Abstract
The biologic plausibility of an association between type 2 diabetes mellitus (T2D) and lung cancer has received increasing attention, but the results of investigations remain largely inconclusive. In the present study we investigated the influence of the anti-diabetic drug metformin on the cytotoxic effects of EGFR targeted therapy and chemotherapy in 7 non-small cell lung cancer (NSCLC) cell lines and a cohort of lung cancer patients with/without T2D. In vitro cell viability assays indicated that metformin didn't potentiate the growth inhibitory effects of erlotinib at different doses in cell lines that are of distinct genetic background. EGFR downstream signaling evaluation further demonstrated that metformin, at its IC50 value, modified apoptosis caused in erlotinib or chemotherapeutic agent-treated cells via AKT activation and the inhibition of caspase 3 and PARP cleavages. These regulations were driven independently from EGFR, LKB1, KRAS, PTEN and p53 status. Metformin triggered autophagy (LC3B expression) was identified to interplay with apoptosis to attenuate the drug effect and postpone cancer cell death. In the retrospective study of 8 NSCLC patients, the administration of metformin did not induce statistically significant changes as assessed by immunohistochemical staining of pERK, pAKT and cleaved PARP. Consequently, the application of metformin for T2D NSCLC patients receiving chemo or EGFR targeted therapy should be considered with caution.
Introduction
Lung cancer leads to the largest number of cancer-related deaths worldwide. More than 85% of those cases are currently classified as non-small-cell lung cancer (NSCLC), with a predicted 5-year survival rate of around 21% [1]. Systemic chemotherapy is still the most widely used treatment for advanced lung cancer, but immunotherapy and targeted therapy are becoming more important. Platinum compounds, such as cis- or carboplatin, are the backbone of most drug combinations in lung cancer [2], [3], [4], [5]. Biomarker directed targeted agents have become the standard of care for a subset of NSCLC patients and have attracted significant attention in the last decade. Erlotinib, gefitinib and afatinib, which are specific inhibitors of the epidermal growth factor receptor (EGFR) tyrosine kinase (TK), are used for patients with classical EGFR mutations [6]. However, more prevalent in NSCLC are mutations in the KRAS oncogene in conjunction with deletion or point mutations in the p53 tumor suppressor or the metabolic sensor LKB1. Regrettably, today there is no targeted therapeutic option for patients with LBK1-p53- or KRAS-mutant tumors.
A number of epidemiologic studies have indicated an increased cancer risk in patients with type 2 diabetes mellitus (T2D) [7], [8], [9], [10]; the overlapping of these two diseases has a devastating impact on the health of patients. Published results suggest T2D and cancer share key metabolic pathways and signaling modules, including AMP activated protein kinase (AMPK) and the members of the insulin receptor family [11]. However, precise molecular genetic links between these two diseases aren't well characterized in terms of a specific denominator or targets which are suitable for drug development.
There is rising interest in the repurposing or repositioning of already approved medications as possible cancer chemotherapeutic agents. Among the medications being evaluated, metformin is one of the most notable. Metformin belongs to the biguanide class of anti-diabetic drugs [12]. In addition to its use in T2D, metformin exerts remarkable anti-cancer properties in tumor cells and prevents spontaneous and induced tumorigenesis in mouse models [13], [14], [15], [16]. The mechanism of action of metformin on cancer cell growth is still not fully elucidated, but it decreases insulin resistance and indirectly reduces the insulin level, thereby inhibiting insulin promoted cancer cell growth [17]. Metformin also activates AMPK, a transducer of cellular energy, which has been proposed as a possible therapeutic target in cancer [18]. Based on the exciting outcomes from in vitro and preclinical studies as well as good physiological tolerance, numerous clinical studies have been launched in order to assess the safety and efficacy of metformin in combination with chemo, radiation and/or targeted therapy for different types of tumors, including lung, breast, ovarian, colon and pancreatic cancer [13], [19], [20]. Unfortunately, metformin has shown conflicting results in in vitro and preclinical studies; the role of its application as a chemo-preventive drug in lung cancer is debatable. Future studies in humans are required to gain insight into the potential of metformin as a suitable candidate to treat NSCLC as a single medication or in combination with chemotherapeutic agents and small molecular tyrosine kinase inhibitors.
The objective of our study was to determine the signaling pathways mediated by metformin in combination with erlotinib and the therapeutic drugs cisplatin and paclitaxel in NSCLC cell lines (Table 1) based on the evaluation of critical modules, namely EGFR, AKT, caspase 3, PARP and LC3B, for necrosis-autophagy-apoptosis programmed cell death development. We observed that metformin, by triggering autophagy, modified programmed cell death in erlotinib or chemotherapeutic agent-treated NSCLC cells in vitro. Metformin associated effects were reflected by AKT activation and the abrogation of PARP and caspase 3 cleavage. Pronounced regulations were manifested in NCI-H2122 and A549 cells with LKB1 deficiency and KRAS mutation. Further data from a retrospective study also suggest a cohort of lung cancer patients didn't benefit from metformin treatment. In this case the clinical necessity for a combinatorial treatment with metformin and chemo or targeted therapy should be appraised with caution.
Table 1.
