuse or worsening infiltrates, and hypoxemic respiratory failure. Bronchoscopy showing hemorrhagic sequential lavage and hemosiderin-laden macrophages support the diagnosis. The latter is usually found after 48–72 hours of hemoptysis [12].
The diagnosis of MP infection is based on serology, particularly IgM detection by ELISA. Sensitivity of IgM assays increases with the duration of symptoms, approaching more than 70% after 16 days of symptoms [13]. The positive predictive value for most of the test ranges from 60 to 80% [13]. Cross-reactivity with Epstein Barr Virus (EBV) is common. Cold agglutinins helps to confirm the diagnosis, they are increased in 50–60% of patients, but may occur in EBV, cytomegalovirus, or Klebsiella infection. Anti-I-specific IgM-cold-agglutinin is more specific for diagnosis [14]. PCR and serological analyses could be good screening tests for the reliable and accurate diagnosis of MP [2]. Bacterial culture is time-consuming and not readily available [2].
The Japanese Respiratory Society scoring system for atypical pneumonias is able to diagnose MP pneumonia with 88.7% sensitivity and 77.5% specificity [15]. The presence of more than four out of six of the following parameters provides high suspicion for MP; age <60 years, absence of or minor underlying diseases, stubborn cough, positive findings in chest auscultation, absence of sputum, or identifiable etiological agent by rapid diagnostic testing and serum white blood cell count <10 × 109/L [15]. Our patient exhibited five parameters, and in addition elevated IgM and high anti-I- specific cold agglutinin levels. Other causes of DAH were ruled out by appropriate serological tests.
It is imperative that an etiological diagnosis for DAH be established to initiate appropriate therapy. In patients with MP infection, macrolides are the drug of choice in adults and children; however, there are growing concerns regarding the development of resistance [1]. Acquired mutations on the ribosomal macrolide target are the only resistance mechanism described [2]. Resistance in Europe and USA may be in upto a quarter of patients, whereas resistance in Japan and China may be approaching more than 90% [16]. Therapeutic alternatives include fluoroquinolones, primarily levofloxacin, and tetracyclines [1], [17]. The management of DAH is supportive. Corticosteroid and immunosuppressive therapies are controversial [11]. Daily or alternate day plasmapharesis may be considered according to the guidelines of the American Society for Apheresis in patients with DAH presenting with severe hypoxemic respiratory failure [18], [19].
4. Conclusions
Diffuse alveolar hemorrhage in a patient with CAP should raise suspicion for severe MP infection. Cases may be missed due to low suspicion. The Japanese Respiratory Society Scoring System may prove useful in these scenarios. Mycoplasma pneumonia should be included as part of the differential diagnosis in patients with CAP and multi-organ involvement. Plasmapheresis may be lifesaving and should be considered for severe DAH associated with infectious causes. Sudden death can occur in MP infection, likely due to thrombotic or cardiac complications.
Conflicts of interest
The authors report no conflicts of interest.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Acknowledgements
No acknowledgements.
Contributor Information
Rashmi Mishra, Email: rashmi_mishra1987@yahoo.com.
Edison Cano, Email: ecano@bronxleb.org.
Sindhaghatta Venkatram, Email: svenkatr@bronxleb.org.
Gilda Diaz-Fuentes, Email: gfuentes@bronxleb.org.
References
- 1.Cdc. Mycoplasma Pneumoniae Infection. https://www.cdc.gov/pneumonia/atypical/mycoplasma/. Accessed on November 18, 2016.
