History
There are approximately 12,500 spinal cord injuries in the US annually [15], with global incidence ranging from 133,000 to 226,000 cases annually [8]. The cost associated with spinal cord injuries is substantial, with estimates of the average lifetime cost of direct care ranging from USD 1.5 to 4.7 million [15]. Motor vehicles are the leading cause of injury, and are becoming an increasingly common cause in developing nations [15]. The demographics of patients with spinal cord injuries have broadened with time, however, males still account for 80% of new patients with spinal cord injuries [15].
The American Spinal Injury Association was created in 1973 to facilitate the exchange of research, data, and ideas among practitioners involved in the treatment of patients with spinal cord injuries. Its founders sought to establish a standardized model of care for the growing number of patients with spinal cord injuries. Before this, the Frankel scale had been developed to categorize spinal cord injuries [3]. However, the Frankel scale had considerable limitations. It did not specify the level of spine injury in its classification. It also did not define the difference between ‘motor useful’ and ‘motor useless’ grades, leading to subjective grading. [17]. In 1982, the American Spinal Injury Association published the International Standards for Neurological Classification of Spinal Injury [1], a grading and classification system that would evolve into the current American Spinal Injury Association Impairment Scale (AIS) [7]. Among its notable contributions, the International Standards for Neurological Classification of Spinal Injury classification helped identify key muscle groups and sensory points that improved practitioners’ precision at identifying neurologic levels of injury. In addition, it was a reproducible classification with detailed descriptions of each sensory and motor grade. This allowed accurate characterization of incomplete and complete spinal cord injuries [9].
The AIS replaced the modified Frankel scale and became the international gold standard for evaluation of spinal cord injuries [17]. Since its inception, the AIS has been revised multiple times as its authors continue to refine the steps of the neurologic examination and details of the classification grades. These revisions have improved reproducibility of the AIS and allowed for better understanding of the scale’s therapeutic and implications [17].
Purpose
The purpose of the AIS is to (1) standardize careful, detailed documentation of spinal cord injuries, (2) guide further radiographic assessment and treatment, and (3) determine whether injuries are complete or incomplete—an important and often subtle neurologic distinction that has tremendous prognostic implications [17].
In addition to standardizing practice and aiding research, the AIS has practical clinical utility. The AIS can help providers answer difficult questions such as “will the patient ever walk again?” [17]. The AIS also may help predict recovery of autonomic functions such as bowel, bladder, cardiovascular, respiratory, and reproductive functions, although this remains a topic for further study [17].
Description of the AIS
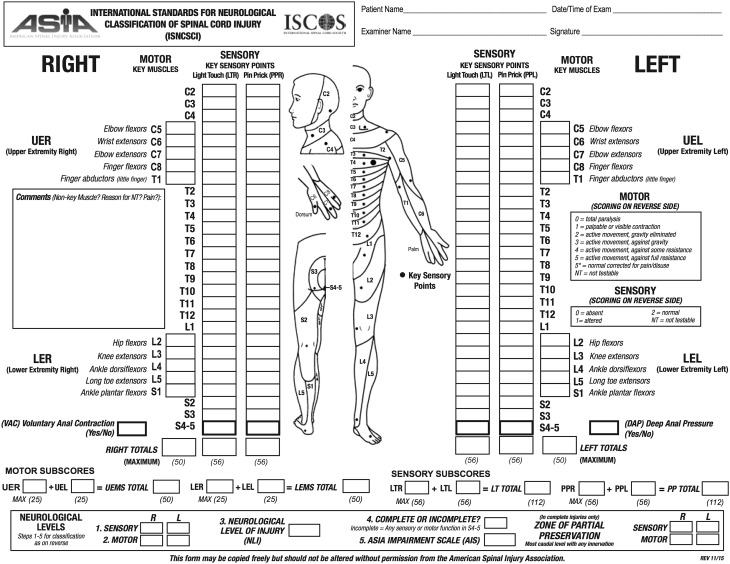
The AIS is a standardized examination consisting of a myotomal-based motor examination, dermatomal based sensory examination, and an anorectal examination. Based on the findings of these examinations, an injury severity or grade and level are assigned (Fig. 1).
Fig. 1.
The American Spinal Injury Association International Standards for Neurological Classification of Spinal Cord Injury form used to evaluate spinal cord injury is presented. (American Spinal Injury Association. International Standards for Neurological Classification of Spinal Cord Injury. Atlanta, GA, Revised 2011, Updated 2015. Published with permission of the American Spinal Injury Association, Richmond, VA, USA.)