Characteristics of the NSCLC Cell Lines Used in This Study
| NSCLC Cell Line | Histology | EGFR Mutation | KRAS Mutation | PTEN Status | P53 Mutation | LKB1 Mutation | IC50s of Erlotinib (μM) |
|---|---|---|---|---|---|---|---|
| HCC4006 | AD | del E746–A750, amplification | wt | 0.25 | |||
| HCC827 | AD | del E746–A750, A750E, E746K, E746A | del V218, del V125 |
0.04 | |||
| NCI-H1650 | AD | del E746–A750 | null | >10 | |||
| NCI-H1975 | AD | L858R and T790 M | wt | R273H | >20 | ||
| NCI-H2122 | AD | wild-type | G12C | wt | Q16L, C176F, C44F, C83F | P281fs*6 | >10 |
| A549 | AD | wild-type | G12S | wt | Q37* | >10 | |
| HCC95 | SCC | wild-type | wt | >20 |
AD, adenocarcinoma; SCC, squamous cell carcinoma.
Materials and Methods
Cell Lines and Chemicals
The human NSCLC cell lines HCC4006, HCC827, NCI-H2122 (H2122), NCI-H1650 (H1650), NCI-H1975 (H1975) and HCC95 were obtained from Dr. Roman Thomas. A549 was obtained from the American Type Culture Collection (ATCC). Cancer cells were cultured in RPMI-1640 medium (Gibco) supplemented with 10% FBS (Gibco), L-glutamine, 100 U/ml penicillin and 50 μg/ml streptomycin (Sigma, St Louis). Erlotinib was purchased from Vichem Chemie (Hungary). Metformin, cisplatin and paclitaxel were purchased from Sigma (St Louis, MO).
Proliferation Assays
Opaque-walled 96-well plates with clear bottoms (Corning, #3603) were used for cell culture. The number of cells used per cell line was determined empirically. Cells from HCC95, H2122, H1650, HCC4006, HCC827, and H1975 were seeded at 1.3 × 105, 2.2 × 105, 1 × 105, 5 × 104, 8 × 104, and 7.5 × 104 cells/well, respectively. Drug treatments were performed the next day after seeding and proliferation was measured 72 hours later using the CellTiter-Glo luminescent cell viability assay (Promega), following manufacturer's instructions. Each point represents as a percentage of the DMSO treated control. Experiments were set up in 4 replicate wells and repeated thrice.
Western Blot Analysis
Protein extracts were prepared as previously described [21]. 35 μg total proteins were denatured, separated on 10% SDS–PAGE, and transferred to nitrocellulose (Protran BA85, GE Healthcare Life Sciences). Membranes were incubated with primary antibodies diluted in NET-gelatin against pEGFR Y1173 (Cell Signaling Technologies, #4407), EGFR (Transduction Laboratories, E12020), pERK1/2 (Cell Signaling Technologies, #9101), ERK1 K23 (Santa Cruz, sc-94), pAKT (Cell Signaling Technologies, #9271), AKT1/2/3 H-136 (Santa Cruz, sc-8312), PARP (Cell Signaling Technologies, #9542), Caspase 3 (Cell Signaling Technologies, #9662), Tubulin (Sigma, T9026). Secondary HRP-conjugated anti-rabbit (Bio-Rad) and anti-mouse (Sigma) antibodies were used and detection was carried out using an ECL reagent (PerkinElmer, Rodgau, Germany) on X-ray films.
Immunofluorescence Assay
Cells were seeded onto 12 mm coverslips in 24-well dishes and allowed to attach for 18 hours before treatment. 72 hours after treatment, cells were fixed in methanol, blocked in 3% BSA, and incubated with rabbit anti-LC3B antibody (Cell Signaling Technologies, #3868). After incubation with secondary antibody Alexa Fluor 488-conjugated goat anti-rabbit (Dianova, #111–545-003), nuclei were stained with Hoechst 33,342. Coverslips were mounted in Prolong Gold Antifade Reagent (Cell Signaling Technologies, #9071). Image acquisition was carried out using a Zeiss Axioplan2 microscope and the Metaview software (Universal Imaging Corp.)
NSCLC Tumor Samples
Eight patients, with locally advanced NSCLC, underwent neoadjuvant chemotherapy and subsequent surgery. The pre- and post-chemotherapy tumor samples were available in Asklepios Institute of pathology, Munich-Gauting, Robert-Koch Allee, Germany. Clinical and pathological features of the patients are listed in Table 2.
Table 2.
Clinical and Pathological Features of the NSCLC Patients
| Diagnose Age | Sex | Treatment | Tissue Sample Location | Metformin Treatment |
|---|---|---|---|---|
| 65 | M | Cisplatin/Gemcitabine | Lobectomy left | Yes |
| 74 | F | Carboplatin/Docetaxel | Lobectomy right | Yes |
| 63 | M | Carboplatin/Paclitaxel + Radiotherapy | Lobectomy right | Yes |
| 63 | M | Cisplatin/Gemcitabine | Lobectomy left | Yes |
| 65 | F | Cisplatin/Gemcitabine | Bi-Lobectomy | No |
| 71 | F | Cisplatin/Navelbine | Lobectomy left | No |
| 61 | F | Cisplatin/Navelbine | Lobectomy right | No |
| 49 | M | Cisplatin/Navelbine | Lobectomy right | No |
F, female; M, male.
