Abstract
Background
Exploration of the complex relationship between prognostic indicators such as tumor grade and size and clinical outcomes such as local recurrence and distant metastasis in patients with cancer is crucial to guide treatment decisions. However, in patients with soft tissue sarcoma, there are many gaps in our understanding of this relationship. Multistate analysis may help us in gaining a comprehensive understanding of risk factor-outcome relationships in soft tissue sarcoma, because this methodology can integrate multiple risk factors and clinical endpoints into a single statistical model. To our knowledge, no study of this kind has been performed before in patients with soft tissue sarcoma.
Questions/purposes
We implemented a multistate model of localized soft tissue sarcoma to statistically evaluate the relationship among baseline risk factors, recurrence, and death in patients with localized soft tissue sarcoma undergoing curative surgery.
Methods
Between 1998 and 2015, our center treated 539 patients for localized soft tissue sarcoma with surgery as curative intent. Of those, 96 patients (18%) were not included in this single-center retrospective study owing to missing baseline histopathology data (n = 3), not yet observed followup (n = 80), or because a neoadjuvant treatment approach in the presence of synchronous distant metastasis was used (n = 13), leaving 443 patients (82%) for the current analysis, of which 40 were lost to followup during the first year after surgery. All patients had tumors of the stages I to III according to the American Joint Committee on Cancer Stages. The median age of the patients was 62 years (range, 16–96 years), and 217 patients (49%) were female. Three hundred-forty-six patients (78%) had tumors of high grade (Grades 2 and 3), and 310 (70%) tumors were greater than 5 cm in maximum diameter. Patients who had died during the first year of followup were included in this analysis. Median followup for the 443 study patients was 6 years, with 84%, 52%, and 23% of patients being followed for more than 1, 5, and 10 years, respectively. The 15-year cumulative incidences of local recurrence, distant metastasis, and death from any cause, using a competing risk analysis, were 16% (95% CI, 11%–22%), 21% (95% CI, 17%–26%), and 55% (95% CI, 44%–67%), respectively. Wide resection with a margin of 1 mm was the preferred treatment for all patients, except for those with Grade 1 liposarcoma where a marginal resection was considered adequate. Multistate models were implemented with the mstate library in R.
Results
In multistate analysis, patients who experienced a local recurrence were more likely to have distant metastasis develop (hazard ratio [HR] = 8.4; 95% CI, 4.3–16.5; p < 0.001), and to die (HR = 3.4; 95% CI, 2.1–5.6; p < 0.001). The occurrence of distant metastasis was associated with a strong increase in the risk of death (HR = 12.6; 95% CI, 8.7–18.3; p < 0.001). Distant metastasis occurring after a long tumor-free interval was not associated with a more-favorable prognosis with respect to mortality than distant metastasis occurring early after surgery (estimated relative decrease in the adverse effect of distant metastasis on mortality for 1-year delay in the occurrence of distant metastasis = 0.9; 95% CI, 0.7-1.1; p = 0.28). High-grade histology (Grades 2 and 3) was associated with a higher risk of overall recurrence (defined as a composite of local recurrence and distant metastasis, HR = 3.8; 95% CI, 1.8–7.8; p = 0.0003) and a higher risk of death after recurrence developed (HR = 4.4; 95% CI, 1.1–18.2; p = 0.04). Finally, the multistate model predicted distinct outcome patterns depending on baseline covariates and how long a patient has remained free from recurrence after surgery.
Conclusions
In patients with localized soft tissue sarcoma undergoing resection, the occurrence of local recurrence and distant metastasis contributes to a dramatically impaired long-term survival outcome. Local recurrences are a substantial risk factor for distant metastasis. Multistate modeling is a very powerful approach for analysis of sarcoma cohorts, and may be used in the future to obtain highly personalized, dynamic predictions of outcomes in patients with localized soft tissue sarcoma.
Level of Evidence
Level III, therapeutic study
Keywords: Local Recurrence, Distant Metastasis, Soft Tissue Sarcoma, High Tumor Grade, Liposarcoma
Introduction
Localized soft tissue sarcoma comprises a heterogenic spectrum of malignant tumors of mesenchymal origin [5]. Although the majority of patients with localized soft tissue sarcoma are cured by surgical resection, long-term survival outcomes are a complex function of baseline tumor risk factors, preexisting medical comorbidities, and the occurrence of local recurrence and/or distant metastasis during followup [13]. A precise quantitative understanding of the epidemiologic relationship between these risk factors and outcomes is a prerequisite for targeting adjuvant treatment strategies to the patients with the greatest clinical benefit while sparing patients at low risk from unnecessary treatment toxicity. A better understanding of the epidemiology of soft tissue sarcoma after surgical resection also may help clinicians in answering patients’ questions regarding prognostic issues, and perhaps to adapt aftercare schedules to a patient’s individual recurrence risk profile.