The sensory examination evaluates 28 specific dermatomes bilaterally for light touch (generally a piece of cotton) and pinprick (generally a clean safety pin) sensation. Each examination component is recorded for each dermatome and laterality. A grade of 0 denotes absent sensation, 1 denotes impaired or altered sensation, and 2 denotes normal sensation. A normal unilateral sensory examination consists of 28 dermatomes each with 2/2 points for light touch and 2/2 points for pinprick, yielding 112 total points. A total score of 224 bilaterally is a fully normal sensory examination. Inability to distinguish pinprick sensation from light touch is technically graded as 0 [11].
The motor examination consists of grading five specific muscle groups in the upper extremities and five specific muscle groups in the lower extremities, representing major cervical and lumbar myotomes (Table 1). Motor strength is graded using a universal six-point scale (graded as 0–5) (Table 2). Motor strength is recorded for each muscle group bilaterally. The maximum bilateral motor score in a healthy individual is 100, 50 for scoring 5/5 in all right upper and lower extremity myotomes, and another 50 for the left.
Table 1.
Key myotomes and dermatomes for extremity neurologic testing
| Upper extremity | Lower extremity | ||||||
|---|---|---|---|---|---|---|---|
| Root | Functional group | Myoterm | Dermatome | Root | Functional group | Myoterm | Dermatome |
| C5 | Elbow flexors | Biceps, brachialis | Lateral shoulder | L2 | Hip flexors | Iliopsoas | Anterior mid-thigh |
| C6 | Wrist extensors | Extensor carpi radialis longus, Extensor carpi radialis brevis | Lateral forearm, dorsum of thumb and index finger | L3 | Knee extensors | Quadriceps | Anterior knee |
| C7 | Elbow extensors | Triceps | Dorsum of middle finger | L4 | Ankle dorsiflexors | Tibialis Anterior | Medial leg and medial malleolus |
| C8 | Finger flexors | Flexor digitorum profundus (middle finger) | Dorsum of ring and small finger | L5 | Long toe extensors | Extensor halluces longus | Lateral leg, medial dorsum foot |
| T1 | Finger abductors | Abductor digiti minimi (small finger) | Medial forearm | S1 | Ankle plantar flexors | Gastrocsoleus complex | Distal calf, lateral plantar foot |
Table 2.
Muscle strength and sensory grading
| Muscle function grading | Sensory grading |
| 0 = total paralysis | 0 = Absent (or inability to tell sharp from dull) |
| 1 = palpable or visible contraction | 1 = Altered, either decreased or impaired sensation or hypersensitivity |
| 2 = active movement, full ROM with gravity eliminated | 2 = Normal |
| 3 = active movement, full ROM against gravity | NT = Not testable |
| 4 = active movement, full ROM against gravity and moderate resistance in a muscle specific position | |
| 5 = (normal) active movement, full ROM against gravity and full resistance in a functional muscle position expected from an otherwise unimpaired person 5* = (normal) active movement, full ROM against gravity and sufficient resistance to be considered normal if identified inhibiting factors (ie, pain, disuse) were not present |
|
| NT = not testable (ie, attributable to immobilization, severe pain such that the patient cannot be graded, amputation of limb, or contracture greater than 50% of the normal ROM) |
The additional anorectal examination is essential for determining the completeness of the injury and evaluating for the presence of spinal shock. The external anal sphincter is examined digitally for voluntary motor contraction and the ability to sense deep anal pressure. Both are graded in a binary fashion, 0 for absent and 1 for present. The bulbocavernosus reflex is assessed via digital rectal examination, during which a palpable internal and external anal sphincter contraction occurs in response to squeezing the glans penis or clitoris. Tugging on an indwelling urinary catheter also may elicit the reflex.
The AIS also includes the level of neurologic injury in its classification. As stated, this is defined as the most caudal functioning root level with intact sensation and Grade 3 or greater motor function; however, the lowest normal sensory level may be substituted in regions without readily testable myotomes (such as in the thoracic spine).
The AIS further classifies injuries as a complete or incomplete spinal cord injury. A complete spinal cord injury is defined as the absence of all motor and sensory functions, including sacral roots, distal to the site of injury. These injuries are designated as being Grade A on the AIS. Incomplete injuries are defined as those with some degree of retained motor or sensory function below the site of injury. These are graded B through E on the AIS (Table 3). Patients with AIS Grade B injuries have some sensory function but no motor function. AIS Grade C injuries have a motor grade less than 3 below the neurologic level of injury while AIS Grade D injuries have a motor grade of at least 3 below the neurologic level of injury. Patients with Grade E injuries have normal motor and sensory examinations, but still may have abnormal reflexes or other neurologic phenomena [17].