Immunohistochemistry Staining
Tissue sections were dehydrated and heated in a microwave oven in citrate buffer 20 min for antigen retrieval. After blocking in 3% hydrogen peroxide (H2O2) in PBS for 15 minutes, the sections were overlaid with 5% normal goat serum and 0.2% Tritonx-100 in TBST. Tissue sections were exposed with the following primary antibodies: rabbit anti-pAKT (1:50, Cell Signaling, #3787), rabbit anti-pERK1/2 (1:50, Cell Signaling, #9101) and rabbit anti-cleaved-PARP form (1:50, Cell Signaling, #5625). After 4 °C overnight incubation, goat anti-rabbit secondary biotinylated antibody (DAKO, Glostrup, Denmark) was applied using a 1:200 dilution for 60 minutes at room temperature. Binding was visualized with DAB (Sigma, St. Louis, Missouri) and H2O2. After the hematoxylin counterstaining, sections were examined by the microscope. Negative controls included the signals omission of the primary antibody. Lung tumor areas were determined by the pathologist and labeled. Additionally, those areas were verified using AxioVision software (Carl Zeiss Microscopy GmbH). For each tumor, two nonconsecutive slides were used for quantification. The intensity (score 3) of the protein signal in tumor cells was automated generated via software for statistical calculations. Finally, all IHC slides were re-evaluated manually by the pathologist and scientist. For microscopy, we employed Zeiss Discovery V8 Stereo microscope (Carl Zeiss MicroImaging GmbH, Göttingen, Germany) integrated with an Axio-Cam ICc3 camera (Spectra Service, Ontario, NY). Images were acquired using AxioVision Rel. 4.7 software provided by Zeiss.
Statistical Analysis
Data were represented as mean ± SD from three independent experiments unless stated otherwise. Statistical analysis was performed by one-way analysis of variance (ANOVA), and statistical significance (*P < .01) was evaluated with the unpaired 2-tailed Student t test to assess differences between treated and control samples.
Results
Metformin Does Not Enhance Erlotinib-Induced NSCLC Cell Growth Inhibition
We have reported earlier that metformin modestly exerts a growth inhibitory effect on various types of NSCLC cell lines [21]; therefore, we conducted an experiment to investigate if its combination with EGFR tyrosine kinase inhibitor (TKI) erlotinib has an additional growth inhibitory effect on NSCLC cells. Seven cell lines were selected according to their genetic status of EGFR and other oncogenes (Table 1).
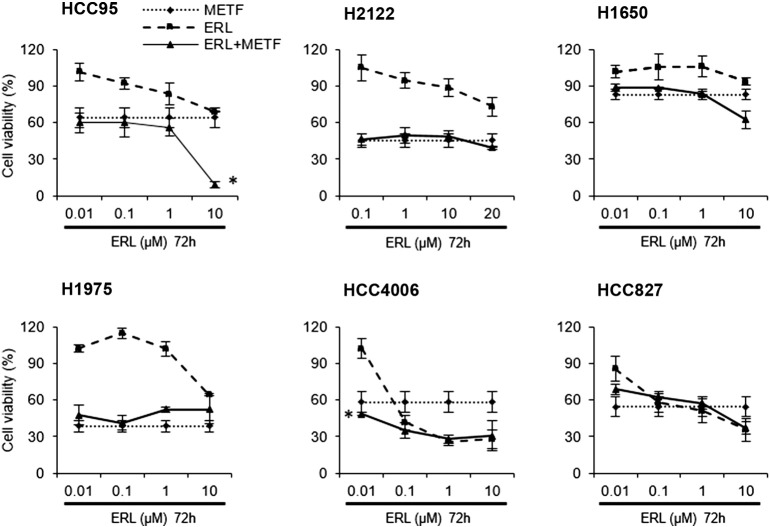
CellTiter-Glo cell viability assay was applied to evaluate the IC50 value of metformin in combination with various concentrations of erlotinib in a panel of NSCLC cell lines with erlotinib resistant (HCC95, H2122, H1650, and H1975) and sensitive genotype (HCC4006 and HCC827). Expectedly, after 72 hours of treatment, erlotinib reduced the viability of HCC4006 and HCC827 cells with an EGFR exon 19 deletion to a large extent, demonstrating a dose-dependent decrease from 100% to 25% and 90% to 30%, respectively. However, this treatment was less effective to the remaining cell lines as indicated in Figure 1. Through the evaluation of four different doses of erlotinib the combined effect was more than likely caused by metformin alone, especially for HCC95 and H2122 (Figure 1, upper left and middle). Among all treatments, enhanced anti-proliferative activity was shown only in HCC95 cells in combination with erlotinib at 10 μM (Figure 1, upper left). Taken together, combinatorial treatment did not influence the effect of either single agent, except for H1975 and HCC827 cell lines where erlotinib attenuates the metformin effect.
Figure 1.