Although clinicians have to integrate multiple prognostic elements of baseline risk factors and future recurrence risk into a rational treatment decision [5], the statistical analysis of soft tissue sarcoma outcomes currently relies on the estimation of event probabilities and regression coefficients for a single endpoint such as time to recurrence or time to death from soft tissue sarcoma [10]. These single endpoint analyses are powerful for defining the relationship between baseline risk factors such as high tumor grade and clinical outcomes, as was recently shown by the development of two nomograms for prediction of distant metastasis and overall survival in patients with soft tissue sarcoma [1]. However, these analyses did not incorporate potentially relevant prognostic information from intermediate events that occur during followup such as local recurrence and distant metastasis [8]. Furthermore, the risk of soft tissue sarcoma recurrence decreases with time elapsed from surgery [9], which also may add information for outcome prediction that is ignored by single-endpoint analyses using only baseline information. Another important quantitative issue is that patients with soft tissue sarcoma are often older and may die from competing nonsoft tissue sarcoma-related causes without progression, thus preventing recurrences from being observed [10]. Together, these competing risks and interdependencies between risk factors and outcomes complicate the interpretation of clinical research data in localized soft tissue sarcoma and impair the translation of these results into better treatment decisions.
In this study, we aim to condense the statistical analysis of baseline risk factors and multiple soft tissue sarcoma-related endpoints into one joint statistical representation, the multistate model. Multistate modeling may help us gain a more comprehensive understanding of the clinical course of patients with soft tissue sarcoma after surgery, because this analysis statistically dissects complex risk-factor endpoint-relationships into its individual components [11]. Modeling results for these components then may be used to quantify the association between two outcomes, or to obtain time-updated, “dynamic” outcome predictions. To our knowledge, no such multistate analysis has been performed in the soft tissue sarcoma setting.
Specifically, we wished to determine whether the clinical course of patients with localized soft tissue sarcoma after curative surgery represents a multistate disease process in which baseline risk factors and intermediate events jointly contribute to long-term survival outcomes. We then implemented multistate models to quantify the associations of (1) local recurrence with the risk of distant metastasis and death; (2) distant metastasis and overall recurrence with the risk of death; and (3) baseline risk factors such as high tumor grade and size greater than 5 cm with the risks of transition between these events.
Patients and Methods
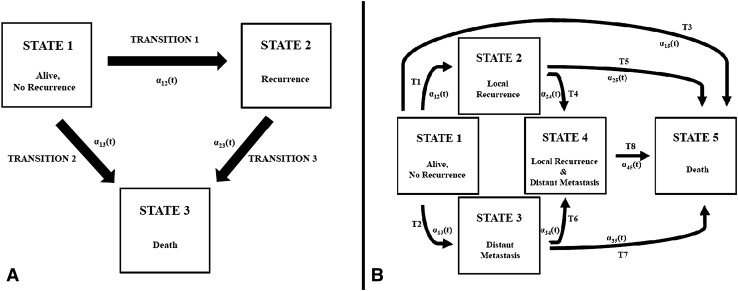
Multistate models are composed of at least three states and the respective transitions between these states [11]. Patients occupy a state until the occurrence of an event, in which they transition to the next state. Multistate modeling is concerned with estimation of the transition hazards, that is, the epidemiologic forces that shift a patient from one state to the other. In this study, we formulate two types of multistate models for localized soft tissue sarcoma. The first model type is a five-state, eight-transition model incorporating local recurrence, distant metastasis, and death (Fig. 1A). This model reflects the full clinical course of patients with soft tissue sarcoma after surgery and is used to estimate transition hazards and state occupation probabilities with time. With this model, we achieved the aim of prediction, that is, to obtain personalized multistate outcome predictions. The second model type is a unidirectional illness death model with three states and three transitions (Fig. 1B). We used this model for estimation. In detail it is used to quantify the contribution of local recurrence toward the risk of distant metastasis and death, and distant metastasis on the risk of death.
Fig. 1A–B.
Prespecification of two types of multistate models for the clinical course of patients with soft tissue sarcoma after curative surgery is presented. The transition hazards for the respective transitions between the states are αxy(t). (A) Multistate model Type 1 is a three-state, three-transition unidirectional illness death model, with States 1, 2, and 3 representing initial, transient, and absorbing states, respectively. In State 1, patients are alive and free from disease after curative surgery. They can remain in this initial state, transition to intermediate State 2 (Transition 1), or transition to the absorbing State 3 (death) either directly from State 1 (Transition 2) or from State 2. No back-transition is allowed between the states. (B) Multistate model Type 2 is a five-state model. The full clinical course of patients with soft tissue sarcoma after curative surgery is specified. T = transition.
Five hundred thirty-nine patients with histologically verified localized soft tissue sarcoma undergoing surgery with curative intent between June 1995 and May 2015 at the Department of Orthopedic Surgery, Medical University of Graz, Graz, Austria, represented the population of this single-center, historical cohort study. Detailed inclusion and exclusion criteria were reported previously [14]. Baseline and outcome data were retrieved retrospectively from our prospectively maintained in-house electronic healthcare database as previously described [6]. Patients who had evidence of distant metastasis at diagnosis (American Joint Committee on Cancer Stage IV) were excluded, even if the (neoadjuvant) treatment goal was cure (n = 13). Furthermore, three patients were excluded because data on baseline histology were missing, and 80 patients were excluded because they did not yet have observed followup time (that is, only baseline data were available at the start of the analysis). All other variables reported in this study were fully observed, and a complete case analysis was performed. Patients who died (n = 24) or were lost to followup (n = 40) during the first year of followup were not excluded from analysis. The study was approved by the local ethics committee before any patient-related activities were performed.