Table 3.
American Spinal Injury Association Impairment Scale
| A | Complete | No motor or sensory function is preserved in the sacral segments S4–S5. |
| B | Incomplete | Sensory function preserved but not motor function is preserved below the neurological level and includes the sacral segments S4–S5. |
| C | Incomplete | Motor function is preserved below the neurological level, and more than half of key muscles below the neurological level have a muscle grade less than 3. |
| D | Incomplete | Motor function is preserved below the neurological level, and at least half of key muscles below the neurological level have a muscle grade of 3 or more. |
| E | Normal | Motor and sensory function are normal. |
The determination of a complete or incomplete spinal cord injury requires resolution of spinal shock. Spinal shock is a physiologic response to trauma that is marked by initial depolarization of axonal tissue immediately after injury. During spinal shock, the patient exhibits a transient period of flaccid paralysis during which time he or she is areflexic. Notably, this includes absence of the bulbocavernosus reflex. After return of this reflex, the patient can be assessed accurately for complete versus incomplete injury. Finally, a complete and meaningful examination cannot be performed in patients with altered or limited consciousness (such as might occur with intoxication, head injury, intubation) or in the presence of an untreated major distracting injury.
Validation
Multiple studies investigating the intra- and interobserver reliability of the AIS show overall good reliability for motor and sensory (pin prick and light touch) testing [2, 12–14, 16, 22]. Correlation coefficients for intra- and interobserver motor and sensory assessment generally are quoted as 0.90 or greater, which reflects generally high agreement [4, 14]. Incomplete injuries tend to exhibit weaker intra- and interobserver correlations than those with more “cut-and-dry” complete injuries [22].
The AIS has strong prognostic value that has been shown across various functional outcomes [18, 19, 21]. van Middendorp et al. [18] reported excellent predictive values of the AIS scores regarding predicting independent ambulation at 1 year. They found that patients with AIS Grade A injuries have a 91.7% (95% CI, 87.4%–94.8%) negative predictive probability for independent ambulatory ability, whereas those with AIS Grade D injuries have a 97.3% (95% CI, 92.2%–99.4%) positive predictive probability of regaining independent ambulation at 1 year [18]. van Middendorp et al. [18, 20] reported superiority of these prognostic factors over the clinical practice of distinguishing injuries as “complete” or “incomplete;” however, the prognostic accuracy of Grades B and C injuries are considerably less consistent. The presence of postinjury somatosensory evoked potentials in the tibial nerve, among others, have been strongly related to ambulatory outcomes. However, neurophysiologic testing does not offer additional prognostic accuracy over information gleaned from the AIS examination [5].
Diagnosis of an AIS Grade A injury after resolution of spinal shock has a remarkably strong—albeit unfortunate—correlation with future inability to regain functional motor capacity [6, 21]. Kirshblum et al. [6] presented a longitudinal study of patients with spinal cord injury and found that only 2.1% patients with a complete injury improved to having an incomplete injury by 5 years.
When examining the AIS by its components, the lowest rates of intra- and interobserver reliability involve assessment of anorectal function and determination of sacral sparing [14, 22]. van Middendorp et al. [20] reported that the anorectal examination and presence of sacral sparing correlated only with prognosis in the setting of chronic injury; in the acute phase, where such functions may be masked by unrecognized spinal shock, reliability is diminished [20].
Limitations
The most fundamental limitation of the AIS may be obvious given its title: it is an impairment scale that does not report the objective anatomic nature of the causal injury. It also does not determine injury severity. For example, a complete AIS Grade A injury in the lower lumbar spine can lead to bowel or bladder dysfunction with foot-drop, but an otherwise ambulatory and independently functional lifestyle. By contrast, the ostensibly less severe AIS Grade C or D injury in the upper cervical spine can still render patients quadriplegic and largely dependent on support. In its defense, use of the AIS grade without an associated level is not its intended application.
A second criticism of the AIS is that it does not account for pain, spasticity, or dysesthesia that might result from spinal cord injury, but only the ability to sense pinprick and light touch. In reality, patients with AIS Grade E injuries may score as having “normal” motor and sensory function but still show marked disability from such neurologic phenomena.