Metformin doesn't enhance in vitro anti-proliferative activity of erlotinib. Cells were cultured in the presence or absence of metformin with the indicated concentrations of erlotinib for 72 hours. The doses of metformin relate to the IC50 values for each cell line, such as 2 mM for H2122 and HCC4006, 5 mM to HCC95, H1650, H1975 and HCC827. The percentages of surviving cells in relation to controls were determined by CellTiter-Glo cell viability assay, *P < .01. METF, metformin; ERL, erlotinib.
Metformin Modifies Erlotinib Induced Apoptosis in NSCLC Cell Lines Independent of EGFR Gene Status and Signaling
Next, we wanted to examine the molecular signaling responsible for the cytotoxic effects of erlotinib after combinatorial treatment with metformin. Consistent with the cell viability assay, 6 NSCLC cell lines with the same validation settings were chosen for investigation. As shown in Figure 2, A–F, EGFR downstream signaling evaluation indicated that at the 48-hour time point, the inhibition of EGFR phosphorylation (pEGFR) and the inactivation of PARP by cleavage (c-PARP) were both dose-dependent in all erlotinib treated NSCLC cell lines. In contrast, metformin alone, at its IC50, strongly inhibited pEGFR and c-PARP over DMSO-treated control. Value changes were analyzed and quantified in histogram dialogs. Treatment with erlotinib and metformin mediated further suppression of pEGFR in all cell lines but HCC827 cells, where the combination of drugs led to a slight up-regulation of this phospho-protein (Figure 2F). Surprisingly, metformin functioned to revert the effect of c-PARP induced by erlotinib treatment to the control level, suggesting its ability to interfere with erlotinib-triggered apoptosis and shift cells to autophagy (data shown in Figure 6). pEGFR independent triggering of phospho-AKT (pAKT), with dual autophagy–apoptosis regulatory potential, was observed after metformin treatment in EGFR wild type (wt) HCC95 and H2122 cells, as well as EGFR mutant H1650 and HCC4006 cells (Figure 2, A–C and E). This observation is not in line with the definition of the classical anti-apoptotic effect of pAKT. At the indicated erlotinib concentrations, pAKT was not detected or changed in H2122 (EGFR wt, KRAS and LKB1 mutations) and H1975 (T790 M mutation) cells (Figure 2, A and B). However, it has been shown to be down-regulated in an erlotinib dose-dependent manner in HCC95 and the two erlotinib sensitive cell lines HCC4006 and HCC827 (Figure 2, A, E and F). Due to a PTEN deletion in H1650 cells, pAKT is constitutively activated, but there was a slight increase under combinatorial treatment relative to erlotinib single agent at the concentrations of 1 and 10 μM (Figure 2C). In HCC95 cells, metformin enhanced inhibition of the pEGFR, pAKT, and pERK was only observed in combination with 20 μM erlotinib (Figure 2A).
Figure 2.
Metformin modifies erlotinib-induced apoptosis via AKT activation and the inhibition of PARP cleavage. Four erlotinib resistant (HCC95, H2122, H1650, H1975) (A-D) and two sensitive (HCC4006, HCC827) (E and F) NSCLC cell lines were exposed for 48 hours to increasing amounts of erlotinib with and without metformin at its IC50 value. Western blots are shown for PARP, phospho- and total EGFR, AKT, ERK1/2 expression. Tubulin served as a loading control. Histograms show the intensity quantification of pAKT, c PARP and pEGFR in the framed lanes. M, metformin; E, erlotinib.
Figure 6.
Metformin triggers autophagy and attenuates cytotoxic effects induced by erlotinib, paclitaxel or cisplatin. (A) Representative images of autophagy-related LC3B staining of H2122, H1650 and H1975 cells following combination treatment with metformin and erlotinib/paclitaxel/cisplatin. IF was performed on methanol fixed cells with LC3B-specific purified IgG (green), and counterstained with Hoechst 33,342 (blue). CQ, chloroquine. M, metformin; P, paclitaxel; C, cisplatin. Scale bar, 20 μm. (B) Summary of IF results upon combinatorial treatment of NSCLC cell lines with drugs and metformin. The intensity of LC3B staining was scored with a scale 0–4. 0 for absence of staining; 4, CQ-like.
We further investigated whether the cell proliferation indicator ERK1/2 (pERK1/2) would also negatively respond to the cytotoxicity effect of erlotinib in the presence of metformin. The data shows only in two of six cell lines (H2122 and H1650) metformin enhances pERK1/2 expression after combinatorial treatment with erlotinib (Figure 2, B and C). Furthermore, this effect was observed in HCC95 cells when treated with low-dose erlotinib (0.1 and 1 μM) (Figure 2A). Thus, our data argue against the involvement of ERK1/2 activity in metformin-mediated proliferation inhibition, indicating metformin attenuates erlotinib cytotoxicity via activation of pAKT, irrespective of EGFR gene status and pERK1/2.
To further confirm the effect of metformin on erlotinib induced apoptosis, we evaluated the status of caspase 3 (c-caspase 3), a mediator of apoptosis signaling, in these 6 cell lines. The results were similar as for PARP cleavage. As shown in Figure 3, after 48 hours of erlotinib treatment, c-caspase 3 was increased in the investigated cell lines, whereas the addition of metformin inhibited this cleavage. Metformin alone didn't induce caspase 3 cleavage, suggesting that in combination with erlotinib, metformin could modify cell death through AKT activation to abrogate PARP cleavage.