Four hundred forty-three patients were included in the analysis (Table 1). At baseline, the median age of the cohort was 62 years (range, 16–96 years), and the distribution of sex was balanced (female: n = 217; 49%). Most patients had high-grade tumors (Grades 2 and 3: n = 346; 78%), and approximately two-thirds of the cohort had tumors larger than 5 cm (n = 310; 70%) and/or deep tumors (n = 284; 64%).
Table 1.
Baseline characteristics of the study population
| Variable | Overall (n = 443) | No recurrence (n = 344) | Recurrence (n = 99) | p value* |
|---|---|---|---|---|
| Age at entry (years) | 62 (47–74) | 61 (46–73) | 68 (56–75) | 0.026 |
| Female gender | 217 (49%) | 170 (49%) | 47 (48%) | 0.733 |
| Nonextremity location | 84 (19%) | 63 (18%) | 21 (21%) | 0.517 |
| Deep tumor | 284 (64%) | 215 (63%) | 69 (70%) | 0.188 |
| Tumor size > 5 cm | 310 (70%) | 231 (67%) | 79 (80%) | 0.016 |
| Histology | 0.02 | |||
| Liposarcoma | 113 (26%) | 100 (29%) | 13 (13%) | NA |
| Myxofibrosarcoma | 131 (30%) | 98 (29%) | 33 (33%) | NA |
| Leiomyosarcoma | 44 (10%) | 30 (9%) | 14 (14%) | NA |
| Other | 109 (25%) | 80 (23%) | 29 (29%) | NA |
| Synovial sarcoma | 31 (7%) | 26 (8%) | 5 (5%) | NA |
| MPNST | 15 (3%) | 10 (3%) | 5 (5%) | NA |
| Resection margins | 0.656 | |||
| Wide | 399 (90%) | 311 (90%) | 88 (89%) | NA |
| Marginal | 44 (10%) | 33 (10%) | 11 (11%) | NA |
| Intralesional | 0 (0%) | 0 (0%) | 0 (0%) | NA |
| Tumor grade | 0.001 | |||
| 1 | 97 (22%) | 89 (26%) | 8 (8%) | NA |
| 2 | 82 (19%) | 61 (18%) | 21 (21%) | NA |
| 3 | 264 (60%) | 194 (56%) | 70 (71%) | NA |
| AJCC stage | < 0.001 | |||
| I | 98 (22%) | 89 (26%) | 9 (9%) | NA |
| II | 175 (40%) | 140 (41%) | 35 (35%) | NA |
| III | 170 (38%) | 115 (33%) | 55 (56%) | NA |
| Adjuvant chemotherapy | 40 (9%) | 26 (8%) | 14 (14%) | 0.041 |
| Adjuvant radiotherapy | 260 (59%) | 200 (58%) | 60 (61%) | 0.661 |
Distribution overall and by total recurrence status; continuous variables are summarized as medians [25th percentile (Q1) to 75th percentile (Q3)], whereas categorical variables are reported as absolute frequencies and percentages; *p values for difference between nonrecurrence and recurrence group are from Pearson’s chi-square tests (categorical variables with expected cell counts ≥ 5), Fisher’s exact tests (categorical variables with expected cell counts < 5), or Wilcoxon rank-sum tests (continuous variables); MPNST = malignant peripheral nerve sheath tumor; AJCC = American Joint Committee on Cancer; NA = not applicable.
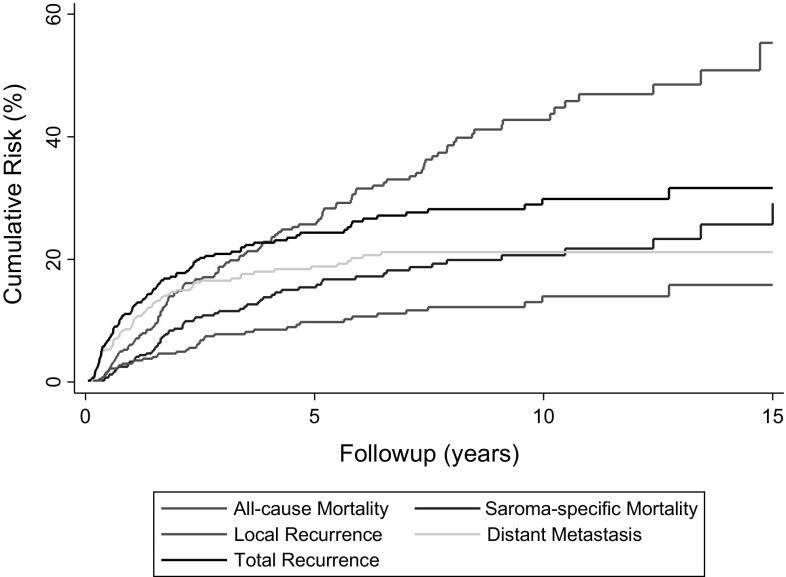
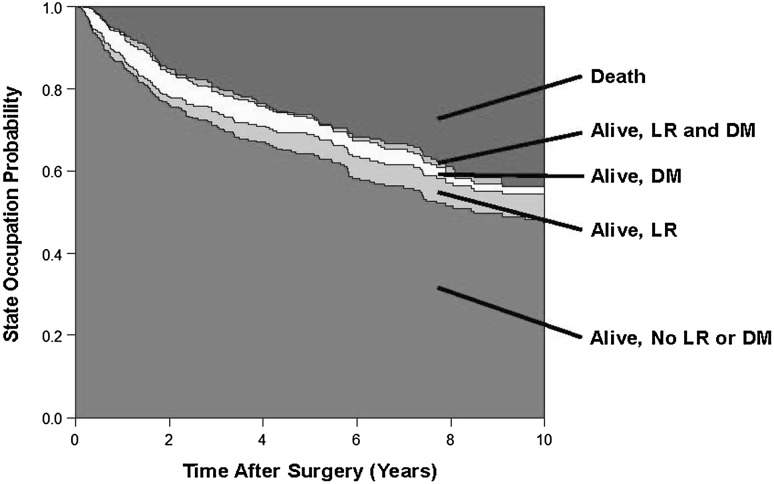
In general, during the period in question, wide resection with a minimal margin of 1 mm (R0) was the preferred surgical treatment approach for all soft tissue sarcoma. This was achieved in 357 (95%) of 377 patients. The remaining 66 patients had Grade 1 liposarcoma (also known as atypical lipomatous tumors) where a marginal resection was considered to be adequate. Adjuvant treatment strategies included radiotherapy (n = 226; 56%), chemotherapy (n = 7; 1.7%), or both (n = 33; 8%). Assessing the influence of aduvant treatments on outcomes in such an observational setting is challenging and was not considered in this analysis as it would require advanced comparative effectiveness methods such as inverse probability of treatment weighting. Our in-house protocol for adjuvant radiotherapy indication has remained consistent since 1998, and encompasses (1) no adjuvant radiotherapy for atypical lipomatous tumors, (2) no adjuvant radiotherapy for other Grade 1 tumors except in case of marginal resection without the possibility of secondary resection, (3) no adjuvant radiotherapy for superficial or epifascial Grade 2 tumors smaller than 5 cm, (4) adjuvant radiotherapy for all deep Grade 2 tumors regardless of size, (5) adjuvant radiotherapy for superficial tumors 5 cm or larger, and (6) adjuvant radiotherapy for all Grade 3 tumors regardless of size and localization. Standard