- 2.Parrott G.L., Kinjo T., Fujita J. A compendium for mycoplasma pneumoniae. Front. Microbiol. 2016;7:513. doi: 10.3389/fmicb.2016.00513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wangondu Rw, Long T. An atypical case of hemoptysis. Conn. Med. 2016;80(3) [PubMed] [Google Scholar]
- 4.Kane J., Shenep J., Krance R., Hurwitz C. Diffuse alveolar hemorrhage associated with mycoplasma hominis respiratory tract infection in a bone marrow transplant recipient. Chest. 1994;105(6):1891–1892. doi: 10.1378/chest.105.6.1891. [DOI] [PubMed] [Google Scholar]
- 5.Narita M. Pathogenesis of extrapulmonary manifestations of Mycoplasma pneumoniae infection with special reference to pneumonia. J. Infect. Chemother.; 16(3): 162–169. [DOI] [PubMed]
- 6.Izumikawa K. Clinical features of severe or fatal mycoplasma pneumoniae pneumonia. Front. Microbiol. 2016;7:800. doi: 10.3389/fmicb.2016.00800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Janney F.A., Lee L.T., Howe C. Cold hemagglutinin crossreactivity with Mycoplasma pneumoniae. Infect. Immun. 1978;22(l):29–33. doi: 10.1128/iai.22.1.29-33.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tanaka H., Narita M., Teramoto S. Role of interleukin- 18 and T-helper type 1 cytokines in the development of Mycoplasma pneumoniae pneumonia in adults. Chest. 2002;121(5):1493–1497. doi: 10.1378/chest.121.5.1493. [DOI] [PubMed] [Google Scholar]
- 9.Yang J., Hooper W.C., Phillips D.J. Cytokines in Mycoplasma pneumoniae infections. Cytokine Growth Factor Rev. 2004;15(2–3):157–168. doi: 10.1016/j.cytogfr.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 10.Afessa B., Tefferi A., Litzow M.R. Diffuse alveolar hemorrhage in hematopoietic stem cell transplant recipients. Am. J. Respir. Crit. Care Med. 2002;166(5):641–645. doi: 10.1164/rccm.200112-141cc. [DOI] [PubMed] [Google Scholar]
- 11.von Ranke F.M., Zanetti G., Hochhegger B., Marchiori E. Infectious diseases causing diffuse alveolar hemorrhage in immunocompetent patients: a state-of-the-art review. Lung. 2013 Feb;191(1):9–18. doi: 10.1007/s00408-012-9431-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lara Ar. Diffuse alveolar hemorrhage. Chest. 2010;137(5):1164–1171. doi: 10.1378/chest.08-2084. [DOI] [PubMed] [Google Scholar]
- 13.Beersma M.F., Dirven K., van Dam A.P. Evaluation of 12 commercial tests and the complement fixation test for Mycoplasma pneumoniae-specific immunoglobulin G (IgG) and IgM antibodies, with PCR used as the “gold standard”. J. Clin. Microbiol. 2005;43(5):2277–2285. doi: 10.1128/JCM.43.5.2277-2285.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Daxböck F., Zedtwitz-Liebenstein K., Burgmann H. Severe hemolytic anemia and excessive leukocytosis masking mycoplasma pneumonia. Ann. Hematol. 2001;80:180. doi: 10.1007/s002770000250. [DOI] [PubMed] [Google Scholar]
- 15.Yin YD, Zhao F, Ren LL, Song SF, Liu YM, Zhang JZ, Cao B. Evaluation of the Japanese Respiratory Society guidelines for the identification of Mycoplasma pneumoniae pneumonia. Respirology, 17: 1131–1136. [DOI] [PubMed]
- 16.Zheng X., Lee S., Selvarangan R., Qin X., Tang Y., Stiles J. Macrolide-Resistant mycoplasma pneumoniae, United States. Emerg. Infect. Dis. 2015;21(8):1470–1472. doi: 10.3201/eid2108.150273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Principi N., Esposito S. Macrolide-resistant Mycoplasma pneumoniae: its role in respiratory infection. J. Antimicrob. Chemother. 2013;68:506–511. doi: 10.1093/jac/dks457. [DOI] [PubMed] [Google Scholar]
- 18.Krause M.L., Cartin-Ceba R., Specks U., Peikert T. Update on diffuse alveolar hemorrhage and pulmonary vasculitis. Immunol. Allergy Clin. North Am. 2012 November;32(4):587–600. doi: 10.1016/j.iac.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Szczepiorkowski Z.M., Winters J.L., Bandarenko N. Guidelines on the use of therapeutic apheresis in clinical practice–evidence-based approach from the apheresis applications committee of the american society for apheresis. J. Clin. Apher. 2010;25(3):83–177. doi: 10.1002/jca.20240. [DOI] [PubMed] [Google Scholar]