Figure 3.
Modulation of cleaved caspase 3 expression after combinatorial treatment of NSCLC cell lines with metformin and erlotinib. Western blots of caspase 3 in NSCLC HCC95 (A), H2122 (B), H1650 (C), H1975 (D), HCC4006 (E), and HCC827 cells (F). Tubulin served as a loading control. Histograms show the quantification of band intensity. M, metformin; E, erlotinib.
Overall, AKT activation and the abrogation of caspase 3 and PARP cleavage suggest a role of metformin in the modification of erlotinib-induced apoptosis in all tested NSCLC cell lines.
Metformin Attenuates Paclitaxel or Cisplatin Induced Apoptosis in NSCLC Cell Lines
Systemic chemotherapy was taken as the first-line treatment for NSCLC; therefore, we applied metformin in combination with the commonly used chemotherapeutic agents paclitaxel or cisplatin for further investigation. Cell proliferation and apoptosis related indicators (pERK1/2, pAKT and c-PARP) were used as the parameters for this evaluation. As shown in Figure 4, Figure 5, paclitaxel or cisplatin induced PARP cleavage in a dose dependent manner in all 6 cell lines at the 48-hour time point, whereas the addition of metformin mediated an abrogation of c-PARP. Value changes have been analyzed and quantified in histogram dialogs. Moreover, the antagonism between metformin and paclitaxel/cisplatin was also reflected via reciprocal activation of pAKT or pERK1/2, depending on the EGFR mutations and distinct genetic background of the cell lines (Table 1, Figure 4, Figure 5). The modification of apoptosis and enhanced cell proliferation upon metformin treatment could minimize the cytotoxic effect caused by TKIs and first-line drugs for NSCLC patient treatment.
Figure 4.
Metformin in combination with paclitaxel activates AKT, ERK1/2 and inhibits PARP cleavage in NSCLC cells. Four erlotinib resistant (A549, H2122, H1650, H1975) (A-D) and two sensitive (HCC4006, HCC827) (E and F) NSCLC cell lines were exposed for 48 hours to increasing amounts of paclitaxel with and without IC50 value of metformin. Western blots are shown for PARP activation, AKT and ERK1/2 expression and phosphorylation. Tubulin served as a loading control. M, metformin; P, paclitaxel.
Figure 5.
Metformin in combination with cisplatin activates AKT, ERK1/2 and inhibits PARP cleavage in NSCLC cells. Cell line preparation and drug application are as described in Figure 4. C, cisplatin.
This data demonstrate that metformin induced modification of apoptosis is not only triggered in combination with erlotinib, but also with chemotherapeutic agents, which warrants caution when considering metformin for T2D NSCLC patients receiving erlotinib or chemotherapeutic agents such as platinum compounds.
Metformin Triggers Autophagy Interplay With Apoptosis Induced by Erlotinib and Chemotherapeutic Agents in NSCLC Cell Lines
Next we investigated which influence on apoptosis the combinatorial treatment exerts to disrupt the balance between cell death and survival. Metformin has been shown to trigger autophagy [22]. Through examining morphological and biological changes, we analyzed the occurrence of autophagy in drug-treated cells in comparison to chloroquine (CQ) treatment as a positive control. Microtubule-associated protein light chain 3 (LC3B) is a specific marker used to monitor autophagy initiation. Immunofluorescence (IF) analysis revealed that after metformin treatment the intensity of LC3B, shown as a punctate pattern, was markedly increased in all tested cell lines over DMSO (Figure 6, A and B).
While treatment with microtubule inhibiting agent paclitaxel dramatically increased multi-nuclei formation, which is a hallmark of cell death through mitotic catastrophe, this effect was prevented by the combination with metformin (Figure 6A). As documented in Figure 6B, this abrogation of multinucleated cells was clearly seen in H1650 and A549 cells.
Taken together, there is evidence that metformin may directly modify and inhibit drug-induced lung cancer cell apoptosis via enhancing LC3B expression and autophagosome formation to shift cells towards autophagy. This effect might temporarily prevent cancer cells from apoptosis via cellular compensation of energy deprivation, or initiate a resistance to drug treatment.
Combinatorial Treatment of NSCLC/T2D Patients With Metformin and Therapeutic Drugs has Minor Effects on pAKT, pERK1/2 and PARP Cleavage
In our in vitro study we found that the simultaneous delivery of metformin with erlotinib, paclitaxel or cisplatin leads to a modification of apoptosis, shifting cells into an autophagy-cell death interplay. This effect of combinatorial treatment, evaluated by expression analysis of the critical protein-modules pERK1/2, pAKT, c-caspase 3 and c-PARP and by cell viability assay, was obvious.