radiation doses were 60 Gy for wide resections and 66 Gy for marginal resections. Adjuvant chemotherapy was indicated only for selected patients whose tumor fulfilled all three criteria of Grade 3 tumor, deep location, and size greater than 5 cm [5]. Median followup for the 443 study patients was 6 years, with 84%, 52%, and 23% of patients being followed for more than 1, 5, and 10 years, respectively. During follow, 41 patients (9%) had local recurrence develop, 74 patients (17%) had distant metastasis develop, 64 patients (15%) died of causes attributed to soft tissue sarcoma, and 59 patients (13%) died of other causes. Overall, this corresponded to 15-year cumulative risks of local recurrence, distant metastasis, overall recurrence, death attributable to soft tissue sarcoma, and death from any cause of 16% (95% CI, 11%–22%), 21% (95% CI, 17%–26%), 32% (95% CI, 26%–38%), 26% (95% CI, 19%–33%), and 55% (95% CI, 44%–67%), respectively (Fig. 2). In contrast to these single-endpoint analyses, the five-state multistate model (Fig. 1A) yielded dynamically changing estimates of state occupation probabilities for each state at any given time after surgery (Fig. 3). These state occupation probabilities also decreased with time as patients changed states. For example, the “distant metastasis” state occupation probabilities at 2, 6, and 10 years after surgery were estimated at 6%, 4%, and 2%, respectively. This decrease with time indicates that patients with distant metastasis intensely transitioned to the death state.
Fig. 2.
The cumulative risks of selected clinical endpoints are shown. Whereas the risk of all-cause mortality was calculated with a 1-Kaplan-Meier estimator, all other endpoints were calculated with competing risk estimators. The y-axis scaling only continues until 60%.
Fig. 3.
The clinical course of patients with soft tissue sarcoma after surgery is shown. The colored boxes represent state occupation probabilities during a 10-year followup. This graph was derived using a stratified hazards model without covariates based on the model design in Fig. 1B. With time, the state occupation probability of State 1 (alive, no recurrence; green) decreases, because patients transition to State 2 (alive, local recurrence; light green), State 3 (alive, distant metastasis; cream), or State 5 (death; red). The state occupation probabilities of the distant metastasis state (State 3) strongly decreases with time, because patients make Transition 7 to the death state (State 5) or Transition 6 to the local recurrence and distant metastasis state (State 4; orange). LR = local recurrence; DM = distant metastasis.
Statistical Analysis
All statistical analyses were performed using Stata® (Windows Version 13.0; Stata Corp, Houston, TX, USA) and R (Windows Version 3.1.1., R Core Team [2014]; The R Foundation for Statistical Computing, Vienna, Austria). Median followup was calculated with the inverse Kaplan-Meier estimator according to Schemper and Smith [12]. The 1-Kaplan-Meier estimator was used for calculating the risk of death from any cause, whereas a competing risk cumulative incidence estimator according to Kalbfleisch and Prentice [7], treating death from any cause as the competing event, was applied for calculating the risks of recurrence endpoints and death attributable to soft tissue sarcoma (Stata® routine stcompet). Multistate analysis was performed with R using the mstate library [2]. To study the effect of recurrence time on mortality, we extended the multistate models by including the time to recurrence as a covariate for Transition 3. In these so-called state arrival extended models, we thus allowed the effect of recurrence to depend on patients’ time that was spent alive and free from recurrence after baseline (the full analysis code is provided on request from the authors). A general model-building framework for multistate analysis, and relationships with competing risk analysis, were discussed in previous studies [2, 11].