A third limitation of the AIS is that, with a few notable exceptions [12, 22], a study of its reliability is limited to adult patients [10]. It has been shown that children as young as 6 years are able to consistently comply with the examination, however, this was evaluated only in patients with chronic spinal cord injuries [13]. It is likely that in patients with an acute injury, the ability of the patient to comply with the examination would be limited by stress, pain, and the presence of medications.
Finally, we do not know the minimal clinically important difference of the AIS [10]. In other words, there is not an agreed-upon threshold above which a given medical or surgical intervention can be clearly said to be beneficial. While numerous authors agree that improvement in a single AIS letter grade is a substantial and desirable improvement [16, 20], gaining a couple of points for one’s numerical American Spinal Injury Association score may not result in tangible benefits. In addition, not all points are created equally: gaining a motor grade or two in a key muscular group may be the difference between ambulating and requiring a wheelchair; however, gaining the same number of points in one’s thoracic sensory levels, for example, is less likely to make a tangible effect on quality of life.
Conclusions and Uses
A spinal cord injury is a relatively common occurrence with devastating complications. When examining a patient with spinal cord injuries, a detailed and carefully performed neurologic assessment is paramount. Testing consists of motor strength and sensory function at various key myotomes and dermatomes. The purpose of the AIS is to (1) standardize careful, detailed documentation of an injury, (2) guide further radiographic assessment and treatment, and (3) determine whether injuries are complete or incomplete, an important and sometimes subtle neurologic distinction that has tremendous prognostic implications. Complete spinal cord injuries are defined by the absence of all motor and sensory functions below the site of injury, whereas incomplete spinal cord injuries will retain variable motor and/or sensory function. Diagnosing complete or incomplete spinal cord injuries requires resolution of spinal shock. The AIS classification has tremendous prognostic value. This allows for better patient counseling regarding expectations of recovery. It also precisely defines the level and degree of a patient’s deficit, allowing treatments and therapy to be tailored to a patient’s individual needs.
Footnotes
Each author certifies that he or she, or a member of his or her immediate family, has no funding or commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research ® editors and board members are on file with the publication and can be viewed on request.
References
- 1.Association American Spinal Injury. Standards for Neurological Classification of Spinal Injury Patients. Chicago, IL: American Spinal Injury Association; 1982. [Google Scholar]
- 2.Chafetz R, Gaughan J, Vogel L, Betz R, Mulcahey MJ. The International Standards for Neurological Classification of Spinal Cord Injury: intra-rater agreement of total motor and sensory scores in the pediatric population. J Spinal Cord Med. 2009;32:157–161. doi: 10.1080/10790268.2009.11760767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frankel HL, Hancock DO, Hyslop G, Melzak J, Michaelis LS, Ungar GH, Vernon JD, Walsh JJ. The value of postural reduction in the initial management of closed injuries of the spine with paraplegia and tetraplegia. I. Paraplegia. 1969;7:179–192. doi: 10.1038/sc.1969.30. [DOI] [PubMed] [Google Scholar]
- 4.Furlan JC, Noonan V, Singh A, Fehlings MG. Assessment of impairment in patients with acute traumatic spinal cord injury: a systematic review of the literature. J Neurotrauma. 2011;28:1445–1477. doi: 10.1089/neu.2009.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jacobs SR, Yeaney NK, Herbison GJ, Ditunno JF., Jr Future ambulation prognosis as predicted by somatosensory evoked potentials in motor complete and incomplete quadriplegia. Arch Phys Med Rehabil. 1995;76:635–641. doi: 10.1016/S0003-9993(95)80632-6. [DOI] [PubMed] [Google Scholar]
- 6.Kirshblum S, Millis S, McKinley W, Tulsky D. Late neurologic recovery after traumatic spinal cord injury. Arch Phys Med Rehabil. 2004;85:1811–1817. doi: 10.1016/j.apmr.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 7.Kirshblum S, Waring W 3rd. Updates for the International Standards for Neurological Classification of Spinal Cord Injury. Phys Med Rehabil Clin N Am. 2014;25:505–517, vii. [DOI] [PubMed]
- 8.Lee BB, Cripps RA, Fitzharris M, Wing PC. The global map for traumatic spinal cord injury epidemiology: update 2011, global incidence rate. Spinal Cord. 2014;52:110–116. doi: 10.1038/sc.2012.158. [DOI] [PubMed] [Google Scholar]
- 9.Marino RJ. Introduction. Reference Manual for the International Standards for Neurological Classification of Spinal Cord Injury. Chicago, IL: American Spinal Injury Association; 2003:1–6.