To investigate whether the observed in vitro effect of modified cell death, reflects the tumor status of NSCLC patients in conjunction with anti-cancer drugs, eight tumor sections from individual patients were used for evaluation (Table 2). Four patients diagnosed with lung cancer were classified as the control group, where chemotherapy was applied as neoadjuvant treatment. The other four lung cancer patients had a preexisting condition of diabetes and were being treated with metformin and chemotherapy at the same time. Paraffin embedded slides were submitted to IHC staining to detect the proliferative marker pERK1/2 and the apoptosis related markers pAKT and c-PARP form. Data show there are no statistically significant differences between the metformin group and the control group (Figure 7). It is worth noting that to meet the surgeon’s requirements, metformin was withdrawn from these patients 2 weeks before their surgery. This is an important distinction between our in vitro data (where all the drugs were given simultaneously) and the analysis of the protein levels from the samples derived after surgery from patients with T2D diabetes.
Figure 7.

Quantification of positive IHC staining of pERK, pAKT and cleaved PARP on primary tumors. Formalin-fixed and paraffin embedded primary tumors from 8 lung cancer patients were IHC-stained to assess the efficiency of pERK, c-PARP and pAKT. The staining with score 3 was taken for evaluation. ns, no significance. METF, metformin.
Discussion
Experimental and clinical studies have shown a survival benefit of metformin used in breast, prostate, and colorectal cancer [13], [20], [23]. However, there is limited and conflicting data regarding the potential effectiveness of this medication for T2D patients with advanced lung cancer in combinatorial chemotherapeutic settings. Previous findings described by Morgillo et al. have shown metformin and gefitinib are synergistic in LKB1 wt NSCLC cells [24]. These data have been supported by Li et al. to show metformin might be used in combination with TKIs in patients with NSCLC harboring EGFR mutations [25].
In our study, assessed by caspase 3 and PARP cleavage, apoptosis induction after metformin treatment was very low, even lower than the control group, which suggested an apoptosis-modification property of metformin (Figure 2, Figure 3). Furthermore, metformin, at its IC50 values, inhibited caspase 3 and PARP cleavage that were induced by different doses of lung cancer drugs, including erlotinib, paclitaxel and cisplatin. Our study reveals that these regulations were tightly connected to metformin dependent AKT activation (Figure 2, Figure 4, Figure 5). pAKT inhibits apoptosis directly through phosphorylation and inactivation of caspase 9, subsequently leading to the abrogation of caspase 3 and 7 activity - initiators of PARP cleavage and apoptosis [26]. These observed combinatorial effects are not dependent on EGFR gene status, suggesting an involvement of other pathways (e.g. insulin receptor, insulin-like growth factor receptor 1, AXL and MET) in activating the molecular signaling of AKT and ERK1/2.
Under basal conditions, the primary function of PARP is to detect and repair DNA damage, resulting in depleted cellular ATP level [27]. AMPK kinase can sensitize low ATP level and be activated in response to metformin treatment, followed by the inactivation of the autophagy inhibitor mTOR, a major nutrient sensor and promoter of growth. In NSCLC cell lines H1650 and A549 metformin has been shown to regulate the balance of death and survival, via strong inhibition of drug-induced multi-nuclei formation (mitotic catastrophe) and simultaneously elevating autophagy marker LC3B expression (Figure 6, A and B). Consequently, these cells have a chance to initiate a transition to resistance. With severe DNA damage, cells have amplified PARP activity that results in high ATP consumption. If unchecked, this activity inevitably leads to passive necrotic cell death [28]. In our case, under drug treatment, this process is blocked by rapid cleavage and inactivation of PARP by the action of caspase 3 (Figure 3).
In this report, we demonstrate for the first time the ability of metformin to modify apoptosis, and even rescue cancer cells from cell death induced by erlotinib, paclitaxel or cisplatin, via autophagy. Our data show that metformin can simultaneously act as a dual mTOR activator and inhibitor in heterogeneous cancer cell populations that were treated with chemotherapeutic drugs. Cancer cell content is playing an even more important role in individual drugs with specific toxicity profiles, like erlotinib, paclitaxel or cisplatin, but metformin can cause unpredictable modifications. These results, obtained in cultured cancer cell lines, should be interpreted with caution, since we didn't observe significant effects of metformin in our pilot study of NSCLC patients with diabetes (Table 2 and Figure 7), where the concentration of metformin was much lower.
At present there are numerous ongoing clinical trials evaluating the response to, and overall survival of, the combination of metformin with standard therapies in patients suffering different cancers. Clinical trials have shown controversial results. A paper published by Smiechowski and colleagues indicated that lung cancer risk is unaffected by metformin in patients with T2D [29]. In our study, we investigated whether the complicated cell-death balance interplay between metformin and chemotherapy also reflects the status of human lung tumors after treatment. Unfortunately, based on the expression of pERK1/2, pAKT and c-PARP, we didn't observe significant differences between our pilot tumor samples (Table 2). This may be because patients were advised to be taken off metformin before surgery. During our investigation, we also found different drug combinations had been used on these cancer patients, and furthermore, the low number of samples would be a drawback for statistical evaluation. Despite strong epidemiological evidence for metformin's anti-cancer properties, there is the possibility that, even if metformin has some ability to prevent cancer, its efficacy may be limited to just several tumor types and it might be beneficent to reconsider the use of phenformin, instead of metformin, particularly in NSCLC with LKB1 deletion and KRAS/p53 mutation [16].