Results
Multistate Association of Local Recurrence With Distant Metastasis and Death
Patients who experienced a local recurrence were more likely to have subsequent distant metastasis develop than patients who experienced no such local recurrence (hazard ratio [HR] = 8.4; 95% CI, 4.3–16.5; p < 0.001). Further, multistate analysis revealed that patients who experienced local recurrence also were more likely to die (HR = 3.4; 95% CI, 2.1–5.6; p < 0.001).
Multistate Association of Distant Metastasis, Overall Recurrence, and Death
Patients who had distant metastasis develop experienced a much higher risk of death (HR = 12.6; 95% CI, 8.7–18.3; p < 0.001). Similarly, overall recurrence (that is, local recurrence and/or distant metastasis) was associated with a strong increase in the risk of death (Fig. 1B) (HR = 8.3; 95% CI, 5.8–11.9; p < 0.001). The time of occurrence of distant metastasis did not appear to modify transition intensities toward the death state (state arrival extended multistate models). In detail, with the numbers we had, distant metastasis that occurred later during followup was not associated with a more-beneficial prognosis than distant metastasis occurring early after surgery (HR for 1-year increase in the occurrence of distant metastasis after surgery = 0.9; 95% CI, 0.7–1.1; p = 0.28). Whereas new-onset distant metastasis 1 year after baseline increased the relative risk of death by a factor of 13 (transition HR = 13.4; 95% CI, 9.1–19.6; p < 0.001), distant metastasis after 3 years was still associated with a strong increase in the risk of death (transition HR = 10.5; 95% CI, 6.3–17.5; p < 0.001). Qualitatively similar results were observed for local recurrence (not shown). In a sensitivity analysis, we excluded the 66 patients with well-differentiated Grade 1 liposarcoma. All modeling results prevailed on exclusion of these patients with atypical lipomatous tumors, including the associations between the onset of local recurrence and mortality (HR = 3.6; 95% CI, 2.2–6.0; p < 0.001), local recurrence and distant metastasis (HR = 8.4; 95% CI, 4.3–16.5; p < 0.001), and distant metastasis and mortality (HR = 11.4; 95% CI, 7.8–16.7; p < 0.001), respectively.
Multistate Contribution of Baseline Risk Factors Toward Clinical Outcomes
After adjusting for older age and high tumor grade, the negative effect of recurrence on survival became slightly weaker but prevailed (adjusted HR of recurrence = 5.0; 95% CI, 1.1–23.8; p = 0.04). We then looked at the transition hazards from the recurrence state to the death state. Here, we observed that age was the dominant risk factor for death without recurrence (HR per 1-year increase in age = 1.1; 95% CI, 1.0–1.1; p < 0.001), whereas high-grade histology was not only associated with a higher risk of recurrence (HR = 3.8; 95% CI, 1.8–7.8; p = 0.0003) but also modified transition rates from the recurrence state to the death state (HR = 4.4; 95% CI, 1.1–18.2; p = 0.04). This effect of high tumor grade on postrecurrence survival implies that patients with high-grade tumors not only have a higher risk of recurrence, but also die sooner after recurrence develops than patients with recurrences from lower-grade tumors. With the numbers we had, we could not show associations between a tumor size greater than 5.0 cm and a higher risk of recurrence (transition HR = 1.6; 95% CI, 1.0–2.6; p = 0.08); and a “deep” tumor location and a worse survival after recurrence (transition HR = 1.7; 95% CI, 0.9–3; p = 0.09).
Multistate Prediction of Clinical Outcomes
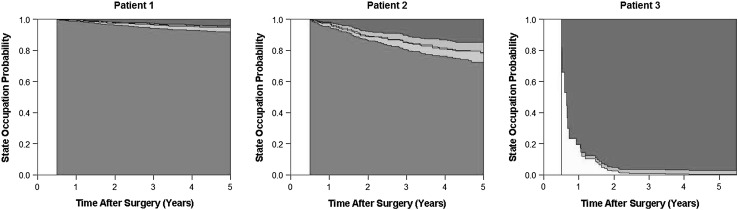
Finally, we used the multistate modeling approach to predict dynamic patient-specific outcomes. To illustrate the concept, we imagined a hypothetical followup at the orthopaedic outpatient department 6 months after surgery and predicted the clinical course for the ensuing 5 years for three archetypical patients: Patient 1 is 45 years old, had a Grade 1 tumor, and is alive and free from recurrence at the followup; Patient 2 is 75 years old, had a Grade 2 tumor, and followup investigations did not reveal any evidence of disease; and Patient 3 is 75 years old, had a Grade 3 tumor, and a followup investigation revealed new-onset pulmonary metastasis. For these three patients, the multistate model identified distinct state occupation probabilities for the 5 years after this clinical visit, leading to individualized joint outcome predictions (Fig. 4).