- 10.Marino RJ, Jones L, Kirshblum S, Tal J, Dasgupta A. Reliability and repeatability of the motor and sensory examination of the international standards for neurological classification of spinal cord injury. J Spinal Cord Med. 2008;31:166–170. doi: 10.1080/10790268.2008.11760707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maynard FM, Jr, Bracken MB, Creasey G, Ditunno JF, Jr, Donovan WH, Ducker TB, Garber SL, Marino RJ, Stover SL, Tator CH, Waters RL, Wilberger JE, Young W. International Standards for Neurological and Functional Classification of Spinal Cord Injury: American Spinal Injury Association. Spinal Cord. 1997;35:266–274. doi: 10.1038/sj.sc.3100432. [DOI] [PubMed] [Google Scholar]
- 12.Mulcahey MJ, Gaughan J, Betz RR, Johansen KJ. The International Standards for Neurological Classification of Spinal Cord Injury: reliability of data when applied to children and youths. Spinal Cord. 2007;45:452–459. doi: 10.1038/sj.sc.3101987. [DOI] [PubMed] [Google Scholar]
- 13.Mulcahey MJ, Gaughan J, Betz R, Vogel L. Rater agreement on the ISCSCI motor and sensory scores obtained before and after formal training in testing technique. J Spinal Cord Med. 2007;30(suppl 1):S146–149. [PMC free article] [PubMed] [Google Scholar]
- 14.Mulcahey MJ, Gaughan JP, Chafetz RS, Vogel LC, Samdani AF, Betz RR. Interrater reliability of the International Standards for Neurological Classification of Spinal Cord Injury in youths with chronic spinal cord injury. Arch Phys Med Rehabil. 2011;92:1264–1269. doi: 10.1016/j.apmr.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 15.National Spinal Cord Injury Statistical Center. Facts and Figures at a Glance. Birmingham, AL: University of Alabama at Birmingham, 2015. Available at: http://www.msktc.org/lib/docs/Data_Sheets_/MSKTC_SCIMS_Fact_Fig_2015.pdf. Accessed June 3, 2016.
- 16.Samdani A, Chafetz R, Vogel L, Betz R, Gaughan J, Mulcahey MJ. The International Standards for Neurological Classification of Spinal Cord Injury: relationship between S4-5 dermatome testing and anorectal testing. Spinal Cord. 2010;49:352–356. doi: 10.1038/sc.2010.144. [DOI] [PubMed] [Google Scholar]
- 17.van Middendorp JJ, Goss B, Urquhart S, Atresh S, Williams RP, Schuetz M. Diagnosis and prognosis of traumatic spinal cord injury. Global Spine J. 2011;1:1–8. doi: 10.1055/s-0031-1296049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Middendorp JJ, Hosman AJ, Donders AR, Pouw MH, Ditunno JF Jr, Curt A, Guerts AC, Van de Meent H; EM-SCI Study Group. A clinical prediction rule for ambulation outcomes after traumatic spinal cord injury: a longitudinal cohort study. Lancet. 2011;377;1004–1010. [DOI] [PubMed]
- 19.van Middendorp JJ, Hosman AJ. Pouw MH; EM-SCI Study Group, Van de Meent H. ASIA impairment scale conversion in traumatic SCI: is it related with the ability to walk? A descriptive comparison with functional ambulation outcome measures in 273 patients. Spinal Cord. 2009;47:555–560. doi: 10.1038/sc.2008.162. [DOI] [PubMed] [Google Scholar]
- 20.van Middendorp JJ, Hosman AJ. Pouw MH; EM-SCI Study Group, Van de Meent H. Is determination between complete and incomplete traumatic spinal cord injury clinically relevant? Validation of the ASIA sacral sparing criteria in a prospective cohort of 432 patients. Spinal Cord. 2009;47:809–816. doi: 10.1038/sc.2009.44. [DOI] [PubMed] [Google Scholar]
- 21.Vazquez XM, Rodriguez MS, Peñaranda JM, Concheiro L, Barus JI. Determining prognosis after spinal cord injury. J Forensic Leg Med. 2008;15:20–23. doi: 10.1016/j.jflm.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 22.Vogel L, Samdani A, Chafetz R, Gaughan J, Betz R, Mulcahey MJ. Intra-rater agreement of the anorectal exam and classification of injury severity in children with spinal cord injury. Spinal Cord. 2009;47:687–691. doi: 10.1038/sc.2008.180. [DOI] [PubMed] [Google Scholar]