Future perspective studies are required in NSCLC patients to better investigate the effect of metformin action on the specific pathways and the best context in which to use metformin in combination with molecularly targeted agents. On the other hand, it is important for further studies to test whether metformin triggered autophagy can mitigate neurotoxicity and nephrotoxicity in combinatorial chemotherapeutic settings.
Authors Declare: there are no conflicts of interest.
Acknowledgements
We thank Dr. Andreas Roidl, and Dr. Franz Kerek for critically reviewing the manuscript and important suggestions; Bianca Sperl and Dr. Emanuele Zanucco for technical support in the IHC study; Dr. Roman Thomas for providing H2122, H1975, H1650, HCC4006, HCC95, and HCC827 NSCLC cell lines.
References
- 1.Society AC. American Cancer Society Atlanta; GA, USA: 2016. Cancer Facts & Figures 2016. [Google Scholar]
- 2.Kim YH, Shin SW, Kim BS, Kim JH, Kim JG, Mok YJ, Kim CS, Rhyu HS, Hyun JH, Kim JS. Paclitaxel, 5-fluorouracil, and cisplatin combination chemotherapy for the treatment of advanced gastric carcinoma. Cancer. 1999;85:295–301. [PubMed] [Google Scholar]
- 3.Ryu JS, Hong YC, Han HS, Lee JE, Kim S, Park YM, Kim YC, Hwang TS. Association between polymorphisms of ERCC1 and XPD and survival in non-small-cell lung cancer patients treated with cisplatin combination chemotherapy. Lung Cancer. 2004;44:311–316. doi: 10.1016/j.lungcan.2003.11.019. [DOI] [PubMed] [Google Scholar]
- 4.Chahlavi A, Todo T, Martuza RL, Rabkin SD. Replication-competent herpes simplex virus vector G207 and cisplatin combination therapy for head and neck squamous cell carcinoma. Neoplasia. 1999;1:162–169. doi: 10.1038/sj.neo.7900016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Watanabe M, Aoki Y, Tomita M, Sato T, Takaki Y, Kato N, Kikuchi M, Kase H, Tanaka K. Paclitaxel and carboplatin combination chemotherapy in a hemodialysis patient with advanced ovarian cancer. Gynecol Oncol. 2002;84:335–338. doi: 10.1006/gyno.2001.6527. [DOI] [PubMed] [Google Scholar]
- 6.Sharma SV, Bell DW, Settleman J, Haber DA. Epidermal growth factor receptor mutations in lung cancer. Nat Rev Cancer. 2007;7:169–181. doi: 10.1038/nrc2088. [DOI] [PubMed] [Google Scholar]
- 7.Everhart J, Wright D. Diabetes mellitus as a risk factor for pancreatic cancer: a meta-analysis. JAMA. 1995;273:1605–1609. [PubMed] [Google Scholar]
- 8.Hu FB, Manson JE, Liu S, Hunter D, Colditz GA, Michels KB, Speizer FE, Giovannucci E. Prospective study of adult onset diabetes mellitus (type 2) and risk of colorectal cancer in women. J Natl Cancer Inst. 1999;91:542–547. doi: 10.1093/jnci/91.6.542. [DOI] [PubMed] [Google Scholar]
- 9.Weiderpass E, Gridley G, Nyrén O, Ekbom A, Persson I, Adami H-O. Diabetes mellitus and risk of large bowel cancer. J Natl Cancer Inst. 1997;89:660–661. doi: 10.1093/jnci/89.9.660. [DOI] [PubMed] [Google Scholar]
- 10.Bowker SL, Majumdar SR, Veugelers P, Johnson JA. Increased cancer-related mortality for patients with type 2 diabetes who use sulfonylureas or insulin. Diabetes Care. 2006;29:254–258. doi: 10.2337/diacare.29.02.06.dc05-1558. [DOI] [PubMed] [Google Scholar]
- 11.Vigneri P, Frasca F, Sciacca L, Pandini G, Vigneri R. Diabetes and cancer. Endocr Relat Cancer. 2009;16:1103–1123. doi: 10.1677/ERC-09-0087. [DOI] [PubMed] [Google Scholar]
- 12.Dunn CJ, Peters DH. Metformin. Drugs. 1995;49:721–749. doi: 10.2165/00003495-199549050-00007. [DOI] [PubMed] [Google Scholar]
- 13.Anisimov VN, Berstein LM, Egormin PA, Piskunova TS, Popovich IG, Zabezhinski MA, Kovalenko IG, Poroshina TE, Semenchenko AV, Provincialic M. Effect of metformin on life span and on the development of spontaneous mammary tumors in HER-2/neu transgenic mice. Exp Gerontol. 2005;40:685–693. doi: 10.1016/j.exger.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 14.Kato K, Gong J, Iwama H, Kitanaka A, Tani J, Miyoshi H, Nomura K, Mimura S, Kobayashi M, Aritomo Y. The antidiabetic drug metformin inhibits gastric cancer cell proliferation in vitro and in vivo. Mol Cancer Ther. 2012;11:549–560. doi: 10.1158/1535-7163.MCT-11-0594. [DOI] [PubMed] [Google Scholar]