Fig. 4.
The dynamic prediction of clinical outcomes in patients with soft tissue sarcoma during followup is shown. Patient 1 is 45 years old with a Grade 1 tumor at baseline, and given that there is no evidence of disease at the 6-month followup after surgery, this patient has an excellent predicted prognosis with a low probability to occupy the recurrence states or the death state. Patient 2 is 75 years old and had a Grade 2 tumor at surgery. Although this patient also has no evidence of disease at the 6-month followup, his older age and higher tumor grade contribute to higher risks of transitioning to the death and recurrence states, respectively. Finally, 60-year-old Patient 3 has new evidence of pulmonary metastasis at his 6-month visit, leading to a dramatically increased risk of transitioning to the death state. State 1 (alive, no recurrence) = green; State 2 (alive, local recurrence) = light green; State 3 (alive, distant metastasis) = cream; State 4 (local recurrence and distant metastasis) = orange; State 5 (death) = red. LR = local recurrence; DM = distant metastasis.
Discussion
Patients who undergo surgery with curative intent for localized soft tissue sarcoma are at risk for local recurrence, distant metastasis, and death. Understanding the relationships between baseline risk factors and these outcomes is crucial for improving prognostic stratification and aftercare planning. Multistate models can integrate baseline risk factors and several clinical endpoints into a single statistical model, however, to our knowledge, they have not yet been used to study outcomes in soft tissue sarcoma. In this study, we showed that the complex clinical course of patients with localized soft tissue sarcoma after surgery can be statistically represented with these multistate models. Multistate analysis provided insight into the disease process and the biology of risk factors beyond what can be observed with single-endpoint analyses. Our analyses confirmed the previously reported adverse association between local recurrence and mortality [4, 8] and also provided quantitative estimates of its magnitude. Local recurrences were observed to be a risk factor for the occurrence of distant metastasis. Furthermore, we obtained dynamic and highly personalized outcome predictions for different clinical scenarios in aftercare of patients with soft tissue sarcoma. Collectively, these results suggest that multistate models are a very powerful tool for studying local recurrence, metastasis, and death in patients with soft tissue sarcoma.
There are limitations of this study including that we only addressed a small subset of potential baseline risk factors to illustrate the multistate approach in a concise manner. Important other variables such as histologic subtype of soft tissue sarcoma, disease location, or adjuvant and palliative treatment strategies were not addressed in the current analysis. The risk of recurrence differs among the many histologic subtypes of soft tissue sarcoma, and some of our multistate associations may be weaker or stronger in selected subtypes. Adjuvant treatment strategies (in particular chemotherapy) may have varied during the time of our study, and patients who had distant metastasis develop were treated by different physicians who may have used variable, nonstandardized palliative treatment strategies ranging from pulmonary metastasectomy to systemic chemotherapy to best supportive care only. Further, we included patients with Grade 1 liposarcoma, also known as atypical lipomatous tumors, in this analysis. Many centers do not routinely follow patients with atypical lipomatous tumors after surgery because the risk of distant dissemination of these low-grade tumors is very small. This means that inclusion of patients with atypical lipomatous tumors in the current study led to absolute risks of distant metastasis that slightly underestimate the “true” distant metastasis risk in a population with purely high-grade soft tissue sarcomas. We have addressed this issue in a sensitivity analysis in which we excluded all 66 patients with atypical lipomatous tumors. In this analysis, all major modeling results prevailed, which is consistent with the assumption that our multistate estimates can be generalized to a wide spectrum of soft tissue sarcoma disease phenotypes. Second, our models did not include the possibility of recovery from local recurrence. Extending the models with a bidirectional transition from the “local recurrence” state back to “alive and disease-free” on successful surgical treatment of local recurrence would be an interesting goal for further multistate analyses. Third, the data underlying this analysis were collected retrospectively, which raises concerns regarding information bias. In detail, we may have underestimated “true” event rates because patients who were lost to followup may have had systematically less compliance with aftercare visits on the basis that they may have been more sick and thus may have had a higher risk of recurrence and/or death. Patients who were lost to followup might have had a more-aggressive disease phenotype, so that our analysis would have underestimated “true” event rates. Because they were lost to followup we do not know whether these patients had more- or less-favorable disease. However, with 40 patients being lost to followup during the first year, we believe loss to followup is not excessive in our study for an observational study, because patients are followed up at our institution, migration in the south of Austria is comparably low, an electronic healthcare linkage to nearly all hospitals in our geographic area serving a population of approximately 1 million patients is in place, and the cohort is being repeatedly updated. We have contacted family physicians of patients where loss to followup was suspected. Therefore, although this retrospective analysis has several limitations, loss to followup is, in our opinion, one of the more-minor concerns in this analysis, reflected by the observation that the covariate profile of the patients who were lost to followup does not differ substantially from that of the patients who were not lost to followup (data not shown, but available on request).