- 15.Sahra IB, Regazzetti C, Robert G, Laurent K, Marchand-Brustel Y, Auberger P, Tanti JF, Giorgetti-Peraldi S, Bost F. Metformin, independent of AMPK, induces mTOR inhibition and cell-cycle arrest through REDD1. Cancer Res. 2011;71:4366–4372. doi: 10.1158/0008-5472.CAN-10-1769. [DOI] [PubMed] [Google Scholar]
- 16.Shackelford DB, Abt E, Gerken L, Vasquez DS, Seki A, Leblanc M, Wei L, Fishbein MC, Czernin J, Mischel PS. LKB1 inactivation dictates therapeutic response of non-small cell lung cancer to the metabolism drug phenformin. Cancer Cell. 2013;23:143–158. doi: 10.1016/j.ccr.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mulligan AM, O’Malley FP, Ennis M, Fantus IG, Goodwin PJ. Insulin receptor is an independent predictor of a favorable outcome in early stage breast cancer. Breast Cancer Res Treat. 2007;106:39–47. doi: 10.1007/s10549-006-9471-x. [DOI] [PubMed] [Google Scholar]
- 18.Dowling RJ, Goodwin PJ, Stambolic V. Understanding the benefit of metformin use in cancer treatment. BMC Med. 2011;9:33. doi: 10.1186/1741-7015-9-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gotlieb WH, Saumet J, Beauchamp M-C, Gu J, Lau S, Pollak MN, Bruchim I. In vitro metformin anti-neoplastic activity in epithelial ovarian cancer. Gynecol Oncol. 2008;110:246–250. doi: 10.1016/j.ygyno.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 20.Iliopoulos D, Hirsch HA, Struhl K. Metformin decreases the dose of chemotherapy for prolonging tumor remission in mouse xenografts involving multiple cancer cell types. Cancer Res. 2011;71:3196–3201. doi: 10.1158/0008-5472.CAN-10-3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xiao Z, Sperl B, Ullrich A, Knyazev P. Metformin and salinomycin as the best combination for the eradication of NSCLC monolayer cells and their alveospheres (cancer stem cells) irrespective of EGFR, KRAS, EML4/ALK and LKB1 status. Oncotarget. 2014;5:12877–12890. doi: 10.18632/oncotarget.2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu X, Chhipa RR, Pooya S, Wortman M, Yachyshin S, Chow LM, Kumar A, Zhou X, Sun Y, Quinn B. Discrete mechanisms of mTOR and cell cycle regulation by AMPK agonists independent of AMPK. Proc Natl Acad Sci U S A. 2014;111:E435–E444. doi: 10.1073/pnas.1311121111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sahra IB, Laurent K, Loubat A, Giorgetti-Peraldi S, Colosetti P, Auberger P, Tanti J, Marchand-Brustel YL, Bost F. The antidiabetic drug metformin exerts an antitumoral effect in vitro and in vivo through a decrease of cyclin D1 level. Oncogene. 2008;27:3576–3586. doi: 10.1038/sj.onc.1211024. [DOI] [PubMed] [Google Scholar]
- 24.Morgillo F, Sasso FC, Della Corte CM, Vitagliano D, D'aiuto E, Troiani T, Martinelli E, Vita FD, Orditura M, Palmaet RD. Synergistic effects of metformin treatment in combination with gefitinib, a selective EGFR tyrosine kinase inhibitor, in LKB1 wild-type NSCLC cell lines. Clin Cancer Res. 2013;19:3508–3519. doi: 10.1158/1078-0432.CCR-12-2777. [DOI] [PubMed] [Google Scholar]
- 25.Li L, Han R, Xiao H, Lin C, Wang Y, Liu H, Li K, Chen H, Sun F, Yang Z. Metformin sensitizes EGFR-TKI–resistant human lung cancer cells in vitro and in vivo through inhibition of IL-6 signaling and EMT reversal. Clin Cancer Res. 2014;20:2714–2726. doi: 10.1158/1078-0432.CCR-13-2613. [DOI] [PubMed] [Google Scholar]
- 26.Pu X, Storr SJ, Zhang Y, Rakha EA, Green AR, Ellis IO, Martin SG. Caspase-3 and caspase-8 expression in breast cancer: caspase-3 is associated with survival. Apoptosis. 2016;22:357–368. doi: 10.1007/s10495-016-1323-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chaitanya GV, Alexander JS, Babu PP. PARP-1 cleavage fragments: signatures of cell-death proteases in neurodegeneration. Cell Commun Signal. 2010;8:31. doi: 10.1186/1478-811X-8-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eguchi Y, Shimizu S, Tsujimoto Y. Intracellular ATP levels determine cell death fate by apoptosis or necrosis. Cancer Res. 1997;57:1835–1840. [PubMed] [Google Scholar]
- 29.Smiechowski BB, Azoulay L, Yin H, Pollak MN, Suissa S. The use of metformin and the incidence of lung cancer in patients with type 2 diabetes. Diabetes Care. 2013;36:124–129. doi: 10.2337/dc12-0740. [DOI] [PMC free article] [PubMed] [Google Scholar]