After surgery, patients with localized soft tissue sarcoma are at risk for local recurrence, distant metastasis, and death [1, 8]. We studied this risk by fitting two different types of multistate models to a large retrospective, single-center cohort study of patients with localized soft tissue sarcoma undergoing surgery with the intent to cure. We aimed to achieve different objectives with these two models. The first type of model, the so-called unidirectional illness-death model, was used to estimate associations between different outcomes, and to quantify the association between baseline risk factors and clinical outcomes. The second model type was a complex five-state model which we used for prediction. With this model we aimed to comprehensively include baseline risk factors and several outcomes in a joint multistate model to obtain dynamic multistate predictions of outcomes conditional on baseline risk factors and patients’ clinical course during followup. Whereas single-endpoint analyses yield risk estimates that can only increase with time or remain constant, multistate modeling yielded time-updated dynamic risk predictions that also could decrease with time as patients transitioned to other states [2]. For example, this was the case for the local recurrence and distant metastasis state occupation probabilities, which decreased with time as patients died. We then used multistate models to quantify the effect of these intermediate events on mortality and on each other. Here, the occurrence of local recurrence and distant metastasis was a strong adverse risk factor for mortality. While the association between local recurrence and mortality in soft tissue sarcoma has been reported [4, 8] our study quantifies its magnitude of association. Furthermore, local recurrences emerged as a potential risk factor for, or at least was associated with distant metastasis. These results show that multistate models can quantify the magnitude of effect attributable to these intermediate events. Our data further suggest that local recurrences appear to be a risk factor for distant metastasis, which allows us to carefully speculate that measures to improve local control, such as improved (neo-)adjuvant radiotherapy protocols, may result in less systemic dissemination of soft tissue sarcoma. Further, in the era of metastasectomy for selected patients with isolated soft tissue sarcoma lung metastasis, this finding supports the approach of centers that follow patients with local recurrence more intensively with CT of the chest to detect pulmonary metastasis as early as possible where it may still be amenable to surgery.
As expected [1], high tumor grade accelerated the time to recurrence. However, multistate analysis revealed that high tumor grade also modified postrecurrence survival in patients with soft tissue sarcoma. Clinically, this interaction can be interpreted in the sense that recurrences in patients with soft tissue sarcoma with Grades 2 and 3 tumors are more aggressive than recurrences in patients with Grade 1 tumors, and the effect of recurrence on mortality can be attributed, at least to some degree, to an aggressive tumor phenotype associated with a high tumor grade rather than to recurrence. In single-endpoint analysis, age was a strong risk factor for mortality. The multistate model could analyze this association according to its components, which revealed that age was a very strong predictor for death without recurrence (that is, most deaths were caused by factors other than soft tissue sarcoma) rather than for death after recurrence. A recently published nomogram that predicts overall survival in patients with soft tissue sarcoma after curative surgery identified higher age as an important negative prognostic factor [1]. According to our multistate results it is likely that this effect of age on mortality is attributable to the association between a higher age and death without recurrence, that is, so-called “background” mortality from other causes than soft tissue sarcoma. Thus, this overall survival nomogram may assign older patients to a higher soft tissue sarcoma risk category, although their risk of death actually may be not related to soft tissue sarcoma but to competing nonmalignant causes such as cardiovascular morbidity. Given clinicians would base their adjuvant treatment decision on this nomogram, older patients may receive more-aggressive adjuvant treatment for reasons unrelated to soft tissue sarcoma. Our results thus encourage an evaluation of this overall survival nomogram using the multistate model, or the use of the concurrently published nomogram for distant metastasis which does not use age for risk prediction.
By incorporating time to recurrence as a covariate, the multistate model further allowed us to estimate the effect of recurrence time on the risk of death. Contrary to our expectation, this analysis suggested that early and late recurrences had a similarly dismal relative effect on mortality.
Finally, we used our multistate model for prediction. Here, we obtained personalized, joint predictions of multiple outcomes for three different hypothetical patients at 6 months after surgery. We found that the risk of local recurrence and distant metastasis declined with time, which confirms previous work on this issue [9]. This declining conditional recurrence risk with time may harbor equally important or even more important information for long-term prognostication than baseline variables. In addition to these known data, we found that individual patients differed regarding this conditional outcome risk according to their baseline risk factors. This is the main difference (and also advantage) of multistate models compared with standard single-endpoint analyses and traditional prediction models, because multistate predictions not only consider baseline covariates [1], but also incorporate time-dependent information on the period a patient has already spent event-free. Although the predictive analysis of this study must not be understood as a ready-for-clinical-use risk prediction tool (whose development would require more patients, more variables, a robust internal validation procedure, and external validation cohorts) [1], it is a proof-of-concept that multistate analysis may improve risk stratification in patients with soft tissue sarcoma. Multiple clinical applications, from enhanced patient counseling to risk-adapted aftercare schedules, can be conceived for such a dynamic prediction model of soft tissue sarcoma. For example, extrapolation of these results to aftercare could lead to a dynamic, risk-adapted plan in which aftercare is terminated for patients who emerge as a good risk during followup, whereas aftercare continues for patients who have a dynamic multistate prediction of a persisting risk of recurrence and/or death. Such a personalized tool could lead to less radiation exposure and less overdiagnosis in patients of low risk, while patients of high risk may benefit for early detection of recurrence. A study by Eichinger et al. [3] in the field of unprovoked venous thromboembolism gives significant precedent to our work. They developed a multistate prediction model based on clinical variables and biomarkers to dynamically assess which patients may be appropriate candidates for discontinuation of anticoagulation. This shows that a transfer of multistate prediction to the clinic is feasible.
We believe multistate models are useful for dissecting the complex epidemiologic relationship between baseline risk factors and clinical outcomes in patients with soft tissue sarcoma. These models can quantify associations between two or more endpoints and dynamically personalize prediction. Our data thus represent the first proof-of-principle that multistate analysis may improve risk stratification of patients with localized soft tissue sarcoma. If these data can be confirmed in larger studies with a more homogeneous patient mix and treatment protocols, multistate models may be useful for future time-to-event analyses and “precision medicine” approaches in the field of orthopaedic oncology.
Footnotes
Each author certifies that he or she, or a member of his or her immediate family, has no funding or commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research ® editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This work was performed at the Medical University of Graz, Graz, Austria.
A comment to this article is available at http://dx.doi.org/10.1007/s11999-017-5295-8.
References
- 1.Callegaro D, Miceli R, Bonvalot S, Ferguson P, Strauss DC, Levy A, Griffin A, Hayes AJ, Stacchiotti S, Pechoux CL, Smith MJ, Fiore M, Dei Tos AP, Smith HG, Mariani L, Wunder JS, Pollock RE, Casali PG, Gronchi A. Development and external validation of two nomograms to predict overall survival and occurrence of distant metastases in adults after surgical resection of localised soft-tissue sarcomas of the extremities: a retrospective analysis. Lancet Oncol. 2016;17:671–680. doi: 10.1016/S1470-2045(16)00010-3. [DOI] [PubMed] [Google Scholar]
- 2.de Wreede LC, Fiocco M, Putter H. The mstate package for estimation and prediction in non- and semi-parametric multi-state and competing risks models. Comput Methods Programs Biomed. 2010;99:261–274. doi: 10.1016/j.cmpb.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 3.Eichinger S, Heinze G, Kyrle PA. D-dimer levels over time and the risk of recurrent venous thromboembolism: an update of the Vienna prediction model. J Am Heart Assoc. 2014;3:e000467. doi: 10.1161/JAHA.113.000467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gronchi A, Lo Vullo S, Colombo C, Collini P, Stacchiotti S, Mariani L, Fiore M, Casali PG. Extremity soft tissue sarcoma in a series of patients treated at a single institution: local control directly impacts survival. Ann Surg. 2010;251:506–511. doi: 10.1097/SLA.0b013e3181cf87fa. [DOI] [PubMed] [Google Scholar]
- 5.ESMO/European Sarcoma Neteork Working Group. Soft tissue and visceral sarcomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25(suppl 3):iii102-112. [DOI] [PubMed]
- 6.Kainhofer V, Smolle MA, Szkandera J, Liegl-Atzwanger B, Maurer-Ertl W, Gerger A, Riedl J, Leithner A. The width of resection margins influences local recurrence in soft tissue sarcoma patients. Eur J Surg Oncol. 2016;42:899–906. doi: 10.1016/j.ejso.2016.03.026. [DOI] [PubMed] [Google Scholar]
- 7.Kalbfleisch JD, Prentice RL. The Statistical Analysis of Failure Time Data. Hoboken, NJ: John Wiley & Sons Inc; 1980. [Google Scholar]
- 8.Lewis JJ, Leung D, Heslin M, Woodruff JM, Brennan MF. Association of local recurrence with subsequent survival in extremity soft tissue sarcoma. J Clin Oncol. 1997;15:646–652. doi: 10.1200/JCO.1997.15.2.646. [DOI] [PubMed] [Google Scholar]
- 9.Parsons HM, Habermann EB, Tuttle TM, Al-Refaie WB. Conditional survival of extremity soft-tissue sarcoma: results beyond the staging system. Cancer. 2011;117:1055–1060. doi: 10.1002/cncr.25564. [DOI] [PubMed] [Google Scholar]
- 10.Pisters PW, Leung DH, Woodruff J, Shi W, Brennan MF. Analysis of prognostic factors in 1,041 patients with localized soft tissue sarcomas of the extremities. J Clin Oncol. 1996;14:1679–1689. doi: 10.1200/JCO.1996.14.5.1679. [DOI] [PubMed] [Google Scholar]
- 11.Putter H, Fiocco M, Geskus RB. Tutorial in biostatistics: competing risks and multi-state models. Stat Med. 2007;26:2389–2430. doi: 10.1002/sim.2712. [DOI] [PubMed] [Google Scholar]
- 12.Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials. 1996;17:343–346. doi: 10.1016/0197-2456(96)00075-X. [DOI] [PubMed] [Google Scholar]
- 13.Sinha S, Peach AH. Diagnosis and management of soft tissue sarcoma. BMJ. 2010;341:c7170. doi: 10.1136/bmj.c7170. [DOI] [PubMed] [Google Scholar]
- 14.Szkandera J, Gerger A, Liegl-Atzwanger B, Stotz M, Samonigg H, Friesenbichler J, Stojakovic T, Leithner A, Pichler M. The derived neutrophil/lymphocyte ratio predicts poor clinical outcome in soft tissue sarcoma patients. Am J Surg. 2015;210:111–116. doi: 10.1016/j.amjsurg.2014.10.021. [DOI] [PubMed] [Google Scholar]